Abstract
Symsagittifera roscoffensis is an intertidal Acoel flatworm that forms a symbiotic relationship with the alga Tetraselmis convolutae. Members of the genus Tetraselmis are known to have a high nutritional value and have been widely used to enrich intermediate prey for fish within the aquaculture industry; therefore, S. roscoffensis could be a good candidate as a trophic shortcut to deliver algal nutrition to fish. In this study, we investigated the likelihood of five ornamental tropical freshwater and six ornamental marine fishes to consume this worm, either as live feed or in freeze-dried form. We also tested the ability of S. roscoffensis to form a symbiotic relationship with alternative algal species, analysing the nutritional profile of S. roscoffensis when grown in different media. All the experimental fish consumed live worms to some degree, with the exception of one species (Meiacanthus grammistes); the response time to the worms ranged from 1.1–68.6 s for freshwater ornamental species to 1–24 s for marine ornamental species, and in most cases, this was comparable to or shorter than their response time to the reference diet Artemia. The fishes showed no negative effects after consuming the worms. We obtained similar results with freeze-dried worms in terms of the number of worms eaten, response time, and feeding time. Symsagittifera roscoffensis was able to form a symbiotic relationship with all the tested algal species of the genus Tetraselmis, but not with members of other genera. Worms grown in nutrient media (f/2 and f/4) had significantly higher contents of protein, pigments, and total and polyunsaturated fatty acids, including eicosapentaenoic acid (20:5n − 3) and α-linolenic acid (18:3n − 3), than those grown in seawater. These results show that S. roscoffensis was acceptable to many ornamental fish species, delivering key algal ingredients that are beneficial to fish health; hence, it is a promising alternative to conventional fish feeds for the ornamental pet trade.
1. Introduction
Globally, it is expected that the pet trade industry will be worth $232.14 B by 2030, while the sale of live ornamental fish will be worth $330 M [1,2]. Within the UK, there are roughly 12,000 people employed across the 3000 pet shops that sell tropical fish and other ornamental aquatic species [3]. It is estimated that 12 million UK households have pets, including ornamental aquatic species, spending £3 billion a year on pet food [3]. Aquatic pet food can be categorised into dry feed and live feed. Most hobbyists feed their fish exclusively on dry feed in the form of flakes or pellets [4]. However, dry feed is not suitable for all fish species, such as those that forage away from the surface, while pellets may sink and be buried in the substrate, thereby contributing to substrate and water fouling over time [4]. Some fish species, especially wild-caught marine fish, will not readily accept dry feeds, due to the fact that in the wild, the only food they would have encountered would be live food [5]. Dry feeds that are left uneaten in the aquarium tend to dissolve quickly, thereby losing their nutritional contents. Dry feeds contain key ingredients, including polyunsaturated fatty acids (PUFAs) and proteins that are often derived from wild-caught fish and shellfish. A suitable alternative to harvesting wild fish and shellfish for dry feeds would help to alleviate the pressure on wild stocks and conserve the marine ecosystem [6,7].
The other type of food for ornamental fish is live feed. Many microalgal species contain polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA, 20:5n − 3) and docosahexaenoic acid (DHA, 22:6n − 3), which are critical for fish health and nutrition [8,9,10]. While a limited number of fish, mainly freshwater species, have the ability to synthesise DHA via a distinct metabolic pathway (∆4, ∆6), provided that the required precursors are available in the diet. Synthesis via these pathways usually results in a low conversion efficiency [11]. However, microalgae can be good sources of DHA, EPA, and ALA, depending on the farming conditions [12,13,14]; they are also rich in key nutrients such as proteins and carotenoids, as well as those potentially beneficial to fish, such as micro-nutrients, carbohydrates, and bioactive compounds (e.g., polyphenols and sterols) [15,16].
One algal genus that has been widely used in fish aquaculture is Tetraselmis (Prasinophyceae, Chlorophyta), as it is rich in PUFAs [8,10]. Microalgal cells are too small for ornamental fish to consume directly, and the presence of the algal cell wall may also hinder digestion [17,18]. To circumvent this problem, an intermediate animal is usually given as live feed, most commonly zooplankton such as Artemia sp. and cladocerans, which are needed to deliver the desired algal constituents to the fish [19,20]. The production of animal live feed can be labour intensive and requires a cultivation system separate from the microalgae, or purchased as a pre-made enrichment paste or powder [21,22]. This increases the likelihood of contamination and operational costs and results in a significant loss of production efficiency. Based on Lindeman’s principle of ecological efficiency, on average, 90% of the energy is lost between the trophic levels, i.e., for every 100% microalgal production, only 10% is incorporated by an intermediate organism, which, in turn, transfers only 1% to the target fish species [23,24]. Finding an alternative live feed organism that is easy to cultivate and can deliver algal nutrients more efficiently to the higher trophic level will be hugely beneficial to the industry.
Symsagittifera roscoffensis is an Acoel flatworm within Xenacoelomorpha, which is found in intertidal areas in South Wales, UK, and the southern tip of Portugal, as well as Roscoff, France, and the Channel Islands [25]. It often occurs in dense patches of millions of individuals along the shore [26]. In the wild, S. roscoffensis contains the algal symbiont Tetraselmis convolutae and relies on it entirely to meet its nutritional requirements [23]. There has been suggestions that the worms can form an early symbiotic relationship with other Tetraselmis spp. when the preferred T. convolutae is unavailable [27,28]. The worm is a hermaphrodite, but reproduces by mating. After successful mating, the worm produces a cocoon with 5–10 embryos inside. New-borns emerge from the cocoon after about 10 days as aposymbiotic individuals and must acquire the algal symbiont from the environment [28,29,30]. After the worm’s initial acquisition, the T. convolutae cells undergo phenotypical changes, such as a loss of eye spot, flagella, and cell wall [31]. It has been estimated that each individual adult worm contains more than 100,000 algal cells within its upper epidermis [26] and using photoassimilates such as lactic acid from the algal symbiont to synthesise other compounds [28,32,33]. Interestingly, S. roscoffensis is unable to make any of its own PUFAs, which are instead synthesised by the algal symbiont [34].
There are several characteristics that make S. roscoffensis a good candidate as a novel fish feed for the aquaculture industry and, in particular, as a live feed within the ornamental fish trade: (1) S. roscoffensis is 3–4 mm in length, similar to that of common live feeds such as Artemia and cladocerans; (2) its symbiotic algae are from the genus Tetraselmis, which is known to have a high nutritional value for fish; (3) as the worm relies solely on its symbiotic algae for its nutrition, its cultivation does not require a separate food source and can be performed similarly to cultivating microalgae [25]; therefore, it may serve as a ‘trophic shortcut’ to delivering microalgal nutrition to the fish more efficiently; and (4) lastly, Douglas (1985) [35] and Mcfarlane (1982) [27] reported that the Welsh population of S. roscoffensis contained different types of algae between the western and eastern fringes of their field site, which suggests that S. roscoffensis can incorporate different algal species if T. convolutae is not available. In the context of a novel feed development, this means the possibility of changing the algal symbiont and customising the biochemical characteristics of S. roscoffensis based on the needs of a given aquaculture industry.
To date, there is no information available on fish predation behaviour toward S. roscoffensis, its use as a fish feed in aquaculture, or its biochemical composition. To explore the suitability of S. roscoffensis as a feed for the marine and freshwater ornamental fish trade, we conducted experiments to (1) observe the fish’s behavioural reactions to the worm; (2) quantify the fish’s consumption of the worm; (3) test the ability of the worm to incorporate different microalgal species; and (4) analyse the biochemical compositions of the worm grown in different media.
2. Materials and Methods
2.1. Symsagittifera roscoffensis Collection and Master Culture
Symsagittifera roscoffensis was collected from a beach in East Aberthaw in South Wales, UK (N 51° 23′ 2.506″ W 3° 22′ 28.004″), in early October 2021. It was found in the upper limit of the intertidal zone at low tide characterised by a vivid green colour upon sand, in small rock pools, and between pebbles (Figure 1). The worms were collected with a pipette into test tubes and returned to the laboratory within 2 h. In the laboratory, the worms were transferred into 300 mL glass containers to establish a master culture. The containers had autoclaved sand that was collected from the same location; the seawater was drawn from Swansea Bay and sterilised using filtration, UV radiation, and autoclaving before use (salinity 30 ppt, pH 8.1). Inorganic nutrients were added in the form of a 0.22 μm filtered Guillard f/2 medium at 10 mL L−1 (f/4 final conc.). The master culture was placed inside an LMSTM (Kent, UK) incubator set to a temperature of 14.5 °C and light was provided by a light panel inside the incubator at an intensity of 69 µmol m−2 s−1 and a photoperiod of 16L:8D. One quarter of the sea water was renewed every 3 days to replenish the nutrients, and the worms were transferred to new containers weekly to avoid a build-up of waste and detritus. The worms were harvested with a pipette and counted to the required number prior to each experiment.
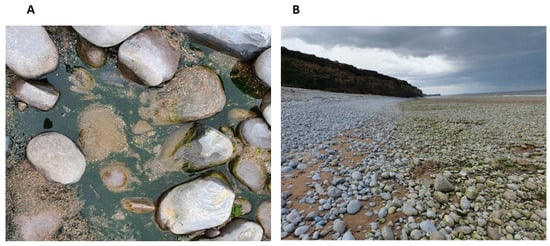
Figure 1.
Symsagittifera roscoffensis in situ at East Aberthaw, Wales. A close up of S. roscoffensis located in shallow pools of water (A), the beach at which the rockpools are located and the worms reside (B). The pools of water are located where there is a distinctive colour change in the rocks (from intertidal to supratidal).
2.2. Feeding Trials with Freshwater Ornamental Fish
The freshwater ornamental fish were acquired from a local aquarium store. Each individual species was approximately the same age (sub adult) and had similar body sizes. We obtained 10 individuals from each freshwater species: Paracheirodon innesi (common name Neon tetras), Xiphophorus maculatus (assorted Platy), Trigonostigma heteromorpha (Harlequin rasbora), Danio margaritatus (Galaxy rasbora), and Danio albolineatus (Pearl danio). We also obtained 30 individuals of Pethia conchonius (Rosy barb) and Devario aequipinnatus (Giant danio), which allowed us to perform repeated trials (see below).
The fish were housed in 10 × 25 L in L37-W27-H35 cm holding tanks connected to a recirculation aquaculture system (RAS). Prior to the feeding trials, the fish were starved for 18 h to standardise their hunger levels. Because the selected fish species are known to live in groups in the wild, to mimic this, we placed ten individuals of each species in a clear 15 L experimental tank. The fish were allowed to acclimate to the experimental tank for 10 min; afterwards, 100 live worms were introduced into the tank at approximately half the tank’s length from the fish. The behavioural responses of the fish were recorded for 10 min using a high-speed camera (Sony RX100 7, Tokyo, Japan). At the end of the 10 min trial, the remaining worms were counted, and the fish were returned to the holding tank, where they were monitored for adverse effects such as a loss of condition and changes in their behaviour, as well as an increased morbidity or mortality. Afterwards, we repeated the trials, but with Artemia sp. that were 72 h old and enriched with SELCO (INVE Aquaculture, Salt Lake City, UT, USA) as the reference diet for comparison. Artemia sp. were cultivated at Swansea University in a salinity of 32 ppt at circa 21 °C and harvested on the day of the experiment. We used Artemia as a reference diet because they are easily accessible, and widely used as a live feed for many ornamental fish species within the aquatic trade. Artemia. was also a part of the diet used at the aquarium store, prior to purchase and when housed in our own holding tanks. The experiment was repeated two more times with Rosy barbs and Giant danio.
2.3. Feeding Trials with Marine Ornamental Fish
From a local aquarium store, we sourced five individuals of each of the following marine ornamental fish species. Each species was of a similar age (sub adult) and had similar body sizes: Chrysiptera parasema (Yellow tail), Meiacanthus grammistes (Striped blenny), Gramma loreto (Royal grammar), Elacatinus oceanops (Neon goby), Amphiprion ocellaris (Clown fish), and Nemateleotris magnifica (Firefish). The marine fish were housed in holding tanks of 6 × 50 L of sea water at 26 °C, with a salinity of 32 ppt. The marine fish experiments used the same set up as that for the freshwater fish experiments. Because the selected marine species can show aggression towards each other, we ran the experiments with one individual at a time. It should be noted that one Clown fish and one Neon goby were removed from the experiment for ethical reasons due to the fish becoming visibly stressed in the experimental tank.
Each marine fish was allowed to acclimate to the experimental tank for 10 min; afterwards, 10 live worms were added, and the fish’s behavioural response was video recorded for 10 min. The remaining worms were counted, and each fish was monitored for any signs of adverse effects. The same procedures were repeated with Artemia sp. as the reference diet.
2.4. Freeze-Dried Worms Feeding Trials
To test the suitability of S. roscoffensis as a dry feed for ornamental fish, we repeated the feeding trials using freeze-dried worms. To produce the dry feed, we first froze the worms in <0.5 mL of sea water at −20 °C overnight, then placed them in a freeze dryer (Edwards super modulyo freeze dryer) at −20 °C under constant vacuum for 24 h. The freeze-dried Artemia were prepared as the reference diet in the same manner.
Only the freshwater ornamental fish species that showed a willingness to eat the live worms in the previous experiments were used for these experiments. Because of the rather low numbers of live worms eaten by the marine ornamental fish, they were not used for these experiments. The experimental set up and procedures were the same as before (see Section 2.2), with the exception that, instead of live worms, 100 freeze-dried worms (or Artemia) were given to the fish.
2.5. Ethical Approval
Prior to the start of any experiments involving either the freshwater fish or the marine species mentioned, the experimental design was approved via Swansea University’s Animal Welfare and Ethics Review Body (AWERB), with the ethics approval number: 190321/3739. This included an acclimation period of two weeks to ensure the fish were healthy prior to the start of the experiments. All the experiments involving fish conformed to the UK Animals (Scientific Procedures) Act 1986.
2.6. Video Processing and Data Analysis
The videos from all the feeding trials (Section 2.1, Section 2.2 and Section 2.3) were processed in VLC media player (formerly VideoLAN Client) to determine the response time and feeding time of the individual fish. The response time was the time taken (in seconds) for each fish to start eating the worms or Artemia (live or freeze-dried) once they were placed into the experimental tank. The feeding time was the total time (in seconds) each fish spent eating the feeds once they had been introduced. The number of animals (live or freeze-dried) eaten was determined by counting the remaining animals at the end of each experiment and with the use of the video data.
2.7. Algae Uptake Experiments
We tested the ability of S. roscoffensis to establish symbiosis with different algal species: Chlorella minutissima, Dunaliella primolecta, Isochrysis galbana, Nannochloropsis oculate, Porphyridium purpureum, Tetraselmis chuii, T. suecica, T. apiculata, T. gracilis, T. convolutae (culture collection), and T. convolutae extracted from the Welsh worms (hereafter referred to as TCW). Except for the TCW, all the algal species were taken from Swansea University’s algal culture collection or procured from the Culture Collection of Algae and Protozoa (CCAP). All the algal stock cultures were maintained in continuous growth in seawater enriched with f/2 medium, inside an LMSTM (Kent, UK) incubator (14 °C, 69 µmol m−2 s−1, and 16/8 light/dark cycle).
We followed the method from [30] to obtain aposymbiotic juvenile worms for these experiments. Multiple adult worms were placed together in a small dish for about two weeks to allow reproduction to occur. We checked the worms every three days under a microscope; any cocoons produced were transferred into another Petri dish. Afterwards, the outer shell of each cocoon was carefully removed with a scalpel and a needle, freeing the embryos inside. Once freed, the embryos were rinsed in sterilised seawater (salinity 30 ppt and pH 8.1) to remove any trace of algae. Ten embryos were then transferred into a small Petri dish and incubated at 14.5 °C for about ten days in the dark until they hatched.
Once hatched, the juvenile worms were checked to confirm their aposymbiotic status under an epifluorescence microscope (Olympus BX43, Tokyo, Japan), a lack of red fluorescence (from chlorophyll) under blue light excitation (480 nm) would indicate an absence of symbiotic algae (Figure 2). Ten aposymbiotic individuals were then placed into a test tube with 100 mL of sterilised seawater enriched with 0.22 μm filtered Guillard f/2 medium at 10 mL L−1 (f/4 final conc.) and 10 mL inoculum of each test algal species; a total of 4 replicates were set up per algal species. The worms were checked every 3 days for 36 days and the number of surviving individuals was counted. Epifluorescence images were taken after 15 days; red fluorescence in the upper epidermis under blue light excitation would indicate that algal symbiosis had been established inside the worm.
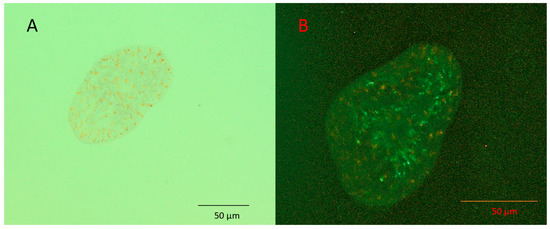
Figure 2.
Aposymbiotic juvenile Symsagittifera roscoffensis visualised using (A) light microscopy, and (B) epifluorescence microscopy. The rhabdoid cells present on the upper surface give off a faint yellow-brown colour under blue light excitation; the lack of red fluorescence indicates the absence of symbiotic algae.
2.8. Nutritional Profiling of Symsagittifera roscoffensis
To evaluate the nutritional value of S. roscoffensis as a potential feed for aquaculture, we analysed the biochemical compositions of S. roscoffensis grown in different media: filtered seawater, f/4, and f/2. The worms were kept in the corresponding medium (approximately 200 worms in 150 mL), which was renewed twice a week. After a 2-week period, the worms were collected and freeze-dried (Edwards super modulyo freeze dryer; −20 °C under vacuum for 24 h), after which, the worms were separated from the remaining salt with tweezers. The biochemical characterisation of the freeze-dried samples followed standard methods, which are briefly described below.
2.8.1. Transesterification, Identification and Quantification of FAMEs
All the chemicals and analytical reagents were of a high-performance liquid chromatography grade (Sigma-Aldrich, Dorset, UK), unless stated otherwise. The worm samples were weighed (~1 to 2 mg), followed by direct transesterification and fatty acid methyl esters (FAMEs) profiling [36]. The dried FAMEs were reconstituted in 300 μL of hexane prior to their identification and quantification on a GC-ToF-MS (Waters Corporation, Milford, MA, USA) using a TR-FAME capillary column [37]. A six-point calibration curve was generated using one internal (C13:0) and two external standards (C17:0 and C19:0) to generate a C17:0/C13:0 calibration curve. The quantification of the FAMEs was performed by comparing the experimentally derived component peak areas with the calibration curve. In total, n = 3 replicates were run, and only the FAMEs identified in at least two replicates were considered to be true hits. The data was reported on a percent dry weight basis.
2.8.2. Proteins, Pigments, and Carbohydrates
A combined colorimetric assay was used to measure the proteins, pigments, and carbohydrates according to [38]. Briefly, the freeze-dried worm samples were treated with BCA reagents; the mixture was then centrifuged at 700× g for 3 min. Afterward, the different chemical fractions were removed to measure the total proteins (absorbance at 562 nm), total carbohydrates (578 nm), chlorophyll a + b (416, 453, and 750 nm), and carotenoids (430, 450, 480, and 750 nm).
2.9. Statistical Analysis
Different statistical tests were applied to different data sets. In the freshwater and marine fish trials, we tested for differences between the feed treatments (worm vs. Artemia) for each fish species and for the number of worms eaten, response time, and total feeding time, using the Kruskal–Wallis test in R studio version 1.41717. Graphs were created using the R package ggplot2.
In the experiments in which we exposed the S. roscoffensis to different algae, we tested the worm survival in the presence of different algal symbionts. This was analysed using the Log rank (Mantel–Cox) test for curve comparisons in GraphPad PRISM v9. When characterising the S. roscoffensis lipids, proteins, and carbohydrates, we tested for differences in the total fatty acids, proteins, pigments, and carbohydrates within the S. roscoffensis when they were kept in different media (seawater, f/4, and f/2). This was performed with a 1-way ANOVA after satisfying the requirement for normality, using the R package DHARMa version 4.1.3 that utilises the Kolmogorov–Smirnov test (R studio version 1.41717).
3. Results
3.1. Algae Uptake Experiments
The aposymbiotic juvenile S. roscoffensis were incubated in the presence of ten different algal species to test whether the worm would incorporate alternative algal symbionts in the absence of the native T. convolutae. Epifluorescence microscopy revealed that S. roscoffensis had incorporated cells of all the tested Tetraselmis species throughout the upper layer of its body, suggesting that successful symbiosis had occurred (Figure 3). On the contrary, the worms incubated with C. minutissima, D. primolecta, I. galbana, N. oculata, and P. purpureum had no algae inside, indicating that there was no uptake of those algal species.
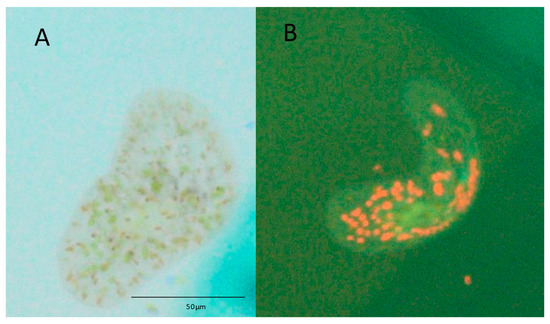
Figure 3.
Symsagittifera roscoffensis visualised using epifluorescence microscopy when cultured in medium containing Tetraselmis suecica for 15 days. (A) Light green patches, i.e., chlorophyll, indicate the presence of algal cells within the worm when viewed under the light microscope. (B) The same worm displays punctate red patches under blue epifluorescent light, which confirms the presence of T. suecica.
Overall, the presence of different Tetraselmis species had a significant impact on the probability of the S. roscoffensis surviving (X2(5) = 33.88, p < 0.001; Figure 4). The differences in overall survival ranged from 5% to 23% over the duration of the experiment (36 days) and the median survival times ranged from 3 to 15 days (Table 1). Compared to T. convolutae, which is naturally found within the worms in situ, T. apiculata led to a significant reduction in survival by the experiment’s end, from 20% to 10%, (day 36) with the shortest median survival time of 3 days. The worms with T. chuii and T. suecica demonstrated median survival times of 15 days each, with survival peaking at 23% for the former.
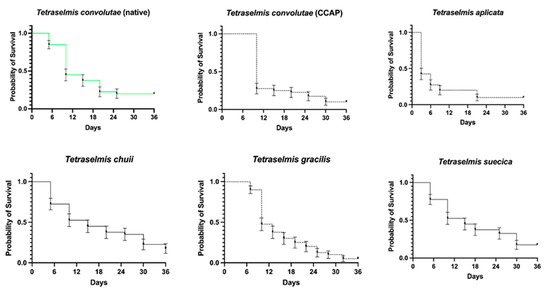
Figure 4.
Survival of juvenile aposymbiotic Symsagittifera roscoffensis when offered Tetraselmis spp. as potential symbionts. Data are plotted as mean ± SE. n = 40 per alga, with 240 in total. CCAP, Culture Collection of Algae and Protozoa.

Table 1.
Survival analysis of Symsagittifera roscoffensis when offered a range of potential Tetraselmis symbionts. Log rank (Mantel–Cox) tests were used to compare curves.
3.2. Nutritional Profiles of S. roscoffensis
The nutritional profiles of the S. roscoffensis grown in different media (f/2, f/4, and filtered seawater) are presented in Figure 5 and Figure 6 and Table 2. The worms had between 7.5 and 8.4% DW of carbohydrates among all the treatments, and there were no significant differences among the treatments (ANOVA; p = 0.2) (Figure 5). The protein contents were significantly different in the S. roscoffensis between the tested media (ANOVA; p > 0.001): it was highest in f/2 (41.0 ± 0.0% DW; mean ± SE) and lowest in the seawater (17.4 ± 0.0% DW). The carotenoid contents varied between 0.4 ± 0.0% DW in f/2, 0.3 ± 0.0% DW in f/4, and 0.1 ± 0.5% DW in seawater; there were significant differences among the tested media types (ANOVA; p = 0.02). The chlorophyll a + b contents of S. roscoffensis varied significantly (ANOVA; p = 0.002), from 0.7 ± 0.0% DW in f/2 to 0.5 ± 0.0% DW in f/4, while it was undetectable in the seawater (Figure 5).
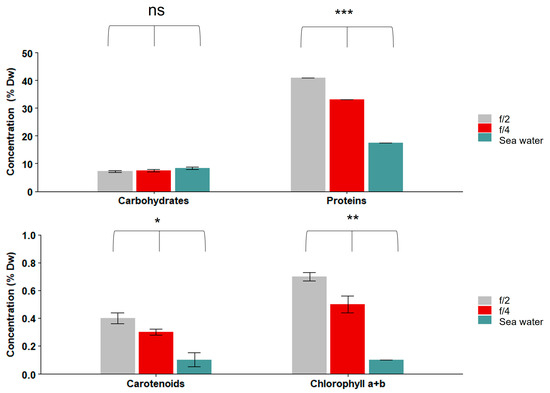
Figure 5.
Carbohydrate, protein, carotenoid, and chlorophyll a + b contents of S. roscoffensis cultured in different media (filtered sea water, f/4, and f/2). Carbohydrate concentrations did not differ significantly. Protein, carotenoids, and chlorophyll a + b were significantly different in S. roscoffensis depending on the medium used. Asterisks indicate significant differences (ANOVA: * p < 0.05; ** p < 0.01; *** p < 0.001; ns not significant). n = 200 worms per experiment. Data are presented as % dry weight (DW; mean ± SE).
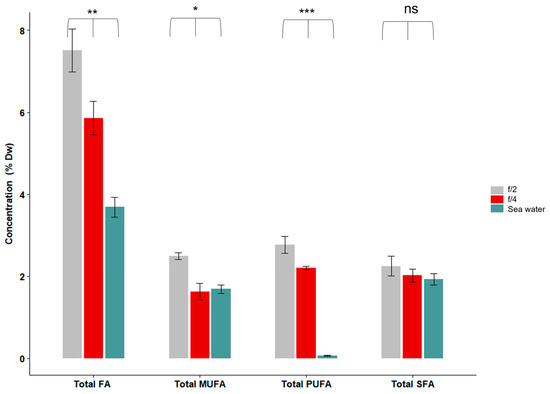
Figure 6.
Total fatty acid content (FA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA) and the saturated fatty acid (SFA) of Symsagittifera roscoffensis cultured in different media (f/2, f/4, or plain seawater). Total fatty acid content, monounsaturated fatty acid, polyunsaturated fatty acid was significantly different when S. roscoffensis was grown in different media. Saturated fatty acid was not significantly different when S. roscoffensis was grown in different media. Asterisks indicate significant differences (ANOVA: * p < 0.05; ** p < 0.01; *** p < 0.001; ns not significant). n = 200 worms per experiment. Data are presented as % dry weight (DW; mean ± SE).

Table 2.
Fatty acids composition of Symsagittifera roscoffensis when grown in different media (f/2, f/4, or plain seawater).
The total fatty acid contents were significantly different among the treatments (ANOVA; p = 0.01): the worms kept in f/2 had the highest total fatty acid content at 7.5 ± 0.52% DW (mean ± SE), followed by those kept in f/4 (5.86 ± 0.41% DW) and seawater (3.69 ± 0.24% DW) (Figure 6). The PUFA contents of S. roscoffensis were also significantly different among the treatments (ANOVA; p < 0.001): it was higher in the f/2 treatment (2.77 ± 0.21% DW) than the f/4 treatment (2.2 ± 0.04% DW) and nearly absent in the seawater treatment (0.07 ± 0.01% DW) (Figure 6).
The fatty acid EPA (20:5n − 3) was present in the worms that were kept in the f/2 (1.75 ± 0.0% DW) and f/4 media (1.36 ± 0.0% DW), but it was nearly absent in the seawater treatment (0.01 ± 0.0% DW) (Table 2). DHA (22:6n − 3) was present, but only at 0.01% DW across all the treatments. ALA (18:3n − 3) was highest in the f/2 treatment (0.55 ± 1.7% DW), followed by the f/4 (0.45± 0.0% DW) and seawater treatments (0.02 ± 0.0% DW) (Table 2).
3.3. Feeding Trials with Freshwater Ornamental Fish
In the first set of freshwater fish feeding trials with live feeds (Figure 7), all the tested fish species consumed significantly more Artemia than the worms. Platy consumed the most worms, at 4.8 ± 1.2 (mean ± SE) worms ind−1, while Pearl danio consumed the least, at 1.0 ± 0.7 worms ind−1. The other fish species ate between one and three worms ind−1. When offered Artemia, Platy also consumed the most, at 10 ± 0 (mean ± SE) Artemia ind−1, Pearl danio consumed 9.5 ± 0.5 Artemia ind−1, and the other fish species consumed, on average, 8.6–8.8 Artemia ind−1.
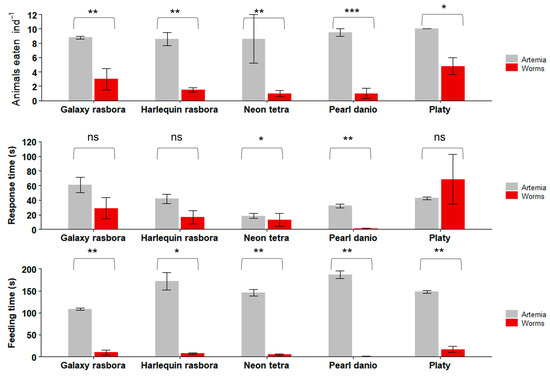
Figure 7.
Feeding trials with freshwater ornamental fishes. Data are plotted as mean ± SE. Asterisks indicate significant differences (Kruskal–Wallis: * p < 0.05; ** p < 0.01; *** p < 0.001; ns not significant). n = 10 fish per experiment.
Platy had a response time of 68.6 ± 34.1 s to the worm, which was not significantly different from its response time to Artemia (42.6 ± 1.7 s) (Kruskal–Wallis chi-squared = 19; p = 0.08). Pearl danio reacted much quicker at 1.1 ± 0.7 s, which was a significantly shorter response time (Kruskal–Wallis chi-squared = 19; p = 0.01) than that towards Artemia (32.1 ± 2.5 s). Harlequin rasbora also responded faster to the worm (16.8 ± 9.1 s) than to Artemia (41.8 ± 6.6 s), but this difference was not statistically significant (Kruskal–Wallis chi-squared = 12.667; p = 0.1). Galaxy rasbora had a longer response time to Artemia (61 ± 10.3 s) than to the worms (28.9 ± 14.7 s), but this difference was also not statistically significant (Kruskal–Wallis chi-squared = 17.1; p = 0.07).
All the tested fish species spent significantly more time eating the Artemia than the worm (Figure 7). Platy spent the longest time eating the worm (16.8 ± 7.4 s) and Pearl danio spent the shortest time (0.6 ± 0.4 s); the remaining fish species spent between 4.9 s and 9.8 s eating the worm. Platy, when offered Artemia, had a total feeding time of between 108.6 s and 186.8 s.
In the second set of experiments with repeated freshwater feeding trials (Figure 8), the Giant danio did not show a significant difference (Kruskal–Wallis chi-squared = 10.666; p = 0.09) in the number of live worms they consumed (9.5 ± 0.1 worms ind−1; mean ± SE) vs. live Artemia (7.5 ± 1.2 ind−1). Rosy barb also did not show a significant difference in the number of live animals consumed: 9.5 ± 0.1 worms ind−1 vs. 10 ± 0 Artemia ind−1 (Kruskal–Wallis chi-squared = 2.3824; p = 0.4). When offered freeze-dried feeds, Giant danio consumed 8 ± 0.1 dried worms ind−1 and 8.1 ± 0.1 dried Artemia ind−1. Rosy barb, on the other hand, consumed slightly more dried Artemia (10.0 ± 0.0 dried Artemia ind−1) than dried worms (9.6 ± 0.1 dried worms ind−1). However, the differences between the freeze-dried worms and freeze-dried Artemia were not statistically significant (Giant danio: Kruskal–Wallis chi-squared = 4.1943, p = 0.1; Rosy barbs: Kruskal–Wallis chi-squared = 4.3333, p = 0.1).
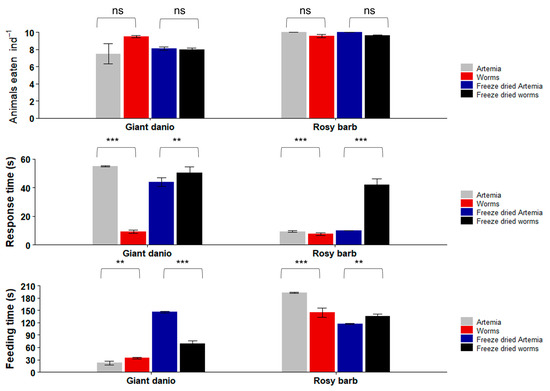
Figure 8.
Repeated feeding trials with Giant danio (Devario aequipinnatus) and Rosy barb (Pethia conchonius) with both live and freeze-dried feeds. Data are plotted as mean ± SE. Asterisks indicate significant differences (Kruskal–Wallis: ** p < 0.01; *** p < 0.001; ns not significant). n = 10 fish per experiment.
The response time of Giant danio to the live worms (9.3 ± 1.1 s) was significantly shorter than that to the live Artemia (54.9 ± 0.4 s) (Kruskal–Wallis chi-squared = 39; p > 0.001). The Giant danio response time to the dried Artemia (43.9 ± 2.9 s) was significantly shorter than its response time to the dried worms (50.3 ± 4.0 s) (Kruskal–Wallis chi-squared = 35.533; p = 0.01). Rosy barb responded to the live worms (7.5 ± 0.9 s) significantly faster than it did to the live Artemia (9.4 ± 0.6 s) (Kruskal–Wallis chi-squared = 26.331; p = 0.001), but it was the opposite when the dried feeds were compared (10.0 ± 0.0 s to dried Artemia; 42.1 ± 4.1 s to dried worms; Kruskal–Wallis chi-squared = 30.333; p = 0.004).
When comparing the total feeding times, Giant danio spent significantly more time eating live worms (34.3 ± 2.2 s) than live Artemia (22.6 ± 4.6 s) (Kruskal–Wallis chi-squared = 24.44; p = 0.006); but this was the opposite when dried feeds were used: 146.0 ± 2.2 s eating Artemia vs. 69.0 ± 6.6 s eating dried worms (Kruskal–Wallis chi-squared = 39; p < 0.001). Rosy barb spent significantly more time eating the live Artemia (193.5 ± 1.5 s) than the live worms (144.9 ± 11.2 s) (Kruskal–Wallis chi-squared = 39; p < 0.001), but the opposite was true when dried feeds were used (135.5 ± 5.4 s eating dried worms vs. 117.4 ± 1.6 s eating dried Artemia; Kruskal–Wallis chi-squared = 16.467; p = 0.05).
3.4. Feeding Trials with Marine Ornamental Fish
In the marine fish feeding trials (Figure 9), Striped blenny did not eat any of the worms, whereas the other tested fish species ate between 0.6 (Royal Gramma) and 3 (Firefish) worms ind−1. Artemia was consumed at 0.6–5 ind−1. There were no significant differences between the two live feeds for any of the tested marine fish (Kruskal–Wallis; p = 0.37 to 0.57), excluding Striped blenny.
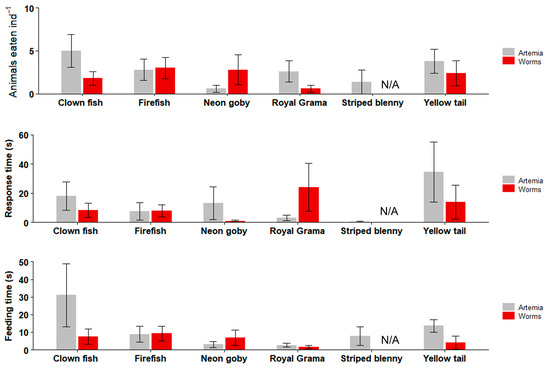
Figure 9.
Feeding trials with marine ornamental fishes. Data are plotted as mean ± SE. There were no significant differences between worm and Artemia treatments in terms of numbers of targets eaten, response time, and feeding time (Kruskal–Wallis: p > 0.05). n = 10 fish per experiment.
Except for the Striped blenny, which did not eat any worms, the response time to the worm was as short as 1 s (Neon goby) and ranged up to 24 s (Royal Grama). The response time to Artemia was between 3.2 (Royal gramma) and 34.6 s (Yellow tail), there were no significant differences between the two live feeds in terms of the response time for all of the tested marine fish (Kruskal–Wallis; p = 0.4 to 0.6), excluding Striped blenny.
The total time spent eating the worm ranged from 1.6 (Royal Gramma) to 9.2 s (Firefish) (excluding Striped blenny), and the total time spent eating the Artemia ranged from 3 (Neon gobies) to 31 s (Clown fish). There were no significant differences between the two live feeds for any of the marine fish tested (Kruskal–Wallis; p = 0.25 to 0.57), excluding the Striped blenny.
4. Discussion
The acoel flatworm S. roscoffensis forms a photosymbiotic relationship with the alga T. convolutae, providing the worm with all its required nutrition via photosynthesis. The Tetraselmis genus is known to be of a good nutritional value for aquaculture applications, and an adult S. roscoffensis is of a comparable size to common live feed for fish. These attributes make S. roscoffensis a good candidate as a novel feed for fish, but until now, there has been no information on its use as a fish feed or its nutritional contents. The purpose of this study was to determine the palatability of S. roscoffensis for ornamental fish and its nutritional profile. We also tested the worm’s ability to establish photosymbiosis with other algal species, which may open up new opportunities to customise the worm for different applications in aquaculture and biotechnology.
The freshwater fish feeding trials showed that all the tested fish species consumed the worm to some degree, and several species appeared to be more attracted to the worm than the Artemia, as indicated by the shorter response times. The fish species that ate the highest number of worms were Giant danio and Rosy barb. Giant danio is an omnivore that lives within fast-flowing water, where it feeds on a variety of insects and plant material [39]. Interestingly, in our experiment, Giant danio consumed 19% more live worms than Artemia per individual; it also consumed as many freeze-dried worms, doing so as quickly as it consumed the freeze-dried Artemia. Rosy barb is also omnivorous and it consumes a varied diet [40]. However, the Rosy barb took more time to consume the same number of live worms as the Giant danio, which may reflect the more passive feeding behaviour of Rosy barb. It also consumed nearly the same number of dried worms as dried Artemia. Taken together, our results suggest that S. roscoffensis was equally, if not more, accepted as a live or dried feed as Artemia by Giant danio and Rosy barb.
Harlequin rasbora consumed four times more Artemia than the worm in our experiment, reflective of its selective feeding behaviour [41]. The worm size (3–4 mm) being larger than the size of the Artemia (1–2 mm) may have imposed some limitation to its relatively small mouth. Galaxy rasbora forages for freshwater invertebrates in its natural habitat [42], which may explain its significantly higher consumption of Artemia, which resembles its natural prey, relative to the worm.
Neon tetra and Pearl danio consumed low numbers of worms, but continued to eat Artemia in comparable amounts to the other freshwater fish species. This was unexpected, since the other danio species in this experiment consumed the largest number of worms. We expected that the Pearl danio would also consume the worms in large amounts, as the diets of danio species tend to overlap, with the consumption of a range of insects and plant material [43]. Neon tetras are not known to be selective feeders and they regularly consume a variety of food when kept in aquaria; they are often recommended as ornamental fish for beginner hobbyists, as they have few special requirements [16]. However, the fish may not have recognised the worms as a food source, resulting in a lower number being eaten. It may also be the case that a repeated encounter could improve their familiarity and increase the number of worms consumed. Moreover, in an aquarium, there is usually a mix of species that would all have different feeding habits, which would ultimately affect their feeding behaviour.
In the marine fish feeding trials, Firefish did not behave any differently to the worm and Artemia, in terms of the animals eaten, response times, and feeding times. Firefish is omnivorous and its natural diet consists of small zooplankton and algae [44,45]; its non-selective feeding habits may have allowed it to consume the worm and Artemia equally well. Among the tested marine fish species, Neon goby had the quickest response to the worm, and it ate more worms than Artemia (albeit this was not statistically significant). In the wild, Neon goby acts as a cleaner fish, removing parasites from other fish. Symsagittifera roscoffensis may resemble the common nematode parasites in marine fish [46], due to a similarly shaped body size, thus triggering a feeding response from the Neon goby. Overall, all the tested marine fish species consumed small amounts of the worm, except for Striped blenny, although the Striped blenny did eat Artemia. Artemia is a common feed for many ornamentals within the aquaculture industry, including the pet store from which the fish were purchased and our in-house holding tanks. Long-term feeding with Artemia may result in the habituation of the fish to a particular feed, even though Artemia is not a natural prey for the fish in the wild. Habituation allows the fish to recognise Artemia as food and eat it more readily [47,48]. It is thought that, if the same fish were offered the worms repeatedly over a longer time period, they too would become habituated, resulting in a higher number of worms eaten. This habituation effect could also be responsible for the larger number of Artemia eaten by our freshwater fish, such as Pearl danios and Neon tetras.
Our data showed that the tested ornamental fish species ate the worm with no obvious ill effects. The differences in the numbers of worms consumed between the fish species could be due to the differences in the morphoanatomical mouth features of different fish species. As some fish found the worms palatable after processing, i.e., freeze dried. Processing the worm could be a method for improving the palatability for fish species with different morphoanatomical mouth features, thus increasing this palatability for a wider range of fish species. However, our results suggest that S. roscoffensis can be used as a suitable alternative feed in the ornamental fish trade. Because the worm is pre-enriched with Tetraselmis as algal symbionts, it can act as a “trophic short-cut” to delivering algal nutrition to the fish more efficiently and cost effectively.
The algae uptake experiments showed that S. roscoffensis successfully incorporated all the Tetraselmis species that we tested. On the other hand, the lack of uptake or ingestion of the non-Tetraselmis species further confirmed that S. roscoffensis lacked a functional digestive tract [26] and did not perform heterotrophic feeding, even in the absence of algal symbionts [28,33,49]. Our observations support some of the literature reports, in that different S. roscoffensis populations in the wild may contain different algal species [27,28], which indicates some flexibility for S. roscoffensis to establish symbiosis with different members of the Tetraselmis genus. However, we noticed that, while symbiosis did occur, the algae were not as abundant throughout the worm’s body as seen in the field-collected samples. Free-living Tetraselmis cells have to undergo drastic structural and physiological changes after being incorporated into S. roscoffensis, such as a loss of cell wall, eyespot, and flagella [31,35], which may require longer than our experimental time to proliferate throughout the worm’s upper epidermis and provide the host with its full nutritional needs. This may also have contributed to the low survival of the worms in the experiment. Longer experiments will be needed to test the stability and long-term physiological benefits of the different S. roscoffensis–Tetraselmis symbiotic pairings.
The nutritional profile of S. roscoffensis in the f/2 medium had similar protein and lipid contents to a closely related algal species, T. suecica grown on f/2 [50]: 38.7% DW of proteins and 12.4% DW of lipids. However, the worms had a much lower carbohydrate content (7.2% DW) than T. suecica (44.3% DW). The carbohydrates associated with the algal cell wall would have been lost as the cell wall was removed, along with other cell features when the cell was incorporated into the worm [31]. Furthermore, the carbohydrates in free-living algae can be used for storage as starch grains [51,52], but for symbiotic algae, excess carbohydrates may be metabolised by the host instead [32].
Without the addition of inorganic nutrients (seawater treatment), the chlorophyll content of S. roscoffensis became nil; its total fatty acid and PUFA concentrations also decreased significantly. The fatty acid compositions of S. roscoffensis, when grown in f/2 and f/4, both contained EPA and DHA, as well as ALA, which can be a precursor to both EPA and DHA [11,14]. Notably, EPA and DHA were close to nil and ALA was absent when S. roscoffensis was grown in seawater. This supports the notion that the host worm relies on algal symbionts for PUFAs via the energy captured from photosynthesis, but this process requires chlorophyll and inorganic nutrients [53]. Therefore, by absorbing inorganic nutrients from its surroundings, the host worm could support its algal symbionts for mutual benefits [54]. The algal symbionts also increased the carotenoid content of the host in the presence of nutrients, which could provide important photoprotection to the worm when it is exposed to strong sunlight in the intertidal environment [55,56].
5. Concluding Remarks
We consider our results to demonstrate the potential of S. roscoffensis as a novel feed—at least for the ornamental aquaculture industry—both in live and freeze-dried forms. The use of S. roscoffensis does not need enrichment and can deliver algae directly to fish species. All the freshwater and marine ornamental fish tested (except Striped blenny) consumed S. roscoffensis and displayed no adverse effects. Additionally, we performed the first nutritional analysis of S. roscoffensis, and in doing so, revealed the presence of ALA, EPA, and DHA, which are essential for fish health [6,7]. Microalgal cells are too small for ornamental fish to directly consume, and the presence of the algal cell wall also reduces digestibility [57,58]. The lack of cell walls in the symbiotic algae within S. roscoffensis theoretically improves its digestibility for fish.
Our own cultures have been maintained for over a year, with only one inoculation of worms collected from the wild and no addition of algae was required. Due to the way the algae are acquired by juvenile S. roscoffensis, no additional algal input would be required for the juveniles to maintain symbiosis in long-term cultures. Therefore, a continuous culture could be maintained with limited inputs. This simplifies the feed production process and reduces waste and the associated costs.
Author Contributions
Conceptualization: N.J.T., K.W.T. and C.J.C.; methodology: N.J.T., K.W.T. and C.J.C.; formal analysis: N.J.T. and C.J.C.; investigation: N.J.T.; resources: K.W.T.; data curation: N.J.T.; writing—original draft: N.J.T. and K.W.T.; writing—review and editing: N.J.T., C.J.C. and K.W.T.; supervision: C.J.C. and K.W.T.; project administration: C.J.C. and K.W.T. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded in part by HEFCW/Swansea University RWIF (RIG1036-125) awarded to C.J.C. and K.W.T.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request to the corresponding author.
Acknowledgments
We thank Rahul Kapoore for assisting with the nutritional analysis, and the Centre for Sustainable Aquatic Research, (notably Craig Pooley) Swansea University for technical assistance and access to the RAS systems.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The Observatory of Economic Complexity. Ornimental live fish. Available online: https://oec.world/en/profile/hs/ornamental-fish (accessed on 1 September 2022).
- Fortune Business Insights. Pet care market size, share & COVID-19 Impact analysis. Available online: https://www.fortunebusinessinsights.com/pet-care-market-104749 (accessed on 1 September 2022).
- OATA. Ornamental Aquatic Trade Association (OATA); Annual review 2018/19; OATA: Westbury, UK, 2019; Volume 11. [Google Scholar]
- Riehl, R.; Baensch, H.A. Aquarium Atlas; Microcosm: Portland, OR, USA, 1997; ISBN 1564651142. [Google Scholar]
- Andrews, C. The Ornamental Fish Trade and Fish Conservation. J. Fish Biol. 1990, 37, 53–59. [Google Scholar] [CrossRef]
- Benemann, J.R. Microalgae Aquaculture Feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Kumar, R.R.; Ganesan, V.; Anbazhagan, C. Microalgae: A Sustainable Feed Source for Aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional Properties of Microalgae for Mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Pratiwy, F.M.; Pratiwi, D.Y. The Potentiality of Microalgae as a Source of DHA and EPA for Aquaculture Feed: A Review. Int. J. Fish. Aquat. Stud. 2020, 8, 39–41. [Google Scholar]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Chini Zittelli, G.; Sampietro, G. Energy Balance of Algal Biomass Production in a 1-Ha “Green Wall Panel” Plant: How to Produce Algal Biomass in a Closed Reactor Achieving a High Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Oboh, A.; Kabeya, N.; Carmona-Antoñanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two Alternative Pathways for Docosahexaenoic Acid (DHA, 22:6n-3) Biosynthesis Are Widespread among Teleost Fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical Marine Macroalgae as Potential Sources of Nutritionally Important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Colombo, M.L.; Risè, P.; Giavarini, F.; De Angelis, L.; Galli, C.; Bolis, C.L. Marine Macroalgae as Sources of Polyunsaturated Fatty Acids. Plant Foods Hum. Nutr. 2006, 61, 67–72. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of Conversion of Alpha-Linolenic Acid to Long Chain n-3 Fatty Acids in Man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Dani, D.; Thirugnanamurthy, S.; Kandasamy, S.; Shalini, B.; Baruah, A.; Kirubasankar, R.; Dam Roy, S. A Review on Microalgae as Potential Fish Feed Ingredient. J. Andaman Sci. Assoc. 2016, 21, 140–144. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Burr, G.S.; Barrows, F.T.; Gaylord, G.; Wolters, W.R. Apparent Digestibility of Macro-Nutrients and Phosphorus in Plant-Derived Ingredients for Atlantic Salmon, Salmo Salar and Arctic Charr, Salvelinus Alpinus. Aquac. Nutr. 2011, 17, 570–577. [Google Scholar] [CrossRef]
- Tibaldi, E.; Chini Zittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth Performance and Quality Traits of European Sea Bass (D. labrax) Fed Diets Including Increasing Levels of Freeze-Dried Isochrysis Sp. (T-ISO) Biomass as a Source of Protein and n-3 Long Chain PUFA in Partial Substitution of Fish Derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Abatzopoulos, T.J.; Beardmore, J.; Clegg, J.S.; Sorgeloos, P. Artemia: Basic and Applied Biology; Springer Science & Business Media: Cham, Switzerland, 2002; Volume 1, ISBN 940170791X. [Google Scholar]
- Coutteau, P.; Lavens, P.; Leger, P.; Sorgeloos, P. Manipulated Yeast Diets as a Partial Algal Substitute for Rearing Bivalve Molluscs Laboratory Trials with Tapes-Semidecussata. Meded. Fac. Landbouwwet. Univ. Gent 1990, 55, 1597–1600. [Google Scholar]
- Treece, G.D. Artemia Production for Marine Larval Fish Culture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2000; Volume 702. [Google Scholar]
- Van Stappen, G.; Sui, L.; Hoa, V.N.; Tamtin, M.; Nyonje, B.; de Medeiros Rocha, R.; Sorgeloos, P.; Gajardo, G. Review on Integrated Production of the Brine Shrimp Artemia in Solar Salt Ponds. Rev. Aquac. 2020, 12, 1054–1071. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Lindeman, R.L. The Trophic-Dynamic Aspect of Ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Thomas, N.J.; Coates, C.J.; Tang, K.W. Environmental Constraints on the Photosynthetic Rate of the Marine Flatworm Symsagittifera Roscoffensis. J. Exp. Mar. Bio. Ecol. 2023, 558, 151830. [Google Scholar] [CrossRef]
- Bailly, X.; Laguerre, L.; Correc, G.; Dupont, S.; Kurth, T.; Pfannkuchen, A.; Entzeroth, R.; Probert, I.; Vinogradov, S.; Lechauve, C. The Chimerical and Multifaceted Marine Acoel Symsagittifera Roscoffensis: From Photosymbiosis to Brain Regeneration. Front. Microbiol. 2014, 5, 498. [Google Scholar] [CrossRef]
- Mcfarlane, A.E. Two Species of Algal Symbiont in Naturally Occurring Populations of Convoluta Roscoffensis. J. Mar. Biol. Assoc. UK 1982, 62, 235. [Google Scholar] [CrossRef]
- Arboleda, E.; Hartenstein, V.; Martinez, P.; Reichert, H.; Sen, S.; Sprecher, S.; Bailly, X. An Emerging System to Study Photosymbiosis, Brain Regeneration, Chronobiology, and Behavior: The Marine Acoel Symsagittifera Roscoffensis. BioEssays 2018, 40, 1800107. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Experimental Studies on Egg Production by Convoluta roscoffensis: Graff, 1882 (Turbellaria, Acoela). Hydrobiologia 1983, 102, 151–154. [Google Scholar] [CrossRef]
- Provasoli, L.; Yamasu, T.; Manton, I. Experiments on the Resynthesis of Symbiosis in Convoluta Roscoffensis with Different Flagellate Cultures. J. Mar. Biol. Assoc. UK 1968, 48, 465–478. [Google Scholar] [CrossRef]
- Douglas, A.E. Establishment of the Symbiosis in Convoluta roscoffensis. J. Mar. Biol. Assoc. UK 1983, 63, 419–434. [Google Scholar] [CrossRef]
- Boyle, J.E.; Smith, D.C. Biochemical Interactions between the Symbionts of Convoluta roscoffensis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1975, 189, 121–135. [Google Scholar]
- Jennings, J.B. Parasitism and Commensalism in the Turbellaria. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 9, pp. 1–32. ISBN 0065-308X. [Google Scholar]
- Meyer, H.; Provasoli, L.; Meyer, F. Lipid Biosynthesis in the Marine Flatworm Convoluta Roscoffensis and Its Algal Symbiont Platymonas Convoluta. Biochim. Biophys. Acta 1979, 573, 464–480. [Google Scholar] [CrossRef]
- Douglas, A.E. Growth and Reproduction of Convoluta roscoffensis Containing Different Naturally Occurring Algal Symbionts. J. Mar. Biol. Assoc. UK 1985, 65, 871–879. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Van Hille, R.P.; Harrison, S.T.L. Selection of Direct Transesterification as the Preferred Method for Assay of Fatty Acid Content of Microalgae. Lipids 2010, 45, 1053–1060. [Google Scholar] [CrossRef]
- Mahanty, A.; Ranjan Maji, S.; Ganguly, S.; Mohanty, B.P. GC-MS Fingerprinting of Fatty Acids of Freshwater Mollusc Lamellidens marginalis Using Different Columns; TR-WaxMS and TR-FAME. J. Anal. Bioanal. Tech. 2015, 6, 238. [Google Scholar] [CrossRef]
- Chen, Y.; Vaidyanathan, S. Simultaneous Assay of Pigments, Carbohydrates, Proteins and Lipids in Microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef]
- Dey, S.; Ramanujam, S.N.; Mahapatra, B.K. Breeding and Development of Ornamental Hill Stream Fish Devario Aequipinnatus (McClelland) in Captivity. Int. J. Fish. Aquat. Stud. 2014, 1, 1–7. [Google Scholar]
- De Magalhães, A.L.B.; Jacobi, C.M. Invasion Risks Posed by Ornamental Freshwater Fish Trade to Southeastern Brazilian Rivers. Neotrop. Ichthyol. 2013, 11, 433–441. [Google Scholar] [CrossRef]
- Robert Woods Fishkeeping World. Available online: https://www.fishkeepingworld.com/harlequin-rasbora/#h24 (accessed on 13 July 2022).
- Roberts, T.R. The “Celestial Pearl Danio”, a New Genus and Species of Colourful Minute Cyprinid Fish from Myanmar (Pisces: Cypriniformes). Raffles Bull. Zool. 2007, 55, 131–140. [Google Scholar]
- McClure, M.M.; McIntyre, P.B.; McCune, A.R. Notes on the Natural Diet and Habitat of Eight Danionin Fishes, Including the Zebrafish Danio rerio. J. Fish Biol. 2006, 69, 553–570. [Google Scholar] [CrossRef]
- Estelita, E.C. Nemateleotris Magnifica, Fire Goby: Aquarium. Available online: https://www.fishbase.se/summary/6629 (accessed on 1 September 2022).
- Kuiter, R.H.; Tonozuka, T. Pictorial Guide to: Indonesian Reef Fishes, Part 3, Jawfishes–Sunfishes, Opistognathidae–Molidae. Zoonetics 2001, 48637, 623–893. [Google Scholar]
- Moravec, F. Some Aspects of the Taxonomy and Biology of Adult Spirurine Nematodes Parasitic in Fishes: A Review. Folia Parasitol. 2007, 54, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, G.; Bui, S.; Oppedal, F.; Dempster, T. Challenges and Benefits of Applying Fish Behaviour to Improve Production and Welfare in Industrial Aquaculture. Rev. Aquac. 2021, 13, 934–948. [Google Scholar] [CrossRef]
- Malison, J.A.; Held, J.A. Habituating pond-reared fingerlings to formulated feed. In Walleye Culture Manual; NCRAC Culture Series 101; North Central Regional Aquaculture Center Publications Office: Ames, IA, USA, 1996; pp. 199–204. [Google Scholar]
- Mamkaev, Y.V.; Kostenko, A.G. On the Phylogenetic Significance of Sagittocysts and Copulatory Organs in Acoel Turbellarians. Hydrobiologia 1991, 227, 307–314. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Abbas, E.; Ashry, O.; Helal, M.; Nazmi, H.; Kelany, M.; Kamel, A.; Hassaan, M.; Rossi, W.; et al. Effects of Dietary Marine Microalgae, Tetraselmis Suecica, on Production, Gene Expression, Protein Markers and Bacterial Count of Pacific White Shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 2216–2228. [Google Scholar] [CrossRef]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Li, X.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving Carbohydrate and Starch Accumulation in Chlorella Sp. AE10 by a Novel Two-Stage Process with Cell Dilution. Biotechnol. Biofuels 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 205–224. ISBN 9789400710382. [Google Scholar]
- Taylor, D.L. Nutrition of Algal-Invertebrate Symbiosis. I. Utilization of Soluble Organic Nutrients by Symbiont-Free Hosts. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1974, 186, 357–368. [Google Scholar]
- Carvalho, L.F.; Rocha, C.; Fleming, A.; Veiga-Pires, C.; Aníbal, J. Interception of Nutrient Rich Submarine Groundwater Discharge Seepage on European Temperate Beaches by the Acoel Flatworm, Symsagittifera Roscoffensis. Mar. Pollut. Bull. 2013, 75, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional Properties of Carotenoids Originating from Algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.; Shcherbakov, D.; Heyer, A.; Brümmer, F.; Schill, R.O. Behaviour of the Plathelminth Symsagittifera Roscoffensis under Different Light Conditions and the Consequences for the Symbiotic Algae Tetraselmis Convolutae. J. Exp. Biol. 2015, 218, 1693–1698. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).