Abstract
Background: A sexually active lifestyle is generally associated with positive effects on physical condition and health. However, engaging in sexual activity prior to a sports competition could affect athletic performance. This systematic review examines the current literature on the impact of pre-exercise sexual activity on sports performance, with particular attention paid to its effects on physiological, hormonal, cognitive, and perceptual markers. Method: Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we systematically reviewed original studies published within the last 25 years. Eligible studies were randomized or non-randomized controlled design and indexed on PubMed, Scopus, Dialnet, and Cochrane. Additional sources were consulted including a network diagram with Connected Papers®. Two methodological quality scales, McMaster University Occupational Therapy Evidence-Based Practice Research Group and Physiotherapy Evidence Database (PEDro), were used. The study was registered in PROSPERO (#CRD42023426555). Results: A total of 244 records were identified through the search process, of which 7 studies met the inclusion criteria. The studies involved 117 (115 men) physically and sexually active subjects including 29 elite top athletes. When comparing the sexual activity condition/group (SexG) to abstinence (AbsG), significant (p < 0.05) decreases were observed in average speed and maximum strength. In contrast, non-significant trends towards improvement (p > 0.05) were observed in exercise capacity, reaction time, and muscular endurance. No significant changes (p > 0.05) were found in physiological and hormonal biomarkers and fatigue perception. However, perceived exertion was significantly higher (p < 0.05) in SexG compared to AbsG. Conclusions: Current evidence does not conclusively support the influence of pre-exercise sexual activity on sports performance, or physiological and hormonal biomarkers. However, it could contribute to increased perception of exercise intensity.
1. Introduction
Sex is a natural human behavior that assists a range of social, psychological, and physiological functions. Sexual activity has been recognized as an key component of optimal Health-Related Quality of Life (HRQOL) [1]. A sexually active lifestyle positively influences physical fitness, immune function, cardiovascular health, circadian rhythms, blood pressure (BP), and stress [2,3]. In addition, regular physical activity contributes to optimal sexual health [4]. However, the potentially beneficial dynamics of sexual behavior (i.e., sexual intercourse, masturbation, and orgasm) seem to be altered in the context of pre-competition situations [5]. Sexual activity before competition may exert either positive or negative effects on sports performance, potentially impacting the central nervous system, sympathetic activation, and cardiovascular function [6]. Psychological states such as aggressiveness, motivation, alertness, and/or competitive attitude may be also influenced [1], along with hormonal fluctuations [7], sleep patterns [8], muscle performance and inflammatory responses [5]. Therefore, managing the athlete’s environment and activities, including sexual behavior, is increasingly viewed as essential. Sexual activity can interfere with conventional training programs and affects performance outcomes [9]. This consideration is particularly relevant in highly competitive sports, where athletic performance is closely tied to commercial and economic interests.
Ancient Greek and Roman cultures considered sexual abstinence to be the optimal condition to maximize sports performance. These cultures considered semen as “a cerebrospinal substance imbued with divine energy”. Consequently, “losing or spilling it” was believed to result in negative consequences such as loss of vital force or a decline in brain function, which could lead to physical and mental health issues, including increased susceptibility to disease [10]. The rational supporting pre-competitive sexual abstinence [11] included the following: increased aggressiveness and mental focus [10,12], preservation of testosterone (T) levels [13], and prevention of energy depletion, and conservation of muscular strength (STR) [14]. Furthermore, sexual activity has been associated with behaviors considered unhealthy and/or detrimental for sports performance, such as insufficient sleep [15], smoking, and alcohol consumption, which may contribute to greater energy loss and reduced sports performance [14,16].
Considering that athletes are often more sexually active than the general population [17], the topic of sexual activity before competitions has generated considerable debate. It has attracted attention from athletes, coaches, and the public alike, as people try to understand its potential impact on sporting outcomes, whether it contributes to victory or defeat. Despite the abundance of conflicting theories regarding the extent to which sexual activity can affect sport performance, this remains a largely under-researched and unresolved issue within the scientific community. Previous review studies [5,14,17] have consistently reported that sexual activity before exercise does not negatively affect sports performance parameters. However, these studies did not include assessment of biological biomarkers. Sexual activity can influence physiological and psychological factors relevant to sports performance. Nonetheless, its specific effects on physiological, hormonal, and psychological biomarkers have yet to be clearly elucidated. To date, no systematic review has examined the potential benefits or harms of sexual activity on health or sports performance in healthy adults. To address this gap, we conducted a systematic review of the current evidence to evaluate how pre-exercise sexual activity affects healthy, physically and sexually active adults. Therefore, the aim was to determine whether sexual activity enhances or impairs physiological, perceptual and cognitive parameters, hormonal profiles, and sports performance.
2. Methods
2.1. Protocol and Registration
The present systematic review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18] (Appendix A). The review protocol can be accessed on the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42023426555).
2.2. Eligibility Criteria
For the study selection, the following inclusion criteria were used: (a) healthy adult men and healthy adult women, without any chronic disease, practicing physical activity and sexually active; (b) clinical trials (randomized controlled trials and other studies) that evaluated the effect of sexual activity compared with a control group; (c) trials that reported effects on variables related to physical performance, physiological biomarkers, hormonal responses, and perceptual and cognitive parameters; (d) studies with a methodological quality ≥ 10 points on the McMaster University Occupational Therapy Evidence-Based Practice Research Group (McMaster) [19] and ≥6 points on the Physiotherapy Evidence Database (PEDro) scale [20]; (e) articles that have been published since 2000.
Studies that did not meet these criteria were excluded. Thus, studies that did not correspond to original research (e.g., editorials, notes, reviews, dissertations, etc.) or did not include adults (e.g., children, elderly, etc.) were also excluded.
2.3. Search Strategy
A research question was structured using the PICO framework, which stands for Patient/Population, Intervention, Comparison, and Outcome, in accordance with the Evidence-Based Medicine (EBM) guidelines [21] as follows: P (population): physically and sexually active healthy adults. I (intervention): “practice of sexual activity before exercise’. C (comparison): “maintaining an abstinence condition before exercise”. O (outcomes): sports performance (musculoskeletal status—strength [STR], power [Pw], endurance; cardiorespiratory physical work capacity, reaction time and speed); physiological biomarkers (heart rate [HR], blood pressure [BP], blood glucose, maximum oxygen volume [VO2max]); hormonal patterns (testosterone [T], cortisol [C]); cognitive assessment (focus); perceptual parameters (ratio of perceived exertion [RPE], fatigue); S (study design): “randomized or non-randomized clinical trials”. These markers were included as outcomes, as they are commonly investigated in biomarker studies on sport and health research.
A structured search was performed between April and June 2025, using 4 databases: Medline/PubMed, Scopus, Dialnet, and Cochrane. In each database, the search strategy used a mixture of Medical Subject Headings (MSHs) and free words for key concepts related to sex, exercise, and the outcomes of the review, which are as follows: (“Coitus” OR “sexual intercourse” OR “sexual activity” OR “sexual relations” OR “masturbation) AND (“physiological” OR “biological” OR “hormonal” OR “physical” OR “sports performance” OR “athletic performance” “heart rate” OR “ power” OR “speed” OR “strength” OR “testosterone” OR “focus” OR “perceived exertion”) AND (“markers” OR “effects” OR “analysis” OR “biomarkers” OR “indicators” OR “activity” OR “behavior”).
The identification of potential studies was enriched thanks to performing a manual search by checking the reference lists of publications eligible for full-text review and using ResearchGate (www.researchgate.net, accessed 23 June 2025) to identify potential articles not included in the four databases mentioned above and used in the study. In addition, a network graph was generated with Connected Papers (http://www.connectedpapers.com, accessed 23 June 2025) to ensure the inclusion of recent publications and to visually find relevant publications.
The study selection took place during April and June 2025, although an updated search was conducted in August 2025 prior to manuscript submission.
2.4. Methodological Quality Assessment Tools
The McMaster University Occupational Therapy Evidence-Based Practice Research Group (McMaster) critical review form [19] and the Physiotherapy Evidence Database (PEDro) (https://pedro.org.au/spanish/ accessed on 19 August 2025) [20] were used as tools to assess the methodological quality of the studies included in this review.
2.5. Data Extraction and Synthesis
Using a spreadsheet (Microsoft Inc., Seattle, WA, USA), two authors independently performed the search for published studies and their inclusion. Disagreements on them were resolved by a third author, to comply with the Consolidated Standards of Reporting Trials (CONSORT) 2025 guidelines [22]. The following data were reported for each study: first author′s name, year of publication, country of study, type of study, sample size, anthropometric measurements of participants, control intervention (sex abstinence), experimental intervention (sexual intercourse), and markers assessed in the studies and the results of these measurements.
3. Results
3.1. Study Selection
The systematic literature search resulted in 249 records. Among these, 244 were initially retrieved from Medline/PubMed, Scopus, Dialnet, and Cochrane, while 5 additional records were identified from additional sources, including ResearchGate and the reference lists of relevant studies. After removing 13 duplicates, a total of 231 records were screened. Of these, 153 studies were excluded for not meeting the eligibility criteria. Seventy-eight records were considered as potential registrations for title and abstract screening. Following full-text review and evaluation, seven studies [23,24,25,26,27,28,29] from databases and complementary sources were included in the final systematic review. Figure 1 presents a flowchart of the literature selection process.
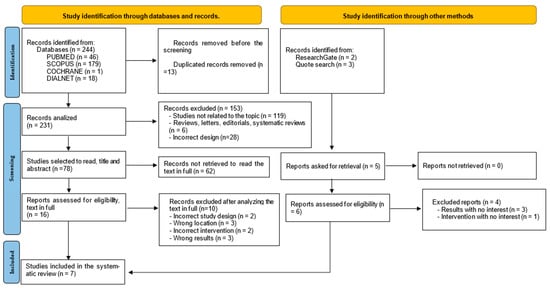
Figure 1.
Flow diagram representing the processes of identification and selection of relevant studies according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
In addition, the verification of key studies on the effects of sexual intercourse is illustrated in Figure 2, which presents a node graph displaying each study. This node graph is adapted from the original proposed by Stefani et al. [14].
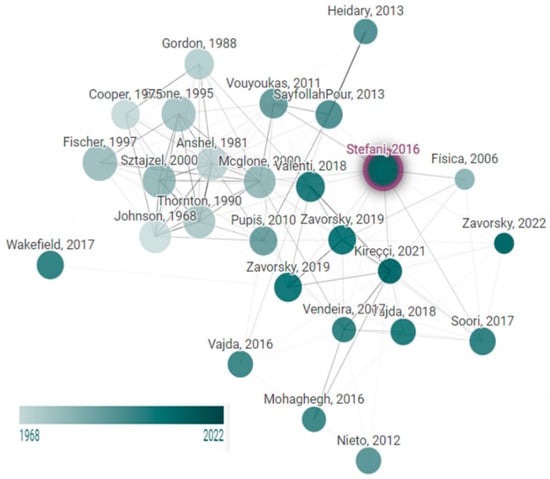
Figure 2.
Network diagram of trials on the influence of sexual relationships on sports performance. Node size is proportional to the number of citations, and the color is the publishing year This graph was developed using www.connectedpapers.com, on 23 June 2025.
3.2. Quality Assessment
Based on the McMaster form criteria [19], four studies [23,24,25,27] were rated as ’very good quality’, while three studies [26,28,29] achieved the ‘good quality’ status. All studies met the minimum quality criteria, although the main deficiencies were identified in items 3, 9 and 11 (Table 1).

Table 1.
Results of the evaluation of the methodological quality of the included studies—McMaster Critical Review Form for Quantitative Studies [19].
Table 2 shows the results of the PEDro [20] scale, the main deficiencies found were in items 5 and 6 of the scale. Three studies [26,27,28] achieved ‘very good quality’, and four studies [23,24,25,29] obtained ‘good quality’. None of the studies were discarded for not reaching the minimum quality.

Table 2.
Results of the evaluation of the methodological quality of the included studies—Physiotherapy Evidence Database (PEDro) [20].
3.3. Characteristics of the Participants and Interventions
We included seven controlled [23,24,25,26,27,28,29] studies, either randomized [25,26,27,28,29] or non-randomized [23,24], using repeated-measures [23,27,29], crossover [24,25,28], or Latin square [26] designs. A total of 117 participants were enrolled: 115 men (♂) [23,24,25,26,27,28,29] and 2 women (♀) [27,29]. These included 14 professional soccer players [24], 15 elite athletes [25] (1 soccer player, 7 hockey players, 3 cyclists, 2 runners, and 2 weightlifters), 50 semiprofessional athletes [23], and 38 (36 ♂) physically active subjects [26,27,28,29]. Sexual activity culminating in orgasm was completed either the night before [27,28,29], 12 h [26], or 24 h [23,24,25,28] before the tests. Periods of abstinence ranged from the previous night [27,29] to 12 h [26], 24 h [23,24,25], or up to 5 days [28] before data collection and biomarker or parameter assessment (Table 3).

Table 3.
Studies included in the systematic review of the effect of sexual activity on sports performance, physiological biomarkers, and hormonal response in sexually and physically active healthy adults.
3.4. Outcomes
Table 3 summarizes the key information and main findings from the seven studies [23,24,25,26,27,28,29] included in the present systematic review. All outcomes were reported by comparing the sexually active condition/group (SexG) with the abstinence study condition/group (AbsG).
3.4.1. Sports Performance
To assess musculoskeletal performance, STR [23,26,27,28,29], Pw [27,28,29] and endurance [27,29] were evaluated. A significant (p < 0.05) [23] decrease in lower extremity (LE) STR was observed in SexG compared to AbsG. Non-significant [26] decreases (p > 0.05) in LE and upper extremity (UE) STR were also reported [27] comparing SexG vs. AbsG. In addition, non-significant (p > 0.05) reductions in UE Pw [28,29] were observed in SexG compared with AbsG. Conversely, marginal improvements in LE STR [28,29], UE Pw [27], and UE muscle endurance [27] were observed in SexG compared to AbsG.
Total cardiorespiratory exercise capacity increased non-significantly (p > 0.05) following sexual activity vs. AbsG in three of the included studies [24,27,28]. However, among professional soccer players [24], non-significant (p > 0.05) reductions were observed in time played and distance covered, along with a significant (p < 0.05) decrease in average speed in the SexG compared to the AbsG. Regarding maximum speed [24] and response time [27,28,29], slight improvements (p > 0.05) were observed in SexG compared to AbsG.
3.4.2. Physiological Biomarkers
The effect of sexual activity on physiological biomarkers was examined in four clinical trials [24,25,27,28]. No significant (p > 0.05) changes were observed for HR [24,25], BP [25], mental focus [25], blood glucose [27], and VO2max [25,28], when comparing SexG vs. AbsG during maximal exertion. However, one study [27] reported a significant increase (p < 0.05) in HR during the early recovery period, followed by a significant decrease (p < 0.05) in a later recovery phase after sexual activity, compared to the day without sexual activity, using a maximal exertion test in elite athletes [25]. In addition, slight non-significant improvements (p > 0.05) in VO2max were observed in elite athletes [25] and individuals with an aerobic fitness percentile between 77 and 99 [28] in SexG vs. AbsG.
3.4.3. Hormonal Pattern
T [25,27] and C [27] hormones showed slight non-significant (p > 0.05) increases in the SexG compared to the abstinence condition.
3.4.4. Perception Parameters
Among elite athletes, Sztajzel et al. [25] reported a significant increase (p < 0.05) in RPE in SexG compared to AbsG during a 1 h exertion test. In contrast, Valentí et al. (2018) [26] found no differences in physical or mental fatigue between participants engaged in sexual activity compared to those who had not.
3.4.5. Cognitive Assessment
Cognitive assessment of mental focus performed during the 1 h physical exertion test [25] decreased non-significantly (p > 0.05) in SexG.
4. Discussion
This systematic review aimed to determine whether engaging in sexual activity culminating in orgasm before physical exercise would be disadvantageous or beneficial for athletic performance. To the best of the authors′ knowledge, this is the first systematic review analysis that critically assesses the impact of sexual activity on physiological biomarkers, hormonal responses, cognitive assessment, and perceptual parameters. Seven studies [23,24,25,26,27,28,29], including one PhD thesis [27], met the inclusion/exclusion criteria specified above. Only the study conducted by Peleg-Sagy et al. [24] investigated the effects of sexual activity during an official competition (a professional soccer match), while the remaining six clinical trials [23,25,26,27,28,29] used different fitness tests. Across parameters such as Pw and muscular endurance, exercise capacity, response time, VO2max, T and C levels, blood glucose, BP, fatigue perception and mental focus, no evidence was found to support a casual influence of sexual activity performed the night before or up to 24 h prior evaluation. However, significant decreases (p < 0.05) were observed in LE STR [23] and the speed measured during the soccer match [24]. In addition, significant increases (p < 0.05) were reported in RPE during long-duration tests and HR during the recovery phase following maximum exertion in SexG compared to AbsG.
Previous research described how sexual activity prior exercise does not alter physiological test outcomes, suggesting it may not impair an athlete′s ability to perform at their peak [4,17,30]. However, this effect appears to depend on the duration and intensity of sexual intercourse, as well as individual variability in sexual response. In this context, highly trained athletes are advised to avoid spending additional hours in bed the night before a competition, not primarily due to the modest energy expenditure (approximately 25–50 Kcal including both pre-orgasmic and orgasmic phases [4]), but more importantly, because of the potential loss of sleep [8], which can negatively affect recovery and performance.
Sztajzel et al. [25] reported markedly elevated HR and BP values during a maximal exertion test, as well as significantly increased HR values (p < 0.05) during the early post-exercise recovery phase, conducted 2 h after sexual activity. These elevations in HR and BP suggest reduced parasympathetic activation and enhanced sympathetic system activity [31]. Elevated HR values may exert a decisive influence on sports performance, particularly when recovery cycles are not optimal [32]. Therefore, athletes participating in sporting events characterized by short recovery periods (multi-stage cycling tours, tennis tournaments, triangular or quadrangular team competitions, or final matches of national or continental cups such as the Country Cup or the European Basketball Cup) require optimal recovery conditions. In such contexts, pre-competition sexual activity could interfere with optimal recovery, potentially compromising performance. Conversely, the same researchers [25] reported significant (p < 0.05) HR reductions during the later phase of recovery following sexual intercourse, compared to the day without sexual activity. This finding suggests enhanced parasympathetic modulation during the late recovery phase [31]. Altogether, these results, using HR as predictive marker, underscore the importance of maintaining an appropriate interval between sexual activity and competitive sports events to avoid disruptions in post-exercise recovery.
However, increases in HR may contribute to significant improvements in overall exercise cardiorespiratory capacity by enhancing cardiac efficiency and pulmonary oxygenation [25,27,28]. Maintaining an elevated HR within a target zone (50–85% of maximum HR) for a sustained period is therefore considered a key mechanism for improving cardiorespiratory fitness. This can enhance VO2max [25,28], considered a key physiological marker for predicting performance and increases in muscular endurance [27], particularly in SexG vs. AbsG. In this sense, sexual arousal and/or response to sexual stimuli involves the release of neurotransmitters such as noradrenaline (NA), which triggers an adrenergic response responsible for elevated HR, BP, and ventilation rates [33]. This sympathetic activation, driven by the adrenergic stimulation during sexual activity, closely resembles the initial physiological response observed at the onset of exercise [27]. Such activation may facilitate demanding physical efforts by promoting intense cardiac output, vasodilation, elevated BP and bronchodilation, thereby improving oxygen delivery and utilization in muscle tissue. In addition, glycogen mobilization and consumption occur, increasing the energy stores available for muscular activity [32].
In a study included in our review, Peleg-Sagy et al. [24] assessed HR and HR variability (reflecting the parasympathetic activity and total training load) in professional soccer players during the first 24 h post-match. Results were similar between both study groups (GSex and AbsG), yet athletic performance declined. Among these athletes [24], playing time, total and acceleration distance, and average match speed decreased significantly. This reduction in performance could suggest that the physiological state following sexual activity does not elicit a sufficiently strong sympathetic response when required, or that the body fails to transition effectively into a strong sympathetic response during exercise. Sympathetic activation involves the secretion of catecholamines such as epinephrine and norepinephrine from the sympathetic nerve endings. These hormones enhance alertness and trigger the “fight-or-flight” response, increasing HR, blood pressure, and glucose availability, while redirecting blood flow to skeletal muscles, preparing the body for intense physical exercise [33]. Therefore, other factors, such as elevated parasympathetic activity, could limit sport performance. Elevated parasympathetic activity can impair the body′s capacity to respond to sympathetic stimulation during exercise [24]. In addition, athletic performance may not improve due to other factors derived from sexual activity, such as sleep and psychological indicators. Lack of rest and psychological factors may also be a factor if the athlete believes it is detrimental or disrupts their usual routine [34]. Additional limiting factors may include insufficient energy intake, muscle fatigue, or the intensity of previous sexual activity [5,14,17]. These mechanisms may help explain the slower response times [26,28,29] observed in SexG vs. AbsG. Altogether, these findings suggest that sexual nervous system activity may influence physical performance by affecting the sequential modulation (activation or inhibition) of sympathetic and/or parasympathetic activity within the autonomous nervous system.
The results reported in the studies included in this systematic review highlight the ongoing controversy regarding the influence of sexual activity on neuromuscular performance, specifically STR [23,24,27,28,29] and Pw [27,28,29], when comparing both conditions (SexG and AbsG). On the one hand, significant (p < 0.05) [23] and substantial [26] decreases in LE STR were observed along with reductions in UE [27]. However, UE STR markedly increased in two studies [27,28]. Pw in the LE showed either an increasing [27,29] or decreasing [28] trend. In addition, musculoskeletal endurance either increased [27] or remained unchanged [28]. Sexual activity culminating in orgasm induces specific neural stimuli that may enhance muscle contractions [33]. These stimuli are repetitive eccentric contractions, such as those occurring during exercises in which muscles lengthen under gravitational load. Similar contractions also occur during the erection process, and the associated movements before and after ejaculation [33]. These stimuli may approximate the phenomenon of post-activation potentiation (PAP), where muscle contractions can temporarily increase the production of STR and/or Pw, as shown in a subsequent exercise [35]. PAP affects the nervous system by increasing motor unit recruitment [36], and influences skeletal muscle by promoting myosin light chain phosphorylation and increasing intracellular calcium (Ca+2) levels, thereby increasing myocyte sensitivity [37]. Altogether, these mechanisms may contribute to improvements in neuromuscular performance. However, the effectiveness of PAP on muscle STR and Pw depends on the balance between potentiation and fatigue, as well as the intensity, volume, and nature of the activating stimulus. This dynamic may result in increases, maintenance, or decreases in neuromuscular performance [36], potentially explaining the divergent findings reported. Stimuli induced by sexual activity prior to an exercise could transmit to the organism the message that it is entering into a physically demanding stage. This can temporarily prime body systems and the central nervous system to initiate adaptive mechanisms required for the upcoming physical effort [35].
Exon et al. [38] reported that prolonged sexual abstinence, particularly over 3 weeks in men, may lead to elevated T levels, a pattern that can be reversed by ejaculation. The average difference in T levels between abstinence and sexual activity is approximately 0.5 ng/mL [38]. Conversely, sexual intercourse and orgasm may also result in transient increases in T levels, peaking around the time of ejaculation and returning to baseline shortly thereafter. These increases are temporary and do not persist chronically [39,40]. Moreover, a lack of sexual activity has been associated with reduced serum T levels [4], further underscoring the relationship between T and orgasm. Sympathetic nervous system activation during sexual activity may contribute to the observed increase in T levels [25]. Another theory that could be challenged is the notion that sexual activity prior to competition negatively affects T levels [8]. T activates satellite cells, promoting myonuclear accretion and replenishment of the satellite cell pool. This also influences pluripotent stem cells, favoring their differentiation to increased muscle mass, enhancing protein synthesis and overall STR [41]. This suggests a positive relationship between total T and muscle STR, particularly in men. However, two studies [25,28] included in this review reported slight increases in T levels, with no corresponding improvements in STR [28]. These findings agree with previous research indicating that post-orgasmic increases in plasma T levels may be insufficient to elicit meaningful changes in STR [42], with the effect being likely too small to produce significant outcomes [6]. Moreover, the duration of sympathetic activation following orgasm varies, but the subsequent calming phase typically lasts from several minutes to a few hours, with the full recovery period potentially extending up to 24 h or more. Following orgasm, this recovery phase is characterized by decreased sympathetic response and increased parasympathetic activity [43], leading to relaxation, temporal fatigue, and a possible decline in STR as the body rebalances. Orgasm also triggers a significant spike in prolactin levels (approximately 10 to 15 ng/mL) peaking within 10–20 min and gradually declining thereafter [44]. Elevated prolactin levels have been associated with lower athletic performance in judokas [45], and post-orgasmic prolactin secretion suppress T levels [46]. Furthermore, recent evidence suggests that although increased serum T levels are associated with greater muscle mass, they do not necessarily correlate with enhanced muscle STR in adult men [47]. Conversely, the substantial improvements in UE STR in the two studies by Zavorsky et al. [28,29] included in this review may be attributed to alternative mechanisms, such as enhanced phosphocreatine availability, increased muscle fiber recruitment [37], or optimized neuromuscular function [36]. These effects may be mediated by sympathetic activation [31] or PAP [35], as previously described.
C hormone was evaluated in the study by Vouyoukas et al. [27], showing a slight tendency to increase in the SexG vs. AbsG. C is released by the adrenal gland in response to sexual activity, particularly in arousal and ejaculation, which constitutes a form of “positive” stress or “eustress” [48]. In this context, both C and T are increased due to sympathetic nervous system activation immediately following orgasm [25], and subsequently returned to baseline levels [33]. These increases in C would facilitate energy metabolism in response to stressors, such as exercise, by enhancing glucose availability and inhibiting glycogen synthesis and insulin secretion [49]. Elevated pre-exercise blood glucose levels allow for greater availability of circulating glucose, a key energy source for working muscles and the central nervous system during exercise [50]. This increase in glucose availability could contribute to improvements in aerobic efficiency during total exercise capacity tests, as well as enhanced musculoskeletal performance in Pw and endurance [27]. In addition, the marked decrease in blood glucose observed in the SexG condition following the exertion test, compared to the AbsG, suggests greater glucose consumption. While the modest increases in T [25,27] appear insufficient to significantly influence athletic performance, the modest increases in C [27] may support energy metabolism under stress by regulating glucose utilization [51]. Another misconception that could be dispelled among athletes and coaches is the belief that sexual activity before competition depletes glycogen stores [8]. A balanced perspective acknowledges C’s dual role: it prepares the body for athletic effort by increasing the availability of energy substrates; however, chronic or excessive C levels may impair recovery and hinder adaptation to exercise stimuli.
Orgasmic sexual activity triggers the immediate release of brain opiates, endocannabinoids (ECBs), and serotonin, which induce feelings of satiety, relaxation, and sedation [47]. Following this, the organism enters a resolution phase or refractory period (RP), returning to its baseline physiological state [33]. This RP, accompanied by the release of serotonin and ECBs, could contribute to reduced mental focus [25] and an increased rating of RPE [25] during exercise, potentially impairing performance. However, total exercise capacity or VO2max do not decrease; in fact, they may increase [25]. The post-orgasmic state does not appear to affect perception of physical or mental fatigue [26] when comparing SexG vs. AbsG. Supporting these findings, a study conducted by Alonso-Aubin et al. [9] reported that the main part of the 616 Spanish amateur athletes (70.9%) who engaged in sexual intercourse the night before a competition indicated that the interval between their sexual activity and the sporting event allowed for full recovery, with no negative impact on performance. Similarly, in sedentary individuals, sexual activity performed 10 h before testing did not affect mental concentration, maximal aerobic power, or oxygen pulse [52]. These results suggest that the influence of ECBs or serotonins released during sexual activity on fatigue perception may be limited, both in terms of physical exertion and concurrent mental load. Altogether, the release of these chemical mediators following sexual activity (serotonin and ECBs) does not appear to significantly alter levels of mental or physical fatigue [26]. However, a decrease in mental focus [25] has been observed, as measured by a test developed by Rey et al. [48]. Additionally, a significant increase in the subjective RPE test has been reported in related studies [25]. Accordingly, 21.4% of athletes reported experiencing fatigue due to sexual activity, with 17.2% perceiving a negative impact on performance [9]. Similar findings have been described in endurance athletes, where 40% of athletes reported decreased sports performance [53]. These findings highlight the importance of self-perception, psychological readiness, and individual confidence in shaping athletic outcomes.
4.1. Limitations
The authors of this review acknowledge some limitations that may have influenced the findings and should be considered when interpreting the results. First, a limited number of manuscripts met the inclusion criteria. Second, the seven studies included predominantly male participants (n = 115), with only one female, which may not be representative of the broader athlete population. Third, the considerable heterogeneity across studies, regarding sports disciplines, physiological outcomes and intervention protocols (e.g., duration of sexual activity, time between orgasm and subsequent physical exertion), precluded the possibility of conducting a meta-analysis. This variability in protocols observed in this systematic review highlights the need for future research employing standardized and controlled trial designs to better determine the effects of sexual activity on biomarkers and sports performance, and to assess its potential health benefits or detriments. Results from more homogeneous studies could be integrated into future meta-analyses, thereby strengthening the evidence in this field. Furthermore, the limited number of studies conducted specifically in athletic populations underscores the need for further research to corroborate and expand upon these findings.
4.2. Strengths
Despite the limitations, the systematic review presents several strengths. It was conducted in accordance with PRISMA guidelines and included a comprehensive search strategy across the four major databases, supported by gray literature (ResearchGate) and a search node diagram generated with Connected Papers. Methodological quality was assessed using the modified McMaster Assessment Tool and PEDro scale, ensuring that all selected records met minimum quality standards. In addition, this review was registered in PROSPERO (#CRD42023426555), reinforcing the originality and transparency. Notably, this is the first systematic review to evaluate the influence of sexual activity on biological biomarkers and athletic performance parameters, assessing potential benefits or detriments to sports performance in physically active healthy adults.
4.3. Future Directions
Although modest changes in the evaluated biomarkers were observed following orgasm, these effects did not translate into measurable differences in athletic performance, suggesting that sexual activity does not negatively affect athletic ability. Therefore, based on the current body of scientific evidence, sexual activity involving orgasm, from the night before up to 24 h prior to a competitive event, cannot be definitively discouraged or recommended. Further investigation would be necessary to evaluate the effects of sexual activity across different sport modalities, including endurance vs. resistance disciplines, and team vs. individual sports. Additional research is also needed to clarify the impact of sexual activity on biological biomarkers and athletic performance parameters in physically active healthy adults. Prospective studies should aim to more thoroughly explore gender-related differences and sport-specific responses within heterogeneous athlete populations. These clinical trials should account for variations in hormonal profiles and other biological factors, and use more robust methodologies to overcome the limitations of existing research. In addition, future studies on sexual activity and sport performance should address methodological constraints, such as small sample sizes and inconsistent study designs, and explore the psychological aspects of this topic, including self-perception, motivation, and mental readiness.
5. Conclusions
The evidence presented in this systematic review indicates that sexual activity involving orgasm, performed from the night before up to 24 h prior to testing, does not produce consistently detrimental or beneficial effects on performance evaluation tests, physiological and hormonal biomarkers, concentration, RPE, or fatigue. Nonetheless, improvements in athletic parameters, such as exercise capacity, reaction time, and muscular endurance, suggest potential benefits for physically active healthy individuals. However, some studies included in this systematic review reported significant decreases in maximal STR and average speed, along with significant increases in RPE, which would negatively impact athletic performance. No significant changes were observed in physiological biomarkers or hormonal responses, further underscoring the complexity and variability of individual reactions to sexual activity prior to exercise.
Author Contributions
D.F.-L.: conceptualization (conceived and designed the study), data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, prepared figures and/or tables, writing—revision and approved the final version submitted for publication. J.S.-C.: analyzed and interpreted the data and critically reviewed the paper. J.M.I.: analyzed and interpreted the data and critically reviewed the paper. J.M.-A.: analyzed and interpreted the data and critically reviewed the paper. E.R.: methodology, investigation, resources, supervision, visualization, writing—revision, validation. G.S.: data curation, formal analysis, investigation, methodology, investigation, prepared figures and/or tables, supervision, visualization, writing—revision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Education of the Junta de Castilla & León and the European Regional Development Fund (ERDF) by TCUE Plan 2023–2027 (grant no. 067/230003). The main investigator was Prof. Dr. Diego Fernández-Lázaro.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors gratefully acknowledge the Neurobiology Research Group, University of Valladolid, for making this review possible. CIBEROBN is an initiative of Instituto de Salud Carlos III, Madrid (Spain).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ♀ | Women |
| ♂ | Men |
| AbsG | Abstinence group |
| BP | Blood pressure |
| Ca+2 | Calcium |
| CG | Control group |
| ECBs | Endocannabinoids |
| HR | Heart rate |
| HRQOL | Health-Related Quality of Life |
| IG | Intervention group (IG) |
| LE | Lower extremities |
| NA | Noradrenaline |
| PAP | Post-activation potentiation |
| Pw | Power |
| RP | Refractory period |
| RPE | Ratio of perceived exertion |
| SexG | Sex group |
| STR | Strength |
| UE | Upper extremities |
Appendix A. Checklist PRISMA 2020 [18]
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstract checklist. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 4 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 4–5 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 5 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | 5 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 4–6 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 4–6 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 4 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | - |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g.,, risk ratio, mean difference) used in the synthesis or presentation of results. | - |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 5 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | 5 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 5 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 4 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | 4 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | 5 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | - |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | - |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 6 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | 6–7 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 9 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | - |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 11–15 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | 11–15 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 11–15 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 11–15 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | 11–15 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | - |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | - |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 16–21 |
| 23b | Discuss any limitations of the evidence included in the review. | 16 | |
| 23c | Discuss any limitations of the review processes used. | 16 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 1416–1417 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | 4 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 4 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | 4 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | 24 |
| Competing interests | 26 | Declare any competing interests of review authors. | 24 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | - |
References
- SayfollahPour, P.; Heidary, M.; Mousavi, M. A Psychological consideration of sexual activity impact upon sporting performance: An overview. Int. J. Acad. Res. Bus. Soc. Sci. 2013, 3, 672–677. [Google Scholar]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection a Scientific Statement from the American Heart Association. Circulation 2021, 143, E763–E783. [Google Scholar] [CrossRef]
- Liu, H.; Waite, L.J.; Shen, S.; Wang, D.H. Is Sex Good for Your Health? A National Study on Partnered Sexuality and Cardiovascular Risk among Older Men and Women. J. Health Soc. Behav. 2016, 57, 276–296. [Google Scholar] [CrossRef]
- Sgrò, P.; Di Luigi, L. Sport and male sexuality. J. Endocrinol. Investig. 2017, 40, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Zavorsky, G.S.; Brooks, R.A. The influence of sexual activity on athletic performance: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 15609. [Google Scholar] [CrossRef] [PubMed]
- Exton, M.S.; Bindert, A.; Krüger, T.; Scheller, F.; Hartmann, U.; Schedlowski, M. Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosom. Med. 1999, 61, 280–291. [Google Scholar] [CrossRef]
- Kajantie, E.; Phillips, D.I.W. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006, 31, 151–178. [Google Scholar] [CrossRef]
- McGlone, S.; Shrier, I. Does sex the night before competition decrease performance? Clin. J. Sport Med. 2000, 10, 233–234. [Google Scholar] [CrossRef]
- Alonso-Aubin, D.A.; Chulvi-Medrano, I.; Picón-Martínez, M.; Gómez-Tomás, C.; Cortell-Tormo, J.M.; Cardozo, L.A.; Rial-Rebullido, T.; Alonso-Aubin, D.A.; Chulvi-Medrano, I.; Picón-Martínez, M.; et al. Impact of sexual activity prior to a sports competition in amateur athletes. J. Phys. Educ. Sport 2023, 23, 929–936. [Google Scholar] [CrossRef]
- Gordon, M. College coaches’ attitudes toward pregame sex. J. Sex Res. 1988, 24, 256–262. [Google Scholar] [CrossRef]
- Oman, R.F.; Vesely, S.K.; Aspy, C.B.; Tolma, E.; Rodine, S.; Marshall, L.D.; Fluhr, J. Youth assets and sexual abstinence in Native American youth. J. Health Care Poor Underserved 2006, 17, 775–788. [Google Scholar] [CrossRef]
- Thornton, J.S. Sexual Activity and Athletic Performance: Is There a Relationship? Phys. Sportsmed. 1990, 18, 148–154. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Kraemer, B.R. The effects of peripheral hormone responses to exercise on adult hippocampal neurogenesis. Front. Endocrinol. 2023, 14, 1202349. [Google Scholar] [CrossRef]
- Stefani, L.; Galanti, G.; Padulo, J.; Bragazzi, N.L.; Maffulli, N. Sexual Activity before Sports Competition: A Systematic Review. Front. Physiol. 2016, 7, 246. [Google Scholar] [CrossRef]
- Venter, R. Role of sleep in performance and recovery of athletes: A review article. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2012, 34, 167–184. [Google Scholar]
- Anshel, M.H. Effects of Sexual Activity on Athletic Performance. Phys. Sportsmed. 1981, 9, 65–68. [Google Scholar] [CrossRef]
- Soori, M.; Mohaghegh, S.; Hajian, M.; Yekta, A.A. Sexual Activity before Competition and Athletic Performance: A Systematic Review. Ann. Appl. Sport Sci. 2017, 5, 5–12. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.E.H. David Sackett and the birth of evidence based medicine: How to practice and teach EBM. BMJ 2015, 350, h3089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. PLoS Med. 2025, 22, e1004587. [Google Scholar] [CrossRef] [PubMed]
- Kirecci, S.L.; Albayrak, A.T.; Yavuzsan, A.H.; Yesildal, C.; Ilgi, M.; Kutsal, C. Sexual intercourse before exercise has a detrimental effect on lower extremity muscle strength in men. Postgrad. Med. J. 2022, 98, E11. [Google Scholar] [CrossRef]
- Peleg-Sagy, T.; Zeller, L.; Perelman, Y.; Bortnik, L.; Maman, T.; Sagy, I. The effect of pre-match sexual intercourse on football players’ performance: A prospective cross over study. J. Sports Med. Phys. Fit. 2023, 63, 250–255. [Google Scholar] [CrossRef]
- Sztajzel, J.; Périat, M.; Marti, V.; Rutishauser, P.K.W. Effect of sexual activity on cycle ergometer stress test parameters, on plasmatic testosterone levels and on concentration capacity. J. Sports Med. Phys. Fit. 2000, 40, 233–239. [Google Scholar] [PubMed]
- Valenti, L.M.; Suchil, C.; Beltran, G.; Rogers, R.C.; Massey, E.A.; Astorino, T.A. Effect of Sexual Intercourse on Lower Extremity Muscle Force in Strength-Trained Men. J. Sex. Med. 2018, 15, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Vouyoukas, E. The Influence of Sexual Activity on Athletic Performance. Ph.D. Dissertation, Concordia University, Montreal, QC, Canada, 2011. Available online: https://spectrum.library.concordia.ca/id/eprint/35939/ (accessed on 12 September 2025).
- Zavorsky, G.S.; Newton, W.L. Effects of sexual activity on several measures of physical performance in young adult males. J. Sports Med. Phys. Fit. 2018, 59, 1102–1109. [Google Scholar] [CrossRef]
- Zavorsky, G.S.; Vouyoukas, E.; Pfaus, J.G. Sexual Activity the Night Before Exercise Does Not Affect Various Measures of Physical Exercise Performance. Sex. Med. 2019, 7, 235–240. [Google Scholar] [CrossRef]
- Chacón Araya, Y.; Moncada Jiménez, J. Sexual intercourse and physical performance: Debunking the myth of the detrimental effect of having sexual intercourse before engaging in strenuous physical activity. Apunt. Educ. Física Deport. 2006, 84, 58–65. [Google Scholar]
- Rosales-Soto, G.; Corsini-Pino, R.; Monsálves-Álvarez, M.; Yáñez-Sepúlveda, R.; Rosales-Soto, G.; Corsini-Pino, R.; Monsálves-Álvarez, M.; Yáñez-Sepúlveda, R. Respuesta del balance simpático-parasimpático de la variabilidad de la frecuencia cardíaca durante una semana de entrenamiento aeróbico en ciclistas de ruta. Rev. Andal. Med. Deport. 2016, 9, 143–147. [Google Scholar] [CrossRef][Green Version]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531. [Google Scholar] [CrossRef]
- Pfaus, J.G. Pathways of sexual desire. J. Sex. Med. 2009, 6, 1506–1533. [Google Scholar] [CrossRef] [PubMed]
- Śliż, D.; Wiecha, S.; Gąsior, J.S.; Kasiak, P.S.; Ulaszewska, K.; Lewandowski, M.; Barylski, M.; Mamcarz, A. Impact of COVID-19 Infection on Cardiorespiratory Fitness, Sleep, and Psychology of Endurance Athletes—CAESAR Study. J. Clin. Med. 2023, 12, 3002. [Google Scholar] [CrossRef]
- Stone, M.H.; Sands, W.A.; Pierce, K.C.; Ramsey, M.W.; Haff, G.G. Power and power potentiation among strength-power athletes: Preliminary study. Int. J. Sports Physiol. Perform. 2008, 3, 55–67. [Google Scholar] [CrossRef]
- Wilson, J.M.; Duncan, N.M.; Marin, P.J.; Brown, L.E.; Loenneke, J.P.; Wilson, S.M.C.; Jo, E.; Lowery, R.P.; Ugrinowitsch, C. Meta-analysis of postactivation potentiation and power: Effects of conditioning activity, volume, gender, rest periods, and training status. J. Strength Cond. Res. 2013, 27, 854–859. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Adams, D.P.; González-Bernal, J.J.; Araque, A.F.; García, A.C.; Fernández-Lázaro, C.I. Electromyography: A Simple and Accessible Tool to Assess Physical Performance and Health during Hypoxia Training. A Systematic Review. Sustainability 2020, 12, 9137. [Google Scholar] [CrossRef]
- Exton, M.S.; Krüger, T.H.C.; Bursch, N.; Haake, P.; Knapp, W.; Schedlowski, M.; Hartmann, U. Endocrine response to masturbation-induced orgasm in healthy men following a 3-week sexual abstinence. World J. Urol. 2001, 19, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Dabbs, J.M.; Mohammed, S. Male and female salivary testosterone concentrations before and after sexual activity. Physiol. Behav. 1992, 52, 195–197. [Google Scholar] [CrossRef]
- Goldey, K.L.; Van Anders, S.M. Sexual thoughts: Links to testosterone and cortisol in men. Arch. Sex. Behav. 2012, 41, 1461–1470. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Morton, R.W.; Oikawa, S.Y.; Wavell, C.G.; Mazara, N.; McGlory, C.; Quadrilatero, J.; Baechler, B.L.; Baker, S.K.; Phillips, S.M. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J. Appl. Physiol. 2016, 121, 129–138. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland, D.L. The physiological basis of human sexual arousal: Neuroendocrine sexual asymmetry. Int. J. Androl. 2005, 28, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Krüger, T.; Exton, M.S.; Pawlak, C.; Mühlen, A.V.Z.; Hartmann, U.; Schedlowski, M. Neuroendocrine and cardiovascular response to sexual arousal and orgasm in men. Psychoneuroendocrinology 1998, 23, 401–411. [Google Scholar] [CrossRef]
- Suay, F.; Salvador, A.; González-Bono, E.; Sanchís, C.; Martínez, M.; Martínez-Sanchis, S.; Simón, V.M.; Montoro, J.B. Effects of competition and its outcome on serum testosterone, cortisol and prolactin. Psychoneuroendocrinology 1999, 24, 551–566. [Google Scholar] [CrossRef]
- Krüger, T.H.C.; Haake, P.; Hartmann, U.; Schedlowski, M.; Exton, M.S. Orgasm-induced prolactin secretion: Feedback control of sexual drive? Neurosci. Biobehav. Rev. 2002, 26, 31–44. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, Z.; Shen, D.; Gao, L.; Li, Q. Testosterone levels positively linked to muscle mass but not strength in adult males aged 20–59 years: A cross-sectional study. Front. Physiol. 2025, 16, 1512268. [Google Scholar] [CrossRef]
- Hamilton, L.D.; Rellini, A.H.; Meston, C.M. Cortisol, sexual arousal, and affect in response to sexual stimuli. J. Sex. Med. 2008, 5, 2111–2118. [Google Scholar] [CrossRef]
- Álvarez Álvarez, A.M.; González Suárez, R.M.; Marrero, M.A. Role of testosterone and cortisol in metabolic syndrome and type 2 diabetes mellitus. Rev. Cuba. Endocrinol. 2010, 21, 80–90. [Google Scholar]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Boone, T.; Gilmore, S. Effects of sexual intercourse on maximal aerobic power, oxygen pulse, and double product in male sedentary subjects. J. Sports Med. Phys. Fit. 1995, 35, 214–217. [Google Scholar] [PubMed]
- Pupiš, M.; Raković, A.; Stanković, D.; Kocić, M.; Savanović, V. Sex and endurance performance. Sport Sci. Pract. Asp. 2010, 7, 21–25. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).