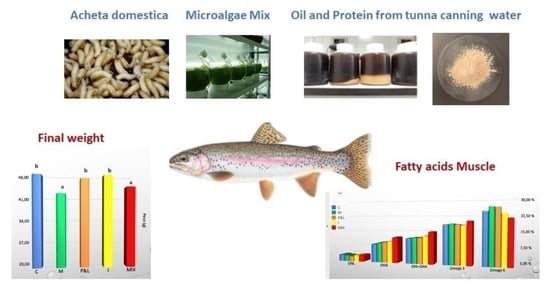
Effects of Alternative and Sustainable Ingredients on Rainbow Trout (Oncorhynchus mykiss) Growth, Muscle Composition and Health
Abstract
:1. Introduction
2. Material and Methods
2.1. Feed Formulation
2.2. Experimental Procedure
2.3. Growth Performance and Somatic Indices
2.4. Biochemical Analyses
2.5. Statistical Analyses
3. Results
3.1. Effect of Alternative Diets on Growth Performance
3.2. Effect of Alternative Diets on Muscle Composition
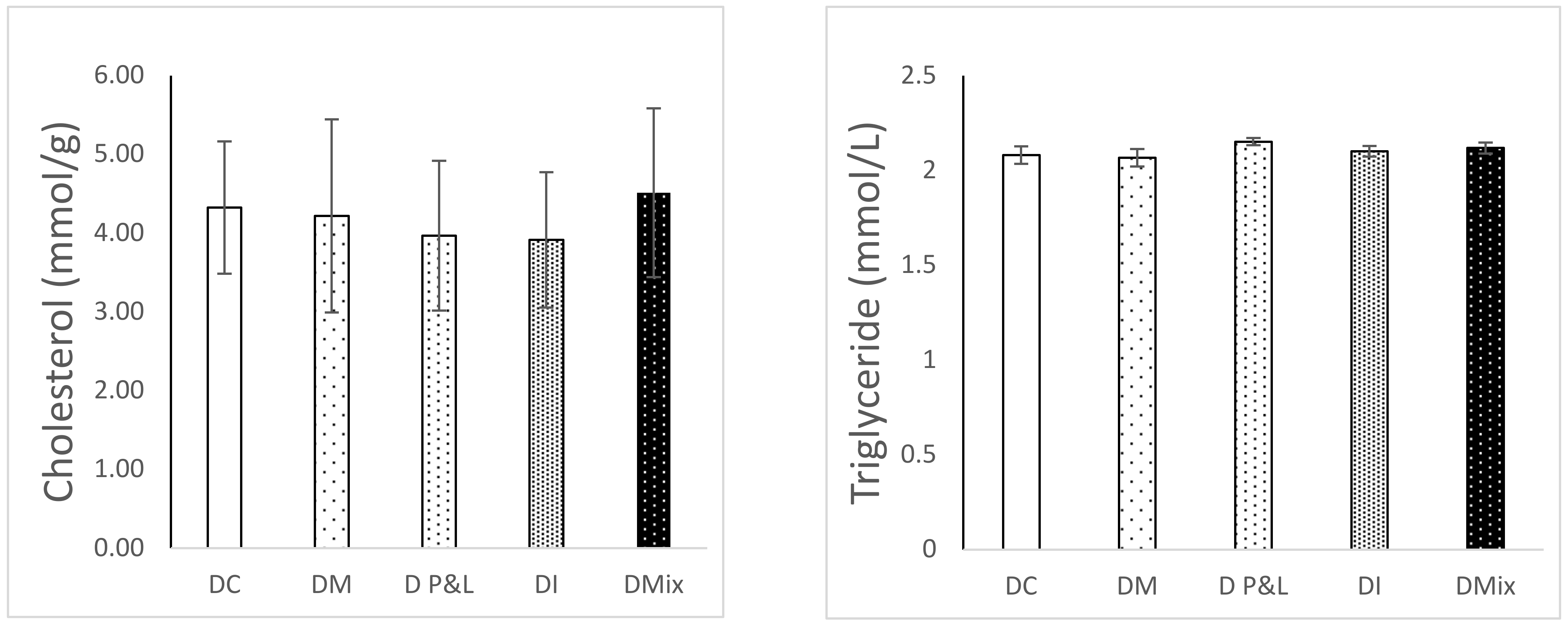
3.3. Effect of Alternative Diets on Fish Health
4. Discussion
4.1. Insect Meal
4.2. Microalgae
4.3. P&L Diet
4.4. DMIX Diet
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandara, T. Alternative feed ingredients in aquaculture: Opportunities and challenges. J. Entomol. Zool. Stud. 2018, 6, 3087–3094. [Google Scholar]
- Konar, M.; Qiu, S.; Tougher, B.; Vause, J.; Tlusty, M.; Fitzsimmons, K.; Barrows, R.; Cao, L. Illustrating the hidden economic, social and ecological values of global forage fish resources. Resour. Conserv. Recycl. 2019, 151, 104456. [Google Scholar] [CrossRef]
- Hodar, A.R.; Vasava, R.J.; Mahavadiya, D.R.; Joshi, N.H. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources a review. J. Exp. Zool. India 2019, 23, 13–21. [Google Scholar]
- Kalhoro, H.; Zhou, J.; Hua, Y.; Ng, W.K.; Ye, L.; Zhang, J.; Shao, Q. Soy protein concentrate as a substitute for fish meal in diets for juvenile Acanthopagrus schlegelii: Effects on growth, phosphorus discharge and digestive enzyme activity. Aquac. Res. 2018, 49, 1896–1906. [Google Scholar] [CrossRef]
- FAO. El Estado Mundial de la Pesca y la Acuicultura 2018. Cumplir los Objetivos de Desarrollo Sostenible; FAO: Rome, Italy, 2018. [Google Scholar]
- Samuelsen, T.A.; Oterhal, Å.; Kousoulaki, K. High lipid microalgae (Schizochytrium sp.) inclusion as a sustainable source of n-3 long-chain PUFA in fish feed—Effects on the extrusion process and physical pellet quality. Anim. Feed. Sci. Technol. 2018, 236, 14–28. [Google Scholar] [CrossRef]
- Gong, Y.; Bandara, T.; Huntley, M.; Johnson, Z.I.; Dias, J.; Dahle, D.; Sørensen, M.; Kiron, V. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 2019, 501, 455–464. [Google Scholar] [CrossRef]
- Berge, G.M.; Grisdale-Helland, B.; Helland, S.J. Soy protein concentrate in diets for Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 1999, 178, 139–148. [Google Scholar] [CrossRef]
- Aksnes, A.; Hope, B.; Albrektsen, S. Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets. II: Flesh quality, absorption, retention and fillet levels of taurine and anserine. Aquaculture 2006, 261, 318–326. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Ai, Q.; Zhang, W.; Wang, X.; Xu, W. Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder (Paralichthys olivaceus). Aquaculture 2006, 258, 503–513. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Biancarosa, I.; Sele, V.; Belghit, I.; Ørnsrud, R.; Lock, I.J.; Amlund, H. Replacing fish meal with insect meal in the diet of Atlantic salmon (Salmo salar) does not impact the amount of contaminants in the feed and it lowers accumulation of arsenic in the fillet. Food Addit. Contam. Part A 2019, 36, 1191–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, P.K.; Kapuscinski, A.R.; Vandenberg, G.W.; Proulx, E.; Sitek, A.J. Towards sustainable and ocean-friendly aquafeeds: Evaluating a fish-free feed for rainbow trout (Oncorhynchus mykiss) using three marine microalgae species. Elem. Sci. Anthr. 2020, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Shaikhiev, I.; Sverguzova, S.; Sapronova, J.; Danshina, E. Using intact and minced Hermetia illucens larvae as a fodder in aquaculture (review of foreign literature). Fisheries 2020, 2020, 86–92. [Google Scholar] [CrossRef]
- García-Sanda, E.; Omil, F.; Lema, J. Clean production in fish canning industries: Recovery and reuse of selected wastes. Clean Technol. Env. Policy 2003, 5, 289–294. [Google Scholar] [CrossRef]
- Estevez, A.; Padrell, L.; Iñarra, B.; Orive, M.; san Martin, D. Brewery by-products (yeast and spent grain) as protein sources in gilthead seabream (Sparus aurata) feeds. Aquaculture 2021, 543, 736921. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Orawattanamateekul, W.; Sentina, J.; Srinivasa Gopal, T.K. By-Products of Tuna Processing. Globefish Research Programme; FAO: Rome, Italy, 2013; Volume 112, 48p, Available online: https://www.fao.org/3/bb215e/bb215e.pdf (accessed on 21 February 2022).
- Bechtel, P.J. Properties of stickwater from fish processing byproducts. J. Aquat. Food Prod. Technol. 2005, 14, 25–38. [Google Scholar] [CrossRef]
- Valdez-Hurtado, S.; Goycolea-Valencia, F.; Márquez-Ríos, E.; Pacheco-Aguilar, R. Effect of complementary centrifugation on chemical and rheological composition from stickwater. Biotecnia 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Jao, C.L.; Ko, W.C. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolysates from tuna cooking juice. Fish. Sci. 2002, 68, 430–435. [Google Scholar] [CrossRef]
- Tremblay, A.; Corcuff, R.; Goulet, C.; Godefroy, S.B.; Doyen, A.; Beaulieu, L. Valorization of snow crab (Chionoecetes opilio) cooking effluents for food applications. J. Sci. Food Agric. 2020, 100, 384–393. [Google Scholar] [CrossRef]
- Martínez-Montaño, E.; Osuna-Ruíz, I.; Benítez-García, I.; Osuna, C.O.; Pacheco-Aguilar, R.; Navarro-Peraza, R.S.; Salazar-Leyva, J.A. Biochemical and Antioxidant Properties of Recovered Solids with pH Shift from Fishery Effluents (Sardine Stickwater and Tuna Cooking Water). Waste Biomass Valoriz. 2020, 12, 1901–1913. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Gilderhus, P.A.; Marking, L.L. Comparative efficacy of 16 anesthetic chemicals on Rainbow Trout. N. Am. J. Fish. Manag. 1987, 7, 288–292. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterloine, N.A.; Little, D.C.; Davies, S.J. Fish and feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aqua-culture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Gas Chromatography. The International Organization for Standardization: Geneva, Switzerland, 2015. Available online: www.iso.org/standard/35454.html (accessed on 17 January 2018).
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; de la Hoz, L. Fatty acid compositions of selected varieties of Spanish ham related to their nutritional implication. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. 2020. Available online: https://www.fao.org/state-of-fisheries-aquaculture/en/ (accessed on 25 January 2019).
- Tacon, A.G.J.; Hasan, M.R.; Metian, M. Demand and supply of feed ingredients for farmed fish and crustaceans: Trends and prospects. In FAO Fisheries Technical Paper; FAO: Rome, Italy, 2011; Volume 564, p. 87. [Google Scholar]
- Davies, S.J.; Laporte, J.; Gouveia, A.; Salim, H.S.; Woodgate, S.M.; Hassaan, M.S.; El-Haroun, E.R. Validation of processed animal proteins (mono-PAPS) in experimental diets for juvenile gilthead sea bream (Sparus aurata L.) as primary fish meal replacers within a European perspective. Aquac. Nutr. 2019, 25, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, N.; Klinger, D.H.; Sims, N.A.; Yoshioka, J.R.; Kittinger, J.N. Nutritional attributes, substitutability, scalability, and environmental intensity of an illustrative subset of current and future protein sources for aquaculture feeds: Joint consideration of potential synergies and trade-offs. Environ. Sci. Technol. 2018, 52, 5532–5544. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Waagbø, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, Å.; Lock, E.J. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- Stamer, A. Insect proteins—A new source for animal feed. EMBO Rep. 2015, 16, 676–680. [Google Scholar] [CrossRef] [Green Version]
- IPIFF. The International Platform of Insects for Food and Feed. 2019. Available online: http://ipiff.org/ (accessed on 24 March 2021).
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Kiron, V.; Phromkunthong, W.; Huntley, M.; Archibald, I.; de Scheemaker, G. Marine micro-algae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012, 18, 521–531. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in aquaculture: A rview with special references to Nutritional value and fish dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Nazzaro, J.; Martin, D.S.; Perez-Vendrell, A.M.; Padrell, L.; Iñarra, B.; Orive, M.; Estévez, A. Apparent digestibility coefficients of brewer’s by-products used in feeds for rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata). Aquaculture 2020, 530, 735796. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Gonçalves, P. Partial replacement of fishmeal by brewers yeast (Saccharomyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2001, 202, 269–278. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, S.; Zou, T.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S.; Zhou, W. Effects of dietary yeast culture on growth performance. immune response and disease resistance of gibel carp (Carassius auratus gibelio CAS). Fish Shellfish Immunol. 2018, 82, 400–407. [Google Scholar] [CrossRef]
- Trushenski, J.; Gause, B. Comparative Value of Fish Meal Alternatives as Protein Sources in Feeds for Hybrid Striped Bass. N. Am. J. Aquac. 2013, 75, 329–341. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Richard, N.; Sergent, L.; Kurz, F.; Skiba-Cassy, S.; Fauconneau, B.; Moing, A. Characterizing alternative feeds for rainbow trout (O. mykiss) by 1H NMR metabolomics. Metabolomics 2018, 14, 155. [Google Scholar] [CrossRef] [Green Version]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 2017, 3, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Józefiak, A.; Nogales-Mérida, S.; Mikołajczak, Z.; Rawski, M.; Kierończyk, B.; Mazurkiewicz, J. The utilization of full-fat insect meal in rainbow trout (Oncorhynchus mykiss) nutrition: The ef-fects on growth performance, intestinal microbiota and gastrointestinal tract histomorphology. Ann. Anim. Sci. 2019, 19, 747–765. [Google Scholar] [CrossRef] [Green Version]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, W.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Acar, U.; Giannetto, A.; Giannetto, D.; Kesbiç, O.S.; Yilmaz, S.; Romano, A.; Tezel, R.; Türker, A.; Güllü, K.; Fazio, F. Evaluation of an innovative and sustainable pre-commercial compound as replacement of fish meal in diets for rainbow trout during pre-fattening phase: Effects on growth per-formances, haematological parameters and fillet quality traits. Animals 2021, 11, 3547. [Google Scholar] [CrossRef] [PubMed]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio Molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Skalli, A.; Firmino, J.P.; Andree, K.B.; Salomón, R.; Estévez, A.; Puig, P.; Gisbert, E. The in-clusion of the microalga Scenedesmus sp. in diets for rainbow trout, Onchorhynchus mykiss, juveniles. Animals 2020, 10, 1656. [Google Scholar] [CrossRef]
- Yarnold, J.; Karan, H.; Oey, M.; Hankamer, B. Microalgal aquafeeds as part of a circular bioeconomy. Trends Plant Sci. 2019, 24, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, M.K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; McKuin, B.; Fitzgerald, D.S.; Nash, H.M.; Greenwood, C. Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of dietary supplement of Spirulina platensis on blood carotenoid concentration and fillet color stability in rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 415, 224–228. [Google Scholar] [CrossRef]
- Dallaire, V.; Lessard, P.; Vandenberg, G.; de la Noüe, J. Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry. Bioresour. Technol. 2007, 98, 1433–1439. [Google Scholar] [CrossRef]
- Goddard, S.; Al-Shagaa, G.; Ali, A. Fisheries by-catch and processing waste meals as ingre-dients in diets for Nile tilapia, Oreochromis niloticus. Aquac. Res. 2008, 39, 518–525. [Google Scholar] [CrossRef]
- Saïdi, S.A.; Azaza, M.S.; Abdelmouleh, A.; van Pelt, J.; Kraïem, M.M.; El-Feki, A. The use of tuna industry waste in the practical diets of juvenile Nile tilapia (Oreochromis niloticus, L.): Effect on growth performance, nutrient digestibility and oxidative status. Aquac. Res. 2010, 41, 1875–1886. [Google Scholar] [CrossRef]
- Hernández, C.; Olvera-Novoa, M.A.; Smith, D.M.; Hardy, R.W.; González-Rodríguez, B. Enhancement of shrimp Litopenaeus vannamei diets based on terrestrial protein sources via the inclusion of tuna by-product protein hydrolysates. Aquaculture 2011, 317, 117–123. [Google Scholar] [CrossRef]
- Kim, K.D.; Jang, J.W.; Kim, K.W.; Lee, B.J.; Hur, S.W.; Han, H.S. Tuna by-product meal as a dietary protein source replacing fishmeal in juvenile Korean rockfish Sebastes schlegeli. Fish. Aquat. Sci. 2018, 21, 29. [Google Scholar] [CrossRef]
- Jeon, G.H.; Kim, H.S.; Hyung, S.H.; Cho, S.H. The effect of the dietary substitution of fishmeal with tuna by-product meal on growth, body composition, plasma chemistry and amino acid profiles of juvenile Korean rockfish (Sebastes schlegeli). Aquac. Nutr. 2014, 20, 753–761. [Google Scholar] [CrossRef]
- Kim, H.S.; Jung, W.G.; Myung, S.H.; Cho, S.H.; Kim, D.S. Substitution effects of fishmeal with tuna byproduct meal in the diet on growth, body composition, plasma chemistry and amino acid profiles of juvenile olive flounder (Paralichthys olivaceus). Aquaculture 2014, 431, 92–98. [Google Scholar] [CrossRef]
- Tekenay, A.A.; Deveciler, E.; Güroy, D. Effects of dietary tuna by-products on feed intake and utilization of rainbow trout Oncorhychus mykiss. J. Fish. Int. 2009, 4, 8–12. [Google Scholar]
- Oncul, F.O.; Aya, F.A.; Hamidoghli, A.; Won, S.; Lee, G.; Han, K.R.; Bai, S.C. Effects of the dietary fermented tuna by-product meal on growth, blood parameters, nonspecific immune response, and disease resistance in juvenile olive flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2019, 50, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.M.; Kim, K.W.; Lee, S.M. Evaluation of rice distillers dried grain as a partial re-placement for fish meal in the practical diet of the juvenile olive flounder Paralichthys olivaceus. Fish. Aquat. Sci. 2015, 18, 151–158. [Google Scholar]
- Sadekarpawar, S.; Parikh, P. Gonadosomatic and hepatosomatic indices of freshwater fish Oreochromis mossambicus in response to a plant nutrient. World J. Zool. 2013, 8, 110–118. [Google Scholar]
- Mikołajczak, Z.; Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. The effect of hydrolyzed insect meals in sea trout fingerling (Salmo trutta m. trutta) diets on growth performance, microbiota and biochemical blood parameters. Animals 2020, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Kostyniuk, D.J.; Culbert, B.M.; Mennigen, J.A.; Gilmour, K.M. Social status affects lipid metabolism in rainbow trout, Oncorhynchus mykiss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubay, G. Biochemistry, 3rd ed.; Brown Publishers: Dallas, TX, USA, 1993; ISBN 0-697-14267-5. [Google Scholar]
- Spiller, G.A. Handbook of Lipids in Human Nutrition; CRS Press, Inc.: Boca Raton, FL, USA, 1996; Volume 33431, p. 54. [Google Scholar]
- Palmegiano, G.B.; Daprá, F.; Forneris, G.; Gai, F.; Gasco, L.; Guo, K.; Peiretti, P.G.; Sicuro, B.; Zocaratto, I. Rice protein concentrate meal as a potential ingredient in practical diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 258, 357–367. [Google Scholar] [CrossRef]
- Garaffo, M.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar]

| Ingredients | DC | DI | DM | DP&L | DMIX |
|---|---|---|---|---|---|
| Squid meal 1 | 8.32 | - | - | - | 8.87 |
| Fish meal 2 | 31.59 | 15.72 | 16.50 | 23.83 | 15.00 |
| Insect meal | - | 15.00 | - | - | 15.00 |
| Microalgae | - | - | 10.00 | - | 10.00 |
| Protein (TWC) | - | - | - | 7.00 | 4.00 |
| Oil (TWC) | - | - | - | 8.00 | 8.00 |
| Starch 1 | 4.50 | 0.79 | 8.11 | 5.75 | 6.13 |
| Wheat gluten 3 | 15.00 | 15.00 | 18.00 | 15.00 | 14.48 |
| Wheat 4 | 15.60 | 15.00 | 5.00 | 10.00 | 10.00 |
| Soy bean 5 | 0.00 | 14.40 | 0.00 | 9.70 | 0.00 |
| Fish oil 6 | 4.00 | 8.00 | 8.00 | - | - |
| Krill oil | 4.00 | - | - | - | - |
| Plant oil 4 | 11.65 | 9.85 | 12.03 | 12.84 | 6.00 |
| AA mix (Aminopro) 7 | 3.00 | 3.00 | 4.14 | 5.00 | - |
| Lysine 8 | 1.00 | 1.00 | 1.00 | 0.66 | 0.75 |
| Threonine 8 | 0.42 | 0.43 | 0.69 | 0.70 | 0.85 |
| Methionine 8 | - | - | - | - | 0.20 |
| Choline 9 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Antibacterian 10 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Antifungal 10 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Antioxidant 11 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Attractant 12 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Anhydrous betaine 13 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Vitamin Conc. 14 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Organic mineral Conc. 14 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin C 14 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Composition (% DW) | DC | DI | DM | DPL | DMIX |
|---|---|---|---|---|---|
| Moisture | 5.71 | 6.18 | 6.49 | 5.72 | 6.08 |
| Protein | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 |
| Fat | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 |
| Fibre | 0.86 | 1.67 | 0.50 | 1.34 | 0.47 |
| Calcium | 1.09 | 1.10 | 1.40 | 1.25 | 1.18 |
| Phosphorus | 1.01 | 1.00 | 1.00 | 1.03 | 1.05 |
| Met | 1.04 | 0.83 | 1.00 | 0.86 | 0.90 |
| Met + Cys | 1.60 | 1.34 | 1.49 | 1.40 | 1.30 |
| Lys | 3.27 | 2.89 | 3.21 | 2.73 | 2.70 |
| Thre | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| Arg | 2.41 | 2.16 | 2.36 | 2.14 | 1.95 |
| His | 1.09 | 0.86 | 0.96 | 1.04 | 0.85 |
| Trp | 0.40 | 0.35 | 0.41 | 0.37 | 0.29 |
| Val | 2.39 | 2.10 | 2.14 | 2.16 | 1.80 |
| Fatty Acids (%TFA) | DC | DI | DM | DP&L | DMIX |
|---|---|---|---|---|---|
| 14:0 | 3.11 | 2.02 | 2.85 | 2.10 | 2.79 |
| 15:0 | 0.25 | 0.22 | 0.59 | 0.40 | 0.58 |
| 16:0 | 12.97 | 14.08 | 18.12 | 12.52 | 17.72 |
| 18:0 | 3.05 | 3.64 | 4.42 | 3.53 | 4.37 |
| Total saturated (SFA) | 21.38 | 21.28 | 27.71 | 19.80 | 27.16 |
| 16:1 | 3.37 | 2.53 | 3.50 | 2.51 | 3.41 |
| 18:1n-9 | 20.58 | 22.09 | 16.98 | 19.49 | 17.09 |
| 18:1n-7 | 3.37 | 2.98 | 2.79 | 2.85 | 2.90 |
| 20:1 | 0.13 | 0.52 | 0.44 | 0.44 | 0.43 |
| 24:1 | 0.21 | 0.21 | 0.28 | 0.23 | 0.30 |
| Total monounsaturated (MUFA) | 28.35 | 28.93 | 24.81 | 25.97 | 24.68 |
| 18:2n-6 | 26.54 | 27.03 | 15.99 | 27.02 | 15.97 |
| 18:3n-6 | 0.21 | 0.28 | 0.32 | 0.41 | 0.33 |
| 20:4n-6 | 0.47 | 0.49 | 0.51 | 0.46 | 0.50 |
| Total n-6 PUFA | 27.64 | 28.19 | 17.51 | 28.57 | 17.49 |
| 18:3n-3 | 6.06 | 6.71 | 6.10 | 5.79 | 6.04 |
| 20:3n-3 | 0.77 | 0.81 | 1.15 | 0.82 | 1.13 |
| 20:5n-3 (EPA) | 4.79 | 3.30 | 4.52 | 3.49 | 4.47 |
| 22:5n-3 | 0.49 | 0.44 | 0.58 | 0.50 | 0.57 |
| 22:6n-3 (DHA) | 4.14 | 4.17 | 11.21 | 8.37 | 11.14 |
| Total n-3 PUFA | 18.92 | 18.33 | 26.42 | 21.59 | 26.14 |
| Total PUFA | 46.94 | 46.52 | 44.40 | 50.54 | 44.08 |
| n-3/n-6 | 0.68 | 0.65 | 1.51 | 0.76 | 1.49 |
| PUFA/SFA | 2.20 | 2.19 | 1.60 | 2.55 | 1.62 |
| EPA + DHA | 8.93 | 7.47 | 15.73 | 11.86 | 15.61 |
| DC | DI | DM | DP&L | DMIX | |
|---|---|---|---|---|---|
| Initial weight (g) | 9.11 ± 1.54 | 9.08 ± 1.81 | 9.27 ± 1.59 | 9.09 ± 1.83 | 9.21 ± 1.68 |
| Initial length (cm) | 9.61 ± 0.63 | 9.46 ± 0.71 | 9.45 ± 0.69 | 9.52 ± 0.72 | 9.49 ± 0.64 |
| Final weight (g) | 47.8 ± 7.2 b | 47.81 ± 7.55 b | 42.23 ± 7.5 a | 46.79 ± 6 b | 44.43 ± 6.65 a |
| Final length (cm) | 15.02 ± 0.8 c | 14.53 ± 0.83 b | 14.11 ± 0.95 a | 14.54 ± 0.68 b | 14.47 ± 0.8 b |
| CF | 1.46 ± 0.1 a | 1.55 ± 0.12 c | 1.49 ± 0.12 ab | 1.52 ± 0.11 bc | 1.46 ± 0.11 a |
| SGR (%) | 2.67 ± 0.08 c | 2.67 ± 0.06 c | 2.44 ± 0.04 a | 2.64 ± 0.02 c | 2.53 ± 0.0 b |
| RGR (%) | 4.24 ± 0.24 c | 4.26 ± 0.2 c | 3.55 ± 0.13 a | 4.14 ± 0.08 c | 3.82 ± 0.01 b |
| PER (%) | 3.19 ± 0.13 | 3.16 ± 0.16 | 2.98 ± 0.87 | 3.03 ± 0.10 | 2.89 ± 0.08 |
| FCR | 0.68 ± 0.03 | 0.7 ± 0.03 | 0.73 ± 0.02 | 0.71 ± 0.03 | 0.74 ± 0.03 |
| FIFO | 0.87 ± 0.04 c | 0.60 ± 0.03 b | 0.65 ± 0.02 b | 0.61 ± 0.02 b | 0.40 ± 0.01 a |
| DC | DI | DM | DP&L | DMIX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Composition | Av | SD | Av | SD | Av | SD | Av | SD | Av | SD |
| Moisture (%) | 76.33 | 5.80 | 76.67 | 5.80 | 77.00 | 0.00 | 77.33 | 5.80 | 77.67 | 5.80 |
| Protein (%) | 73.58 | 3.60 | 72.60 | 2.90 | 73.72 | 2.50 | 75.57 | 5.30 | 73.96 | 2.00 |
| Fat (%) | 16.32 | 0.45 | 18.38 | 0.75 | 15.79 | 0.40 | 15.11 | 0.76 | 16.58 | 0.10 |
| Carbohydrates (%) | 4.21 | 0.30 | 3.42 | 0.35 | 4.49 | 1.15 | 3.21 | 0.40 | 3.26 | 0.32 |
| Ash (%) | 5.89 a | 0.10 | 5.60 b | 0.30 | 6.01 a | 0.40 | 6.11 a | 0.20 | 6.20 a | 0.20 |
| Fatty Acids (% TFA) | ||||||||||
| 14:0 | 2.28 | 0.32 | 2.06 | 0.36 | 1.88 | 0.07 | 1.81 | 0.04 | 1.91 | 0.17 |
| 15:0 | 0.25 | 0.01 | 0.28 | 0.09 | 0.28 | 0.05 | 0.30 | 0.07 | 0.34 | 0.11 |
| 16:0 | 13.37 | 0.49 | 13.99 | 0.47 | 12.43 | 0.08 | 13.30 | 0.92 | 14.72 | 0.65 |
| 18:0 | 3.63 | 0.06 | 3.81 | 0.18 | 3.57 | 0.19 | 3.92 | 0.19 | 4.10 | 0.19 |
| 20:0 | 0.33 | 0.01 | 0.32 | 0.05 | 0.33 | 0.02 | 0.34 | 0.04 | 0.32 | 0.04 |
| 24:0 | 0.12 | 0.01 | 0.08 | 0.07 | 0.12 | 0.01 | 0.13 | 0.01 | 0.03 | 0.06 |
| Total saturated | 21.69 ab | 0.81 | 22.24 ab | 0.26 | 20.35 b | 0.18 | 21.43 ab | 1.01 | 23.17 a | 1.13 |
| 16:1 | 3.19 | 0.14 | 3.13 | 0.31 | 2.90 | 0.28 | 2.63 | 0.12 | 3.16 | 0.36 |
| 18:1n-9 | 22.02 | 0.56 | 21.54 | 2.14 | 22.11 | 1.07 | 22.03 | 1.57 | 21.21 | 3.22 |
| 18:1n-7 | 2.82 | 0.21 | 2.61 | 0.30 | 2.52 | 0.14 | 2.39 | 0.05 | 2.46 | 0.05 |
| 20:1 | 2.82 | 0.10 | 2.82 | 0.20 | 2.83 | 0.15 | 2.78 | 0.14 | 2.69 | 0.09 |
| 24:1 | 0.38 | 0.01 | 0.35 | 0.03 | 0.36 | 0.01 | 0.38 | 0.04 | 0.35 | 0.02 |
| Total monounsaturated | 32.05 | 0.43 | 31.29 | 1.97 | 31.52 | 1.65 | 31.01 | 1.78 | 30.70 | 2.86 |
| 18:2n-6 | 22.36 | 0.52 | 21.46 | 3.06 | 24.09 | 0.52 | 23.95 | 0.59 | 19.50 | 3.08 |
| 18:3n-6 | 0.37 | 0.07 | 0.33 | 0.06 | 0.45 | 0.06 | 0.39 | 0.05 | 0.31 | 0.08 |
| 20:4n-6 | 0.49 | 0.01 | 0.47 | 0.03 | 0.47 | 0.02 | 0.46 | 0.02 | 0.45 | 0.01 |
| Total n-6 PUFA | 24.17 | 0.64 | 23.22 | 3.09 | 26.05 | 0.46 | 25.89 | 0.60 | 21.25 | 3.15 |
| 18:3n-3 | 4.55 | 0.09 | 4.74 | 0.09 | 4.59 | 0.21 | 4.49 | 0.06 | 4.60 | 0.10 |
| 20:3n-3 | 1.11 | 0.11 | 1.11 | 0.17 | 1.17 | 0.08 | 1.04 | 0.03 | 1.22 | 0.17 |
| 20:5n-3 | 3.03 | 0.22 | 2.91 | 0.85 | 2.69 | 0.08 | 2.33 | 0.26 | 3.02 | 0.90 |
| 22:5n-3 | 0.90 | 0.07 | 0.85 | 0.22 | 0.78 | 0.03 | 0.75 | 0.08 | 0.86 | 0.16 |
| 22:6n-3 | 8.56 | 0.30 | 9.87 | 4.08 | 9.25 | 1.59 | 9.61 | 2.15 | 11.73 | 3.63 |
| Total n-3 PUFA | 18.15 | 0.21 | 17.88 | 7.38 | 18.47 | 1.28 | 18.21 | 2.37 | 19.86 | 4.46 |
| Total PUFA | 43.63 | 0.89 | 42.43 | 4.26 | 45.85 | 1.69 | 45.54 | 2.68 | 42.24 | 2.92 |
| n-3/n-6 | 0.75 | 0.02 | 0.81 | 0.44 | 0.71 | 0.04 | 0.70 | 0.08 | 0.96 | 0.31 |
| PUFA/SFA | 2.01 a | 0.12 | 1.91 ab | 0.20 | 2.25 a | 0.07 | 2.13 a | 0.22 | 1.83 b | 0.18 |
| EPA + DHA | 11.59 | 0.19 | 12.78 | 4.75 | 11.94 | 1.54 | 11.94 | 2.39 | 14.75 | 4.52 |
| AI | 0.3 | 0.31 | 0.26 | 0.27 | 0.31 | |||||
| TI | 0.12 | 0.13 | 0.12 | 0.13 | 0.12 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estévez, A.; Frade, P.; Ferreira, M.; Regueiro, L.; Alvarez, M.; Blanco, B.; Fernández, L.; Soula, M. Effects of Alternative and Sustainable Ingredients on Rainbow Trout (Oncorhynchus mykiss) Growth, Muscle Composition and Health. Aquac. J. 2022, 2, 37-50. https://doi.org/10.3390/aquacj2020004
Estévez A, Frade P, Ferreira M, Regueiro L, Alvarez M, Blanco B, Fernández L, Soula M. Effects of Alternative and Sustainable Ingredients on Rainbow Trout (Oncorhynchus mykiss) Growth, Muscle Composition and Health. Aquaculture Journal. 2022; 2(2):37-50. https://doi.org/10.3390/aquacj2020004
Chicago/Turabian StyleEstévez, Alicia, Paula Frade, Martiña Ferreira, Leticia Regueiro, Mayte Alvarez, Belen Blanco, Laura Fernández, and Mohamed Soula. 2022. "Effects of Alternative and Sustainable Ingredients on Rainbow Trout (Oncorhynchus mykiss) Growth, Muscle Composition and Health" Aquaculture Journal 2, no. 2: 37-50. https://doi.org/10.3390/aquacj2020004