Recovery of Haemal Lordosis in European Seabass Dicentrarchus labrax (Linnaeus 1758) †
Abstract
:1. Introduction
2. Materials and Methods
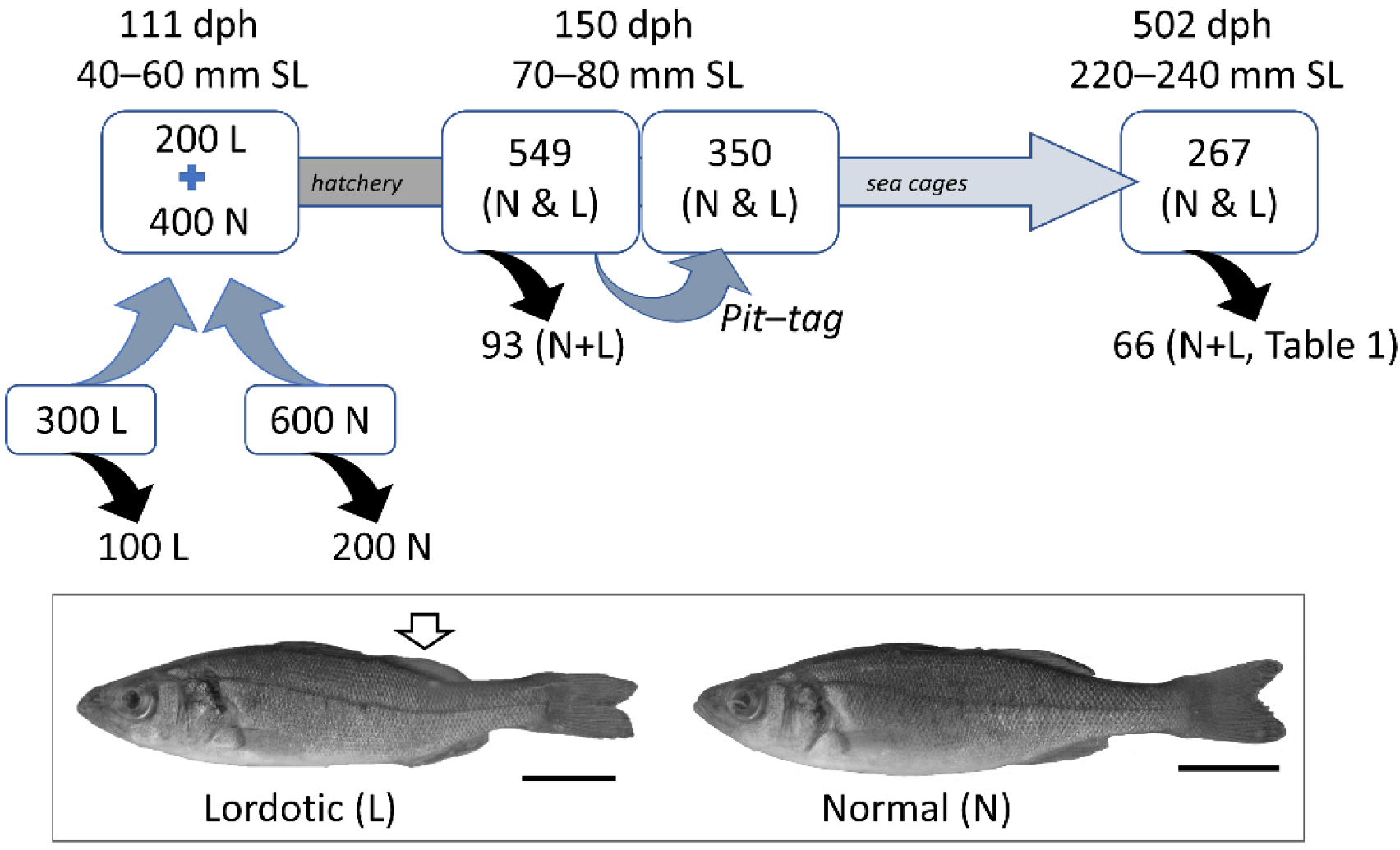
2.1. Experimental Design and Fish Origin
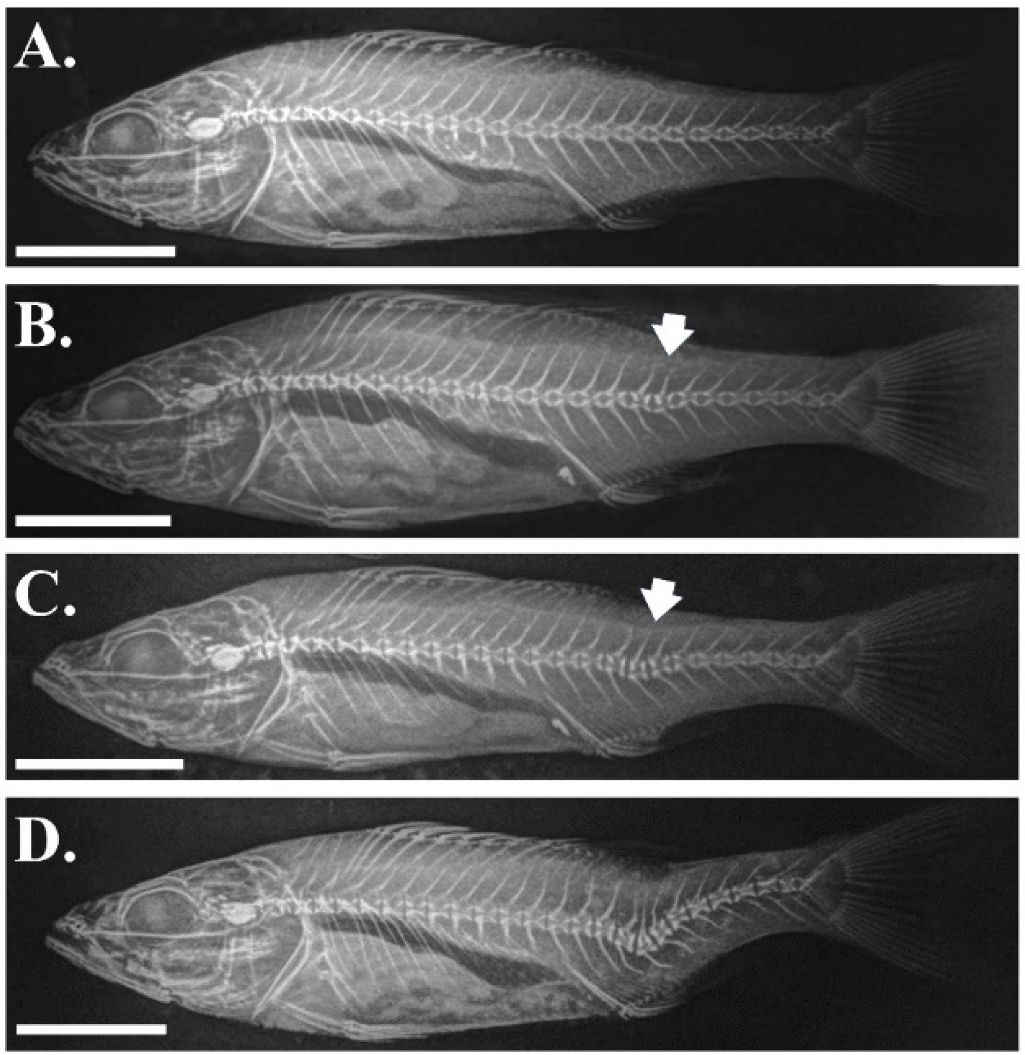
2.2. Categorization of the External Morphology during the On-Growing Period
2.3. Geometric Morphometric and Meristic Analyses
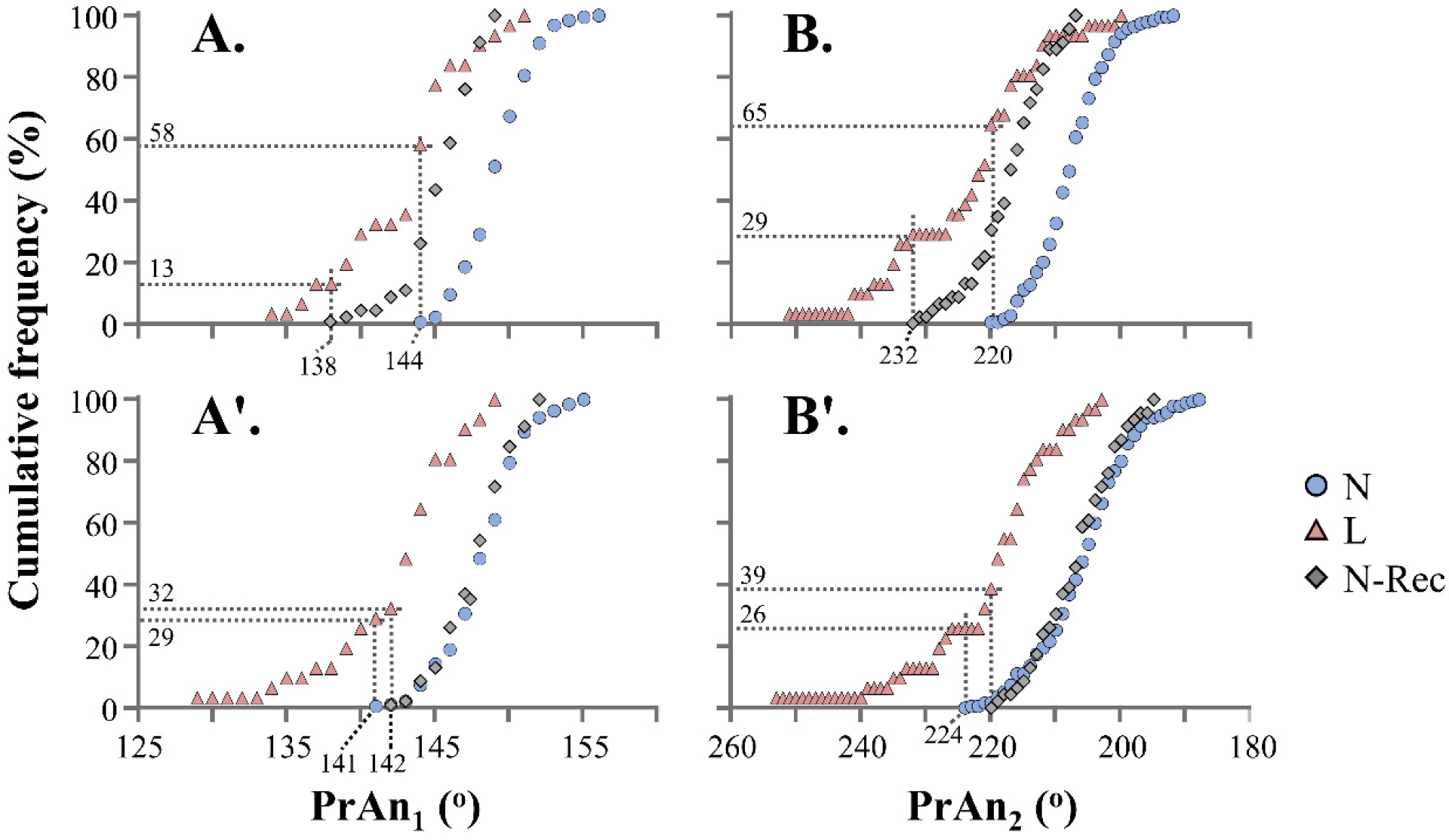
2.4. Simple Morphometric Descriptors of Lordosis-Induced Body-Shape Deviations
3. Results
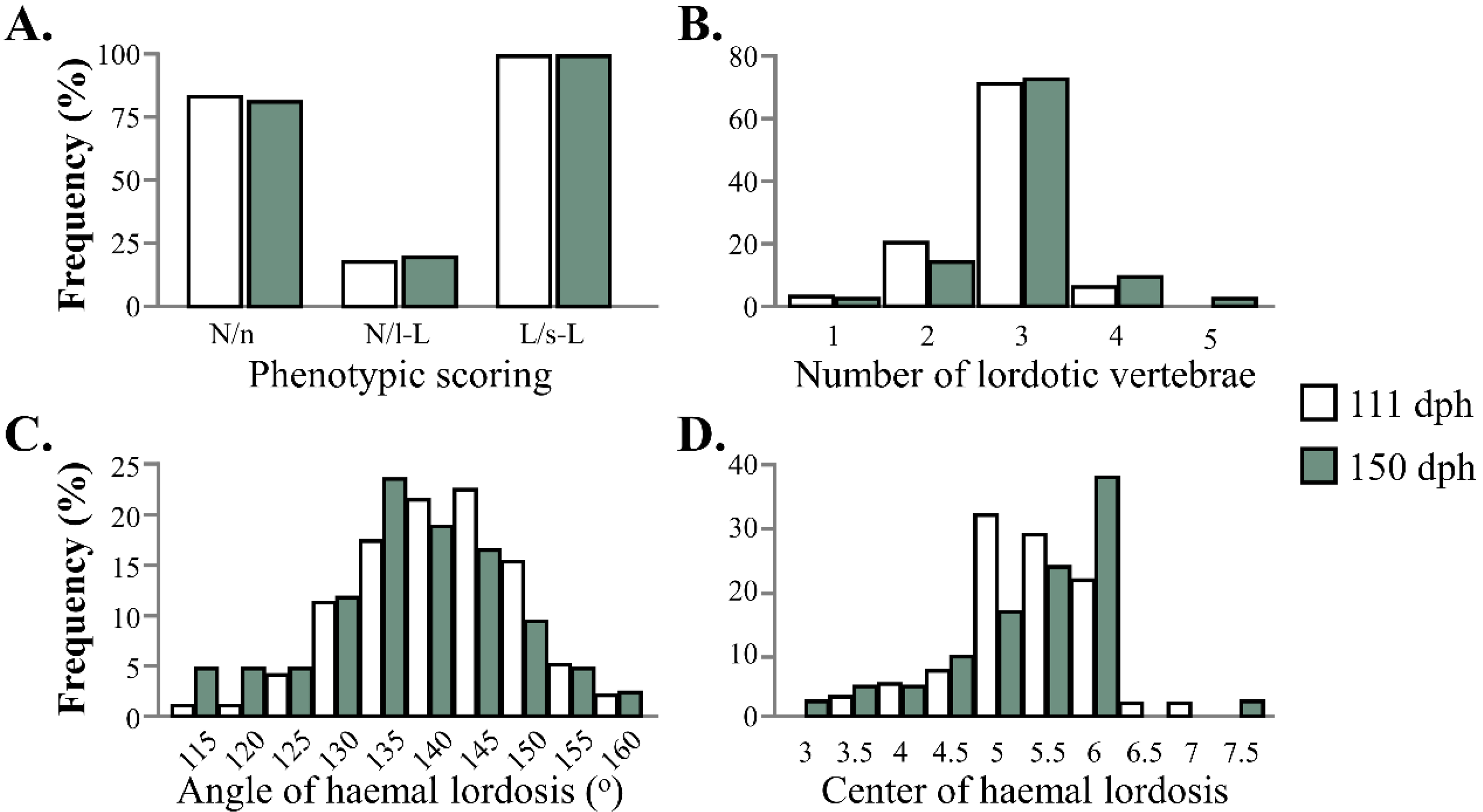
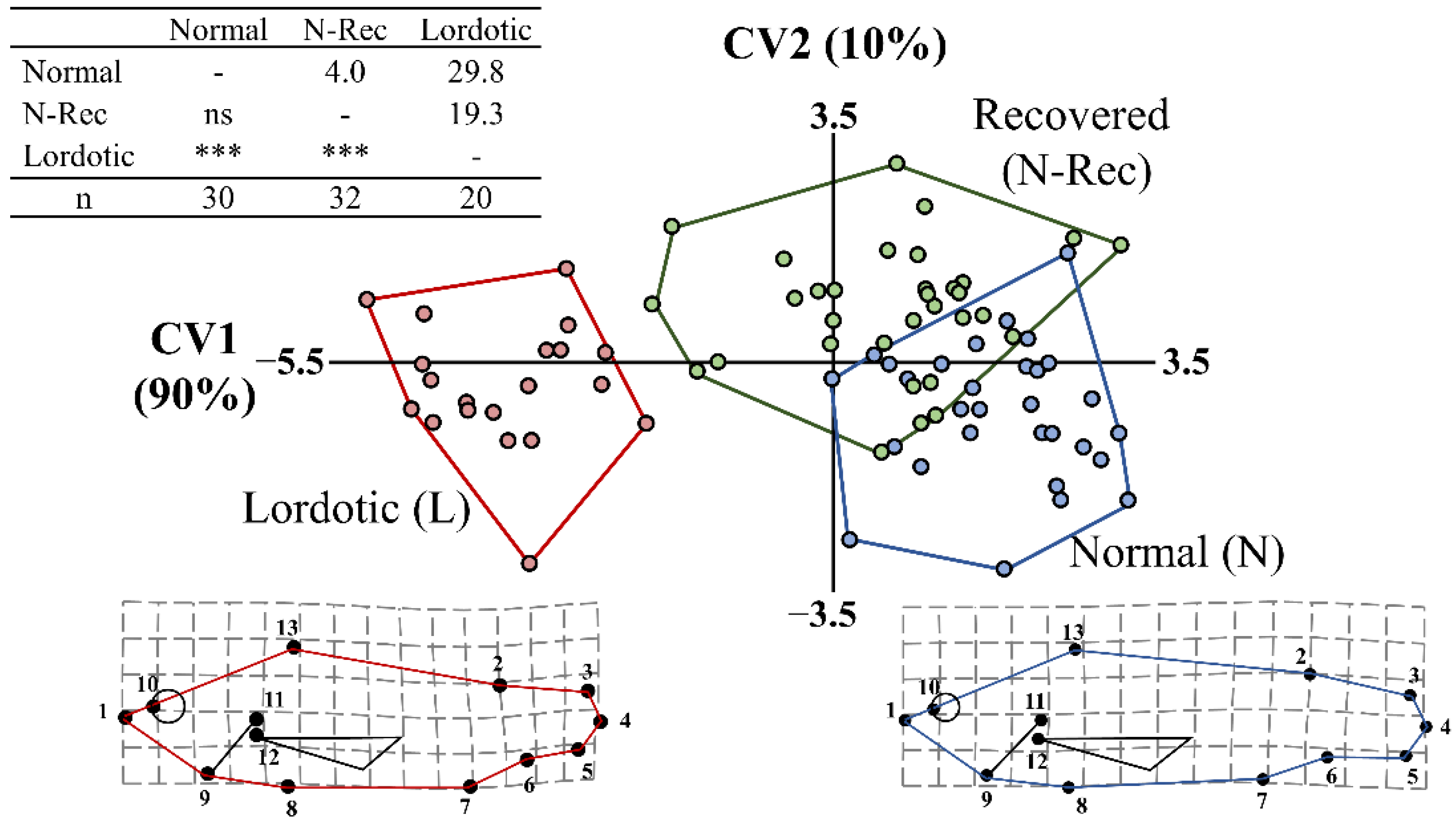
3.1. Validation of the Phenotypic Categorization by Means of External Phenotype
3.2. Recovery of Haemal Lordosis during the On-Growing Period (150–502 dph)
3.3. Morphometric Analysis of Haemal Lordosis and Its Recovery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beraldo, P.; Pinosa, M.; Tibaldi, E.; Canavese, B. Abnormalities of the operculum in Gilthead sea bream (Sparus aurata): Morphological description. Aquaculture 2003, 220, 89–99. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Oran, G.; Divanach, P.; Stefanakis, S.; Kentouri, M. The Opercular complex deformity in intensive Gilthead sea bream (Sparus aurata L.) larviculture. Moment of apparition and description. Aquaculture 1997, 156, 165–177. [Google Scholar] [CrossRef]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval performance and skeletal deformities in farmed Gilthead sea bream (Sparus aurata) fed with graded levels of vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Fragkoulis, S.; Batargias, C.; Kolios, P.; Koumoundouros, G. Genetic parameters of the upper-jaw abnormalities in Gilthead seabream Sparus aurata. Aquaculture 2018, 497, 226–233. [Google Scholar] [CrossRef]
- Sawada, Y.; Sasaki, T.; Nishio, K.; Kurata, M.; Honryo, T.; Agawa, Y. Positive Phototaxis as the cause of jaw malformations in larval greater amberjack, Seriola dumerili (Risso, 1810): Mitigation by rearing in tanks with low-brightness walls. Aquac. Res. 2020, 51, 2261–2274. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Boglione, C.; Cataudella, S.; Kentouri, M. Normal and abnormal osteological development of caudal fin in Sparus aurata L. fry. Aquaculture 1997, 149, 215–226. [Google Scholar] [CrossRef]
- Kourkouta, C.; Printzi, A.; Geladakis, G.; Mitrizakis, N.; Papandroulakis, N.; Koumoundouros, G. Long Lasting Effects of Early Temperature Exposure on the Swimming Performance and Skeleton Development of Metamorphosing Gilthead Seabream (Sparus Aurata L.) Larvae. Sci. Rep. 2021, 11, 8787. [Google Scholar] [CrossRef] [PubMed]
- Fragkoulis, S.; Paliogiannis, H.; Kokkinias, P.; Chiers, K.; Adriaens, D.; Koumoundouros, G. Saddleback syndrome in European sea bass Dicentrarchus labrax (Linnaeus, 1758): Anatomy, ontogeny and correlation with lateral-line, anal and pelvic fin abnormalities. J. Fish Dis. 2017, 40, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Loizides, M.; Georgiou, A.N.; Somarakis, S.; Witten, P.E.; Koumoundouros, G. A new type of lordosis and vertebral body compression in gilthead sea bream, Sparus aurata L.: Aetiology, anatomy and consequences for survival. J. Fish Dis. 2014, 37, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Honryo, T.; Agawa, Y.; Kurata, M. Teratogenic Effects of isolated and combined short-term hypercapnia and hypoxia on red sea bream (Pagrus major) embryos. Aquac. Res. 2018, 49, 3176–3186. [Google Scholar] [CrossRef]
- Giebichenstein, J.; Giebichenstein, J.; Hasler, M.; Schulz, C.; Ueberschär, B. Comparing the performance of four commercial microdiets in an early weaning protocol for European seabass larvae (Dicentrarchus labrax). Aquac. Res. 2021. [Google Scholar] [CrossRef]
- Printzi, A.; Kourkouta, C.; Fragkoulis, S.; Dimitriadi, A.; Geladakis, G.; Orfanakis, M.; Mazurais, D.; Zambonino-Infante, J.L.; Koumoundouros, G. Balancing between Artemia and microdiet usage for normal skeletal development in zebrafish (Danio rerio). J. Fish Dis. 2021, 44, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Fragkoulis, S.; Economou, I.; Moukas, G.; Koumoundouros, G.; Batargias, C. Caudal fin abnormalities in Gilthead seabream (Sparus aurata L.) have a strong genetic variance component. J. Fish Dis. 2020, 43, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5, S121–S167. [Google Scholar] [CrossRef] [Green Version]
- Divanach, P.; Papandroulakis, N.; Anastasiadis, P.; Koumoundouros, G.; Kentouri, M. Effect of water currents on the development of skeletal deformities in sea bass (Dicentrarchus labrax L.) with functional swimbladder during postlarval and nursery phase. Aquaculture 1997, 156, 145–155. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Georgakopoulou, E.; Papadakis, I.E.; Divanach, P.; Kentouri, M.; Koumoundouros, G. Environmental determinants of haemal lordosis in European sea bass, Dicentrarchus labrax (Linnaeus, 1758). Aquaculture 2006, 254, 54–64. [Google Scholar] [CrossRef]
- Palstra, A.P.; Roque, A.; Kruijt, L.; Jéhannet, P.; Pérez-Sánchez, J.; Dirks, R.P. Physiological effects of water flow induced swimming exercise in seabream Sparus aurata. Front. Physiol. 2020, 11, 1605. [Google Scholar] [CrossRef]
- Printzi, A.; Fragkoulis, S.; Dimitriadi, A.; Keklikoglou, K.; Arvanitidis, C.; Witten, P.E.; Koumoundouros, G. Exercise-induced lordosis in zebrafish Danio rerio (Hamilton, 1822). J. Fish Biol. 2021, 98, 987–994. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Katharios, P.; Divanach, P.; Koumoundouros, G. Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 2010, 308, 13–19. [Google Scholar] [CrossRef]
- Mazurais, D.; Glynatsi, N.; Darias, M.J.; Christodoulopoulou, S.; Cahu, C.L.; Zambonino-Infante, J.L.; Koumoundouros, G. Optimal levels of dietary vitamin A for reduced deformity incidence during development of European sea bass larvae (Dicentrarchus labrax) depend on malformation type. Aquaculture 2009, 294, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Kihara, M.; Ogata, S.; Kawano, N.; Kubota, I.; Yamaguchi, R. Lordosis induction in juvenile red sea bream, Pagrus major, by high swimming activity. Aquaculture 2002, 1–4, 149–158. [Google Scholar] [CrossRef]
- Fragkoulis, S.; Printzi, A.; Geladakis, G.; Katribouzas, N.; Koumoundouros, G. Recovery of haemal lordosis in Gilthead seabream (Sparus aurata L.). Sci. Rep. 2019, 9, 9832. [Google Scholar] [CrossRef] [Green Version]
- Fragkoulis, S.; Koumoundouros, G. Simple morphometrics for predicting lordosis-induced deviations of body shape in reared Gilthead seabream (Sparus aurata L.). J. Fish Dis. 2021, 44, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Kranenbarg, S.; Waarsing, J.H.; Muller, M.; Weinans, H.; van Leeuwen, J.L. Lordotic vertebrae in sea bass (Dicentrarchus labrax L.) are adapted to increased loads. J. Biomech. 2005, 38, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, D.G.; Georgakopoulou, E.; Kentouri, M.; Koumoundouros, G. Geometric quantification of lordosis effects on body shape in European sea bass, Dicentrarchus labrax (Linnaeus, 1758). Aquaculture 2006, 256, 27–33. [Google Scholar] [CrossRef]
- Bardon, A.; Vandeputte, M.; Dupont-Nivet, M.; Chavanne, H.; Haffray, P.; Vergnet, A.; Chatain, B. What is the heritable component of spinal deformities in the European sea bass (Dicentrarchus labrax)? Aquaculture 2009, 294, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; W. H. Freeman and Company: San Francisco, CA, USA, 1981; 859p. [Google Scholar]
- Rohlf, F.J. tpsDig, Digitize landmarks and outlines, version 2.31; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2017. [Google Scholar]
- Sheets, H.D. Integrated Morphometrics Package (IMP) 8. 2014. Available online: https://www.animal-behaviour.de/imp/ (accessed on 10 May 2021).
- Rohlf, F.J. tpsRelw, Relative Warps Analysis, version 1.62; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2016. [Google Scholar]
- Rohlf, F.J. TpsRegr, Shape Regression, version 1.43; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2016. [Google Scholar]
- Witten, P.E.; Obach, A.; Huysseune, A.; Baeverfjord, G. Vertebrae fusion in Atlantic salmon (Salmo salar): Development, aggravation and pathways of containment. Aquaculture 2006, 258, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Beraldo, P.; Canavese, B. Recovery of opercular anomalies in Gilthead sea bream, Sparus aurata L.: Morphological and morphometric analysis. J. Fish Dis. 2011, 34, 21–30. [Google Scholar] [CrossRef]
- Amoroso, G.; Cobcroft, J.M.; Adams, M.B.; Ventura, T.; Carter, C.G. Concurrence of lower jaw skeletal anomalies in triploid Atlantic salmon (Salmo salar L.) and the effect on growth in freshwater. J. Fish Dis. 2016, 39, 1509–1521. [Google Scholar] [CrossRef]
- Witten, P.E.; Huysseune, A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol. Rev. 2009, 84, 315–346. [Google Scholar] [CrossRef]
- Printzi, A.; Mazurais, D.; Witten, P.E.; Zambonino-Infante, J.-L.; Koumoundouros, G. Recovery of haemal lordosis in juvenile zebrafish (Danio rerio). In Proceedings of the EAS 2021, Oceans of Opportunity, Madeira, Portugal, 4–7 October 2021; European Aquaculture Society: Madeira, Portugal, 2021; pp. 1028–1029. [Google Scholar]
- Ortiz-Delgado, J.B.; Fernández, I.; Sarasquete, C.; Gisbert, E. Normal and histopathological organization of the opercular bone and vertebrae in Gilthead sea bream Sparus aurata. Aquat. Biol. 2014, 21, 67–84. [Google Scholar] [CrossRef] [Green Version]
- Chatain, B. Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 1994, 119, 371–379. [Google Scholar] [CrossRef]
- de Azevedo, A.M.; Losada, A.P.; Barreiro, A.; Barreiro, J.D.; Ferreiro, I.; Riaza, A.; Vázquez, S.; Quiroga, M.I. Skeletal anomalies in reared Senegalese Sole Solea senegalensis Juveniles: A radiographic approach. Dis. Aquat. Org. 2017, 124, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loy, A.; Boglione, C.; Gagliardi, F.; Ferrucci, L.; Cataudella, S. Geometric morphometrics and internal anatomy in sea bass shape analysis (Dicentrarchus labrax L., Moronidae). Aquaculture 2000, 186, 33–44. [Google Scholar] [CrossRef]

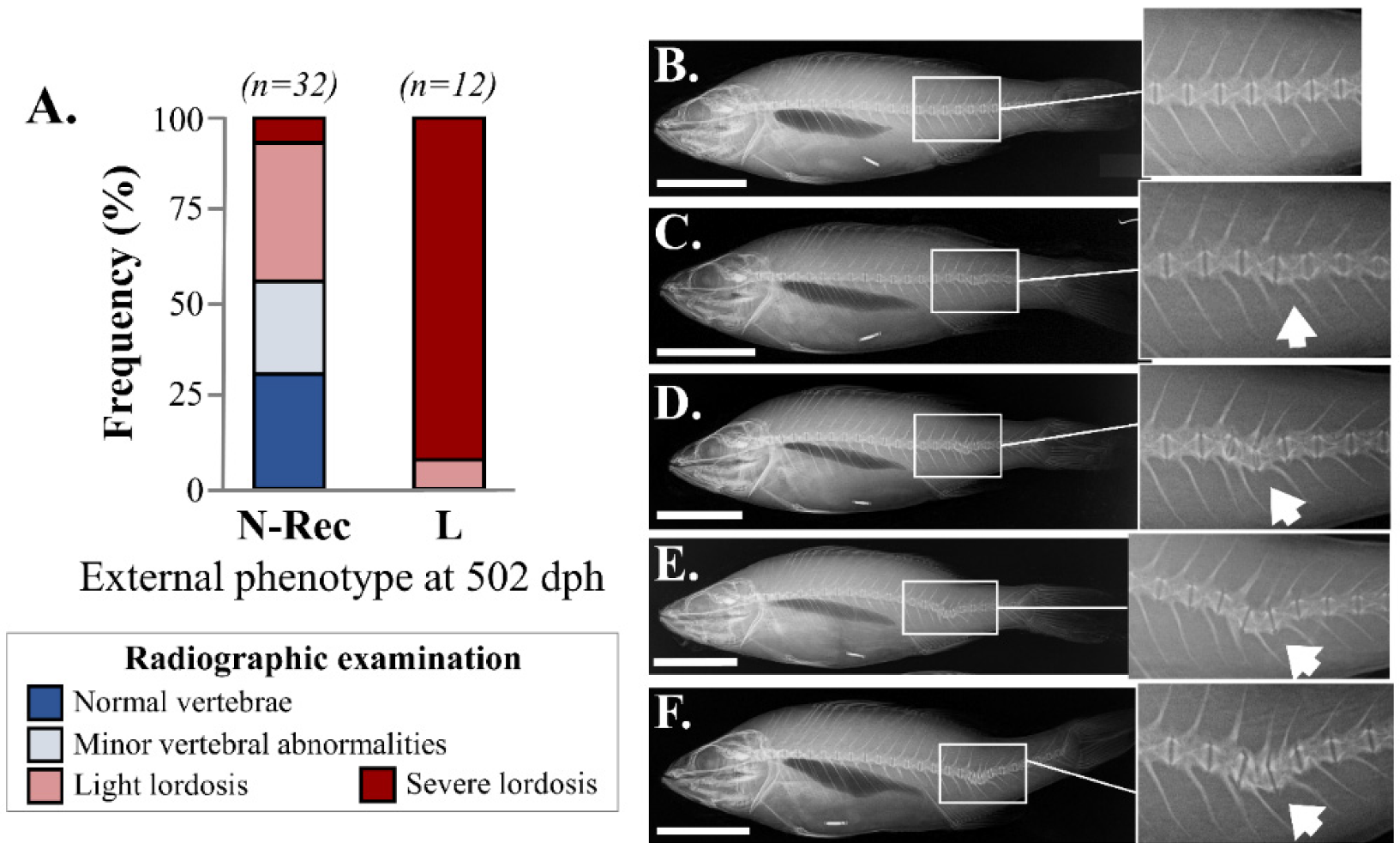

| External Phenotype at Tagging (150 dph) | Final External Phenotype (502 dph) | Total | Number of Radiographed Fish | % of Fish Radiographed |
|---|---|---|---|---|
| N | N | 190 | 22 | 12 |
| L | - | - | - | |
| L | N | 46 | 32 | 70 |
| L | 31 | 12 | 39 | |
| Total | 267 | 66 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragkoulis, S.; Kourkouta, C.; Geladakis, G.; Printzi, A.; Glaropoulos, A.; Koumoundouros, G. Recovery of Haemal Lordosis in European Seabass Dicentrarchus labrax (Linnaeus 1758). Aquac. J. 2022, 2, 1-12. https://doi.org/10.3390/aquacj2010001
Fragkoulis S, Kourkouta C, Geladakis G, Printzi A, Glaropoulos A, Koumoundouros G. Recovery of Haemal Lordosis in European Seabass Dicentrarchus labrax (Linnaeus 1758). Aquaculture Journal. 2022; 2(1):1-12. https://doi.org/10.3390/aquacj2010001
Chicago/Turabian StyleFragkoulis, Stephanos, Chara Kourkouta, George Geladakis, Alice Printzi, Alexis Glaropoulos, and George Koumoundouros. 2022. "Recovery of Haemal Lordosis in European Seabass Dicentrarchus labrax (Linnaeus 1758)" Aquaculture Journal 2, no. 1: 1-12. https://doi.org/10.3390/aquacj2010001
APA StyleFragkoulis, S., Kourkouta, C., Geladakis, G., Printzi, A., Glaropoulos, A., & Koumoundouros, G. (2022). Recovery of Haemal Lordosis in European Seabass Dicentrarchus labrax (Linnaeus 1758). Aquaculture Journal, 2(1), 1-12. https://doi.org/10.3390/aquacj2010001








