Abstract
Adipose tissue is an organ with a high metabolic rate, functioning as a storage site for potential energy derived from food. It is a heterogeneous tissue composed of various cell types that respond differently to stimuli. Polyunsaturated fatty acids are lipids characterized by the presence of multiple double bonds in their molecular structure. These fatty acids are particularly vulnerable to oxidation by Reactive Oxygen Species, a process known as lipoperoxidation. While the oxidized lipids can serve important physiological roles within adipose tissue, they can also enter the bloodstream, where they associate with lipoproteins, leading to cellular damage and increased systemic oxidative stress. In cases of obesity, adipose tissue displays an exaggerated inflammatory and immune response that can affect multiple body systems, contributing to the onset of chronic degenerative diseases. Therefore, adipose tissue is a complex organ in which metabolic, endocrine, and immune response processes are intricately regulated and coordinated. This paper explains the role of alterations in redox balance, lipogenic, and inflammatory functions in adipose tissue as important risk factors for the development of chronic degenerative diseases, including those affecting the central nervous system. For this study, we searched multiple databases, including PubMed, Scopus, Google Scholar, the Cochrane Library, and Medscape, from 2015 to the present.
1. Introduction
Adipose tissue functions as a metabolically active endocrine organ and serves as a reservoir of energy in the form of lipids for the body [1]. The primary form of energy storage in adipose tissue is triglycerides (TG), which are synthesized and stored in adipocytes through a process called lipogenesis. TG are mobilized to provide energy as needed through the process of lipolysis [2,3]. Storage and release of lipids are regulated by the energy demands of various organs and systems, including the brain, liver, skeletal muscles, cardiac muscles, and pancreas [4].
Adipose tissue comprises a diverse population of cells, including adipocytes and preadipocytes, as well as non-adipose cells such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and hematopoietic progenitor cells [5]. This tissue also contains a significant number of immune cells, including B and T lymphocytes, macrophages, myeloid cells, and mesenchymal stem cells (Figure 1). All these cells are embedded in a connective tissue matrix and surrounded by vascular tissue and nerve innervations, forming a complex communication network that regulates the activity and function of adipose tissue [6]. The metabolic, hormonal, immune, and nervous system regulatory mechanisms in adipose tissue are crucial for maintaining the body’s homeostasis [7] and require precise coordination to function correctly.
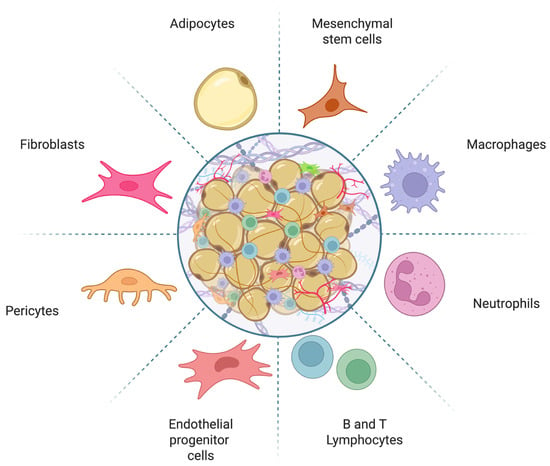
Figure 1.
Schematic representation of adipose tissue composition. Adipose tissue consists of a diverse array of cells, including preadipocytes, mature adipocytes, mesenchymal stem cells, fibroblasts, pericytes, immune cells, and vascular-associated cells. Image created with Biorender.com.
Distinct cellular features characterize adipose tissue, which can be classified into three types: white adipose tissue (WAT), brown adipose tissue (BAT), and beige adipose tissue (BeAT) [8]. WAT consists of large adipocytes, ranging from 25 to 200 µm in diameter. These cells are characterized by a single lipid droplet that can occupy up to 90% of the cell’s volume, as well as limited vascularization and a low number of mitochondria [9,10]. In contrast, BAT is composed of smaller adipocytes, measuring between 15 and 60 µm in diameter. These cells contain smaller and more numerous lipid compartments, a greater number of mitochondria, which gives them their brown appearance, high expression of Uncoupling Protein 1 (UCP-1), and greater vascularization [10,11]. BeAT exhibits characteristics of both white and brown adipose tissues. It contains both large and small lipid droplets, reflecting a multilocular structure, and has a greater number of UCP-1 in its mitochondria. This enhancement increases its ability to generate heat from fat and glucose compared to WAT [9,12]. Beige adipocytes develop through two main processes: transdifferentiation and de novo adipogenesis. Transdifferentiation involves the transformation of fully mature white adipocytes found in subcutaneous WAT into beige adipocytes. In contrast, de novo adipogenesis occurs when progenitor cells within WAT give rise to beige adipocytes [13,14]. Transdifferentiation of white adipocytes is primarily triggered by cold exposure. However, research has also shown that several factors, such as β-adrenergic stimulation, diet, exercise, prebiotics and probiotics, certain medications, and even adipokines—especially adiponectin—can promote transformation of WAT into BeAT [15].
Adipose tissue can be classified into two types based on its location in specific anatomical regions of the body: subcutaneous and visceral [16]. In cases of obesity, visceral adipose tissue often undergoes hypertrophy in the adipocytes, which is associated with increased angiogenic and inflammatory activity, leading to significant metabolic changes [17]. In contrast, subcutaneous obesity is marked by hyperplasia, where adipocytes tend to secrete lower levels of pro-inflammatory molecules [18]. This distinction is important because different types of adipose tissue exhibit unique patterns in the expression and synthesis of adipokines, as well as varying metabolic functions, levels of vascularization, and innervation throughout the body [19].
In humans, WAT is distributed throughout the body, with the largest deposits located in the abdominal cavity, buttocks, thighs, and subcutaneous regions of the abdomen [5]. Smaller amounts of WAT can also be found in areas such as the pericardial, perivascular, periarticular, retroorbital, intramuscular, bone marrow, and facial tissues [20]. BAT, which is present in newborns, is in the axillary, cervical, perirenal, and periadrenal areas and decreases in quantity after birth. While BAT was previously thought to be of minor importance in adults, increased deposits of BAT have been observed in individuals exposed to cold climates [21,22] and in those who participate in regular physical activity (Figure 2) [23]. Additionally, adipose tissue exhibits marked sexual dimorphism, reflected in morphological, metabolic, and hormonal differences between men and women [24]. In women, gluteofemoral subcutaneous adipose tissue predominates, associated with greater insulin sensitivity and cardioprotective effects [25,26], while in men, visceral adipose tissue prevails, linked to greater lipolysis, insulin resistance, and elevated cardiovascular risk [27]. These differences are modulated mainly by estrogens, androgens, and nuclear receptors that regulate adipogenesis, lipolysis, and adipokine expression [26,28]. Furthermore, the thermogenic activity of BAT and BeAT is more prevalent in women, contributing to higher energy expenditure [28,29]. Sexual dimorphism of adipose tissue significantly influences susceptibility to obesity, type 2 diabetes, and metabolic diseases as well as in longevity [24,30].
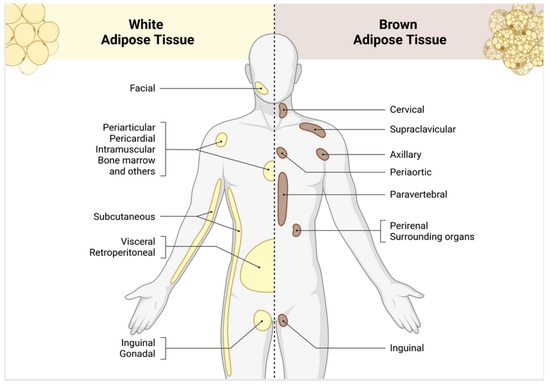
Figure 2.
Anatomical distribution of white and brown adipose tissue deposits in the human body. White adipose tissue is found predominantly in the subcutaneous, visceral, and gonadal areas, where it acts as the main energy reserve in body and participates in endocrine signaling and metabolic regulation. Brown adipose tissue is specifically located in the cervical, supraclavicular, axillary, and paravertebral regions and facilitates non-shivering thermogenesis through the action of mitochondrial uncoupling protein 1 (UCP-1). Image created with Biorender.com.
2. Lipid Metabolism in Adipose Tissue
Lipids are a diverse group of molecules, including TG, cholesterol, ceramides, phospholipids, sphingolipids, and others [31]. These lipids and their metabolites serve various functions, including energy storage, structural components, and signal transduction. Cells can either produce lipids endogenously or acquire them from their environment [32]. De novo lipogenesis (DNL) is the process through which fatty acids are synthesized from carbohydrates and other substrates, which are subsequently stored as TG [31]. DNL occurs in almost all cells throughout the body, but it is most prevalent in the liver, skeletal muscle, and adipose tissues, where it has significant metabolic relevance [33,34]. Under normal physiological conditions, DNL in adipocytes and hepatocytes is synergistically regulated by signals from peripheral tissues and the central nervous system [35]. However, in pathological conditions, this balance can be disrupted, resulting in increased hepatic DNL and decreased DNL in adipose tissue. This imbalance contributes to hepatic steatosis and other related metabolic diseases [32].
Dietary lipids are primarily ingested and absorbed in the intestine; monoacylglycerides and fatty acids are re-esterified and assembled for secretion into the lymph, which then enters the blood [36]. In circulation, lipoprotein lipase hydrolyzes TG, releasing fatty acids to muscle and adipose tissue for energy use or storage [37,38]. Due to their amphipathic nature, lipids are packaged and transported along with cholesterol and different types of lipoproteins. These include chylomicrons, very low-density lipoproteins (VLDL) rich in triglycerides, intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL) rich in cholesterol, and high-density lipoproteins (HDL) [39].
Oxidative stress can affect lipid synthesis, function, degradation, and transport, resulting in alterations in lipid metabolism [40]. Abnormalities during lipogenesis can result in systemic lipid metabolism failure, contributing to conditions such as dyslipidemia, metabolic syndrome, obesity, type 2 diabetes mellitus (DM2), non-alcoholic fatty liver disease, cardiovascular diseases, cancer [41,42], and neurodegenerative disorders. This is particularly concerning as the brain requires a significant amount of lipids to maintain its structure and function [43]. These conditions highlight the critical role of lipogenesis in maintaining metabolic homeostasis and the consequences of its dysregulation.
3. Adipose Tissue as an Endocrine Organ
The discovery of leptin revealed the adipose tissue’s role as an endocrine organ. Following this discovery, a diverse group of peptides known as adipokines, secreted by adipocytes, was identified. These peptides have important local and systemic functions, contributing to the balance between appetite and satiety, regulation of body fat stores and energy expenditure, glucose tolerance, insulin release and sensitivity, cell growth, immunity, reproduction, adipogenesis, angiogenesis, extracellular matrix restructuring, steroid metabolism, hemostasis, and maintenance of body temperature [15,44,45]. Additionally, adipose tissue releases inflammatory and anti-inflammatory molecules, as well as steroid hormones [6]. The primary target organs for adipokines include the liver, muscle, heart, pancreas, thymus, spleen, lymph nodes, and various areas of the central nervous system [45,46].
Synthesis and release of adipokines depend on the type of adipose tissue that produces them. In WAT secretome includes molecules such as adiponectin, leptin, adipsin, omentin, tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), resistin, visfatin, and retinol-binding protein 4 (RBP4). Together, these adipokines modulate glucose and lipid metabolism, regulate appetite and energy expenditure, and influence tissue and systemic inflammation [44,47]. In contrast, BAT produces a thermogenic secretome that includes fibroblast growth factor 21 (FGF21), bone morphogenetic protein 7 (BMP-7), vascular endothelial growth factor A (VEGF-A), irisin, neuregulin 4 (NRG4), nesfatin-1, meteorin-like protein (METRNL), chemerin, IL-6, interleukin-8 (IL-8), and interleukin-10 (IL-10). These cytokines are associated with thermogenesis and energy expenditure, glucose homeostasis, lipid metabolism, and insulin sensitivity. Additionally, they promote angiogenesis and exert anti-inflammatory effects [47,48]. In BAT, IL-6 may have favorable metabolic effects during sympathetic activation [49], while IL-10, produced by macrophages in the tissue, supports metabolic processes [50]. Furthermore, VEGF-A helps synchronize angiogenesis with the mitochondrial oxidative capacity of BAT, enhancing its thermogenic efficiency [51].
BeAT contains inducible adipocytes and shares a large part of the secretory repertoire with BAT and participates in the conversion of WAT. FGF21 and METRNL are highlighted, linked to thermogenic activation and signaling that facilitates the recruitment of cells with anti-inflammatory capacity [52,53]. NRG4 contributes to endocrine communication with the liver and other organs. In summary, the BeAT secretome induces thermogenesis, increases energy expenditure, and enhances insulin sensitivity, while also providing anti-inflammatory effects and indirect support for angiogenesis as shown in Table 1. The information gathered on adipokines has enabled their use as prognostic markers and in the treatment of metabolic diseases, including insulin resistance (IR), DM2, and cardiovascular disease [54].

Table 1.
Comparative Overview of Adipokines by Adipose Tissue Type.
4. Oxidative Stress in Adipose Tissue
Various cell types in adipose tissue produce free radicals. Reactive oxygen species (ROS) are generated primarily during glucose metabolism and ATP production [55,56], as well as by innate immune system cells, including macrophages, monocytes, and neutrophils. These free radicals play essential roles in normal cell function, serving as signaling molecules and second messengers in processes such as phagocytosis and cell proliferation, and they facilitate the activation of immune system cells (Figure 3) [50,51].
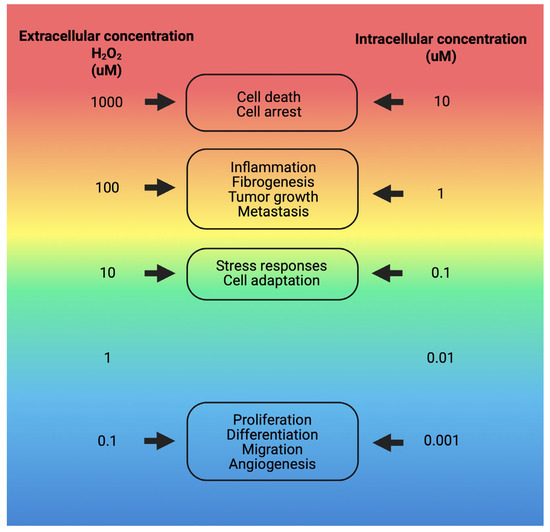
Figure 3.
Physiological responses to various intra- and extracellular concentrations of H2O2. Responses to H2O2 concentrations range from 10 nM, which elicits normal cellular activities, to higher levels, where concentrations around 100 nM trigger cell death processes. The specific cellular responses and the extent of damage depend on the type of cell. They are influenced by factors such as the individual’s health condition, genetic predispositions, age, and other relevant variables [57]. Image created with Biorender.com.
Oxidative stress occurs when the increased production of ROS in the cell exceeds the capacity of antioxidant systems to neutralize them [58,59]. Excess ROS, such as superoxide (O2●−) and hydroxyl radicals (●OH), covalently modify numerous biomolecules, including lipids, proteins, and nucleic acids [60]. With this, ROS can cause damage to cellular organelles within the adipocyte. Mitochondria, being the primary producers of ROS within the cell, are highly susceptible to oxidative damage, primarily affecting the electron transport chain, which leads to increased mitochondrial fragmentation and damage to mitochondrial DNA [61]. Furthermore, ROS also causes damage to nuclear DNA, particularly affecting telomeres, which contributes to cellular aging and apoptosis [62].
Cells possess a protective mechanism to counteract the effects of oxidative stress on the endoplasmic reticulum (ER), which leads to protein misfolding and redox imbalance. This adaptive process, known as the unfolded protein response (UPR), restores cellular homeostasis by reducing protein synthesis, increasing chaperone expression, and promoting antioxidant defense pathways to mitigate both ER dysfunction and oxidative damage [63]. Furthermore, under conditions of oxidative stress, ER promotes the production of ceramides [64] and diacylglycerol (DAG) [65], which are lipid products associated with a phenomenon called lipotoxicity [66,67,68]. Ceramides have a critical role in IR, linking changes in the ER with disruptions in insulin signaling pathways and, consequently, affecting uptake and utilization of glucose in various tissues [69]. Additionally, due to adipocyte hypertrophy, there is a deregulated release of ceramides and DAG, which can be ectopically deposited in the liver or skeletal muscle, thereby altering their metabolism [67,68]. Ireland et al. demonstrated that the Golgi apparatus is also vulnerable to oxidative stress, as protein trafficking is disrupted following exposure to H2O2 [70].
Increased production of mitochondrial ROS in adipocytes leads to alteration of polyunsaturated fatty acids (PUFAs). This occurs when free radicals attack the double bonds in PUFAs through non-enzymatic processes, resulting in byproducts such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) [57,71]. These lipid byproducts are highly electrophilic and can attack the side chains of lysine, histidine, and cysteine residues in proteins, leading to their carbonylation and the formation of covalent adducts. Additionally, these byproducts modify the double bonds of lipids, which perpetuates the formation of advanced lipoperoxidation products and further ROS [72,73]. Moreover, LDL can be oxidized either directly by lipoperoxidation products or through the enzymatic actions of phospholipases and lipoxygenases [74]. Unlike ROS, oxidation byproducts can propagate and cause harmful effects in tissues distant from their site of production [75].
On the other hand, there is evidence that adipocyte hypertrophy has been associated with increased ROS in WAT [76]. Furthermore, increased ROS is also a factor involved in the pathophysiology of hypertension and atherosclerosis, as it oxidizes LDL that adheres to endothelial cells [77]. LDL and its oxidized forms (oxLDL) bind to Pattern Recognition Receptors (PRRs), such as Toll-like receptors (TLRs) 2 and 4, which are responsible for detecting the presence of microorganisms and inducing the secretion of pro-inflammatory interleukins, including IL-1β, IL-6, and TNF-α [78]. Circulating oxLDL possesses antigenic potential and significantly contributes to inflammatory processes, activating both innate and adaptive immunity, and affecting different tissues [79]. Similarly, ROS are increased in adipose tissue by bacterial and viral infections, exposure to stimuli such as tobacco smoke, electromagnetic and solar radiation, ozone, herbicides, xenobiotics, food additives, nanoplastics, among others [80,81]. Although the physiological activity of ROS has traditionally been related to adverse metabolic events, they act as signalers in adipose tissue and play essential roles, such as the inactivation of Protein Phosphatase and Tensin Homolog (PTEN), Protein Tyrosine Phosphatase 1B (PTP1B) and Protein Phosphatase 2 (PP2A) favoring insulin signaling pathways [82] or regulating the activity of UCP-1 that uncouples oxidative phosphorylation, allowing the flow of protons and dissipating the gradient as heat [83].
Loss of redox balance generally impacts various cellular organelles in adipocytes, including mitochondria, ER, and Golgi apparatus, as well as the functions they perform within the cell. As a result, oxidative stress becomes a significant risk factor and plays a role in the development of various chronic diseases. These include DM2, atherosclerosis, heart disease, kidney disease, cancer, and central nervous system disorders such as Parkinson’s disease and Alzheimer’s disease [65].
5. Inflammation in Adipose Tissue
Excessive lipid storage in adipose tissue leads to adipocyte hypertrophy, increased lipolysis, accelerated cell death, and the development of local inflammatory and hypoxic environments [84]. The ability of adipose tissue to store fat is essential for metabolic health, as it serves as a physiological mechanism to prevent fat accumulation in ectopic locations, such as the liver, muscles, and heart [85]. Hypertrophic adipocytes release MCP-1, which attracts monocytes that differentiate into macrophages, primarily accumulating in visceral adipose tissue. These macrophages play a role in removing apoptotic cells and cellular debris, initiating tissue remodeling and repair [86]. When monocytes differentiate into M1 macrophages, they acquire pro-inflammatory properties, whereas M2 macrophages are anti-inflammatory, supportive of repair, and contribute to resolving the inflammatory response [87]. Distinctive markers of the M1 macrophage phenotype include TNF-α, IL-6, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), MCP-1, matrix metalloproteinase-9 (MMP-9), and vascular endothelial growth factor alpha (VEGF-α). In contrast, M2 macrophages are characterized by the presence of interleukin-10 (IL-10) and transforming growth factor beta (TGF-β) [88]. The infiltration of adipose tissue by pro-inflammatory macrophages is a defining feature of obesity [89].
T lymphocytes are present in adipose tissue and exhibit phenotypic changes during lipid accumulation [90]. They are divided into cytotoxic CD8+ cells, which recognize antigens presented by major histocompatibility complex I (MHC I), and CD4+ cells, which interact with antigens presented by MHC II [90,91]. In turn, CD4+ cells are divided into regulatory T cells (Treg) and helper T cells (Th), the latter of which are classified into three main subsets: Th1, Th2, and Th17 cells. Th1, Th17, and CD8+ cells can promote the development of adipose tissue inflammation. In contrast, Th2 and Treg cells maintain immune tolerance, limit excessive inflammatory responses, and prevent autoimmune diseases that could harm the body; however, their proportion decreases with lipid accumulation (Table 2) [92,93].

Table 2.
Immune populations of adipose tissue, phenotypic markers, and inflammatory effects.
Pro-inflammatory cytokines alter signaling pathways and affect the functionality and nutrient flow in and out of adipose tissue, impacting high-metabolic organs such as the liver, skeletal muscle, and pancreas [4,94]. This indicates that lipid accumulation in adipose tissue directly contributes to a systemic state of inflammation [95] and influences inflammatory and immune response factors that promote the progression of various diseases [96]. In cases of adipocyte hyperplasia and hypertrophy, the formation of new blood vessels does not keep pace with the expansion of tissue cells, leading to local hypoxia [68]. This hypoxia in adipose tissue activates Hypoxia-Inducible Factor-1 (HIF-1), which promotes an increase in extracellular matrix synthesis [97]. The resulting mechanical stress, cellular necrosis, and accumulation of immune cells perpetuate the inflammatory state, negatively affecting tissue flexibility and its ability to expand [98].
In summary, excessive accumulation of oxidized lipids leads to cellular changes that create a pro-oxidant and inflammatory environment within adipocytes. This environment affects the secretion profiles of adipokines and alters the endocrine, metabolic, antioxidant, and immunological functions of adipose tissue. As a result, these negative effects extend to other organs, compromising the organism’s overall functioning [99].
6. Cholesterol and Lipotoxicity
Adipose tissue plays a significant role in cholesterol metabolism. Cholesterol is a vital structural lipid component of cell membranes, essential for cell growth and viability [100]. It can also be found in organelles that contain lipid bilayers, such as mitochondria and the ER. Additionally, cholesterol serves as a precursor to steroid hormones, bile acids, and vitamin D [101]. While virtually all cells can produce cholesterol, the liver, intestine, and brain are the primary organs responsible for its synthesis [102]. The processes of biosynthesis, uptake, transport, storage, utilization, and excretion regulate cholesterol homeostasis. Approximately 25% of the body’s cholesterol is stored in adipose tissue. In cases of obesity, an imbalance in cholesterol levels can trigger an inflammatory response from adipocytes and immune cells within the tissue, disrupting metabolic homeostasis [103,104]. In the early stages of obesity, WAT expands primarily due to adipocyte hypertrophy [105]. This remodeling is necessary to store excess energy. It requires the formation of new blood vessels and the recruitment of immune cells that produce cytokines and chemokines as part of a temporary adaptive inflammatory response [106,107]. This increase in pro-inflammatory cytokines, such as IL-6, TNF-α, and MCP-1, reduces the expression of cholesterol transporters, ATP-Binding Cassette Transporter A1 (ABCA1) [108,109], which are responsible for transferring free cholesterol from cells to HDL in a mechanism called reverse cholesterol transport. The decrease in ABCA1 promotes intracellular accumulation of cholesterol, which can affect the structure of adipocyte membranes and mitochondrial function [109]. In contrast, Angiopoietin-Like Protein 4 (ANGPTL4), whose expression increases due to hypoxia [97] and inflammation [110], inhibits lipoprotein lipase (LPL) activity, preventing the degradation of triglycerides [111]. This raises plasma concentrations of VLDL and LDL, promoting dyslipidemia and prolonging tissue exposure to lipids, generating oxidative stress, endothelial inflammation, and lipotoxicity (Figure 4) [110,112].
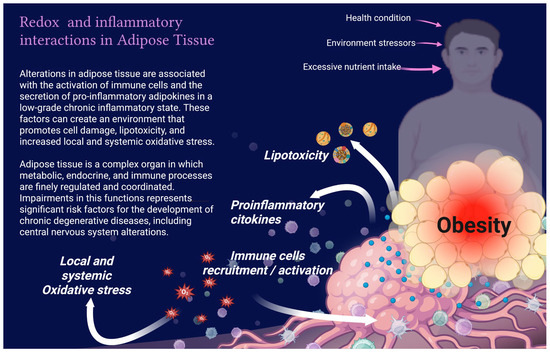
Figure 4.
Redox and inflammatory interactions in adipose tissue.
When there is an excess of lipids and local hypoxia persists, cholesterol can crystallize within adipocytes. When adipocytes undergo cell death, cholesterol crystals are released to the extracellular space. Immune system recognizes these abnormal cholesterol crystals as Damage Associated Molecular Patterns (DAMPs) [113]; macrophages in the tissue attempt to break down these cholesterol crystals from dead cells through phagocytosis. Upon entering the macrophages, the cholesterol crystals can destabilize lysosomal membranes [114]. This release of lysosomal contents into the cytoplasm acts as an intracellular signal that activates the NLRP3 inflammasome [104]. This process causes the cleavage of Caspase-1 and the maturation of interleukins IL-1β and IL-18, thereby amplifying inflammation, recruiting more immune cells to the area, and forming crown-like structures (CLS) around necrotic adipocytes. These structures also facilitate the formation of macrophage aggregates around dead adipocytes, which phagocytose lipid debris, including cholesterol crystals [115]. Activation of signaling pathways through TLRs by ROS or DAMPs favors the production of extracellular matrix components, such as collagen, which renders adipose tissue fibrotic [86], contributing to the perpetuation of the inflammatory response [116] and dysfunctional tissue growth.
Cholesterol accumulates in the inner membranes of mitochondria, leading to a reduction in membrane fluidity. This change promotes the oxidation of cardiolipin and alters the assembly of respiratory complexes I, II, and III, which results in decreased oxidative phosphorylation and lower ATP production [117]. Furthermore, this accumulation restricts the transport of reduced glutathione from the cytosol, causing an increase in ROS within the mitochondria [118]. Cholesterol also influences the physiology of BeAT and BAT, because an increase in cholesterol within mitochondrial membranes may affect the activity of uncoupling protein 1 (UCP-1) and hinder mitochondrial biogenesis. This limits the transformation of WAT into BeAT or BAT, ultimately reducing thermogenesis and energy expenditure [119], contributing to IR and chronic low-grade systemic inflammation.
It is important to note that cholesterol can be oxidized through non-enzymatic mechanisms by ROS. This process generates oxysterols, including 7-oxocholesterol, 7β-hydroxycholesterol, cholesterol epoxides, 27-hydroxycholesterol, and 24S-hydroxycholesterol [120,121,122]. These oxysterols can modify ionic fluxes across the plasma membrane, significantly alter membrane properties, induce oxidative stress in ER, activate signaling pathways that promote inflammation, and ultimately lead to cell death [123,124]. Moreover, experimental evidence indicates that adipose tissue not only accumulates traditional lipids but also acts as a significant reservoir of oxysterols. This may have important implications for chronic inflammation and metabolic dysfunction associated with obesity [125]. Ultimately, oxysterols play crucial roles in maintaining cholesterol homeostasis, regulating immune function, and controlling bone health [126]. They also facilitate neurogenesis in the brain [127] and modulate lipid metabolism in various cells and tissues [128].
In general, a dysfunctional relationship between cholesterol metabolism and oxidative stress in adipose tissue is marked by the buildup of cholesterol and its oxidized derivatives, leading to mitochondrial dysfunction, increased ROS production, and, consequently, cellular damage and necrosis.
Adipose tissue dysfunction in obesity is characterized by chronic low-grade inflammation and oxidative stress. Excessive nutrient intake, pollutants, and external systemic stressors promote adipocyte hypertrophy, immune cell recruitment, and secretion of pro-inflammatory cytokines. These processes generate local and systemic oxidative stress, lipotoxicity, leading to metabolic dysregulation and failure in different organs. Image created with Biorender.com.
7. Conclusions
Adipose tissue should be considered more than just an energy reservoir, but rather an organ that participates in redox, endocrine, and immune processes, which are crucial for overall health. Excessive nutrient intake and various stressors trigger an increase in ROS production and the accumulation of lipotoxic intermediates. These changes alter mitochondrial structure and function, activate the NLRP3 complex, and reprogram innate and adaptive immune cells, promoting a chronic inflammatory response and the development of fibrosis. In contrast, thermogenesis processes in BAT and BeAT, along with anti-inflammatory signals, mitigate adverse effects and improve the body’s health. It can be stated that many metabolic and chronic degenerative diseases originate from the dynamics of redox balance and thermogenic and immune adaptive mechanisms, highlighting the importance of controlling oxidative load and lipotoxic flux in adipose tissue.
Author Contributions
Conceptualization, A.M.-M. and S.R.-A.; methodology, A.M.-M.; investigation, A.M.-M., E.R.-M., P.B.-R. and S.R.-A.; resources, S.R.-A.; writing—original draft preparation, A.M.-M. and E.R.-M.; writing—review and editing, S.R.-A.; supervision, S.R.-A.; funding acquisition, S.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Dirección General de Asuntos de Personal Académico de la Universidad Nacional Autónoma de México, grant number (PAPIIT IN204324) for S.R.A.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.M.M. received funding from Secretaría de Ciencias, Humanidades, Tecnología e Innovación (SECIHTI) CVU 385286.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| •OH | Hydroxyl radical |
| 4-HNE | 4-Hydroxynonenal |
| ABCA1 | ATP-Binding Cassette Transporter A1 |
| ANGPTL4 | Angiopoietin-Like Protein 4 |
| ATP | Adenosine triphosphate |
| BAT | Brown adipose tissue |
| BeAT | Beige adipose tissue |
| BMP-7 | Bone morphogenetic protein 7 |
| CD4+ | CD4+ T lymphocytes (Helper T cells) |
| CD8+ | CD8+ T lymphocytes (Cytotoxic T Cells) |
| CLS | Crown-like structures |
| COX-2 | Cyclooxygenase-2 |
| CXCL14 | C-X-C motif chemokine ligand 14 |
| DAG | Diacylglycerol |
| DAMP | Damage-associated molecular patterns |
| DM2 | Type 2 diabetes mellitus |
| DNL | De novo lipogenesis |
| FGF21 | Fibroblast growth factor 21 |
| H2O2 | Hydrogen peroxide |
| HDL | High-density lipoprotein |
| HIF-1 | Hypoxia-inducible factor 1 |
| IDL | Intermediate-density lipoprotein |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| iNOS | Inducible nitric oxide synthase |
| IR | Insulin Resistance |
| LDL | Low-density lipoprotein |
| LPL | Lipoprotein lipase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| METRNL | Meteorin-like protein |
| MHC-I | Major histocompatibility complex class I |
| MHC-II | Major histocompatibility complex class II |
| MMP-9 | Matrix metalloproteinase-9 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NRG4 | Neuregulin 4 |
| O2•− | Superoxide anion |
| oxLDL | Oxidized low-density lipoprotein |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PRR | Pattern recognition receptors |
| PP2A | Protein Phosphatase 2 |
| PTEN | Phosphatase and Tensin Homolog |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| PUFA | Polyunsaturated fatty acids |
| RBP4 | Retinol-binding protein 4 |
| RE | Endoplasmic reticulum |
| ROS | Reactive oxygen species |
| TG | Triglycerides |
| Th1 | T helper 1 cells |
| Th2 | T helper 2 cells |
| Th17 | T helper 17 cells |
| TGF-β | Transforming growth factor beta |
| TLR | Toll-like receptors |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cells |
| UCP-1 | Uncoupling protein 1 |
| UPR | Unfolded Protein Response |
| VEGF-A | Vascular endothelial growth factor A |
| VLDL | Very-low-density lipoprotein |
| WAT | White adipose tissue |
References
- Harvey, I.; Boudreau, A.; Stephens, J.M. Adipose tissue in health and disease. Open Biol. 2020, 10, 200291. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F. Adipose Tissue Lipid Metabolism During Exercise, in Exercise Metabolism; Springer: Cham, Switzerland, 2022; pp. 137–159. [Google Scholar]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Trevor, L.V.; Riches-Suman, K.; Mahajan, A.L.; Thornton, M.J. Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing. J. Clin. Med. 2020, 9, 2161. [Google Scholar] [CrossRef]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef]
- Hsiao, W.Y.; Guertin, D.A. De Novo Lipogenesis as a Source of Second Messengers in Adipocytes. Curr. Diab Rep. 2019, 19, 138. [Google Scholar] [CrossRef]
- Pilkington, A.-C.; Paz, H.A.; Wankhade, U.D. Beige adipose tissue identification and marker Specificity—Overview. Front. Endocrinol. 2021, 12, 599134. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue—A Translational Perspective. Endocr. Rev. 2022, 44, 143–192. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Brown, Z.; Yoneshiro, T. Brown Fat and Metabolic Health: The Diverse Functions of Dietary Components. Endocrinol. Metab. 2024, 39, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Bielczyk-Maczynska, E. White Adipocyte Plasticity in Physiology and Disease. Cells 2019, 8, 1507. [Google Scholar] [CrossRef] [PubMed]
- Sepa-Kishi, D.M.; Ceddia, R.B. White and beige adipocytes: Are they metabolically distinct? Horm. Mol. Biol. Clin. Investig. 2018, 33, 20180003. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The colors of adipose tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef]
- Parra-Peralbo, E.; Talamillo, A.; Barrio, R. Origin and development of the adipose tissue, a key organ in physiology and disease. Front. Cell Dev. Biol. 2021, 9, 786129. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Cierzniak, A.; Pawełka, D.; Kaliszewski, K.; Rudnicki, J.; Dobosz, T. Metabolic differences between subcutaneous and visceral adipocytes differentiated with an excess of saturated and monounsaturated fatty acids. Genes 2020, 11, 1092. [Google Scholar] [CrossRef]
- Mora, I.; Puiggròs, F.; Serras, F.; Gil-Cardoso, K.; Escoté, X. Emerging models for studying adipose tissue metabolism. Biochem. Pharmacol. 2024, 223, 116123. [Google Scholar] [CrossRef]
- Lim, K.; Haider, A.; Adams, C.; Sleigh, A.; Savage, D.B. Lipodistrophy: A paradigm for understanding the consequences of “overloading” adipose tissue. Physiol. Rev. 2021, 101, 907–993. [Google Scholar]
- Huo, C.; Song, Z.; Yin, J.; Zhu, Y.; Miao, X.; Qian, H.; Wang, J.; Ye, L.; Zhou, L. Effect of Acute Cold Exposure on Energy Metabolism and Activity of Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 917084. [Google Scholar] [CrossRef]
- U-Din, M.; de Mello, V.D.; Tuomainen, M.; Raiko, J.; Niemi, T.; Fromme, T.; Klåvus, A.; Gautier, N.; Haimilahti, K.; Lehtonen, M.; et al. Cold-stimulated brown adipose tissue activation is related to changes in serum metabolites relevant to NAD+ metabolism in humans. Cell Rep. 2023, 42, 113131. [Google Scholar] [CrossRef]
- Nirengi, S.; Stanford, K. Brown adipose tissue and aging: A potential role for exercise. Exp. Gerontol. 2023, 178, 112218. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.K.; Scheele, C. Human adipose depots’ diverse functions and dysregulations during cardiometabolic disease. npj Metab. Health Dis. 2024, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B. Adipose Tissue and Endocrine-Disrupting Chemicals: Does Sex Matter? Int. J. Environ. Res. Public Health 2020, 17, 9403. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Viguerie, N.; Massier, L.; Rydén, M.; Astrup, A.; Blaak, E.; Langin, D.; Andersson, D.P. Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1. Int. J. Obes. 2024, 48, 934–940. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-E.; Sung, M.-K. Sex and Gender Differences in Obesity: Biological, Sociocultural, and Clinical Perspectives. World J. Mens. Health 2025, 43, 758–772. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Valencak, T.G.; Osterrieder, A.; Schulz, T.J. Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017, 12, 806–813. [Google Scholar] [CrossRef]
- Jeon, Y.G.; Kim, Y.Y.; Lee, G.; Kim, J.B. Physiological and pathological roles of lipogenesis. Nat. Metab. 2023, 5, 735–759. [Google Scholar] [CrossRef]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Wallace, M.; Metallo, C.M. Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin. Cell Dev. Biol. 2020, 108, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Taher, J.; Farr, S.; Adeli, K. Central nervous system regulation of hepatic lipid and lipoprotein metabolism. Curr. Opin. Lipidol. 2017, 28, 32–38. [Google Scholar] [CrossRef]
- Cook, J.R.; Kohan, A.B.; Haeusler, R.A. An Updated Perspective on the Dual-Track Model of Enterocyte Fat Metabolism. J. Lipid Res. 2022, 63, 100278. [Google Scholar] [CrossRef]
- Vergès, B. Intestinal lipid absorption and transport in type 2 diabetes. Diabetologia 2022, 65, 1587–1600. [Google Scholar] [CrossRef]
- Wit, M.; Trujillo-Viera, J.; Strohmeyer, A.; Klingenspor, M.; Hankir, M.; Sumara, G. When fat meets the gut—Focus on intestinal lipid handling in metabolic health and disease. EMBO Mol. Med. 2022, 14, e14742. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism during Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef]
- Li, L.; Guo, Z.; Zhao, Y.; Liang, C.; Zheng, W.; Tian, W.; Chen, Y.; Cheng, Y.; Zhu, F.; Xiang, X. The impact of oxidative stress on abnormal lipid metabolism-mediated disease development. Arch. Biochem. Biophys. 2025, 766, 110348. [Google Scholar] [CrossRef]
- Akyol, O.; Akyol, S.; Chou, M.-C.; Chen, S.; Liu, C.-K.; Selek, S.; Soares, J.C.; Chen, C.-H. Lipids and lipoproteins may play a role in the neuropathology of Alzheimer’s disease. Front. Neurosci. 2023, 17, 1275932. [Google Scholar] [CrossRef]
- de Lima, E.P.; Moretti, R.C.; Torres Pomini, K.; Laurindo, L.F.; Sloan, K.P.; Sloan, L.A.; Castro, M.V.M.d.; Baldi, E.; Ferraz, B.F.R.; de Souza Bastos Mazuqueli Pereira, E.; et al. Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways. Biology 2024, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. Febs J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Wu, J.; Chang, J.; Peng, H.; Liu, Z.; Liu, P.; Han, X.; Sun, T.; Shang, D.; Yang, Y.; et al. Deciphering endocrine function of adipose tissue and its significant influences in obesity-related diseases caused by its dysfunction. Differentiation 2025, 141, 100832. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Hemat Jouy, S.; Mohan, S.; Scichilone, G.; Mostafa, A.; Mahmoud, A.M. Adipokines in the Crosstalk between Adipose Tissues and Other Organs: Implications in Cardiometabolic Diseases. Biomedicines 2024, 12, 2129. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 11. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef]
- Mishra, D.; Richard, J.E.; Maric, I.; Porteiro, B.; Häring, M.; Kooijman, S.; Musovic, S.; Eerola, K.; López-Ferreras, L.; Peris, E.; et al. Parabrachial Interleukin-6 Reduces Body Weight and Food Intake and Increases Thermogenesis to Regulate Energy Metabolism. Cell Rep. 2019, 26, 3011–3026.e5. [Google Scholar] [CrossRef]
- Sulen, A.; Aouadi, M. Fed Macrophages Hit the Liver´s Sweet Spot with IL-10. Mol. Cell 2020, 79, 1–3. [Google Scholar] [CrossRef]
- Mahdaviani, K.; Chess, D.; Wu, Y.; Shirihai, O.; Aprahamian, T.R. Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism 2016, 65, 26–35. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Z.; Sun, T.; Zhang, S.; Yang, S.; Zheng, M.; Shen, H. Meteorin-like/Metrnl, a novel secreted protein implicated in inflammation, immunology, and metabolism: A comprehensive review of preclinical and clinical studies. Front. Immunol. 2023, 14, 1098570. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Wu, X.; Zhu, R.; Sun, Y.; Zou, S.; Zhang, D.; Yang, X. Molecular Regulation of Thermogenic Mechanisms in Beige Adipocytes. Int. J. Mol. Sci. 2024, 25, 6303. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2023, 204, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Abou-Rjeileh, U.; Contreras, G.A. Redox Regulation of Lipid Mobilization in Adipose Tissues. Antioxidants 2021, 10, 1090. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Hernandez-Orozco, E.; Rodriguez-Martinez, E.; Valdes-Fuentes, M.; Cornejo-Trejo, V.; Perez-Pacheco, N.; Dorado-Martinez, C.; Zequeida-Carmona, D.; Espinosa-Caleti, I. Ozone Pollution, Oxidative Stress, Regulatory T Cells and Antioxidants. Antioxidants 2022, 11, 1553. [Google Scholar] [CrossRef]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef]
- Qian, W.; Kumar, N.; Roginskaya, V.; Fouquerel, E.; Opresko, P.L.; Shiva, S.; Watkins, S.C.; Kolodieznyi, D.; Bruchez, M.P.; Van Houten, B. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 18435–18444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Li, G.; Fu, T.; Zhang, T.; Lu, X.; Li, N.; Geng, Q. Ceramides and mitochondrial homeostasis. Cell. Signal. 2024, 117, 111099. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Demir, S.Ç.; Sezer, H. Insulin Resistance, Obesity, and Lipotoxicity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 391–430. [Google Scholar]
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef]
- Engin, A.B. What is lipotoxicity? In Obesity and Lipotoxicity; Springer: Cham, Switzerland, 2017; pp. 197–220. [Google Scholar]
- Liu, F.; He, J.; Wang, H.; Zhu, D.; Bi, Y. Adipose Morphology: A Critical Factor in Regulation of Human Metabolic Diseases and Adipose Tissue Dysfunction. Obes. Surg. 2020, 30, 5086–5100. [Google Scholar] [CrossRef]
- Chaurasia, B.; Talbot, C.L.; Summers, S.A. Adipocyte Ceramides—The Nexus of Inflammation and Metabolic Disease. Front. Immunol. 2020, 11, 576347. [Google Scholar] [CrossRef]
- Ireland, S.C.; Huang, H.; Zhang, J.; Li, J.; Wang, Y. Hydrogen peroxide induces Arl1 degradation and impairs Golgi-mediated trafficking. Mol. Biol. Cell 2020, 31, 1931–1942. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Salvayre, R. Reactive Carbonyl Species and Protein Lipoxidation in Atherogenesis. Antioxidants 2024, 13, 232. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Soulage, C.O.; Pelletier, C.C.; Florens, N.; Lemoine, S.; Dubourg, L.; Juillard, L.; Guebre-Egziabher, F. Two toxic lipid aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), accumulate in patients with chronic kidney disease. Toxins 2020, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Álvarez-Indo, J.; Cifuentes, M.; Morselli, E.; Kerr, B.; Burgos, P.V. Enhancing adipose tissue functionality in obesity: Senotherapeutics, autophagy and cellular senescence as a target. Biol. Res. 2024, 57, 51. [Google Scholar] [CrossRef] [PubMed]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of cardiovascular diseases: New insights into molecular mechanisms of atherosclerosis, arterial hypertension, and coronary artery disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Shyy, J.Y.J. Context-Dependent Role of Oxidized Lipids and Lipoproteins in Inflammation. Trends Endocrinol. Metab. 2017, 28, 143–152. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Antony, V.; Sun, H.; Liang, G. Metabolism-associated molecular patterns (MAMPs). Trends Endocrinol. Metab. 2020, 31, 712–724. [Google Scholar] [CrossRef]
- Lolescu, B.M.; Furdui-Lința, A.V.; Ilie, C.A.; Sturza, A.; Zară, F.; Muntean, D.M.; Blidișel, A.; Crețu, O.M. Adipose tissue as target of environmental toxicants: Focus on mitochondrial dysfunction and oxidative inflammation in metabolic dysfunction-associated steatotic liver disease. Mol. Cell. Biochem. 2025, 480, 2863–2879. [Google Scholar] [CrossRef]
- Revilla Flores, E.M. Especies reactivas de oxígeno, importancia e implicación patológica. Rev. Científica Cienc. Médica 2021, 24, 125–132. [Google Scholar]
- Lennicke, C.; Cochemé, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 101964. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016, 532, 112–116. [Google Scholar] [CrossRef]
- Li, Q.; Spalding, K.L. The regulation of adipocyte growth in white adipose tissue. Front. Cell Dev. Biol. 2022, 10, 1003219. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, N.; Tercero-Alcázar, C.; Malagón, M.M.; Garcia-Roves, P.M.; Guzmán-Ruiz, R. Interrelation of adipose tissue macrophages and fibrosis in obesity. Biochem. Pharmacol. 2024, 225, 116324. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Sung, J.H.; Huh, J.Y. Diverse Functions of Macrophages in Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease: Bridging Inflammation and Metabolism. Immune Netw. 2025, 25, e12. [Google Scholar] [CrossRef]
- Stevens, H.Y.; Bowles, A.C.; Yeago, C.; Roy, K. Molecular Crosstalk Between Macrophages and Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 600160. [Google Scholar] [CrossRef]
- Caër, C.; Rouault, C.; Le Roy, T.; Poitou, C.; Aron-Wisnewsky, J.; Torcivia, A.; Bichet, J.-C.; Clément, K.; Guerre-Millo, M.; André, S. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 2017, 7, 3000. [Google Scholar] [CrossRef]
- Weinstock, A.; Moura Silva, H.; Moore, K.J.; Schmidt, A.M.; Fisher, E.A. Leukocyte heterogeneity in adipose tissue, including in obesity. Circ. Res. 2020, 126, 1590–1612. [Google Scholar] [CrossRef]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef]
- Park, C.-S.; Shastri, N. The role of T cells in obesity-associated inflammation and metabolic disease. Immune Netw. 2022, 22, e13. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T helper cell subsets and related human immunological disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef]
- Varra, F.-N.; Varras, M.; Varra, V.-K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options. Mol. Med. Rep. 2024, 29, 1–27. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 505887. [Google Scholar] [CrossRef]
- Chakarov, S.; Blériot, C.; Ginhoux, F. Role of adipose tissue macrophages in obesity-related disorders. J. Exp. Med. 2022, 219, e20211948. [Google Scholar] [CrossRef]
- Huynh, P.M.; Wang, F.; An, Y.A. Hypoxia signaling in the adipose tissue. J. Mol. Cell Biol. 2024, 16, mjae039. [Google Scholar] [CrossRef]
- Sun, K.; Li, X.; Scherer, P.E. Extracellular matrix (ECM) and fibrosis in adipose tissue: Overview and perspectives. Compr. Physiol. 2023, 13, 4387–4407. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The interplay between adipose tissue and vasculature: Role of oxidative stress in obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Zhang, Y.; Liu, J.; Jiang, L.; Hu, L.; Yao, K.; Yu, Y.; Chen, X. Cholesterol metabolism: Physiological regulation and diseases. MedComm (2020) 2024, 5, e476. [Google Scholar] [CrossRef]
- Long, T.; Debler, E.W.; Li, X. Structural enzymology of cholesterol biosynthesis and storage. Curr. Opin. Struct. Biol. 2022, 74, 102369. [Google Scholar] [CrossRef]
- Ho, W.Y.; Hartmann, H.; Ling, S.C. Central nervous system cholesterol metabolism in health and disease. IUBMB Life 2022, 74, 826–841. [Google Scholar] [CrossRef]
- Bays, H.E.; Kirkpatrick, C.; Maki, K.C.; Toth, P.P.; Morgan, R.T.; Tondt, J.; Christensen, S.M.; Dixon, D.; Jacobson, T.A. Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. Obes. Pillars 2024, 10, 100108. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- White, U. Adipose tissue expansion in obesity, health, and disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 2022, 25, 439–453. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.W.; Zeng, P.H.; Zhou, Y.J.; Yin, W.J. Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 2022, 21, 1121–1139. [Google Scholar] [CrossRef]
- Bacchetti, T.; Morresi, C.; Simonetti, O.; Ferretti, G. Effect of Diet on HDL in Obesity. Molecules 2024, 29, 5955. [Google Scholar] [CrossRef]
- Zuo, Y.; He, Z.; Chen, Y.; Dai, L. Dual role of ANGPTL4 in inflammation. Inflamm. Res. 2023, 72, 1303–1313. [Google Scholar] [CrossRef]
- Leth-Espensen, K.Z.; Kristensen, K.K.; Kumari, A.; Winther, A.M.L.; Young, S.G.; Jørgensen, T.J.; Ploug, M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc. Natl. Acad. Sci. USA 2021, 118, e2026650118. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Saito, A.; Sunagawa, Y.; Sari, N.; Funamoto, M.; Shimizu, S.; Shimizu, K.; Akimoto, T.; Ueki, C.; Kitano, M.; et al. ANGPTL4 Expression Is Increased in Epicardial Adipose Tissue of Patients with Coronary Artery Disease. J. Clin. Med. 2022, 11, 2449. [Google Scholar] [CrossRef]
- Ma, Q.; Lim, C.S. Molecular Activation of NLRP3 Inflammasome by Particles and Crystals: A Continuing Challenge of Immunology and Toxicology. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 417–433. [Google Scholar] [CrossRef]
- Liang, J.J.; Fraser, I.D.C.; Bryant, C.E. Lipid regulation of NLRP3 inflammasome activity through organelle stress. Trends Immunol. 2021, 42, 807–823. [Google Scholar] [CrossRef]
- Lindhorst, A.; Raulien, N.; Wieghofer, P.; Eilers, J.; Rossi, F.M.V.; Bechmann, I.; Gericke, M. Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis. 2021, 12, 579. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Goicoechea, L.; Conde de la Rosa, L.; Torres, S.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease. Redox Biol. 2023, 61, 102643. [Google Scholar] [CrossRef]
- Rauchbach, E.; Zeigerman, H.; Abu-Halaka, D.; Tirosh, O. Cholesterol Induces Oxidative Stress, Mitochondrial Damage and Death in Hepatic Stellate Cells to Mitigate Liver Fibrosis in Mice Model of NASH. Antioxidants 2022, 11, 536. [Google Scholar] [CrossRef]
- Brunner, S.; Höring, M.; Liebisch, G.; Schweizer, S.; Scheiber, J.; Giansanti, P.; Hidrobo, M.; Hermeling, S.; Oeckl, J.; Prudente de Mello, N.; et al. Mitochondrial lipidomes are tissue specific—Low cholesterol contents relate to UCP1 activity. Life Sci Alliance 2024, 7. [Google Scholar] [CrossRef]
- Baila-Rueda, L.; Cenarro, A.; Lamiquiz-Moneo, I.; Marco-Benedi, V.; Gracia-Rubio, I.; Casamayor-Franco, M.C.; Arbones-Mainar, J.M.; Civeira, F.; Laclaustra, M. Association of Cholesterol and Oxysterols in Adipose Tissue with Obesity and Metabolic Syndrome Traits. J. Clin. Endocrinol. Metab. 2022, 107, e3929–e3936. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Yutuc, E.; Abdel-Khalik, J.; Crick, P.J.; Hearn, T.; Dickson, A.; Bigger, B.W.; Hoi-Yee Wu, T.; Goenka, A.; Ghosh, A.; et al. Metabolism of Non-Enzymatically Derived Oxysterols: Clues from sterol metabolic disorders. Free. Radic. Biol. Med. 2019, 144, 124–133. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef]
- Nury, T.; Yammine, A.; Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Brahmi, F.; Samadi, M.; Rup-Jacques, S.; Vervandier-Fasseur, D.; Pais de Barros, J.P.; et al. Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases. Ageing Res. Rev. 2021, 68, 101324. [Google Scholar] [CrossRef]
- Pariente, A.; Pérez-Sala, Á.; Ochoa, R.; Bobadilla, M.; Villanueva-Martínez, Á.; Peláez, R.; Larráyoz, I.M. Identification of 7-Ketocholesterol-Modulated Pathways and Sterculic Acid Protective Effect in Retinal Pigmented Epithelium Cells by Using Genome-Wide Transcriptomic Analysis. Int. J. Mol. Sci. 2023, 24, 7459. [Google Scholar] [CrossRef]
- Nguyen, C.; Saint-Pol, J.; Dib, S.; Pot, C.; Gosselet, F. 25-Hydroxycholesterol in health and diseases. J. Lipid Res. 2024, 65, 100486. [Google Scholar] [CrossRef]
- Choi, I.A.; Umemoto, A.; Mizuno, M.; Park-Min, K.-H. Bone metabolism—An underappreciated player. npj Metab. Health Dis. 2024, 2, 12. [Google Scholar] [CrossRef]
- De La Fuente, D.C.; Tamburini, C.; Stonelake, E.; Andrews, R.; Hall, J.; Owen, M.J.; Linden, D.E.J.; Pocklington, A.; Li, M. Impaired oxysterol-liver X receptor signaling underlies aberrant cortical neurogenesis in a stem cell model of neurodevelopmental disorder. Cell Rep. 2024, 43, 113946. [Google Scholar] [CrossRef]
- Brown, A.J.; Sharpe, L.J.; Rogers, M.J. Oxysterols: From physiological tuners to pharmacological opportunities. Br. J. Pharmacol. 2021, 178, 3089–3103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).