1. Introduction
Nowadays, food products are chosen by consumers not only based on their nutritive value, but also based on their beneficial effects on health. Therefore, food manufacturers have enriched their products with bioactive food ingredients, such as vitamins, minerals, functional lipids, proteins, probiotics and so on [
1]. Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [
2]. While it is a widely known fact that probiotics have a beneficial influence on the functioning of the gastrointestinal tract, they also have other positive effects on the human body, e.g., they might enhance immunity or decrease cholesterol levels [
3]. The viability of probiotic bacteria can be negatively affected by manufacturing and storage conditions, as well as through their passage in the gastrointestinal tract [
4]. Therefore, delivery systems that are able to protect probiotics against adverse conditions, such as hydrogels, have constantly been developed [
5,
6,
7].
In our previous work, we described hydrogels based on poly-γ-glutamic acid (γ-PGA), which were successfully tested as probiotic-delivery vehicles able to improve the viability of entrapped strains of probiotic bacteria under acidic conditions. Those hydrogels were obtained in a reaction between a γ-PGA biopolymer and poly(ethylene glycol)s (PEGs) cross-linkers [
8]. Poly-γ-glutamic acid, a naturally occurring polymer, is water-soluble, edible, biodegradable and non-toxic toward humans or environment [
9]. Currently, fermentative production of γ-PGA from waste biomass is carried out on an industrial scale [
10]. This biopolyamide can be used in various industrial fields, such as in medicine, pharmaceuticals, cosmetics, food or agriculture [
11]. Moreover, hydrogels based on γ-PGA exhibit properties that make them suitable for medical and pharmaceutical applications [
12,
13].
Previously, different PEGs were selected as cross-linkers due to their availability, relatively low price and widespread applications in the pharmaceutical and food industries [
8]. However, it seemed reasonable to develop fully biobased hydrogels for probiotic oral-delivery systems. Therefore, in our current work, we decided to use sugar alcohols as cross-linkers. Sugar alcohols, a class of polyols, are naturally present in fruits, vegetables and mushrooms. Some of them, such as erythritol, xylitol, mannitol, sorbitol or isomalt, are used in the food industry as additives, which act as sweeteners, stabilizers, anti-caking agents and so on [
14]. Erythritol is produced on an industrial scale from glucose derived from corn or wheat through biological processes. Other sugar alcohols are mostly produced from corresponding mono- and disaccharides by catalytic hydrogenation [
15,
16]. However, alternative routes of obtaining various sugar alcohols, e.g., biotechnological, have constantly been developed. For example, it is known that xylitol could be produced by different yeast strains from various agricultural industry wastes, such as sugar cane bagasse, corncob, wood sawdust or sunflower stalks [
17]. Sorbitol, another sugar alcohol commonly used in the food industry, might be obtained during the fermentation of glucose and fructose by bacteria strains [
18,
19].
In the present work, the γ-PGA-based hydrogels, obtained in a reaction between a γ-PGA biopolymer and selected sugar alcohols as cross-linkers, were tested as probiotic-delivery vehicles. The obtained hydrogels were investigated for their ability to provide entrapped probiotics with proper protection against acidic conditions, as well as improve the viability of probiotic bacteria during freeze drying.
2. Materials and Methods
2.1. Materials
Poly-γ-glutamic acid (γ-PGA), Mw = 200,000–500,000 g/mol, was purchased from FUJIFILM Wako Chemicals Europe GmbH (Neuss, Germany). 4-Dimethylaminopyridine (DMAP), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), phthalate buffer solution (pH = 3.0), erythritol and xylitol were purchased from Acros Organics (Geel, Belgium). Sorbitol was purchased from Fisher Scientific U.K. Ltd. (Loughborough, UK). DMSO, acetone and phosphate buffered saline (PBS, pH = 7.4) were purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland). Dialysis membrane Spectra/Por (MWCO 6000–8000) was purchased from Carl Roth (Karlsruhe, Germany). A mixture of Lactobacillus strains (L. acidophilus, L. casei and L. rhamnosus) was obtained from Holland & Barrett Retail Ltd. (Nuneaton, UK). Streptococcus thermophilus was obtained from the University of Wolverhampton culture collection. Tryptic Soy Agar (TSA) was purchased from Lab M Ltd. (Heywood, UK).
2.2. Synthesis of Hydrogels
Hydrogels were synthesized in a manner similar to the method previously described [
8]. Briefly, the 1.5 g of γ-PGA was placed in a round bottom flask equipped with a magnetic stirring bar and dissolved in 80 mL of DMSO. Then, the remaining reagents were added (mol % was calculated per glutamic acid residue of γ-PGA): sugar alcohol (30 mol %), EDC (30 mol %) and DMAP (10 mol %). The reaction mixture was stirred for 2 h at room temperature. Then, the mixture was precipitated in acetone and centrifuged for 3 min at 8000 rpm. Obtained hydrogels were purified by putting them into dialysis membranes and kept overnight in distilled water at room temperature. Then, swollen hydrogels were lyophilised.
2.3. Nuclear Magnetic Resonance (NMR) Characterisation
Nuclear magnetic resonance (NMR) analyses were performed at room temperature using NMR Varian 300 MHz (Palo Alto, CA, USA). Each spectrum was recorded with 32 scans, a 1.71 s acquisition time and a 10 s relaxation delay. Before the analyses, samples were hydrolyzed in D2O solution of NaOH. Samples of dry hydrogels (15 mg) were put into vials containing 2.5 mL of D2O solution of NaOH (pH 10.0) and mixed at 60 °C for 6 h. Trimethylsilylpropanoic acid (TSP) was used as an internal standard.
2.4. Preliminary Hydrolitic Degradation
Studies of hydrolytic degradation of hydrogels were carried out under laboratory conditions in two aqueous solutions: phthalate buffer (pH = 3.0) and phosphate buffered saline (pH = 7.4). Glass vials containing 150 mg of hydrogel and 10 mL of buffer solution were placed in a thermostatically controlled incubator set at 37 °C. Vials with samples were withdrawn in duplicate from the incubator after 1, 3, 7, 14 and 21 days. For samples containing undissolved hydrogels, prior to lyophilization, fragments of hydrogel were removed by filtration. Lyophilised samples were dissolved in D2O, and NMR analyses were performed.
2.5. Swelling Studies
Samples of dried hydrogels (with mass of 9–15 mg each) were put into 4.0 mL of tested medium: phthalate buffer and phosphate-buffered saline. After 24 h of incubation at room temperature, samples of swollen hydrogels were placed on mesh sheets (Flow-Mesh
TM; Diversified Biotech, Boston, MA, USA) to remove excess of buffer and weighed. Then, hydrogels were lyophilised and dried samples were weighted. Each swelling experiment was carried out in triplicate. Swelling ratio SR (g/g) was calculated according to the formula:
where Ws is the weight of the swollen sample (g) and Wd is the weight of the dry sample (g).
2.6. Probiotic Survival in Acid Environments
The protective effects of the γ-PGA-based hydrogels was investigated under pH = 1.5 using a mixture of three Lactobacillus strains (L. acidophilus, L. casei, and L. rhamnosus) and under pH = 2.0 using Streptococcus thermophilus. Samples of hydrogels (25 mg each) absorbed 0.5 mL of the suspension of probiotic strains in Ringer’s solution. The 0.5 mL of suspension of free cells in Ringer’s solution was used as a control. Control and samples of hydrogels with bacteria were subjected to an acidic solution and incubated at 37 °C. Triplicate samples were withdrawn after 2 and 4 h. Hydrogel samples were put into Ringer’s solution and shaken on a vortex mixer to release the bacteria. A serial dilution of bacteria suspension was prepared up to 10−8; then, 20 μL of each cell suspension was aseptically plated out in duplicate on the TSA plates. Plates were incubated at 37 °C. Then, cell viability was determined by counting the CFU/mL.
2.7. Probiotic Survival after Freeze Drying
The 0.5 mL of suspension of probiotic strains in Ringer’s solution was absorbed by the 25 mg sample of hydrogel at room temperature. The 0.5 mL of suspension of free cells in Ringer’s solution was used as a control. Samples of hydrogels with entrapped cells and suspension of free cells were frozen at −20 °C overnight. Triplicate samples were lyophilised at 0.52 mbar for 48 h. The freeze-dried control was mixed with Ringer’s solution to prepare a bacteria suspension. Freeze-dried samples of hydrogels were put into Ringer’s solution, allowed to swell for 1 h and shaken on a vortex mixer to release the bacteria. A serial dilution of bacteria suspension was prepared up to 10−6; then, 20 μL of each cell suspension was aseptically plated out in duplicate on the TSA plates. Plates were incubated at 37 °C. Then, cell viability was determined by counting the CFU/mL.
2.8. Statistical Analysis
The data were expressed as mean ± standard deviation. Statistically significant differences of the samples were assessed using a Student’s t-test, and p < 0.05 was considered to be statistically significant.
3. Results
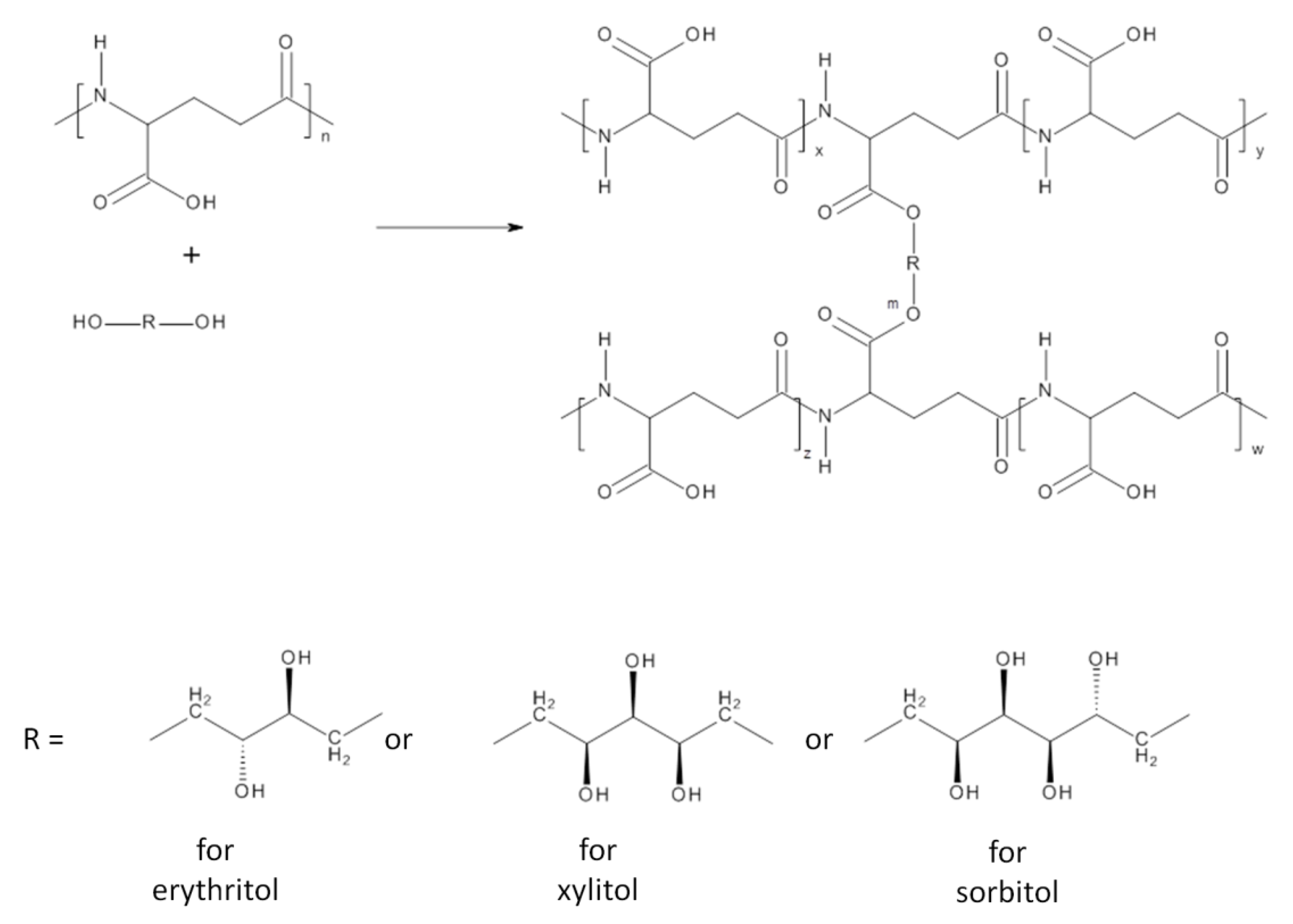
Hydrogels: γ-PGA-erythritol (PGA-E), γ-PGA-xylitol (PGA-X) and γ-PGA-sorbitol (PGA-S) were obtained via an esterification reaction between carboxyl groups present along the γ-PGA chain and hydroxyl groups of the selected sugar alcohol, erythritol, xylitol andsorbitol, respectively (
Scheme 1).
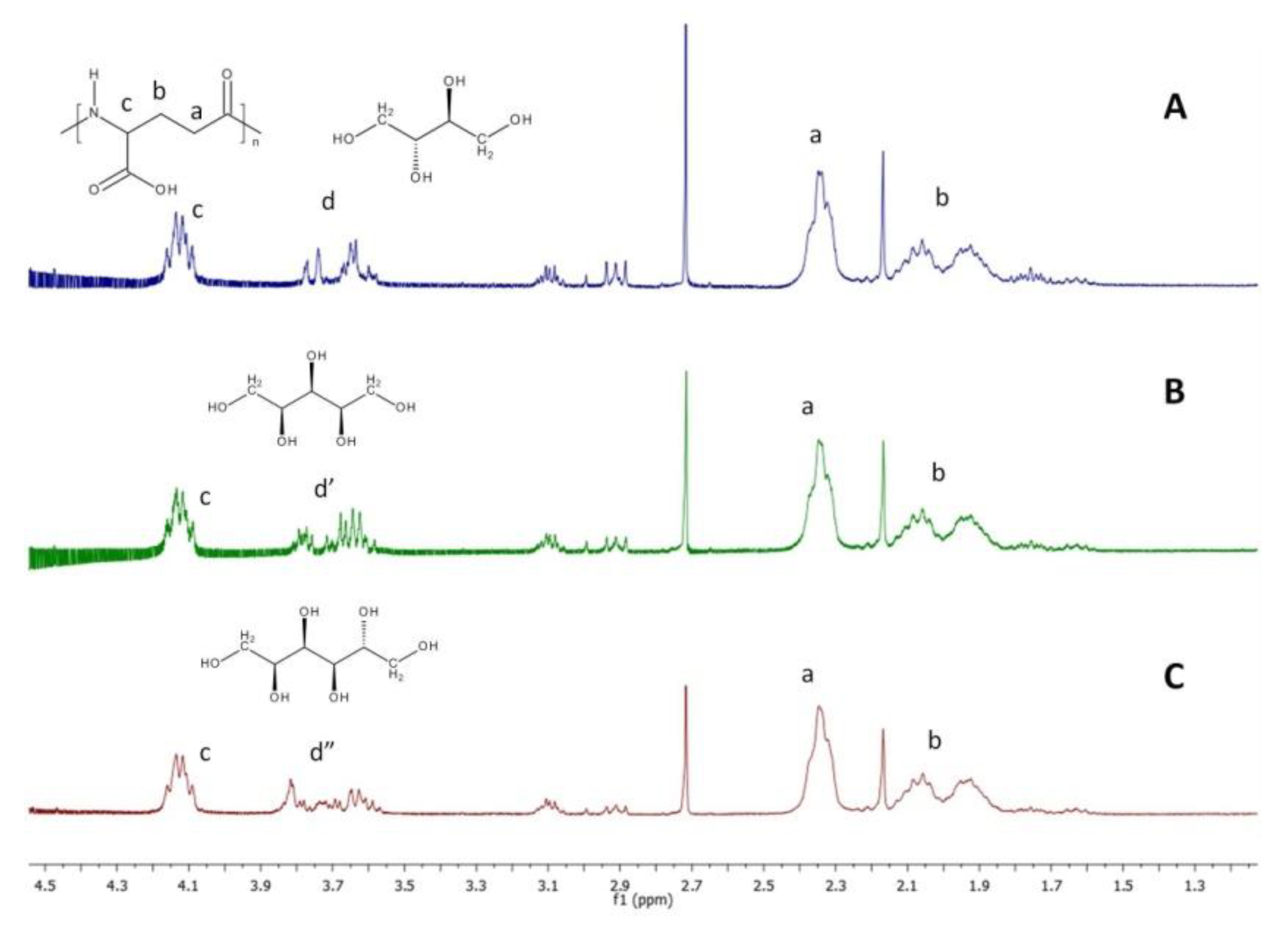
Composition of hydrogels was determined using
1H NMR. In preparing solution for analysis, samples of dried hydrogels were hydrolyzed with a solution of NaOH in D
2O. As a result of contact with the NaOH solution, samples of hydrogels first swelled visibly and then dissolved simultaneously with the progress of the hydrolysis of ester bonds between γ-PGA and sugar alcohol. The
1H NMR spectra of the hydrolyzed samples are presented in
Figure 1.
In each
1H NMR spectra, signals corresponding to protons of γ-PGA chain (signals a–c) are visible. Signals corresponding to –CH– and –CH
2– protons of each sugar-alcohol cross-linker overlap each other and are labeled as d, d’ and d’’ for erythritol, xylitol and sorbitol, respectively. Based on signals corresponding to sugar alcohol protons (signal d, d’ and d’’ in
Figure 1) and the γ-proton of PGA (signal c in
Figure 1), it was calculated that content of the cross-linker was 12 mol % in PGA-E hydrogel and 11 mol % in PGA-X and PGA-S hydrogels.
Preliminary hydrolytic degradation of each hydrogel was carried out at 37 °C for 21 days in two aqueous solutions: phthalate buffer (pH = 3.0) and phosphate buffered saline (pH = 7.4). Samples were withdrawn in duplicates after 1, 3, 7, 14 and 21 days. For each hydrogel, it was observed that samples swelled visibly (tested vials contained hydrogel and solution), and in synchrony with time of the experiment, samples dissolved completely (vials contained only transparent solution) (
Table 1). PGA-E dissolved completely after 21 days at pH 3.0, while at pH 7.4, after 21 days small fragments of swollen hydrogel could be observed. PGA-X dissolved completely after 14 days at pH 3.0 and 21 days at pH 7.4. PGA-S dissolved completely after 21 days at pH 3.0 and at pH 7.4.
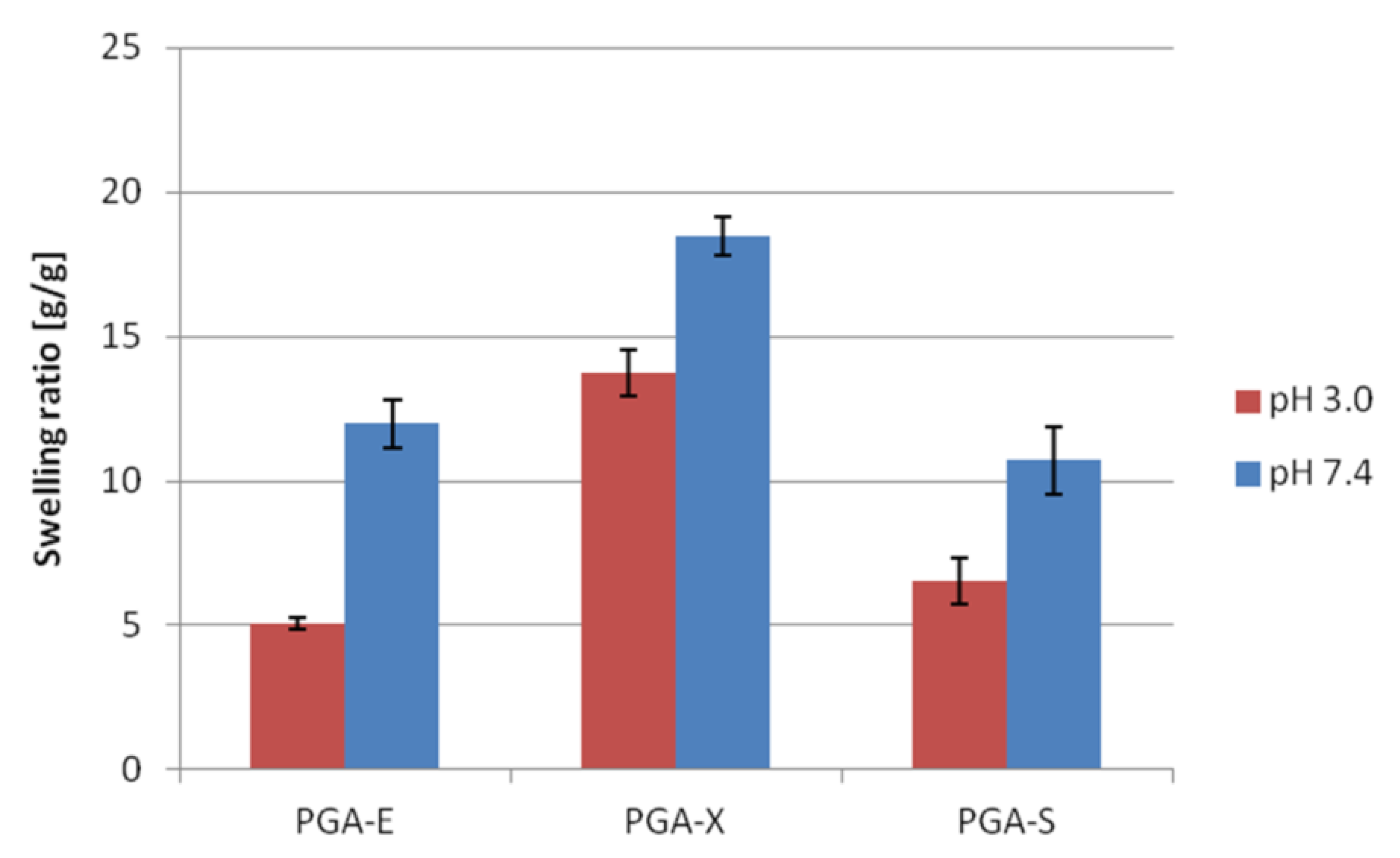
Swelling studies of PGA-E, PGA-X and PGA-S hydrogels were carried out in aqueous solutions at pH = 3.0 (phthalate buffer) and at pH = 7.4 (phosphate buffered saline). For each tested hydrogel, swelling ratio (SR) after soaking in buffer at pH = 7.4 was higher than SR observed after soaking in buffer at pH = 3.0 (
Figure 2). The highest SR in both media was observed for PGA-X (13.74 ± 0.80 at pH = 3.0 and 18.48 ± 0.64 at pH = 7.4).
3.1. Protective Effects of γ-PGA-Based Hydrogels on Probiotic Strains under Low pH
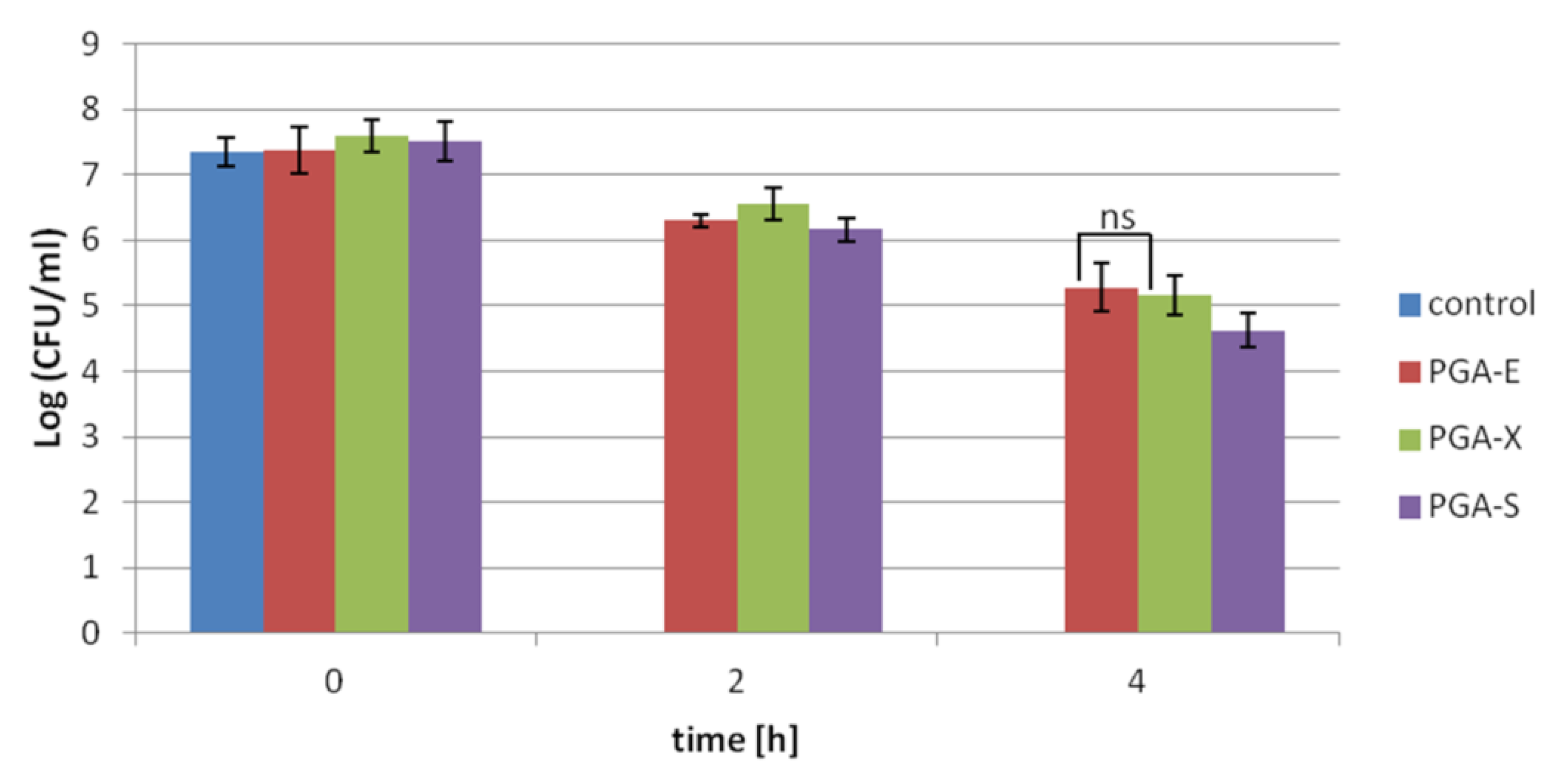
The results of protective effects of γ-PGA-based hydrogels on
Lactobacillus strains subjected to pH 1.5, expressed as Log
10 CFU/mL, is presented in
Figure 3. Control (free cells) and probiotic bacteria entrapped in hydrogel samples were subjected to pH 1.5 up to 4 h. For free cells, total loss in viability was observed after 2 h of incubation under acidic condition. While after 2 h of incubation under these conditions, PGA-E, PGA-X and PGA-S hydrogels allowed the maintenance of probiotic viability at 6.30 ± 0.11, 6.56 ± 0.25 and 6.16 ± 0.20 Log CFU/mL, respectively. After 4 h of the experiment, the viable cell count of the cells entrapped in 25 mg of PGA-E, PGA-X and PGA-S hydrogels further decreased, reaching values of 5.27 ± 0.37, 5.15 ± 0.31 and 4.62 ± 0.26 Log CFU/mL, respectively.
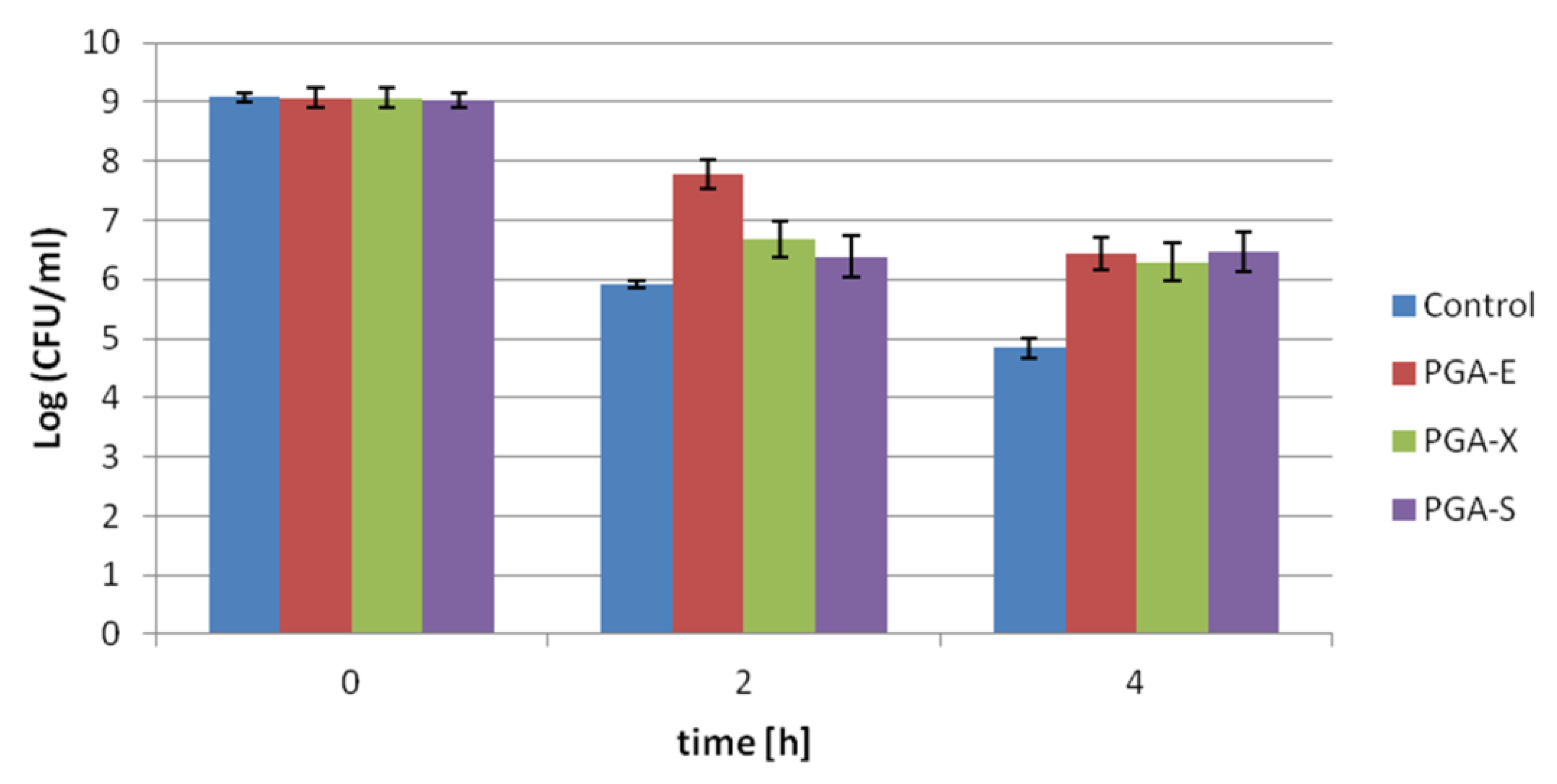
The results of protective effects of γ-PGA-based hydrogels on
Streptococcus thermophilus subjected to pH 2.0, expressed as Log10 CFU/mL, is presented in
Figure 4. Probiotic bacteria entrapped in hydrogel samples, as well as free cells (control), were subjected to acid conditions up to 4 h. After incubation for 2 h, the highest loss in cell viability (3.15 Log CFU/mL) was observed for free cells, while the lowest loss in cell viability (1.28 Log CFU/mL) was recorded for bacteria entrapped in PGA-E hydrogel. After 4 h, for free cells kept in pH 2.0, the decrease in cell number from 9.07 ± 07 Log CFU/mL to 4.84 ± 0.17 Log CFU/mL was observed. In cells protected by hydrogels under the same time of incubation in low pH, less than a 2.8 Log CFU/mL reduction in viable cell count was recorded. PGA-E, PGA-X and PGA-S hydrogels allowed the maintenance of probiotic viability at 6.44 ± 0.27, 6.29 ± 0.33 and 6.47 ± 0.34 Log CFU/mL, respectively. Number of cells entrapped in all tested hydrogels after 4 h in pH 2.0 were comparable and there was no significant difference between PGA-E/PGA-X, PGA-E/PGA-S or PGA-X/PGA-S (
p > 0.05).
3.2. Cryoprotective Effects of γ-PGA-Based Hydrogels on the Viability of Probiotic Bacteria during Freeze Drying
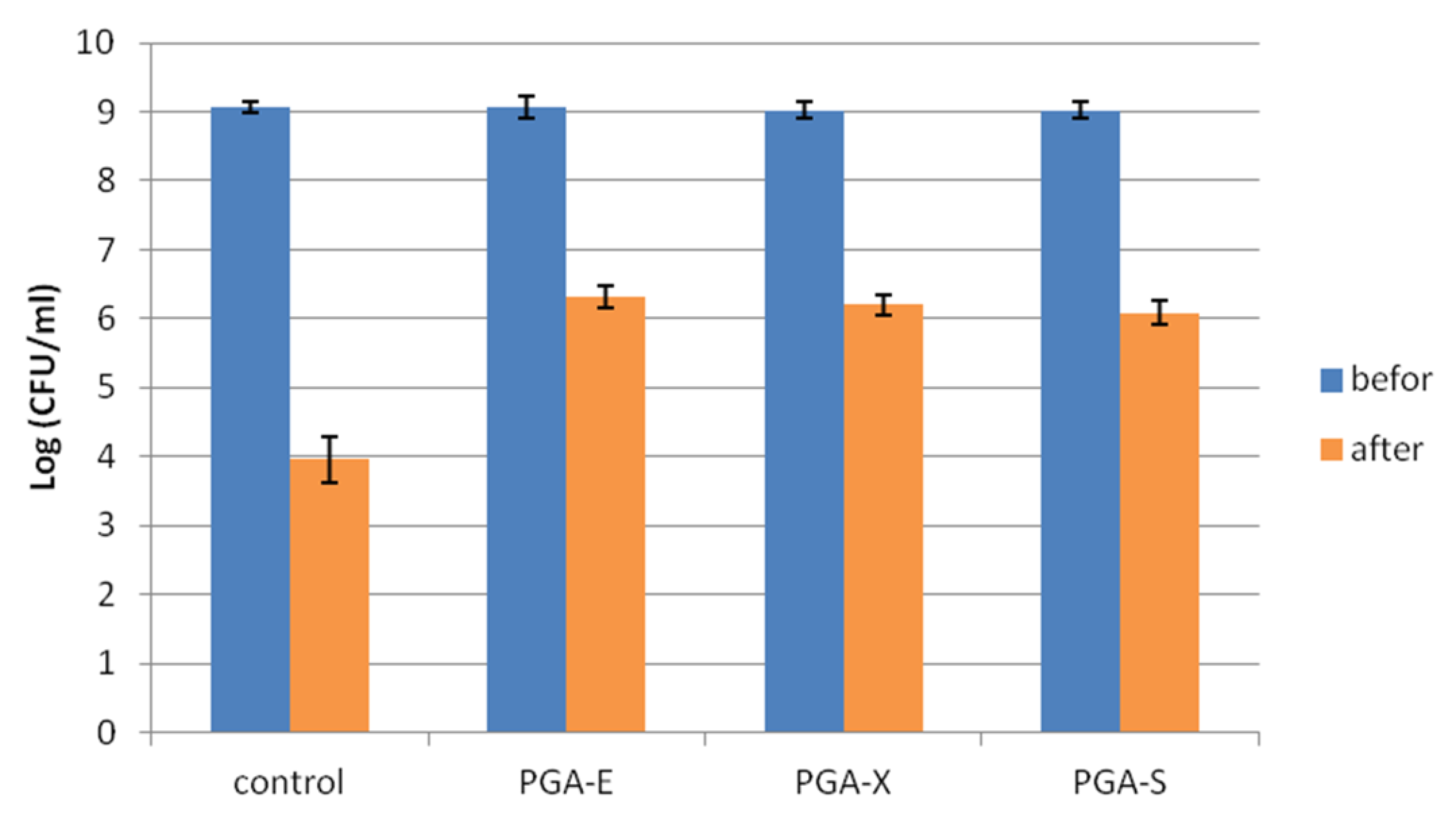
Cryoprotective effects of PGA-E, PGA-X and PGA-S hydrogels on the viability of
Streptococcus thermophilus during freeze drying was investigated, and results are presented in
Figure 5. After freeze drying, the highest loss in cell viability (from 9.07 ± 0.07 Log CFU/mL to 3.96 ± 0.32 Log CFU/mL) was recorded for free cells (control). For probiotic cells entrapped in hydrogels, less than a 3 Log CFU/mL reduction in viable cell count was observed after freeze drying. PGA-E, PGA-X and PGA-S hydrogels allowed the maintenance of probiotic viability at 6.31 ± 0.16, 6.20 ± 0.15 and 6.08 ± 0.18 Log CFU/mL, respectively. There was no significant difference between PGA-E/PGA-X and PGA-X/PGA-S (
p > 0.05).
4. Discussion
Numerous hydrogels have been developed constantly to obtain materials that are able to improve the survivability of entrapped probiotic bacteria under gastric conditions, during heat treatment or storage at various temperatures [
20]. Previously, we have established that γ-PGA-based hydrogels, developed by some of us, were able to improve the viability of entrapped strains of probiotic bacteria during exposure to low pH [
8]. Those outcomes encouraged further research on γ-PGA-based hydrogels. In the present work, we report hydrogels made of a γ-PGA biopolymer and selected sugar alcohol. Sugar alcohols used as food additives and consumed in moderate doses could be beneficial to human health because they act as a prebiotic [
21]. Prebiotics are compounds that support the growth or activity of bacteria that colonize the gastrointestinal tract [
22].
PGA-E, PGA-X and PGA-S hydrogels were obtained in an esterification reaction between a γ-PGA biopolymer and selected sugar alcohol: erythritol, xylitol and sorbitol, respectively. Based on
1H NMR spectra of the hydrolyzed hydrogels samples, it was established that the content of sugar alcohol was similar in each hydrogel (12 mol % in PGA-E and 11 mol % in PGA-X and PGA-S). During swelling studies, it was observed that for each tested hydrogel, the swelling ratio (SR) was lower after soaking in buffer at pH = 3.0 than after soaking at pH = 7.4. Differences in SR values at tested buffers might be associated with the influence of pH on swelling behavior. Swelling behavior of γ-PGA-based hydrogels is associated with repulsive forces between –COO
− groups. At lower pH, the –COO
− groups are protonated, which led to a depressed swelling of the hydrogel [
23]. For probiotic delivery vehicles, low swelling ratio under acidic conditions is desirable since contact between entrapped probiotic cells and acidic medium in the stomach should be limited. Then, a probiotic vehicle subjected to the gradually increasing pH (from pH = 5.5 in the small intestine to about pH = 7.5 in the ileo-colonic region [
24]) would swell so probiotic cells could be released. A preliminary hydrolytic degradation test carried out under laboratory conditions at pH = 3.0 and at pH = 7.4 showed that hydrogels dissolved completely after 14 or even 21 days of experiment. However, under gastrointestinal tract conditions, there are considerably more factors that affect degradation rate. It is known that presence of enzymes accelerate degradation of ester bonds [
25,
26]. Therefore, it can be assumed that in the human gastrointestinal tract, degradation of hydrogels would perform faster.
The protective effects of developed γ-PGA-based hydrogels was investigated under low pH. In the performed experiment,
Lactobacillus strains subjected to pH 1.5 were not able to survive for 2 h (
Figure 3). Under the same time frame, 25 mg of hydrogels made of γ-PGA biopolymer and selected sugar alcohol facilitated in maintaining the viability of the entrapped probiotic cells higher than 6.0 Log CFU/mL. Moreover, even after 4 h of experiment, the viability rate of cells entrapped in PGA-E and PGA-X hydrogels were higher than 5.0 CFU/mL. Notably, in our previous work, the highest viable cell count of the same
Lactobacillus strain cells entrapped in 25 mg of hydrogels made of γ-PGA and PEG, after 4 h of incubation under pH = 1.5, reached values of 2.88 ± 1.86 Log CFU/mL for PEG400 and 3.34 ± 2.61 Log CFU/mL for PEG1000-3arm. It was previously established that increasing the hydrogel amount (from 25 to 50 mg per sample) visibly improved the cell survival rate. The viable cell count of
Lactobacillus cells entrapped in 50 mg of hydrogels made of γ-PGA and PEG, after 4 h of incubation under pH = 1.5, was higher than 4.0 Log CFU/mL (4.16 ± 0.37 Log CFU/mL for PEG400 or 4.29 ± 0.24 Log CFU/mL for PEG1000-3arm) [
8]. Based on the obtained results, it was established that the smaller amount (25 mg) of hydrogels made of γ-PGA and sugar alcohol were able to maintain a higher viable cell count of
Lactobacillus strains subjected to low pH than a larger amount (50 mg) of γ-PGA-PEG hydrogels. As a result, we managed to develop hydrogels (made of γ-PGA and sugar alcohol) that provide better protection than previously reported hydrogels made of γ-PGA and PEG for the same probiotic bacteria subjected to low pH.
It was established that hydrogels made of γ-PGA and sugar alcohol improve the survivability of
Lactobacillus strains subjected to acidic conditions. In the next step, the protective effects of developed hydrogels were investigated towards another probiotic strain sensitive to gastrointestinal tract conditions—
Streptococcus thermophilus [
27]. Free cells of
Streptococcus thermophilus and cells entrapped into PGA-E, PGA-X and PGA-S hydrogels were subjected to pH 2.0 up to 4 h (
Figure 4). After 2 h of experiment, as well as after 4 h, the viability of free cells was lower than the viability of cells protected by hydrogels. Although certain loss in the viability of cells entrapped in all hydrogels was recorded, the number of viable cells ≥6.00 Log CFU/mL after 4 h should be still enough to carry out health benefits on the host [
28]. Based on literature data, it could be explained that developed hydrogels enhanced the cells’ survival rate by providing a physical barrier between entrapped probiotic cells and the harmful environment [
4,
5,
6,
7].
Probiotic bacteria are subjected to freeze drying to prolong their storage time, as well as to reduce costs of transport and storage. Cell dehydration during the freeze drying process may cause damages on the cellular structures; therefore, a decrease in cell viability is commonly observed [
29]. The viability of free cells of
Streptococcus thermophilus visibly decreased during a freeze-drying experiment (
Figure 5). It is known that the negative effects of freeze drying could be overcome by using cryoprotectants, substances such as skim milk, ascorbic acid or mono and disaccharides, which are capable of protecting the cells from membrane injuries [
30]. The γ-PGA has already been successfully tested as a cryoprotectant for probiotic bacteria during freeze drying [
31,
32]. Moreover, it was found that some sugar alcohols, such as xylitol or sorbitol, were able to stabilize the viability of probiotic bacteria during freeze drying, as well as during storage at refrigerated conditions [
33,
34]. Taking that into consideration, it seems reasonable to develop cryoprotectants for probiotics containing both materials, γ-PGA and selected sugar alcohol. Obtained results (
Figure 5) showed that all tested hydrogels were able to improve the viability of entrapped cells during freeze drying in comparison to free cells.