Abstract
Motile aeromonad septicemia (MAS), caused by the Aeromonas species, has been a serious problem in fish health management, particularly in Nile tilapia (Oreochromis niloticus). This study characterized an Aeromonas species isolated from farmed tilapia fingerlings in Binangonan, Rizal, Philippines, and tested for its pathogenicity in tank trials. The isolate, designated as Aeromonas veronii DFR01 (Diseased Fish Rizal), was identified based on 16S rRNA phylogenetic analysis, 16S rRNA homology, and MALDI-TOF mass spectrometry. Its biochemical profile was generated from API and Biolog Gen III systems. A median lethal dose of A. veronii DFR01 was determined to be 107 CFU/mL in tank trials and was utilized as a whole-cell inactivated antigen for oral vaccine development. The immunized tilapia fingerlings produced elevated levels of immunoglobulin M (IgM) in the blood as determined by an enzyme-linked immunosorbent assay (ELISA). There was a significant increase in IgM levels 14 days post-vaccination. A quantitative polymerase chain reaction (qPCR) showed increasing levels of IgM gene expression after vaccination until 38 days of culture. Vaccinated fish showed 25–35% cumulative mortality after the challenge, while non-vaccinated-challenged fish showed 75% mortality. The findings of this research suggest that the fish oral vaccine may prove beneficial for farmed tilapia populations. The vaccine elicited improved immune responses in the fish and resulted in higher survival rates.
1. Introduction
Increasing fish morbidity and mortality associated with motile aeromonad septicemia (MAS) [1,2] have been attributed to various Aeromonas species such as A. hydrophila [1], A. sobria [3], A. veronii [2], and others [2,4]. These bacteria have affected a wide variety of freshwater fish species and occasionally marine fish [5], as well as humans [2,6,7,8]. Several strains of the motile aeromonads, such as A. hydrophila, A. veronii, A. caviae, A. dhakensis, and A. jandei, have been reported from the diseased O. niloticus collected from aquaculture farms in the Philippines [9,10,11]. With the goal to develop a fish oral vaccine against bacterial pathogens of Nile tilapia, an Aeromonas species that caused serious morbidities and mortalities with signs of MAS was isolated and characterized. This study resulted in the development of an oral vaccine against A. veronii with a potential to be used against the other motile aeromonads.
Motile aeromonad septicemia or fatal hemorrhagic septicemia has been identified as one of the major diseases of aquatic organisms, particularly in tilapia [2,12]. The diseases caused by Aeromonas ranged from fatal septicemia to latent infections and has been referred to as hemorrhagic septicemia or MAS [2,12]. The fatal hemorrhagic septicemia has caused high mortality rates in reared O. mossambicus, O. niloticus, and Tilapia zillii [13]. The clinical signs of MAS are furunculosis or external ulcerations, abdominal swelling, and hemorrhagic septicemia [12]. The course of the disease usually progresses in an acute manner.
In aquaculture farms, this pathogen caused major disease outbreaks that resulted in high economic losses. The prolonged use of antibiotics for treatment may lead to the emergence of antimicrobial resistance (AMR) in bacteria. There have been reports of Aeromonas species that are resistant to ampicillin, penicillin, rifampicin, cephalosporin, sulfonamide, and erythromycin [14].
Vaccines are preventive measures to stimulate fish adaptive and innate immune responses. The oral route of immunization is a non-invasive, stress- and cold-chain free method for mass vaccination of fish of all sizes. Most of the oral vaccines against bacteria are currently at their various experimental stages [15,16]. Attempts at oral vaccination of fish against MAS, vibriosis, yersiniosis, and furunculosis have either yielded mild and short-lived or inadequate responses [17]. A major problem in the poor response to oral vaccination is the digestive degradation of antigens in the foregut before the vaccine can reach the immune-responsive areas in the hind gut and other lymphoid organs [16,18]. Several strategies have been investigated to enhance oral vaccination, such as encapsulating antigens to shield them from degradation [16,18] and utilizing A. hydrophila biofilm to induce high antibody titers and protect carps [19,20]. A suspension of V. anguillarum bacterin was coated onto commercial feed pellets for application as vaccine carriers [20].
Studies have shown that fish immunization via oral or mucosal route conferred 80% to 100% protection [21,22], with a higher systemic immune response than other routes [23,24].
The application of nanomaterials (<1000 nm), such as immunostimulating complexes (ISCOMs), polymeric materials (D,L lactide-co-glycolic acid/PLGA), chitosan, and non-degradable nanospheres have been reported as delivery systems for fish oral vaccines [16,25]. These nanoparticles can be targeted for delivery to specific cells, improve bioavailability, enable controlled release, work as adjuvants, and protect vaccines from degradation in the gut when administered orally [26].
A variety of naturally occurring and synthetic polymers are used in the encapsulation of antigens, usually through the formation of covalent bonds. Moreover, nanomaterials have been shown to be more efficient than microparticles, since microparticles vary in size, with variations in production conditions [27]. Several research studies have previously explored the possibility of a naturally occurring phyllosilicate material as a drug carrier due to its special intercalation activity. Drug and phyllosilicate interaction slows down drug release and absorption into the body, which may be beneficial when a controlled release is necessary for proper therapeutic action. In addition, due to the clay minerals’ high specific surface area, adsorption capacity, rheology, chemical inertness, and low toxicity, clay minerals have also been used in many pharmaceutical formulations beneficial to human health [28]. Consequently, an oral vaccine was developed using a phyllosilicate nanomaterial as carrier.
This study isolated and characterized an Aeromonas species from Nile tilapia (Oreochromis niloticus) exhibiting symptoms of fatal motile aeromonad septicemia, determined its pathogenicity, and developed an oral vaccine based on this isolate.
2. Materials and Methods
2.1. Bacterial Isolation, Characterization, and Identification
2.1.1. Isolation
The Aeromonas sp. DFR01 was isolated from Nile tilapia fingerlings from a fish farm in Binangonan, Rizal, using Alkaline Peptone Salt Water (APSW) as the enrichment medium and Starch-Ampicillin Agar [29] as the selective medium. White-to-yellowish-colored colonies, 3 to 5 mm in diameter and amylase-positive (with a clear zone surrounding the colony) were considered presumptive Aeromonas. Nutrient Agar and Glutamine-Starch-Phenol Red (GSP) agar medium (Sigma-Aldrich, St. Louis, MO, USA) were used for the maintenance of the microorganism. A modified GSP medium (without phenol red and starch) supplemented with glucose (1%), peptone (1%), and beef extract (0.1%) was also used to grow the organism.
Basic morphological characterization of the isolate was performed by the Gram staining method using the Hucker staining method [30]; oxidase, catalase, and hanging drop motility tests were conducted on the isolate [31].
2.1.2. Characterization Using API and Biolog
Biochemical characterization was employed using the Analytical Profile Index—API 20E and API 20 NE system (Biomerieux, Craponne, France) following the manufacturer’s instructions. Appropriate incubation temperatures and periods were observed (API 20E strip at 35 °C for 24 h and 20 NE strip at 29 °C for 48 h). The profiles of the organism were compared to the database for identification.
Biolog GEN III microplate (Biolog Inc., Hayward, CA, USA) was also used to generate characteristics of the isolate including utilization of carbon sources, resistance to inhibitory chemicals, growth at pH 5 and 6, and NaCl concentrations of 1%, 4%, and 8%. Positive results were indicated by color development in a 96-well microtiter plate. The metabolic profile of the isolate was compared to the Biolog database for identification using the SIM index, which refers to the similarity of the isolate to the other strains deposited in the Biolog database, and DIST, which refers to the mismatch(es) between the tested isolate and the database pattern. A SIM value of >0.5 gives a strong ID, and DIST should be <5.00 and >2 when compared to the next matched ID (Biolog Microstation™ System/MicroLog User’s Guide).
2.1.3. Molecular Identification (16S rRNA Gene)
The genomic DNA was isolated using a phenol chloroform method. Briefly, the bacterial cells were aseptically weighed, mixed with 200 µL 0.1 N NaOH and boiled to lyse the cells. About 100 µL sterile distilled water and 300 µL chloroform–isoamyl alcohol (24:1) were added. The cell suspension was vortexed, then centrifuged at 13,000 rpm. The pellet was washed with 500 µL 70% ice-cold ethanol and centrifuged at 13,000 rpm to remove salts and small organic molecules, after which ethanol was carefully removed. The resulting DNA pellets were air-dried at 45 °C, re-suspended in DNAse/RNAse free water and boiled at 100 °C. The DNA extract was stored in a −20 °C freezer.
PCRs were run using Promega GoTaq® Flexi DNA polymerase (Promega, Fitchburg, WI, USA), in accordance with the manufacturer’s specifications. The PCR mixtures contained 0.2 µL of 10 mM each dNTP, 1 µL of 25 mM MgCl2, 100–300 ng genomic DNA, 2 µL of 5× GoTaq® Flexi buffer, 0.5 µL of 10 µM of each primer, and 0.05 µL GoTaq® DNA polymerase and water to bring the reaction volume to 20 µL. Cycling conditions were set at 96 °C for 5 min followed by 30 cycles of 96 °C for 1 min, 55 °C for 1 min, and 74 °C for 1 min. The primers used to amplify the 16S rDNA gene were Aero I forward 5′-TAATGGCTCACCAAGGCGACGATCC-3′ and reverse 5′-CGTGCTGGCAACAAAGGACAG-3′ and Aero II forward 5′-CTTCGGGCCTTGCGCGATTGGATA-3′ and reverse 5′-GACGGGCGGTGTGTACAA-3′. The PCR products were checked using electrophoresis through a 1.5% TAE/agarose gel and visualized under UV after ethidium bromide staining.
The amplified 16S rDNA was sent to 1st Base Team (Singapore) for sequencing. Multiple sequence alignments were performed using ClustalW [32]. Basic Local Alignment Search Tool (BLAST) was used to align the sequence with homologous sequence from the Genbank [33].
The final sequence alignment of the 1043 bp 16S rRNA gene was generated using MEGA X version 10.1.7 [34]. Aeromonas schubertii (X60416) was selected as an outgroup. The phylogenetic tree was calculated using the Hasegawa-Kishino-Yano +I+G model, which was selected by jModelTest 2 [35]. The Bayesian Inference (BI) was used to reconstruct the tree using MrBayes v.3.2.7a [36]. Statistical support for each node of the BI tree was based on two Markov Chain Monte Carlo (MCMC) runs (nchains = 4) for 40,000,000 generations with sampling at every 20,000 generations, after discarding 90% of the resulting trees (i.e., the trees below the convergence, with a standard deviation of split frequencies above 0.01) as ‘burn-in’. In addition, Maximum Likelihood (ML) phylogenies were generated using MEGA X version 10.1.7 [34]. The phylogenetic trees were visualized using FigTree.v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/ accessed on 12 July 2024).
2.1.4. MALDI-TOF MS Identification
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (EXS 2600 Zybio) was used to clarify the identity of the bacterial samples according to the manufacturer’s protocol (Zybio, Chongqing, China). Briefly, the spectrometer was calibrated with the Zybio microbiology calibrator solvent. A blank matrix solution (alpha-cyano-hydroxycinnamic acid) was prepared as control. Triplicates of each sample were prepared for analysis.
A direct smear method was used from bacterial colonies from the agar plate culture. Fresh 24 h cultures of bacterial isolates were prepared using this method. The single colony from the culture was smeared on the sample plate. One (1) µL of the matrix solution was dropped onto the bacterial smear and air-dried.
Alternatively, for the extraction method, 10 µL of bacterial cells were placed into a microcentrifuge tube and mixed with 300 µL ultrapure water and 900 µL ethanol. The tube was then centrifuged at 12,000 rpm, and the supernatant was discarded. The pellet was dried at room temperature for 5 min. Following the drying process, 20 µL of formic acid was added to the sample, mixed thoroughly, and incubated at room temperature for 5 min. Subsequently, 20 µL of acetonitrile was added, and the mixture was centrifuged at 12,000 rpm for 2 min. From the resulting supernatant, 1 µL was transferred onto the sample plate and allowed to air-dry. After this, 1 µL of the matrix solution was added and air-dried in preparation for MALDI-TOF MS analysis. For the standard, Escherichia coli (ATCC 25922) was prepared using the same extraction method.
2.2. LD50 Bacterial Challenge Experiment
A total of 370 healthy Nile tilapia fingerlings, with an average body weight of 6 ± 0.5 g, were utilized to assess the pathogenicity of Aeromonas veronii DFR01. These fish were produced at the hatchery of the Binangonan Freshwater Station, Southeast Asian Fisheries Development Center Aquaculture Department (SEAFDEC/AQD). The fingerlings were acclimated for 14 days before the experiment. To ensure that the fish were free from bacteria, ten fish were randomly selected, and their kidneys were aseptically dissected for bacterial isolation. All tested fish were confirmed to be free of bacteria. Subsequently, five different concentrations of A. veronii DFR01 were prepared: 109.8 CFU/fish, 108.8 CFU/fish, 107.8 CFU/fish, 106.8 CFU/fish, and 105.8 CFU/fish. Each group, consisting of twenty fish with three replicates per group, was subjected to intraperitoneal injection with the respective bacterial concentrations. The control group was injected with saline (normal saline solution or NSS). Mortalities and signs of disease were monitored for 15 days. Re-isolation of bacteria from the kidneys of the experimentally infected fish was conducted to verify infection.
2.3. Oral Vaccine Preparation, Vaccination and Challenge Experiment
Aeromonas veronii DFR01 was grown in Nutrient Broth (HiMedia Laboratories, Maharashtra, India) for 24 h at 28 °C. The broth was then used to inoculate GSP (Glutamate Starch Phenol Red Agar; Sigma-Aldrich, St. Louis, MO, USA) agar plates, which were incubated for 24 h at 28 °C. The cells were harvested into sterile tubes, resuspended in phosphate-buffered saline (PBS, pH 7.2), pelleted at 14,000 rpm at 10 °C, and washed with PBS. The other steps in the preparation of the oral vaccine were based on the proprietary oral fish vaccine methods [37]. Briefly, the cells were freeze-dried and inactivated. Inactivated cells were microencapsulated with a phyllosilicate carrier, mixed evenly with the feeds in a biosafety cabinet (BSL2) and air-dried for at least three hours under the UV light to prevent contamination.
A total of 240 healthy tilapia fingerlings were used for the vaccination and challenge experiments. The fish were acclimated in a holding tank for 14 days before the start of the vaccination phase. The fingerlings were then divided into four groups, each containing 20 fish maintained in a 100 L aquarium, with three replicates per group. The first group, called the Vaccinated group (Vaccine), was fed a SEAFDEC/AQD formulated diet (FD) supplemented with the microencapsulated inactivated A. veronii DFR01 at 3.3 × 109 cells per fish for five days at 5% body weight for primary vaccination. The second group, the Carrier group, received FD supplemented with the carrier. The third group, the Bacterial Control, (Non-vaccinated) and the fourth group, the Negative Control (NSS, mock challenge), were both fed FD. A booster vaccination was given after two weeks for another five (5) days. During the interval, the fish were fed with formulated diets.
Fish in the Vaccinated, Carrier, and Bacterial Control groups were intraperitoneally challenged with A. veronii DFR01 30 days post-vaccination. The fingerlings were anesthetized with MS222 (Tricaine methanesulfonate) before injecting with A. veronii DFR01 at 9.2 × 106 CFU/fish. For the Negative Control group, NSS was injected into the fish. Fish were returned to their respective aquaria after injection, and mortality rates were monitored periodically over the following 14 days. Post-mortem fish specimens were collected for bacterial re-isolation from their kidneys. At the end of the experiment, the kidneys of all surviving and freshly dead fish were subjected to bacterial re-isolation using GSP (Glutamate Starch Phenol Red Agar; Sigma-Aldrich, St. Louis, MO, USA). This procedure aimed to determine the efficacy of the vaccination by assessing the presence of A. veronii in the kidney tissues of the fish. The relative percent survival (RPS) rate was calculated as follows [38]:
RPS = [1 − (% vaccinate mortality/% control mortality)] × 100
2.4. IgM Determination by Enzyme-Linked Immunosorbent Assay (ELISA)
The immune response of the experimental fish was confirmed at different time intervals by measuring IgM levels in fish blood sera. Fish serum samples were obtained at three key stages: (a) pre-treatment (prior to administration of vaccine), (b) 14 days post-booster vaccination, and (c) 14 days post-infection. ELISA was used to determine the relative IgM levels of the different treatments. Fish blood sera were diluted tenfold with PBS and added to sulfhydryl-bind strip well plates at a volume of 100 µL/well and blocked with 3% casein. After the casein was washed off, diluted (1:50) tilapia IgM antibody-HRP (C4-HRP Aquatic Diagnostics, Ltd., Scotland, UK) was loaded into the wells and washed off using degassed PBS. The wells were then allowed to color react with the substrate at 100 µL well−1. The color reaction was arrested with 5 M H2SO4, and absorbances were read at 450–490 nm (StatFax 2100 Microplate Reader).
2.5. IgM Gene Expression Analysis by RT-PCR
The IgM gene expression across various tissues in both vaccinated and unvaccinated fish was assessed by extracting total RNA from the spleens of fish that survived the pre-vaccination and vaccination phases, 22 days post-challenge. This extraction was performed using Trizol reagent, following the manufacturer’s guidelines provided by Life Technologies. The RNA template was used to synthesize the first strand of complementary DNA (cDNA) using the RT Maxime premix kit (Intron, Seoul, South Korea), following the manufacturer’s instructions. The reverse transcription (RT) products were utilized for quantitative polymerase chain reaction (qPCR). RT-PCR was conducted using Biotium EVA Green in conjunction with Promega GoTaq®® Flexi DNA polymerase (Promega, Fitchburg, WI, USA), adhering strictly to the manufacturer’s specifications. The primers used for the IgM gene were the forward primer 5′-GCAGCAAGTTTTCTCACAGTA-3′ and the reverse primer 5′-CCTCAAAGGCTCAATCAAGTC-3′, which were expected to amplify a 485-base pair (bp) product. The housekeeping gene β-actin (AY116536.1) was used as an internal control, with the forward primer 5′-CGTGACATCAAGGAGAAGC-3′ and reverse primer 5′-ACATCTGCTGGAAGGTGGAC-3′ designed to yield a 321 bp amplicon [39]. The primer efficiency was 100% and the correlation coefficient (R2) was 0.99. Cycling conditions were set at 96 °C for 5 min, followed by a series of 30 cycles consisting of 1 min at 96 °C, 1 min at 60 °C, and 1 min at 74 °C. Quantitative PCR was performed using the Eco™ Real-Time PCR system (Illumina, San Diego, CA, USA). The fold change was calculated relative to the non-vaccinated control after normalization to β-actin. This was achieved using the formula 2−∆∆Ct, where ∆Ct was defined as (IgM Ct) − (β-actin Ct), and ∆∆Ct was calculated as (∆Ct treated) − (∆Ct control) [40,41].
2.6. Statistical Analyses
The absorbance readings underwent statistical analyses to identify significant differences among the various treatments. Data were evaluated using one-way Analysis of Variance (ANOVA) with GraphPad Prism version 6.01 for Windows, developed by GraphPad Software in La Jolla, California, USA. Additionally, Tukey’s Honest Significant Difference post hoc tests were applied to further explore the results.
3. Results
3.1. Isolation and Biochemical Characterization/Identification
The bacterial isolate was characterized as Gram-negative motile rods, measuring between 3 and 5 μm in length, as observed under the microscope (Figure 1A). Bacterial colonies displayed whitish, rounded morphologies when cultured in the modified GSP medium (Figure 1B). By contrast, smaller colonies with yellowish clearing zones were observed on GSP agar plates (Figure 1C).
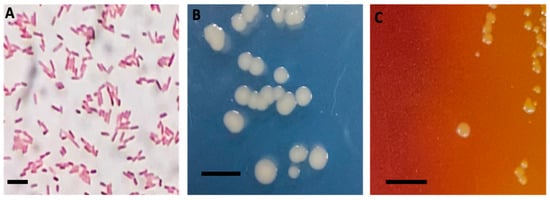
Figure 1.
Cell and colony morphology of A. veronii DFR01. (A) Gram staining image showing Gram-negative rods (bar = 10 microns); (B) Colony morphology in modified GSP agar medium (bar = 10 mm) and (C) in GSP medium (bar = 10 mm).
The biochemical analysis conducted using the API 20E and API 20 NE systems revealed several characteristics of the DFR01 isolate. Notably, it demonstrated the production of β-galactosidase (ONPG), arginine dihydrolase (ADH), lysine decarboxylase (LDC), tryptophanase (IND), and gelatinase. Additionally, DFR01 was capable of acetoin production via the butylene glycol pathway during D-glucose fermentation (VP).
The isolate exhibited fermentation of both glucose and sucrose, as well as the reduction of nitrates to nitrites. It also assimilated a variety of substrates, including mannose, mannitol, N-acetyl-glucosamine, maltose, potassium gluconate, capric acid, malate, and citrate. However, it is worth noting that DFR01 tested negative for ornithine decarboxylase (ODC), hydrogen sulfide (H2S) production, and urease activity (Tables S1 and S2).
When compared to the API database, the biochemical profile of the DFR01 in API 20E showed good identification as Aeromonas hydrophila/caviae/sobria 2 (96.4%) and, in API 20NE, showed very good identification (99.2%) as Aeromonas sobria.
Verification of the identification was also achieved using the Biolog Gen III system, 16S rRNA gene sequencing, and MALDI-TOF MS. Table S3 presents the biochemical profiles of DFR01 as assessed by the Biolog Gen III system. The organism exhibited positive growth on several substrates, including α-D-glucose, D-mannose, D-fructose, D-galactose, glycerol, L-arginine, and L-glutamic acid. DFR01 demonstrated the ability to grow within a pH range of 5 to 6 and showed tolerance to NaCl concentrations of up to 1%. However, it was found to be sensitive to antibiotics, such as vancomycin and rifampicin.
The Biolog Gen III system successfully identified the isolate at the genus level, suggesting A. veronii/sobria as potential species candidates (DNA group 8; SIM 0.429, DIST 4.590).
3.2. Molecular and MALDI-TOF Identification of the Aeromonas DFR01 Isolate
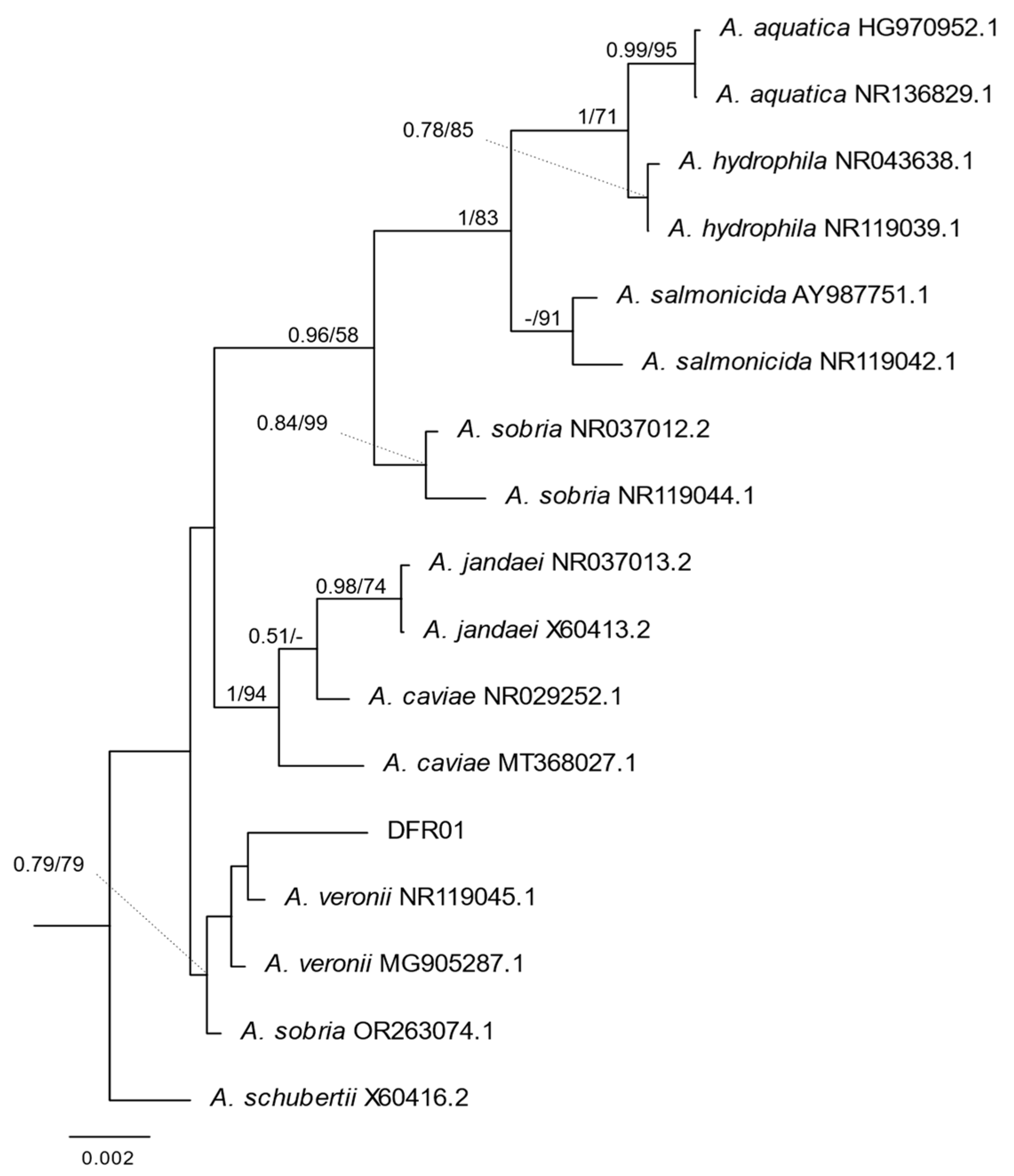
A phylogenetic tree was used to understand the relationship between the DFR01 isolate and representative Aeromonas species. Based on the nucleotide sequence of the 16S rRNA gene, the DFR01 isolate was found to cluster most closely with known strains of the species A. veronii and A. sobria (Figure 2).
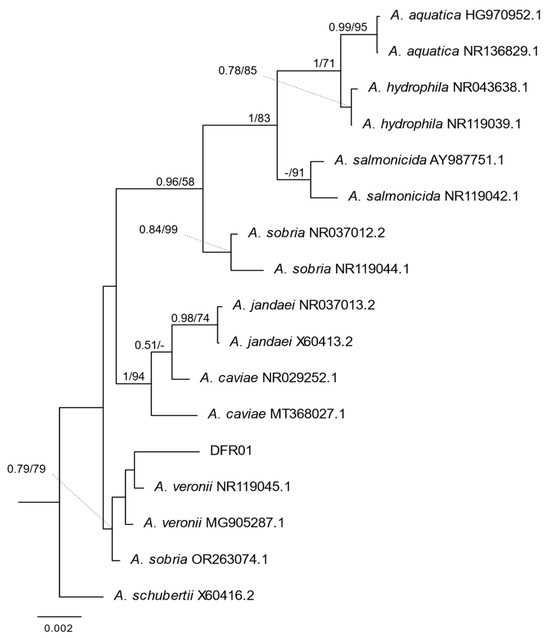
Figure 2.
Bayesian Inference (BI) phylogeny of Aeromonas based on 16S rRNA. Nodal support: posterior probabilities from BI/bootstrap percentage from Maximum Likelihood. Only the posterior probabilities (≥0.5) and bootstrap percentages (≥50) were shown. The scale bar represents nucleotide changes per site.
Based on the 16S rRNA homology using BLAST, DFR01 (JBEHWV000000000.1) showed 99.14% homology with A. veronii strain JCM 7375, A. veronii bv. veronii strain 35624, and 16S ribosomal RNA partial sequence, and 98.67% homology with A. sobria strain JCM 2139, ATCC 43979.
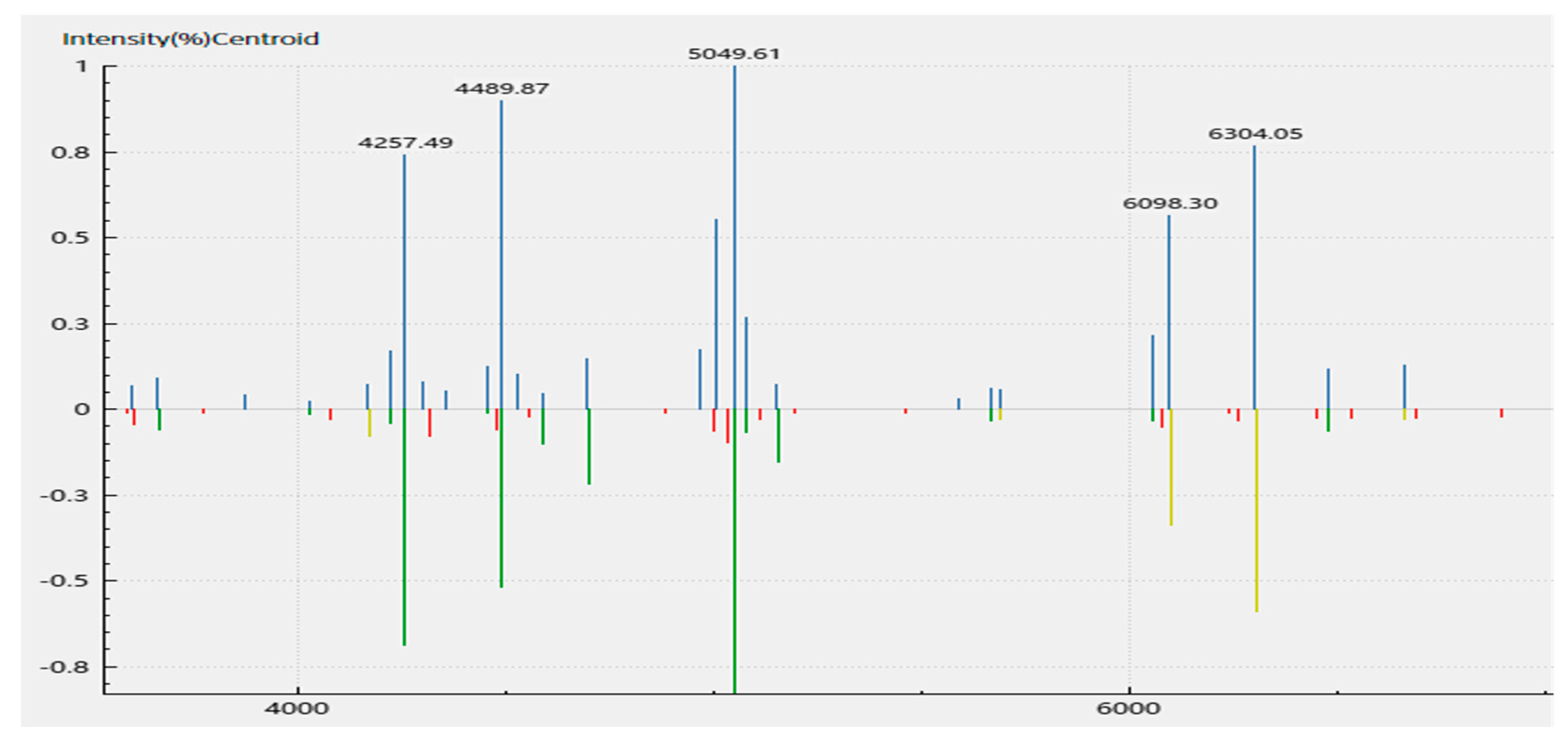
A MALDI-TOF MS analysis of the isolate suggested A. veronii identity based on the cut-off score of above 2.0 generated from the mass-to-charge (m/z) ratio of the ribosomal protein fragments. The scores generated an average identification score of 2.32 ± 0.12 for A. veronii. One of the representative spectra of the peaks of the m/z of the fragments generated is shown (Figure 3, Table S4), with a score of 2.42 for sample S16_2.
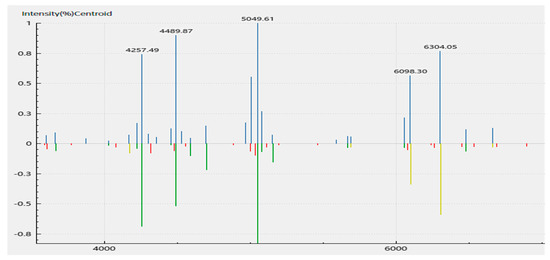
Figure 3.
MALDI-TOF spectrum of the ribosomal protein fragments of the Aeromonas veronii DFR01 mass cultivated in beef extract–peptone–glucose (BPG) broth media. A direct smear method of a bacterial colony was performed. The spectra of six (6) replicates gave an average identification score of 2.32 ± 0.12.
3.3. Experimental Infection
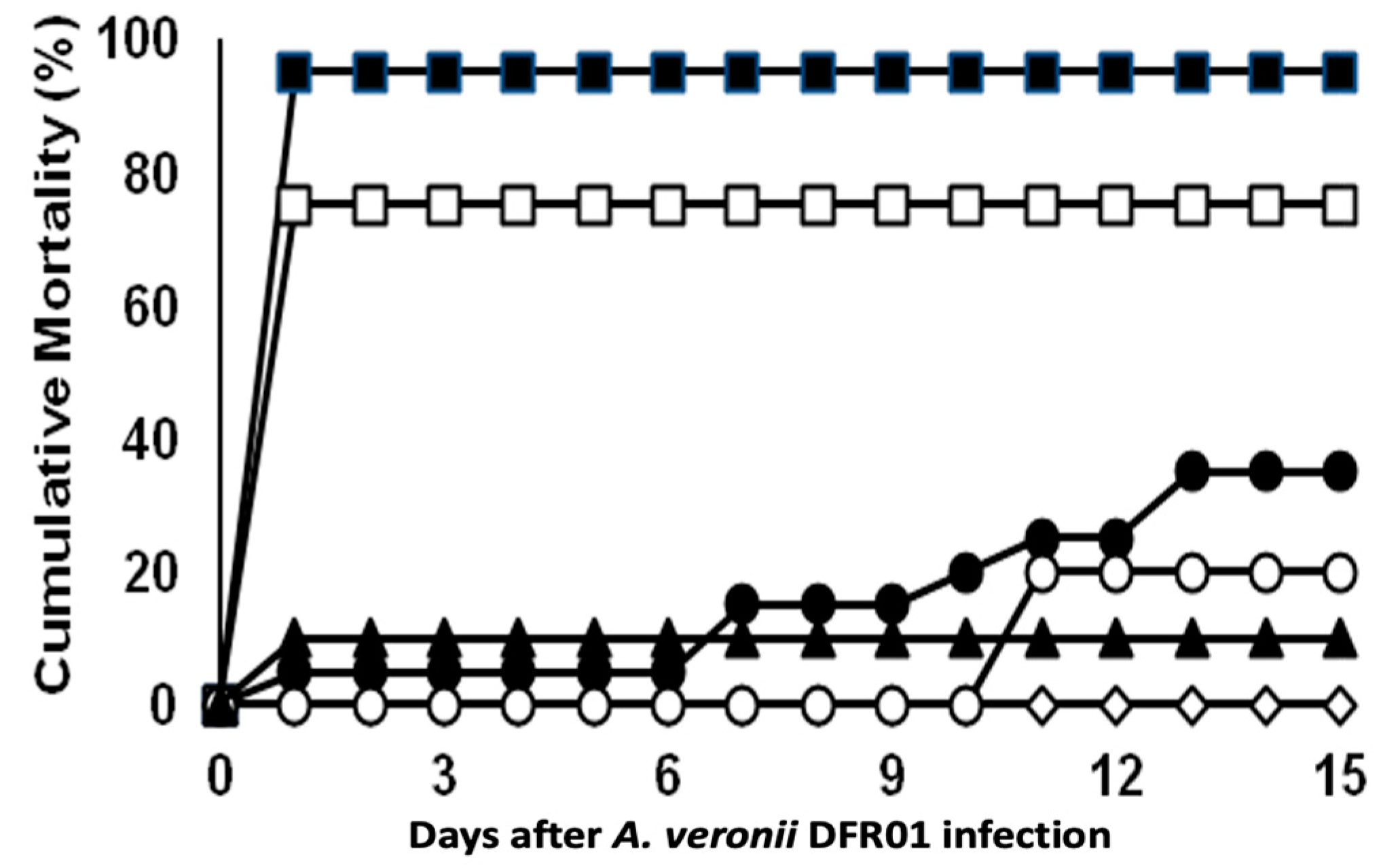
The mortality of fish infected with the DFR01 isolate is shown in Figure 4 and summarized in Table S5. The cumulative mortality rates in the three groups infected with 1 × 109.8, 1 × 108.8 and 1 × 107.8 CFU/fish, were 95%, 75% and 65%, respectively. The other groups infected with 1 × 106.8 and 1 × 105.8 CFU/fish showed 20% and 10% cumulative mortality rates, respectively. The LD50 was calculated as 1.31 × 107 CFU/mL.
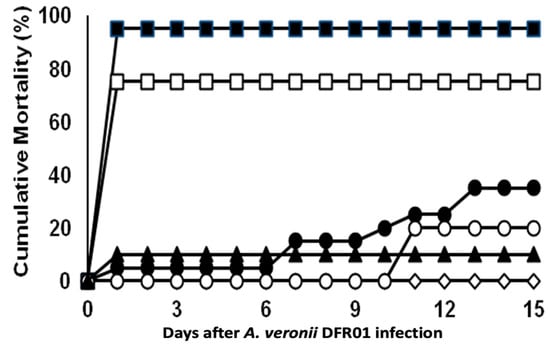
Figure 4.
Cumulative mortalities of tilapia juveniles (ABW: 6 ± 0.5 g; n = 20 fish/inoculum dose) experimentally infected by intraperitoneal injection with A. veronii DFR01. (■) 109.8 CFU/fish; (□) 108.8 CFU/fish; (●) 107.8 CFU/fish; (○) 106.8 CFU/fish; (▲) 105.8 CFU/fish; (◊) control (NSS buffer only).
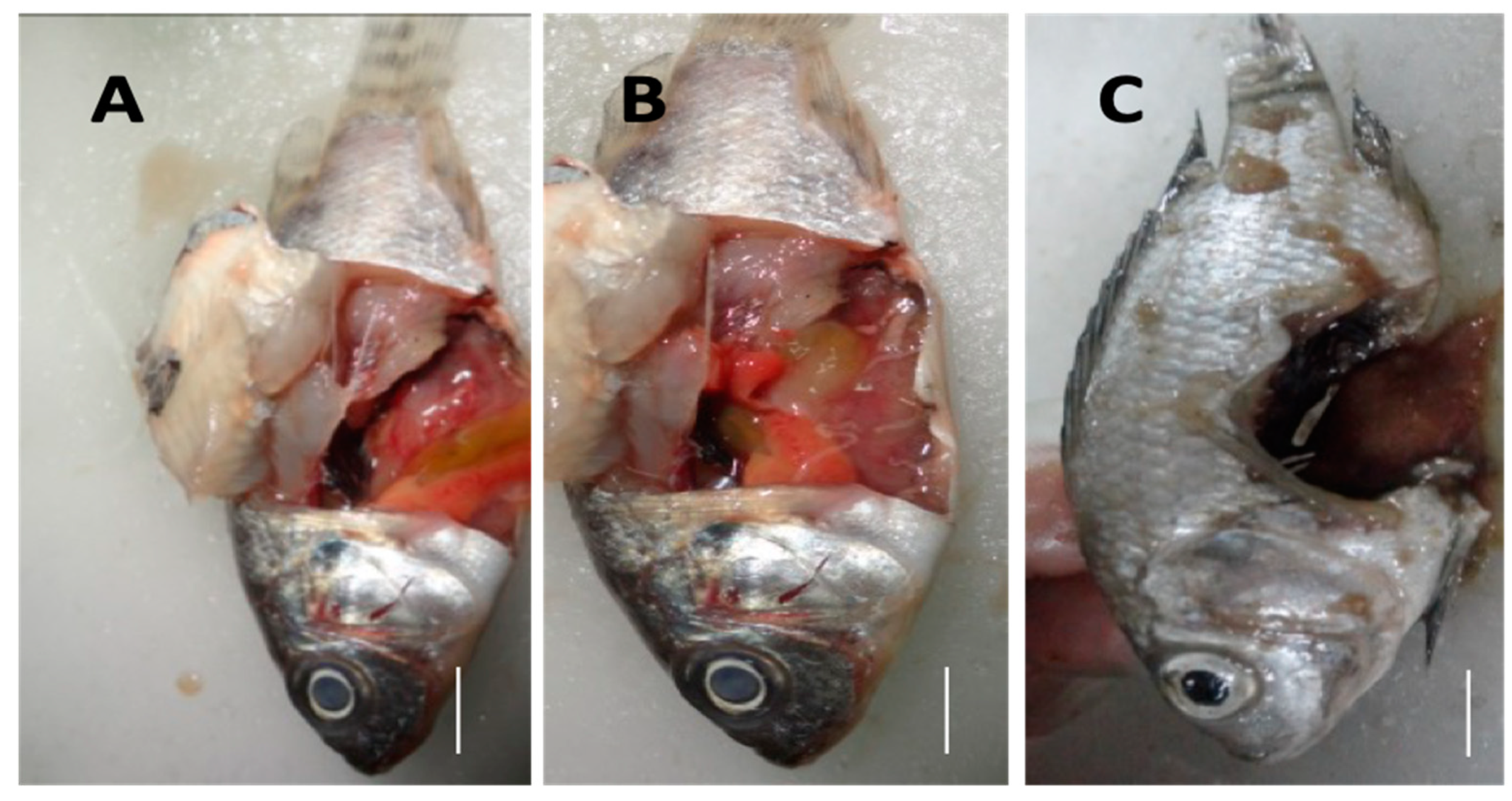
Gross clinical signs of MAS infection were observed, such as discoloration or bleaching of the skin (Figure 5A–D) and hyperemia or blood build up in the vascular tissues of the operculum (Figure 5B,D,E), in the radials of the pectoral fin (Figure 5A,C), and at the base of the dorsal and pelvic fins (Figure 5C). Moreover, swollen abdomen (Figure 5A), corneal opacity (Figure 6A,B), and caudal fin rot (Figure 5A and Figure 6C) were also documented. In severe cases, a ruptured abdominal wall (Figure 6C) was also observed in some diseased fish samples. Gross internal clinical pathologies that were observed include ascites (Figure 6A,B), pale liver with patches of greenish coloration (Figure 6A,B), and bloody fluid in the visceral organs (Figure 6A,B). Infected moribund fish displayed erratic swimming while others became lethargic. Infected fish died within two days post-infection (Figure 6C). Mortalities were seen within 18 h after injection. Bacterial isolates were re-isolated from the infected fish; the re-isolation rate from the kidneys is indicated in Table S5.
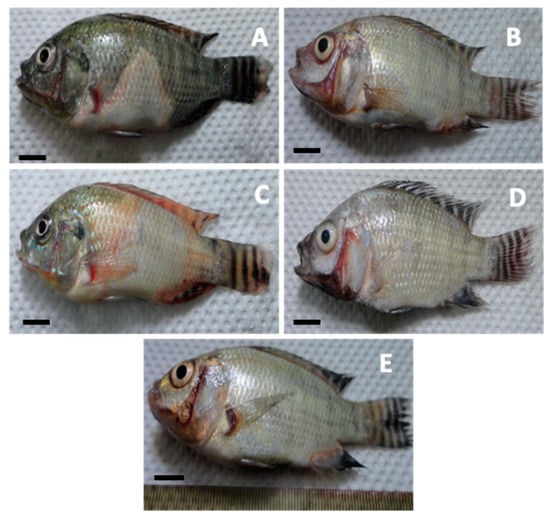
Figure 5.
Gross clinical signs of motile aeromonad septicemia infection observed in diseased Nile tilapia (O. niloticus) infected with A. veronii DFR01 through intraperitoneal injection: (A) discolored or bleached skin at the abdominal area, (B) depressed abdominal cavity and caudal fin rot, (C) hyperemia at the bases of pectoral and dorsal fins, (D) hemorrhagic areas in the operculum and loss of scales around the base of the dorsal fins, and (E) skin lesions at the base of the pelvic fins (bar = 1 cm).
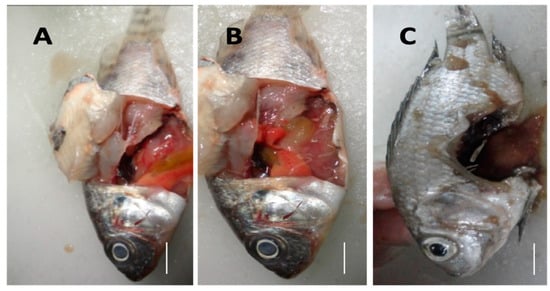
Figure 6.
The internal clinical signs of MAS observed at post-mortem included pale liver, greenish coloration of the liver with hemorrhagic spots, and occurrence of ascites are shown (A,B). Corneal opacity was also observed in the diseased fish (A,B). A ruptured abdominal wall with dark-colored exudate is shown (C).
3.4. Efficacy of Vaccine
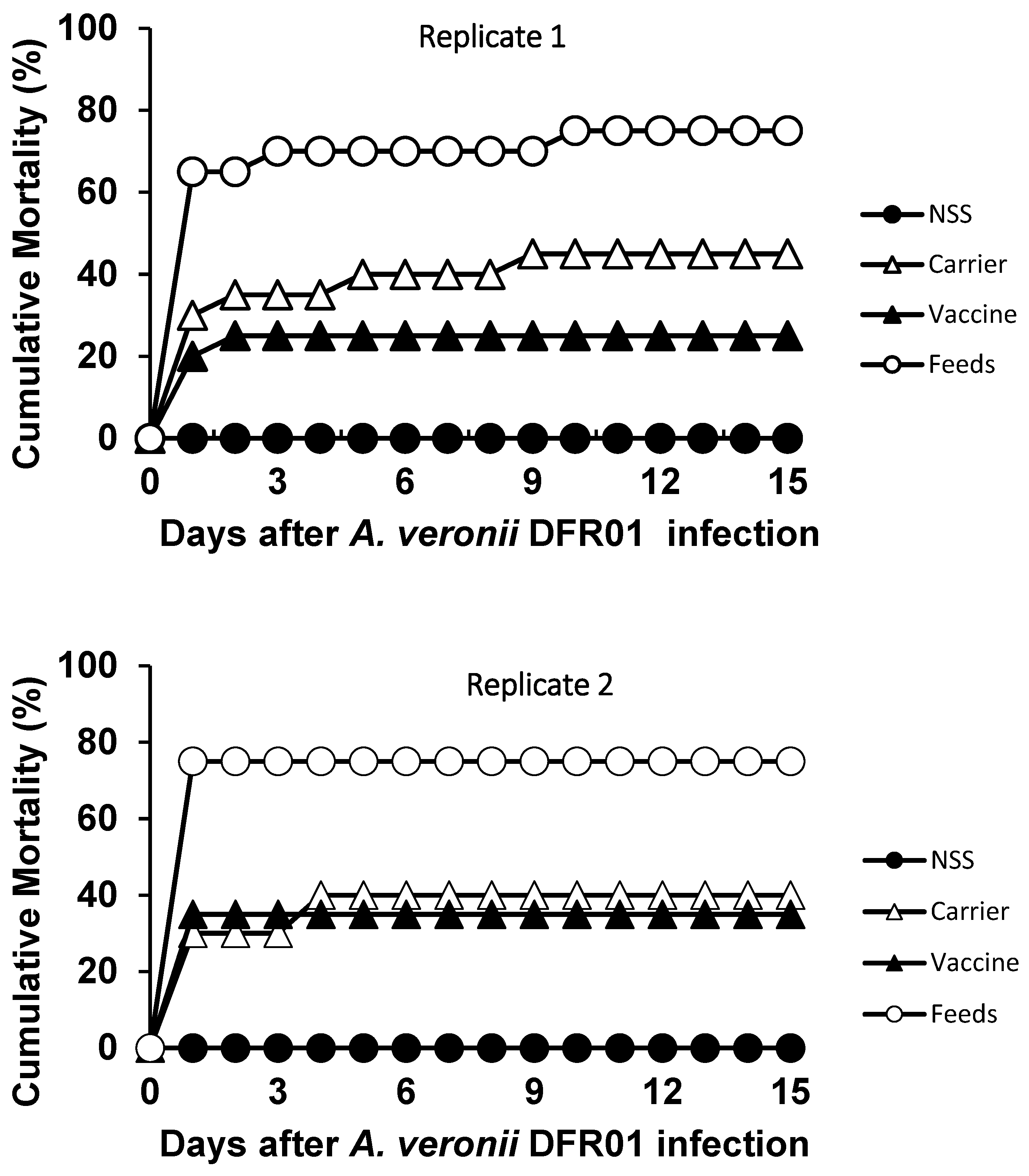
After the bacterial challenge, the mortality rates were lower in the vaccinated groups compared to the control groups. The relative percent survival (RPS) rate was 67% (Replicate 1) and 53% (Replicate 2) for the vaccinated groups (Table 1, Figure 7). The cumulative mortality of the vaccine group was only 25%. All the post-mortem fish specimens tested were found positive for the organism upon bacterial re-isolation. This bacterium was also detected from the challenged surviving fish. Based on the Koch’s postulate, the re-isolation of the bacteria from the pathogenicity tests confirmed that Aeromonas DFR01 was the causative agent of the motile aeromonad septicemia (Table 1).

Table 1.
Mortality rate, relative percent survival (RPS) rate, and bacterial re-isolation data from A. veronii DFR01-challenged tilapia at different treatments: Carrier, Vaccine, and Control (challenged and mock challenged) in two replicates.
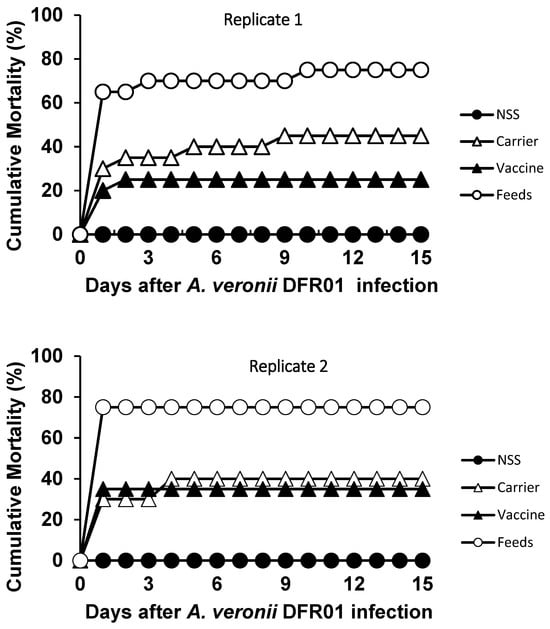
Figure 7.
Cumulative mortalities of the different groups of tilapia juveniles intraperitoneally challenged with A. veronii DFR01 at a dose of 107 CFU/mL for replicates 1 and 2. Carrier group: 45% and 40%; Vaccine group: 25% and 35%; Control (Non-vaccinated)—challenged group: 75%; Control (NSS)—mock challenged: 0%.
At the end of the challenge test, all the unvaccinated survivors displayed clinical signs of MAS disease such as skin discoloration, abnormal swimming behavior, and other clinical signs. However, none of the vaccinated fish challenged exhibited any clinical signs.
3.5. Antibody Response by ELISA and Real Time PCR
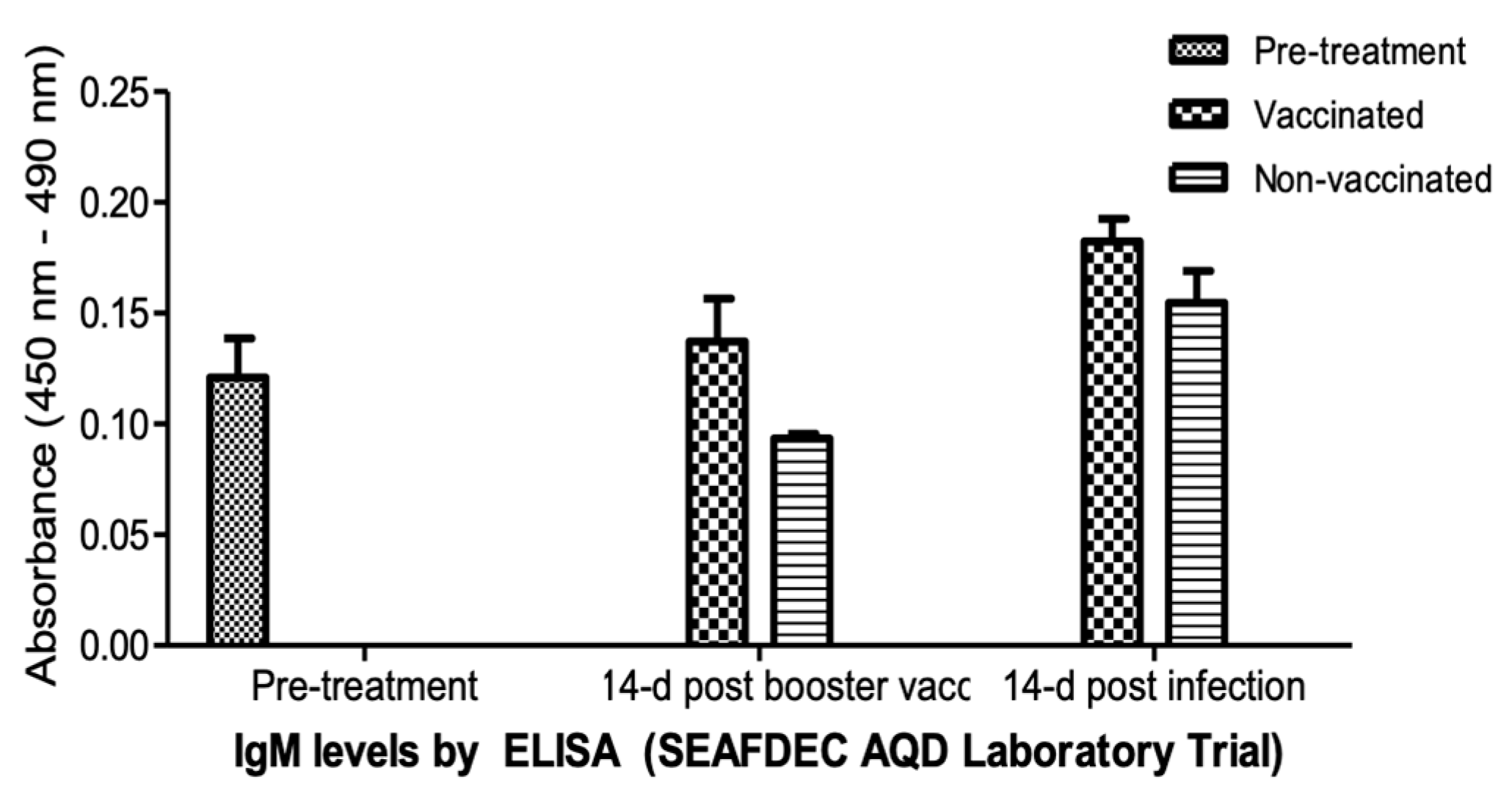
An ELISA was used to determine the level of systemic IgM antibodies at pre-vaccination (pre-treatment), 14 days post-booster vaccination, and 14 days post-infection, as measured by optical density at 450–490 nm (Figure 8). The vaccinated fingerlings showed elevated levels of circulating IgM in the blood. A significant increase in IgM levels (p < 0.05) was observed 14 days post-booster vaccination, and the levels continued to increase for two weeks after infection. By contrast, the IgM levels of the non-vaccinated fingerlings were lower.

Figure 8.
IgM levels of the experimental O. niloticus samples during the laboratory trial of the vaccine, detected by ELISA.
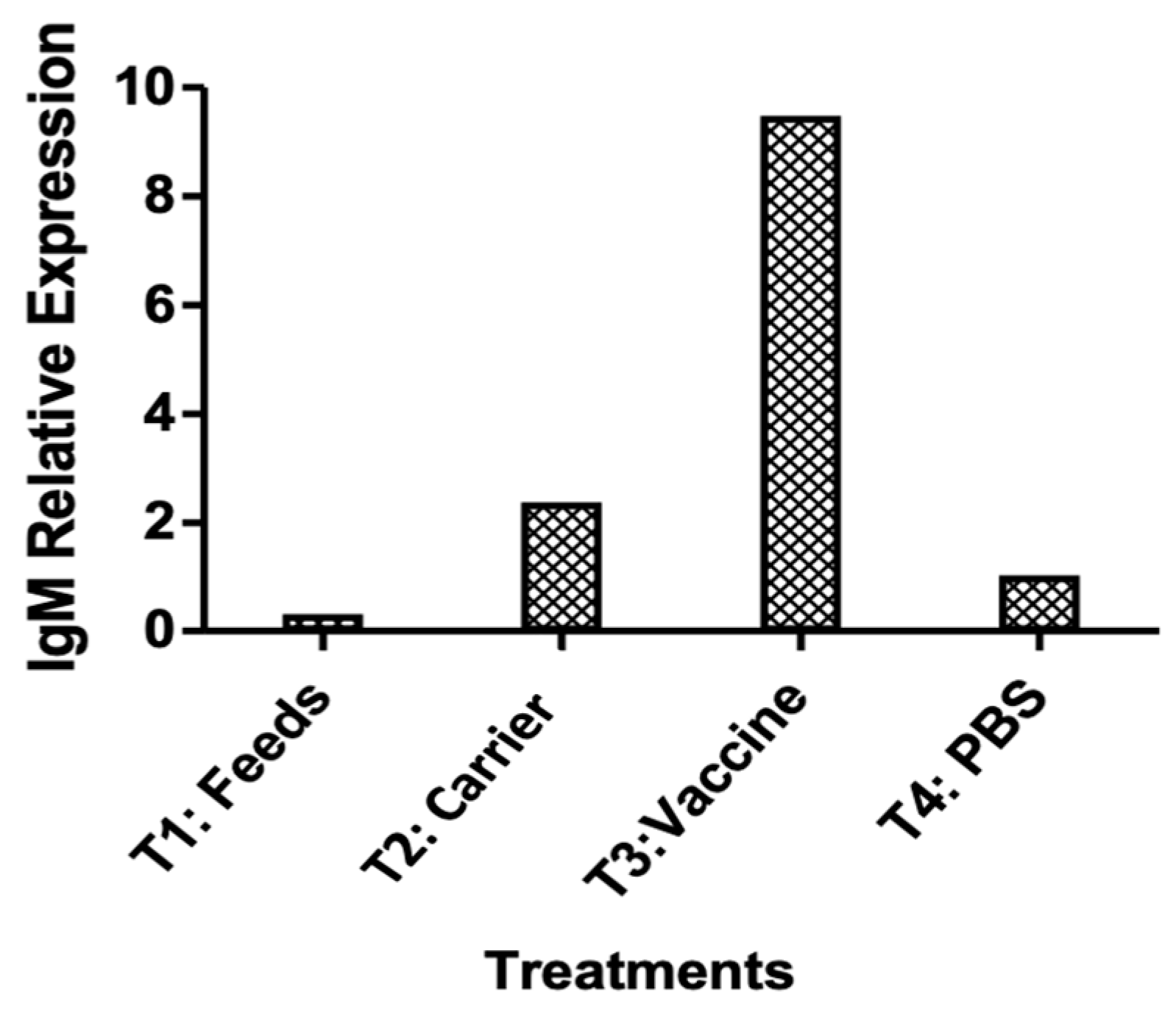
Based on RT-PCR analysis, the oral immunization effectively increased the IgM levels, which conferred protection against bacterial challenge (Figure 9).
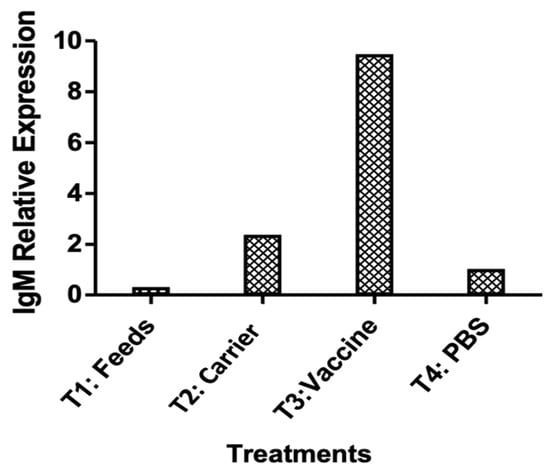
Figure 9.
qRT-PCR relative IgM expression analysis in pre-vaccinated and vaccinated survivors. Gene expression in pooled spleen samples was normalized to β-actin and calibrated to the pre-vaccinated group.
4. Discussion
An important step in the successful development of the vaccine was the isolation and characterization of A. veronii from tilapia fingerlings, which experienced high mortality following transport to the laboratory. This strategy could effectively isolate bacterial pathogens in fish from nursery cultures, which may lead to fatal septicemia (MAS) infections in grow-out farms. This study was benchmarked against reports concerning A. hydrophila, where a vaccine was initially documented to provide significant protection, achieving a relative percentage survival (RPS) of 53–61% [42]. Notably, we successfully isolated and characterized a strain of A. veronii, which exhibited high mortality rates in fish following challenge exposure.
The conventional techniques employed to identify bacteria, including morphological, biochemical, and molecular methods, proved insufficient for identifying Aeromonas at the species level. Aeromonas spp. have been recognized as emerging pathogenic species, and their taxonomy continues to change. For example, the A. veronii biogroup sobria was formerly assigned as A. sobria [43]. Aeromonas veronii was proposed as a separate species due to its ornithine decarboxylase-positive trait, which distinguishes it from the other aeromonads [43,44]. Our MALDI-TOF analysis was consistent with the identification of A. veronii. The negative ornithine decarboxylase activity of the isolate indicated that it belonged to A. veronii biovar sobria, which is a characteristic feature of the DNA/DNA hybridization group or cluster. However, the biochemical characteristics of the DFR01 isolate were not entirely consistent with those of the A. veronii type strain ATCC 25604 [44], which has a positive reaction to ornithine decarboxylase and negative reaction to arginine dihydrolase.
Upon re-isolating A. veronii DFR01 after an experimental infection, we obtained samples from both the water and the tissues of diseased fish, including the kidney, liver, and spleen. The combined biochemical properties of these isolates provided strong evidence that motile aeromonad septicemia was indeed caused by this particular strain.
The proposed identification of the isolate as A. veronii was based on 16S rRNA phylogenetic analysis, homology assessments, and MALDI-TOF MS. The MALDI-TOF analysis produced a profile consistent with A. veronii, based on the sizes of the ribosomal protein fragments generated, which were compared against a comprehensive database. However, API 20 E, API 20 NE and Biolog Gen III assays did not give a definite identification to the species level. Using biochemical techniques to identify motile Aeromonas isolates at the genospecies level is imprecise because of the absence of a sharp dividing line between species [45]. For example, difficulties in trying to separate A. caviae from A. hydrophila using biochemical tests have been encountered.
Although a positive ornithine decarboxylase may be a key characteristic of the species [7,46] and DFR01 showed negative results for this enzyme, it is still possible that the isolate is A. veronii. The isolate was submitted for whole-genome sequencing for definite identification and for the mining and analyses of genes relevant to its pathogenicity.
When intramuscular challenge was used, A. hydrophila isolates were found to be virulent; however, mortality never exceeded 60% when intraperitoneal injection was used. This suggests that the strains studied lacked the protective specific surface characteristics as previously described by other authors [47,48,49] for highly virulent strains. In this way, the peritoneal phagocytic barrier and natural host defenses were overcome, permitting easier tissue invasion. It is possible that known virulence factors of A. hydrophila for warm-blooded animals may not be attributable to its fish pathogenicity, as previously suggested [50], and a different mechanism of virulence may be involved in the invasion of the bacteria in poikilothermic and homoeothermic hosts [45].
Increased IgM levels in the blood after immunization with the oral vaccine containing inactivated A. veronii DFR01 were associated with lower mortalities after the bacterial challenge. The rise in antibody levels observed two weeks following the administration of the booster may indicate an activation of systemic immunity. In addition, the elevated IgM levels recorded 14 days after the challenge suggest that a significant number of the live DFR01 cells injected contributed to an enhanced and sustained immune response. These findings are supported by the IgM gene expression analyses conducted on spleen samples from the immunized survivors. It is also noteworthy that a study involving a non-virulent strain, A. veronii, when used as a live vaccine showed 95% RPS in Nile tilapia [51]. On the other hand, the hybrid red tilapia that received an oral vaccine containing formalin-fixed A. hydrophila (with FIA) exhibited a higher IgM level compared to the unvaccinated group following a bacterial challenge at the 96 h mark [1]. However, vaccine efficacy may not necessarily be correlated with serological analysis, as when Nile tilapia immunized with Flavobacterium columnare oral and parenteral vaccines produced high absorbance values in ELISA for circulating antibodies but failed to provide effective protection after vaccination and challenge [17].
Blood chemistry profiles can reveal adverse effects of vaccine treatments, but analyses of ions, liver enzymes, and proteins show that the treated fish have healthy blood chemistry profiles (Table S6). These values demonstrated that the inactivated or killed bacteria encapsulated with the naturally occurring phyllosilicate material were safe for delivery.
After an intraperitoneal injection of the pathogen, survival rates of immunized fingerlings were significantly higher than those of non-immunized fingerlings. Given the vaccine’s exposure to the acidic environment of the fish’s stomach, the increase in the relative percent survival of the vaccinated group may be attributed to successful encapsulation of the antigen and release in immune relevant mucosal tissues in the small intestines. The increase in circulating IgM levels demonstrated the effectiveness of the oral vaccine for tilapia aquaculture systems. The observed duration of protection, lasting several weeks to at least a month, was consistent with the measured IgM levels. For oral vaccine technologies such as this, a convenient application of an oral booster could be administered to enhance protection until the fish reach market size (approximately 250 g−1 kg per fish) during field trials or commercial grow-out operations.
The LD50 that was obtained for the isolate was 1.31 × 107 CFU/mL. Highly virulent fish bacterial pathogens can have an LD50 of about 106 CFU/fish while the weakly virulent Aeromonas register an LD50 of >107 CFU/fish [47]. Based on these reference values, the DFR01 isolate may be considered a weakly virulent pathogen. It was thought that part of the efficacy of the developed oral vaccine against MAS in Nile tilapia was partly contributed by the virulence of the bacterial strain used. For instance, a more virulent strain of A. veronii (HY2), which has an LD50 of 5 × 106, exhibited an RPS of 90% and 80% when inactivated by autoclaving or formaldehyde treatment, respectively, and subsequently injected into Nile tilapia as a vaccine [51].
The observed ruptured abdominal wall in the diseased fish could be a severe case of infection, while skin and muscle lesions in discolored moribund fish were typical pathologies of MAS [1,12]. Moreover, clinical signs in tilapia infected with Aeromonas spp. such as A. veronii showed ulcerations, pale spots, and hemorrhages along their body [12]. Virulence test results may sometimes be variable when conducted in non-optimal conditions, which are affected by the temperature, dissolved oxygen, and source. In addition, Aeromonas virulence can also be strain-specific [52].
Despite the fact that the natural route of infection is through the mouth, gills, skin, gut, and anus, the intraperitoneal injection used in the study was reported as a more direct, reliable, and reproducible experimental method of infection [53]. Infection via the natural oral route and vaccination via a carrier on coated feeds may result in better protection after vaccination. Oral administration of the antigen has been found to stimulate both systemic and mucosal responses compared to parenteral delivery where gut responses will be almost absent [16].
In this study, the efficacy of an oral vaccine coupled with a naturally occurring phyllosilicate material was evaluated in Nile tilapia. Data suggest that the Aeromonas vaccine could activate a specific antibody response through the oral mucosal route. It sufficiently produced and increased the levels of the specific antibodies (IgM) and resulted in significant protection after the bacterial challenge in tank trials.
The observed 67% RPS in tank trials indicate an acceptable application for pre-commercial deployment in fish farms. Additional booster vaccination is suggested to increase the protection and survival rates. Although the A. veronii DFR01 used to develop the oral vaccine has given significant protection, other virulent Aeromonas species or strains [11,12] may be explored to improve the survival rates of Nile tilapia, especially in nurseries and grow-out areas, and prevent outbreaks in motile aeromonad infection.
5. Conclusions
In conclusion, the A. veronii DFR01 strain isolated from the fingerlings with Aeromonas infections showed pathogenicity for Nile tilapia and was utilized as a whole-cell inactivated antigen for oral vaccine development. Vaccination increased the fish’s blood and spleen IgM levels, suggesting a systemic and adaptive immune response. The RPS data obtained from the tank trials challenged with the bacterial isolate indicate potential for large-scale deployment in ponds or cages for pre-commercial trials. For mass immunization of fish for aquaculture, oral vaccines remain the most effective delivery method. These have promising advantages in improving fish health to increase food production and food security and in mitigating the emergence of antibiotic-resistant pathogens for One Health.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/aquacj4030012/s1, Table S1: Biochemical characteristics of A. veronii DFR01 using API 20E; Table S2: Biochemical characteristics of A. veronii DFR01 using API 20 NE; Table S3: Characteristics of A. veronii DFR01 in Biolog Gen III; Table S4: MALDI-TOF scores of the A. veronii DFR01 isolated from a diseased O. niloticus from Binangonan, Rizal. Table S5: Inoculum dose, cumulative mortality, and re-isolation rate of A. veronii DFR of experimentally infected O. niloticus juveniles. Table S6: Blood chemistry values for vaccinated and non-vaccinated O. niloticus.
Author Contributions
Conceptualization: A.M.A.; Investigation and Methodology: A.M.A., M.N.M.S., V.B.A., R.V.P.J., W.B., M.L.S. and R.E.T.; Funding acquisition, administration and supervision: A.M.A.; Visualization and Validation: A.M.A., M.N.M.S. and V.B.A.; Writing—original draft: A.M.A., M.N.M.S. and V.B.A.; Writing—Review and editing: A.M.A., M.N.M.S., V.B.A. and R.V.P.J. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this study came from a research grant to AMA from the Department of Science and Technology (DOST) Philippine Council for Aquatic and Marine Research and Development (PCAMRD), now the Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (PCAARRD; Program Code: 2021-01-A1-CRADLE-3042; approved 3 November 2021). Many thanks to the Trinity University of Asia and Santeh Feeds Corporation for the support and counterpart funding.
Institutional Review Board Statement
All animal experiments were conducted in accordance with the institutional guidelines of the Southeast Asian Fisheries Development Center Aquaculture Department (SEAFDEC/AQD), Tigbauan, Iloilo.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are included in the article and Supplementary Material; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank the following: Trinity University of Asia and the Institute of Biology, U.P. Diliman for the administrative support; Technology Transfer and Business Development Office (TTBDO), U.P. Diliman for their assistance on the DOST-CRADLE project; RainPhil Inc. for the use of MALDI-TOF equipment, and the College of Fisheries, Central Luzon State University for the 16sRNA primer sequences. Thanks are extended to Chelo Pascua, the late Florentino Sumera, John Anthony Yason, and Alpha Rae Espigar as co-inventors in the development of the fish oral vaccine technology. We are grateful to Jacques Zarate, Ava Zamora, and Tim McGloin for proofreading the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests or personal relationships that may have influenced this paper.
References
- Monir, W.; Abdel-Rahman, M.A.; El-Din Hassan, S.; Mansour, E.S.; Awad, S.M.M. Pomegranate Peel and Moringa-Based Diets Enhanced Biochemical and Immune Parameters of Nile Tilapia against Bacterial Infection by Aeromonas Hydrophila. Microb. Pathog. 2020, 145, 104202. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.T.; Techatanakitarnan, C.; Jindakittikul, P.; Thaiprayoon, A.; Taengphu, S.; Charoensapsri, W.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Aeromonas jandaei and Aeromonas veronii Caused Disease and Mortality in Nile Tilapia, Oreochromis niloticus (L.). J. Fish. Dis. 2017, 40, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, S.-H. Identification and Pathogenicity of Aeromonas Sobria on Tail-Rot Disease in Juvenile Tilapia Oreochromis Niloticus. Curr. Microbiol. 2011, 62, 623–627. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Lozano-Olvera, R.; Garcia-Gasca, M.T.; Abad-Rosales, S.M.; Gomez-Gil, B.; Ayala-Arellano, J. Virulence of the Fish Pathogen Aeromonas dhakensis: Genes Involved, Characterization and Histopathology of Experimentally Infected Hybrid Tilapia. Dis. Aquat. Organ. 2018, 129, 107–116. [Google Scholar] [CrossRef]
- Larsen, J.L.; Jensen, N.J. An Aeromonas Species Implicated in Ulcer-Disease of the Cod (Gadus morhua). Nord. Vet. Med. 1977, 29, 199–211. [Google Scholar]
- Daily, O.P.; Joseph, S.W.; Coolbaugh, J.C.; Walker, R.I.; Merrell, B.R.; Rollins, D.M.; Seidler, R.J.; Colwell, R.R.; Lissner, C.R. Association of Aeromonas sobria with Human Infection. J. Clin. Microbiol. 1981, 13, 769–777. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.B.G.; de Oliveira, W.F.; Correia, M.T.D.S.; Fontes, A.; Coelho, L.C.B.B. Aeromonas and Human Health Disorders: Clinical Approaches. Front. Microbiol. 2022, 13, 868890. [Google Scholar] [CrossRef]
- Yambot, A.V. Isolation of Aeromonas hydrophila from Oreochromis niloticus During Fish Disease Outbreaks in the Philippines. Asian Fish. Sci. 1998, 10, 347–354. [Google Scholar] [CrossRef]
- Pakingking, R.; Palma, P.; Usero, R. Quantitative and Qualitative Analyses of the Bacterial Microbiota of Tilapia (Oreochromis niloticus) Cultured in Earthen Ponds in the Philippines. World J. Microbiol. Biotechnol. 2015, 31, 265–275. [Google Scholar] [CrossRef]
- Legario, F.S.; Choresca, C.H., Jr.; Grace, K.; Turnbull, J.F.; Crumlish, M. Identification and characterization of motile Aeromonas spp. isolated from farmed Nile tilapia (Oreochromis niloticus) in the Philippines. J. Appl. Microbiol. 2023, 134, 12:lxad279. [Google Scholar] [CrossRef] [PubMed]
- Haenen, O.L.M.; Dong, H.T.; Hoai, T.D.; Crumlish, M.; Karunasagar, I.; Barkham, T.; Chen, S.L.; Zadoks, R.; Kiermeier, A.; Wang, B.; et al. Bacterial Diseases of Tilapia, Their Zoonotic Potential and Risk of Antimicrobial Resistance. Rev. Aquac. 2023, 15, 154–185. [Google Scholar] [CrossRef]
- Lio-Po, G.D.; Pascual, J.P.; Santos, J.G. Fish Quarantine and Fish Diseases in South East Asia; International Development Research Centre: Ottawa, ON, Canada, 1983; pp. 35–46. Available online: https://digitallibrary.un.org/record/53612?ln=es (accessed on 5 August 2024).
- Dubey, S.; Ager-Wick, E.; Kumar, J.; Karunasagar, I.; Karunasagar, I.; Peng, B.; Evensen, Ø.; Sørum, H.; Munang’andu, H.M. Aeromonas Species Isolated from Aquatic Organisms, Insects, Chicken, and Humans in India Show Similar Antimicrobial Resistance Profiles. Front. Microbiol. 2022, 13, 1008870. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Areechon, N.; Unajak, S. Development of Fish Vaccine in Southeast Asia: A Challenge for the Sustainability of SE Asia Aquaculture. Fish. Shellfish. Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Mutoloki, S.; Munang’andu, H.M.; Evensen, Ø. Oral Vaccination of Fish—Antigen Preparations, Uptake, and Immune Induction. Front. Immunol. 2015, 6, 519. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.A.G.; Carvalho-Castro, G.A.; Sacchetin, P.S.C.; Lopes, C.O.; Moraes, A.M.; Figueiredo, H.C.P. Oral and Parenteral Vaccines against Flavobacterium columnare: Evaluation of Humoral Immune Response by ELISA and in Vivo Efficiency in Nile Tilapia (Oreochromis Niloticus). Aquacult. Int. 2010, 18, 657–666. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Forlenza, M. Oral Vaccination of Fish: Lessons from Humans and Veterinary Species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef]
- Azad, S.; Shankar, K.M.; Mohan, C.; Kalita, B. Protective Response of Common Carp Orally Vaccinated with Biofilm and Free Cells of Aeromonas hydrophila Challenged by Injection and Immersion Routes. J. Aquac. 2000, 15, 65–70. [Google Scholar]
- Vervarcke, S.; Ollevier, F.; Kinget, R.; Michoel, A. Oral Vaccination of African Catfish with Vibrio anguillarum O2: Effect on Antigen Uptake and Immune Response by Absorption Enhancers in Lag Time Coated Pellets. Fish. Shellfish. Immunol. 2004, 16, 407–414. [Google Scholar] [CrossRef]
- Palm, R.C.; Landolt, M.L.; Busch, R.A. Route of Vaccine Administration: Effects on the Specific Humoral Response in Rainbow Trout Oncorhynchus mykiss. Dis. Aquat. Org. 1998, 33, 157–166. [Google Scholar] [CrossRef]
- Esteve-Gassent, M.D.; Barrera, R.; Amaro, C. Vaccination of Market-Size Eels against Vibriosis Due to Vibrio vulnificus serovar E. Aquaculture 2004, 241, 9–19. [Google Scholar] [CrossRef]
- Akhlaghi, M. Immunogenicity of Aeromonas hydrophila in Common Carp (Cyprinus carpio, L.). J. Vet. Med. Tehran 2000, 55, 55–62. [Google Scholar]
- Rodrigues, A.P.; Hirsch, D.; Figueiredo, H.C.P.; Logato, P.V.R.; Moraes, A.M. Production and characterisation of alginate microparticles incorporating Aeromonas hydrophila designed for fish oral vaccination. Process Biochem. 2006, 41, 638–643. [Google Scholar] [CrossRef]
- Adams, A. Progress, Challenges and Opportunities in Fish Vaccine Development. Fish. Shellfish. Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Harshitha, M.; Nayak, A.; Disha, S.; Akshath, U.S.; Dubey, S.; Munang’andu, H.M.; Chakraborty, A.; Karunasagar, I.; Maiti, B. Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges. Vaccines 2023, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Sinyakov, M.S.; Dror, M.; Lublin-Tennenbaum, T.; Salzberg, S.; Margel, S.; Avtalion, R.R. Nano- and Microparticles as Adjuvants in Vaccine Design: Success and Failure Is Related to Host Natural Antibodies. Vaccine 2006, 24, 6534–6541. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay Minerals and Their Beneficial Effects upon Human Health. A Review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Palumbo, S.A.; Maxino, F.; Williams, A.C.; Buchanan, R.L.; Thayer, D.W. Starch-Ampicillin Agar for the Quantitative Detection of Aeromonas hydrophila. Appl. Environ. Microbiol. 1985, 50, 1027–1030. [Google Scholar] [CrossRef]
- Murray, R.; Doetsch, R.; Robinow, C. Determinative and Cytological Light Microscopy; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Collins, C.H.; Lyne, P.M. Microbilogical Methods, 4th ed.; Butterworths: London, UK, 1976. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Argayosa, A.M.; Pascua, C.S.; Sumera, F.; Yason, J.A.D.L.; Espigar, A.R. Oral Vaccine. Available online: https://onlineservices.ipophil.gov.ph/patgazette/IPASJournal/V18N25_INV_1st.pdf (accessed on 6 February 2024).
- Amend, D. Potency Testing of Fish Vaccines.International Symposium in Fish Biologics:Serodiagnostics and Vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Harms, C.A.; Howard, K.E.; Wolf, J.C.; Smith, S.A.; Kennedy-Stoskopf, S. Transforming growth factor-b response to mycobacterial infection in striped bass Morone saxatilis and hybrid tilapia Oreochromis spp. Vet. Immunol. Immunopathol. 2003, 95, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ruangpan, L.; Kitao, T.; Yoshida, T. Protective Efficacy of Aeromonas hydrophila Vaccines in Nile Tilapia. Vet. Immunol. Immunopathol. 1986, 12, 345–350. [Google Scholar] [CrossRef]
- Joseph, S.W.; Carnahan, A.; Fanning, G.R. Aeromonas jandaei (Formerly Genospecies DNA Group 9 A. sobria), a New Sucrose-Negative Species Isolated from Clinical Specimens. J. Clin. Microbiol. 1991, 29, 560–564. [Google Scholar] [CrossRef]
- Hickman-Brenner, F.W.; MacDonald, K.L.; Steigerwalt, A.G.; Fanning, G.R.; Brenner, D.J.; Farmer, J.J. Aeromonas veronii, a New Ornithine Decarboxylase-Positive Species That May Cause Diarrhea. J. Clin. Microbiol. 1987, 25, 900–906. [Google Scholar] [CrossRef]
- Paniagua, C.; Rivero, O.; Anguita, J.; Naharro, G. Pathogenicity Factors and Virulence for Rainbow Trout (Salmo gairdneri) of Motile Aeromonas Spp. Isolated from a River. J. Clin. Microbiol. 1990, 28, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Cheung, W.K.W.; Janda, J.M. The Genus Aeromonas: Biochemical Characteristics, Atypical Reactions, and Phenotypic Identification Schemes. J. Clin. Microbiol. 2003, 41, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.R.; Lalonde, G.; Leblanc, D.; Olivier, G.; Lallier, R. Aeromonas hydrophila in Rainbow Trout: Relation between Virulence and Surface Characteristics. Can. J. Microbiol. 1980, 26, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Oshiro, L.S.; Abbott, S.L.; Duffey, P.S. Virulence Markers of Mesophilic Aeromonads: Association of the Autoagglutination Phenomenon with Mouse Pathogenicity and the Presence of a Peripheral Cell-Associated Layer. Infect. Immun. 1987, 55, 3070–3077. [Google Scholar] [CrossRef] [PubMed]
- Dooley, J.S.; Trust, T.J. Surface Protein Composition of Aeromonas hydrophila Strains Virulent for Fish: Identification of a Surface Array Protein. J. Bacteriol. 1988, 170, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Santos, Y.; Toranzo, A.E.; Barja, J.L.; Nieto, T.P.; Villa, T.G. Virulence Properties and Enterotoxin Production of Aeromonas Strains Isolated from Fish. Infect. Immun. 1988, 56, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.A.; Ayoub, H.F.; Soror, E.I.; Matter, A.F. Virulence Genes Contributing to Aeromonas veronii Pathogenicity in Nile Tilapia (Oreochromis niloticus): Approaching the Development of Live and Inactivated Vaccines. Aquac. Int. 2023, 31, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016, 7, 1337. [Google Scholar] [CrossRef]
- Angelidis, P.; Karagiannis, D.; Crump, E.M. Efficacy of a Listonella anguillarum (Syn. Vibrio anguillarum) Vaccine for Juvenile Sea Bass Dicentrarchus labrax. Dis. Aquat. Organ. 2006, 71, 19–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).