Prospecting the Photosynthetic Flatworm Symsagittifera roscoffensis as a Novel Fish-Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Symsagittifera roscoffensis Collection and Master Culture
2.2. Feeding Trials with Freshwater Ornamental Fish
2.3. Feeding Trials with Marine Ornamental Fish
2.4. Freeze-Dried Worms Feeding Trials
2.5. Ethical Approval
2.6. Video Processing and Data Analysis
2.7. Algae Uptake Experiments
2.8. Nutritional Profiling of Symsagittifera roscoffensis
2.8.1. Transesterification, Identification and Quantification of FAMEs
2.8.2. Proteins, Pigments, and Carbohydrates
2.9. Statistical Analysis
3. Results
3.1. Algae Uptake Experiments
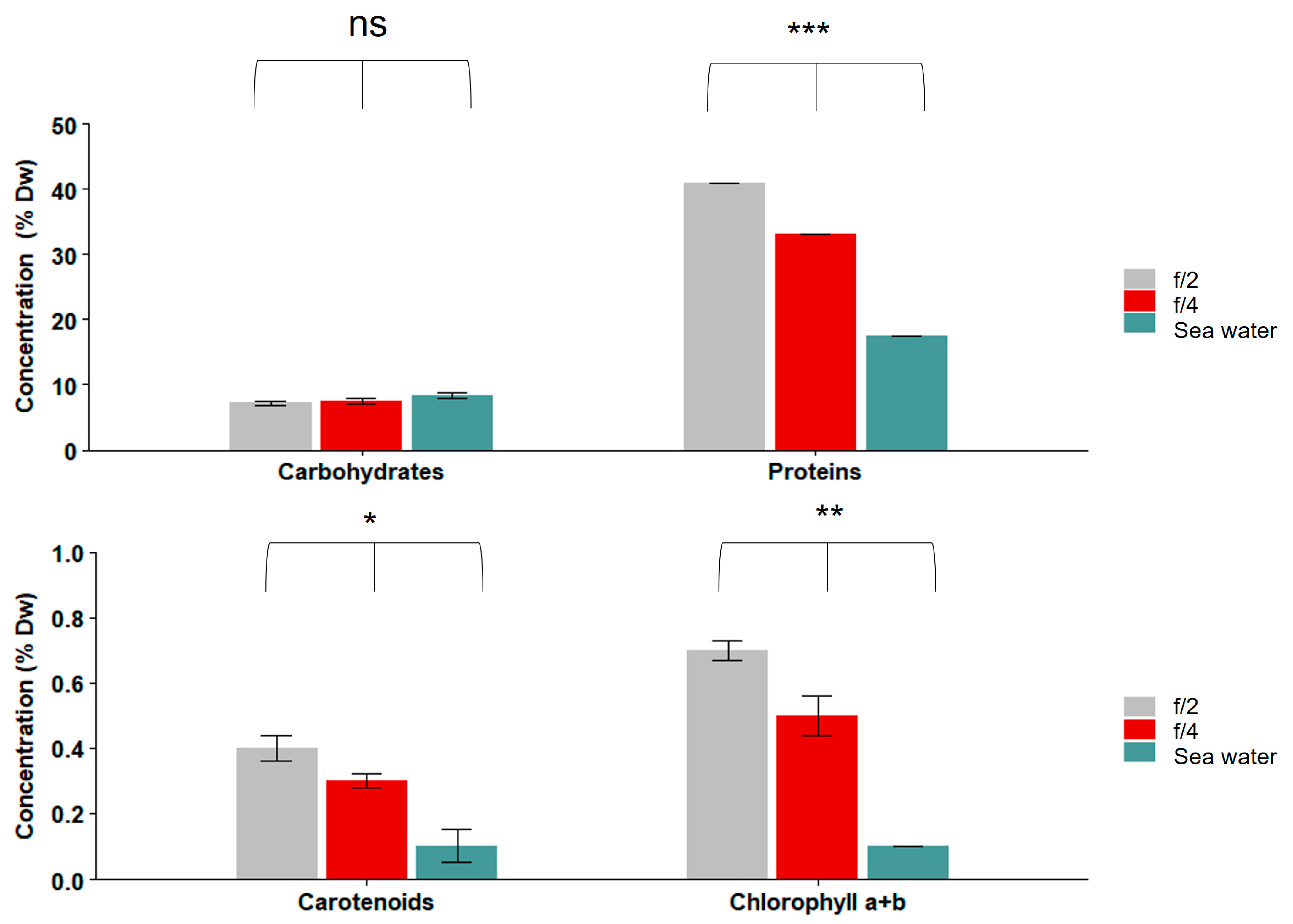
3.2. Nutritional Profiles of S. roscoffensis
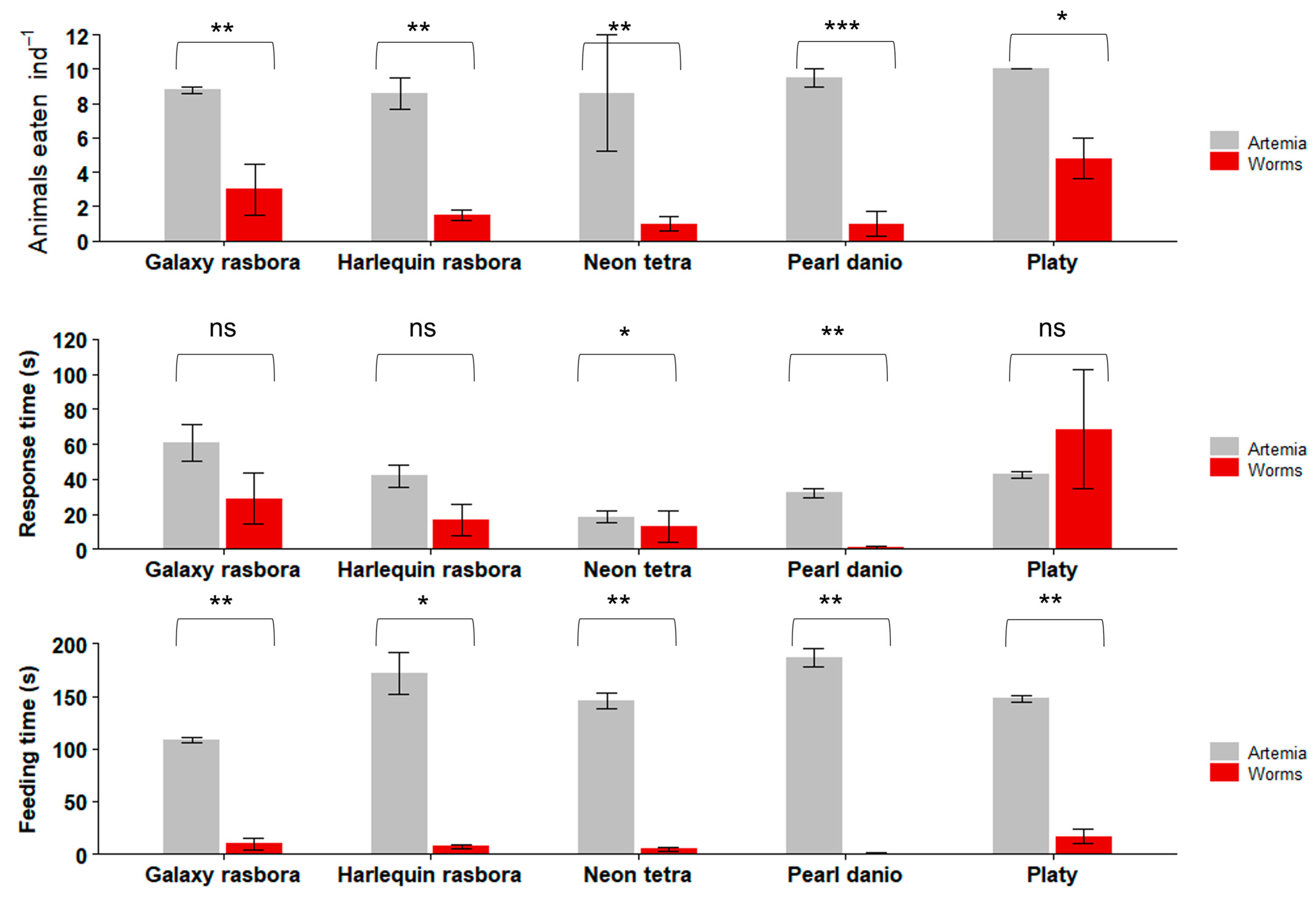
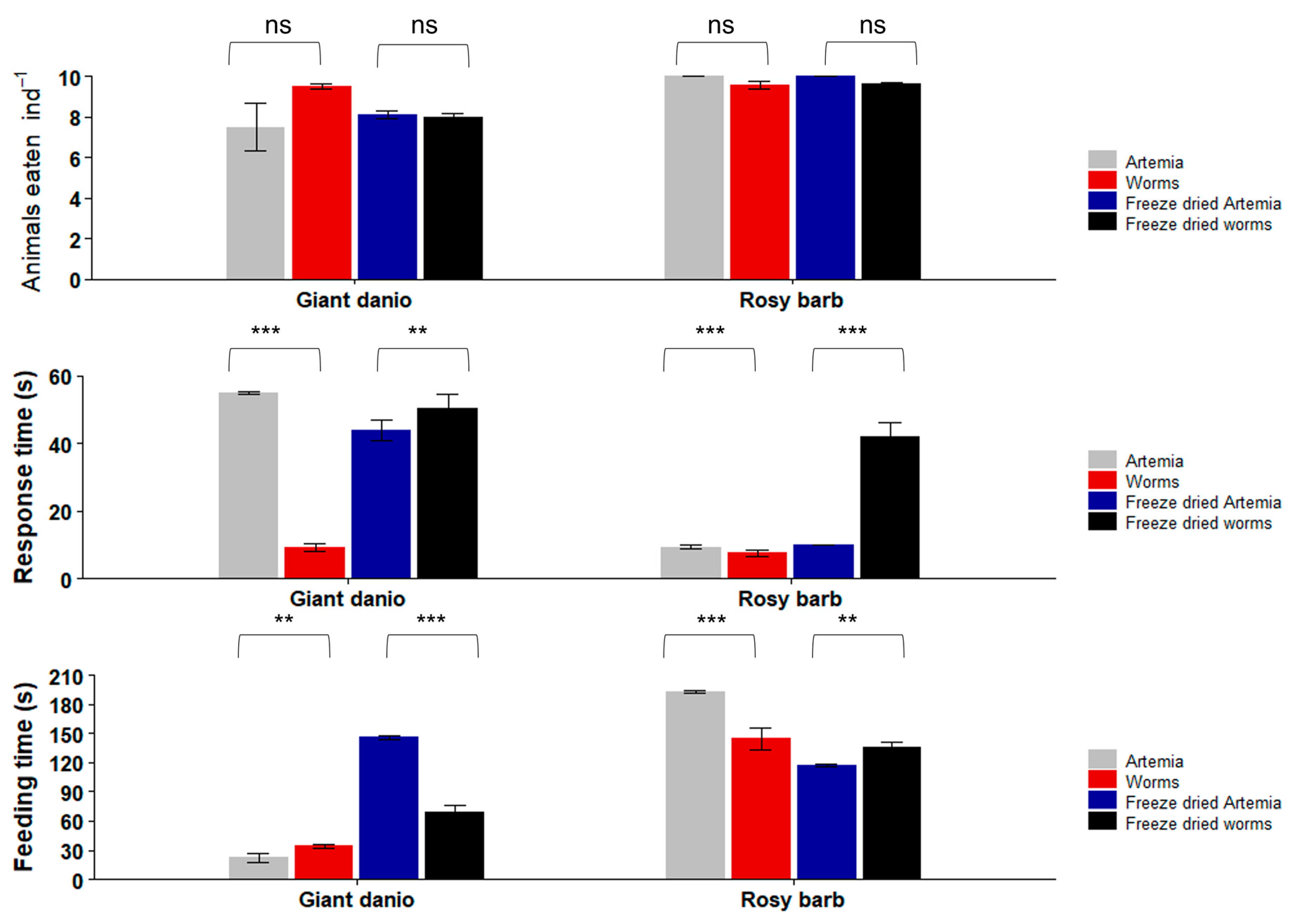
3.3. Feeding Trials with Freshwater Ornamental Fish
3.4. Feeding Trials with Marine Ornamental Fish
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Observatory of Economic Complexity. Ornimental live fish. Available online: https://oec.world/en/profile/hs/ornamental-fish (accessed on 1 September 2022).
- Fortune Business Insights. Pet care market size, share & COVID-19 Impact analysis. Available online: https://www.fortunebusinessinsights.com/pet-care-market-104749 (accessed on 1 September 2022).
- OATA. Ornamental Aquatic Trade Association (OATA); Annual review 2018/19; OATA: Westbury, UK, 2019; Volume 11. [Google Scholar]
- Riehl, R.; Baensch, H.A. Aquarium Atlas; Microcosm: Portland, OR, USA, 1997; ISBN 1564651142. [Google Scholar]
- Andrews, C. The Ornamental Fish Trade and Fish Conservation. J. Fish Biol. 1990, 37, 53–59. [Google Scholar] [CrossRef]
- Benemann, J.R. Microalgae Aquaculture Feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Kumar, R.R.; Ganesan, V.; Anbazhagan, C. Microalgae: A Sustainable Feed Source for Aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional Properties of Microalgae for Mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Pratiwy, F.M.; Pratiwi, D.Y. The Potentiality of Microalgae as a Source of DHA and EPA for Aquaculture Feed: A Review. Int. J. Fish. Aquat. Stud. 2020, 8, 39–41. [Google Scholar]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Chini Zittelli, G.; Sampietro, G. Energy Balance of Algal Biomass Production in a 1-Ha “Green Wall Panel” Plant: How to Produce Algal Biomass in a Closed Reactor Achieving a High Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Oboh, A.; Kabeya, N.; Carmona-Antoñanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two Alternative Pathways for Docosahexaenoic Acid (DHA, 22:6n-3) Biosynthesis Are Widespread among Teleost Fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical Marine Macroalgae as Potential Sources of Nutritionally Important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Colombo, M.L.; Risè, P.; Giavarini, F.; De Angelis, L.; Galli, C.; Bolis, C.L. Marine Macroalgae as Sources of Polyunsaturated Fatty Acids. Plant Foods Hum. Nutr. 2006, 61, 67–72. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of Conversion of Alpha-Linolenic Acid to Long Chain n-3 Fatty Acids in Man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Dani, D.; Thirugnanamurthy, S.; Kandasamy, S.; Shalini, B.; Baruah, A.; Kirubasankar, R.; Dam Roy, S. A Review on Microalgae as Potential Fish Feed Ingredient. J. Andaman Sci. Assoc. 2016, 21, 140–144. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Burr, G.S.; Barrows, F.T.; Gaylord, G.; Wolters, W.R. Apparent Digestibility of Macro-Nutrients and Phosphorus in Plant-Derived Ingredients for Atlantic Salmon, Salmo Salar and Arctic Charr, Salvelinus Alpinus. Aquac. Nutr. 2011, 17, 570–577. [Google Scholar] [CrossRef]
- Tibaldi, E.; Chini Zittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth Performance and Quality Traits of European Sea Bass (D. labrax) Fed Diets Including Increasing Levels of Freeze-Dried Isochrysis Sp. (T-ISO) Biomass as a Source of Protein and n-3 Long Chain PUFA in Partial Substitution of Fish Derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Abatzopoulos, T.J.; Beardmore, J.; Clegg, J.S.; Sorgeloos, P. Artemia: Basic and Applied Biology; Springer Science & Business Media: Cham, Switzerland, 2002; Volume 1, ISBN 940170791X. [Google Scholar]
- Coutteau, P.; Lavens, P.; Leger, P.; Sorgeloos, P. Manipulated Yeast Diets as a Partial Algal Substitute for Rearing Bivalve Molluscs Laboratory Trials with Tapes-Semidecussata. Meded. Fac. Landbouwwet. Univ. Gent 1990, 55, 1597–1600. [Google Scholar]
- Treece, G.D. Artemia Production for Marine Larval Fish Culture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2000; Volume 702. [Google Scholar]
- Van Stappen, G.; Sui, L.; Hoa, V.N.; Tamtin, M.; Nyonje, B.; de Medeiros Rocha, R.; Sorgeloos, P.; Gajardo, G. Review on Integrated Production of the Brine Shrimp Artemia in Solar Salt Ponds. Rev. Aquac. 2020, 12, 1054–1071. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Lindeman, R.L. The Trophic-Dynamic Aspect of Ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Thomas, N.J.; Coates, C.J.; Tang, K.W. Environmental Constraints on the Photosynthetic Rate of the Marine Flatworm Symsagittifera Roscoffensis. J. Exp. Mar. Bio. Ecol. 2023, 558, 151830. [Google Scholar] [CrossRef]
- Bailly, X.; Laguerre, L.; Correc, G.; Dupont, S.; Kurth, T.; Pfannkuchen, A.; Entzeroth, R.; Probert, I.; Vinogradov, S.; Lechauve, C. The Chimerical and Multifaceted Marine Acoel Symsagittifera Roscoffensis: From Photosymbiosis to Brain Regeneration. Front. Microbiol. 2014, 5, 498. [Google Scholar] [CrossRef]
- Mcfarlane, A.E. Two Species of Algal Symbiont in Naturally Occurring Populations of Convoluta Roscoffensis. J. Mar. Biol. Assoc. UK 1982, 62, 235. [Google Scholar] [CrossRef]
- Arboleda, E.; Hartenstein, V.; Martinez, P.; Reichert, H.; Sen, S.; Sprecher, S.; Bailly, X. An Emerging System to Study Photosymbiosis, Brain Regeneration, Chronobiology, and Behavior: The Marine Acoel Symsagittifera Roscoffensis. BioEssays 2018, 40, 1800107. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Experimental Studies on Egg Production by Convoluta roscoffensis: Graff, 1882 (Turbellaria, Acoela). Hydrobiologia 1983, 102, 151–154. [Google Scholar] [CrossRef]
- Provasoli, L.; Yamasu, T.; Manton, I. Experiments on the Resynthesis of Symbiosis in Convoluta Roscoffensis with Different Flagellate Cultures. J. Mar. Biol. Assoc. UK 1968, 48, 465–478. [Google Scholar] [CrossRef]
- Douglas, A.E. Establishment of the Symbiosis in Convoluta roscoffensis. J. Mar. Biol. Assoc. UK 1983, 63, 419–434. [Google Scholar] [CrossRef]
- Boyle, J.E.; Smith, D.C. Biochemical Interactions between the Symbionts of Convoluta roscoffensis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1975, 189, 121–135. [Google Scholar]
- Jennings, J.B. Parasitism and Commensalism in the Turbellaria. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 9, pp. 1–32. ISBN 0065-308X. [Google Scholar]
- Meyer, H.; Provasoli, L.; Meyer, F. Lipid Biosynthesis in the Marine Flatworm Convoluta Roscoffensis and Its Algal Symbiont Platymonas Convoluta. Biochim. Biophys. Acta 1979, 573, 464–480. [Google Scholar] [CrossRef]
- Douglas, A.E. Growth and Reproduction of Convoluta roscoffensis Containing Different Naturally Occurring Algal Symbionts. J. Mar. Biol. Assoc. UK 1985, 65, 871–879. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Van Hille, R.P.; Harrison, S.T.L. Selection of Direct Transesterification as the Preferred Method for Assay of Fatty Acid Content of Microalgae. Lipids 2010, 45, 1053–1060. [Google Scholar] [CrossRef]
- Mahanty, A.; Ranjan Maji, S.; Ganguly, S.; Mohanty, B.P. GC-MS Fingerprinting of Fatty Acids of Freshwater Mollusc Lamellidens marginalis Using Different Columns; TR-WaxMS and TR-FAME. J. Anal. Bioanal. Tech. 2015, 6, 238. [Google Scholar] [CrossRef]
- Chen, Y.; Vaidyanathan, S. Simultaneous Assay of Pigments, Carbohydrates, Proteins and Lipids in Microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef]
- Dey, S.; Ramanujam, S.N.; Mahapatra, B.K. Breeding and Development of Ornamental Hill Stream Fish Devario Aequipinnatus (McClelland) in Captivity. Int. J. Fish. Aquat. Stud. 2014, 1, 1–7. [Google Scholar]
- De Magalhães, A.L.B.; Jacobi, C.M. Invasion Risks Posed by Ornamental Freshwater Fish Trade to Southeastern Brazilian Rivers. Neotrop. Ichthyol. 2013, 11, 433–441. [Google Scholar] [CrossRef]
- Robert Woods Fishkeeping World. Available online: https://www.fishkeepingworld.com/harlequin-rasbora/#h24 (accessed on 13 July 2022).
- Roberts, T.R. The “Celestial Pearl Danio”, a New Genus and Species of Colourful Minute Cyprinid Fish from Myanmar (Pisces: Cypriniformes). Raffles Bull. Zool. 2007, 55, 131–140. [Google Scholar]
- McClure, M.M.; McIntyre, P.B.; McCune, A.R. Notes on the Natural Diet and Habitat of Eight Danionin Fishes, Including the Zebrafish Danio rerio. J. Fish Biol. 2006, 69, 553–570. [Google Scholar] [CrossRef]
- Estelita, E.C. Nemateleotris Magnifica, Fire Goby: Aquarium. Available online: https://www.fishbase.se/summary/6629 (accessed on 1 September 2022).
- Kuiter, R.H.; Tonozuka, T. Pictorial Guide to: Indonesian Reef Fishes, Part 3, Jawfishes–Sunfishes, Opistognathidae–Molidae. Zoonetics 2001, 48637, 623–893. [Google Scholar]
- Moravec, F. Some Aspects of the Taxonomy and Biology of Adult Spirurine Nematodes Parasitic in Fishes: A Review. Folia Parasitol. 2007, 54, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, G.; Bui, S.; Oppedal, F.; Dempster, T. Challenges and Benefits of Applying Fish Behaviour to Improve Production and Welfare in Industrial Aquaculture. Rev. Aquac. 2021, 13, 934–948. [Google Scholar] [CrossRef]
- Malison, J.A.; Held, J.A. Habituating pond-reared fingerlings to formulated feed. In Walleye Culture Manual; NCRAC Culture Series 101; North Central Regional Aquaculture Center Publications Office: Ames, IA, USA, 1996; pp. 199–204. [Google Scholar]
- Mamkaev, Y.V.; Kostenko, A.G. On the Phylogenetic Significance of Sagittocysts and Copulatory Organs in Acoel Turbellarians. Hydrobiologia 1991, 227, 307–314. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Abbas, E.; Ashry, O.; Helal, M.; Nazmi, H.; Kelany, M.; Kamel, A.; Hassaan, M.; Rossi, W.; et al. Effects of Dietary Marine Microalgae, Tetraselmis Suecica, on Production, Gene Expression, Protein Markers and Bacterial Count of Pacific White Shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 2216–2228. [Google Scholar] [CrossRef]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Li, X.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving Carbohydrate and Starch Accumulation in Chlorella Sp. AE10 by a Novel Two-Stage Process with Cell Dilution. Biotechnol. Biofuels 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 205–224. ISBN 9789400710382. [Google Scholar]
- Taylor, D.L. Nutrition of Algal-Invertebrate Symbiosis. I. Utilization of Soluble Organic Nutrients by Symbiont-Free Hosts. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1974, 186, 357–368. [Google Scholar]
- Carvalho, L.F.; Rocha, C.; Fleming, A.; Veiga-Pires, C.; Aníbal, J. Interception of Nutrient Rich Submarine Groundwater Discharge Seepage on European Temperate Beaches by the Acoel Flatworm, Symsagittifera Roscoffensis. Mar. Pollut. Bull. 2013, 75, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional Properties of Carotenoids Originating from Algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.; Shcherbakov, D.; Heyer, A.; Brümmer, F.; Schill, R.O. Behaviour of the Plathelminth Symsagittifera Roscoffensis under Different Light Conditions and the Consequences for the Symbiotic Algae Tetraselmis Convolutae. J. Exp. Biol. 2015, 218, 1693–1698. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]

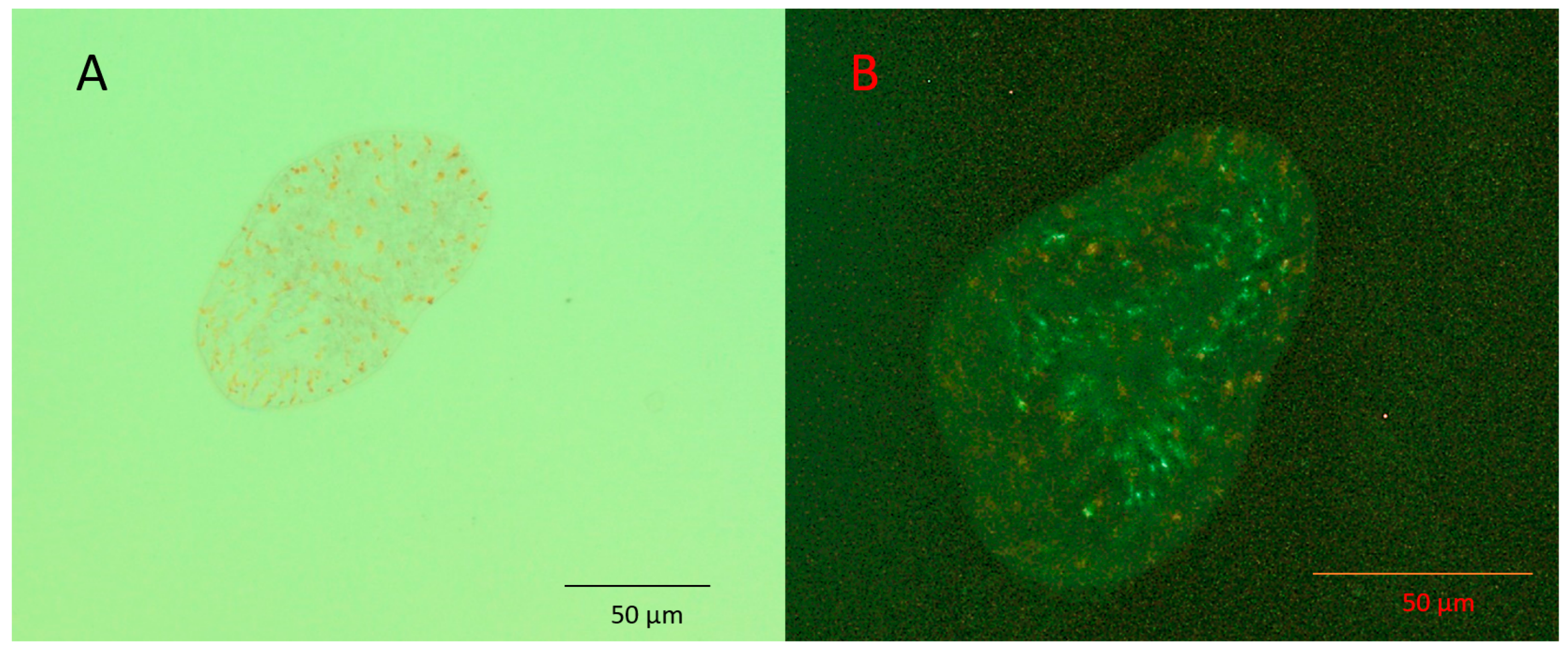
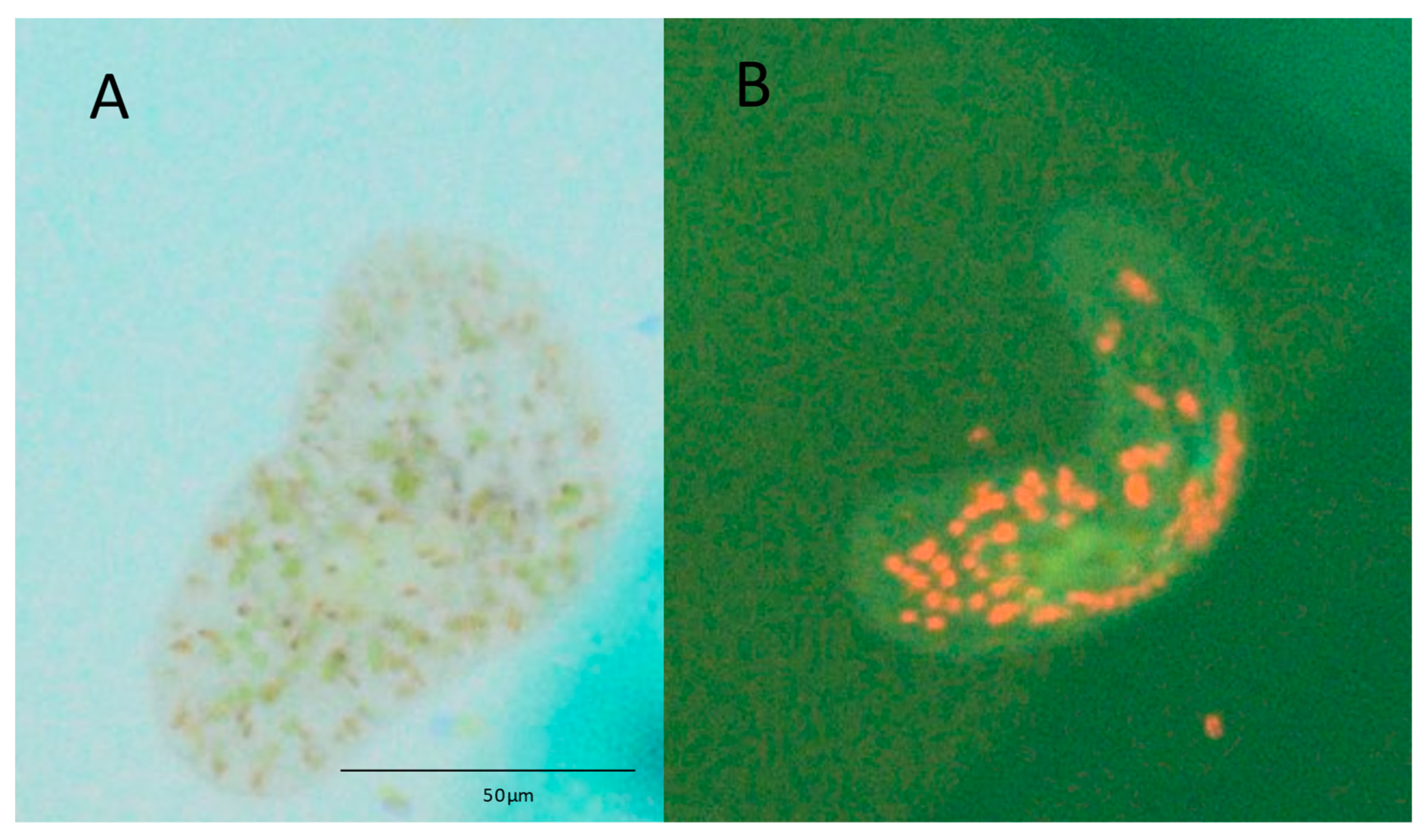



| Survival (%) at Day 36 (Median Survival Time *) | Curve Comparison with T. convolutae (Native) | |
|---|---|---|
| T. convolutae (native) | 20% (10 days) | N/A |
| T. convolutae (CCAP) | 10% (10 days) | X2(1) = 0.23, p = 0.632 |
| T. apiculata | 10% (3 days) | X2(1) = 12.23, p < 0.001 |
| T. chuii | 23% (15 days) | X2(1) = 0.003, p = 0.959 |
| T. gracilis | 5% (10 days) | X2(1) = 1.069, p = 0.301 |
| T. suecica | 18% (15 days) | X2(1) = 0.011, p = 0.918 |
| f/2 Medium | f/4 Medium | Sea Water | |||
|---|---|---|---|---|---|
| Fatty Acid | % DW | SE | % DW | % DW | SE |
| Caproic acid (C6:0) | 0.01 | 0.0011 | 0.02 | 0 | 0 |
| Caprylic acid (C8:0) | 0.03 | 0.0012 | 0.05 | 0.01 | 0.00132 |
| Capric acid (C10:0) | 0.02 | 0.0034 | 0.03 | 0 | 0 |
| Undecanoic acid (C11:0) | 0 | 0 | 0.01 | 0 | 0 |
| Lauric acid (C12:0) | 0.02 | 0.0005 | 0.01 | 0.01 | 0.00057 |
| Tridecanoic acid (C13:0) | 0 | 0 | 0 | 0 | 0 |
| Myristic acid (C14:0) | 0.01 | 0.0001 | 0.08 | 0.03 | 0.0007 |
| Pentadecanoic acid (C15:0) | 0 | 0 | 0.01 | 0.01 | 0.00203 |
| Palmitic acid (C16:0) | 1.12 | 0.1625 | 1.09 | 1.47 | 0.03302 |
| Heptadecanoic acid (C17:0) | 0.02 | 0.0006 | 0.01 | 0 | 0 |
| Stearic acid (C18:0) | 0.23 | 0.0156 | 0.19 | 0.2 | 0 |
| Arachidic acid (C20:0) | 0.43 | 0.0604 | 0.3 | 0.16 | 0.10386 |
| Heneicosanoic acid (C21:0) | 0.31 | 0.0191 | 0.2 | 0.03 | 0.0016 |
| Behenic acid (C22:0) | 0.02 | 0.0002 | 0.02 | 0.01 | 0.00131 |
| Tricosanoic acid (C23:0) | 0.03 | 0.001 | 0.02 | 0 | 0 |
| Lignoceric acid (C24:0) | 0 | 0 | 0 | 0 | 0 |
| Myristoleic acid (C14:1) | 0 | 0 | 0 | 0.01 | 0.00055 |
| Cis-10-Pentadecenoic acid methyl ester (C15:1) | 0 | 0 | 0 | 0 | 0 |
| Palmitoleic acid (C16:1) | 0.06 | 0.0068 | 0.17 | 0.04 | 0.0037 |
| Cis-10-Heptadecenoic acid methyl ester (C17:1) | 0 | 0 | 0 | 0.01 | 0.00239 |
| Elaidic acid (C18:1n9t) | 0 | 0 | 0.01 | 0.01 | 0.00365 |
| Oleic acid (C18:1n9c) | 0.91 | 0.0387 | 0.65 | 0.97 | 0.03979 |
| Cis-11-eicosenoic acid (C20:1n9) | 0.93 | 0.001 | 0.47 | 0.44 | 0.00239 |
| Erucic acid (C22:1n9) | 0.38 | 0.0256 | 0.23 | 0.13 | 0.04778 |
| Nervonic acid (C24:1n9) | 0.2 | 0.0083 | 0.11 | 0.1 | 0.0067 |
| Linoleic acid (C18:2n6c) | 0 | 0 | 0 | 0 | 0 |
| Linolelaidic acid methyl ester (C18:2n6t) | 0.06 | 0.0066 | 0.05 | 0.01 | 0.00332 |
| γ-Linolenic acid (C18:3n6) | 0.01 | 0.0023 | 0.01 | 0 | 0 |
| α-Linolenic acid (C18:3n3) | 0.55 | 0.1793 | 0.45 | 0.02 | 0.00062 |
| cis-11, 14-eicosadienoic acid (C20:2) | 0.05 | 0.0011 | 0.05 | 0 | 0 |
| cis-8, 11, 14-eicosatrienoic acid (C20:3n6) | 0 | 0 | 0 | 0 | 0 |
| Arachidonic acid (C20:4n6) | 0.01 | 0.0039 | 0.01 | 0 | 0 |
| cis-11, 14, 17-eicosatrienoic acid (C20:3n3) | 0.35 | 0.007 | 0.26 | 0.01 | 0.00054 |
| Eicosapentaenoic acid (C20:5n3) | 1.73 | 0.0005 | 1.36 | 0.01 | 0.00045 |
| Eicosadienoic acid | 0.02 | 0.0001 | 0.02 | 0 | 0 |
| Docosahexaenoic acid (C22:6n3) | 0.01 | 0.0001 | 0.01 | 0.01 | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, N.J.; Tang, K.W.; Coates, C.J. Prospecting the Photosynthetic Flatworm Symsagittifera roscoffensis as a Novel Fish-Feed. Aquac. J. 2023, 3, 149-167. https://doi.org/10.3390/aquacj3020013
Thomas NJ, Tang KW, Coates CJ. Prospecting the Photosynthetic Flatworm Symsagittifera roscoffensis as a Novel Fish-Feed. Aquaculture Journal. 2023; 3(2):149-167. https://doi.org/10.3390/aquacj3020013
Chicago/Turabian StyleThomas, Nathan J., Kam W. Tang, and Christopher J. Coates. 2023. "Prospecting the Photosynthetic Flatworm Symsagittifera roscoffensis as a Novel Fish-Feed" Aquaculture Journal 3, no. 2: 149-167. https://doi.org/10.3390/aquacj3020013
APA StyleThomas, N. J., Tang, K. W., & Coates, C. J. (2023). Prospecting the Photosynthetic Flatworm Symsagittifera roscoffensis as a Novel Fish-Feed. Aquaculture Journal, 3(2), 149-167. https://doi.org/10.3390/aquacj3020013






