Environmental Hydrogen Concentration as a Novel Factor Determining Changes in Redox Potential
Abstract
1. Introduction
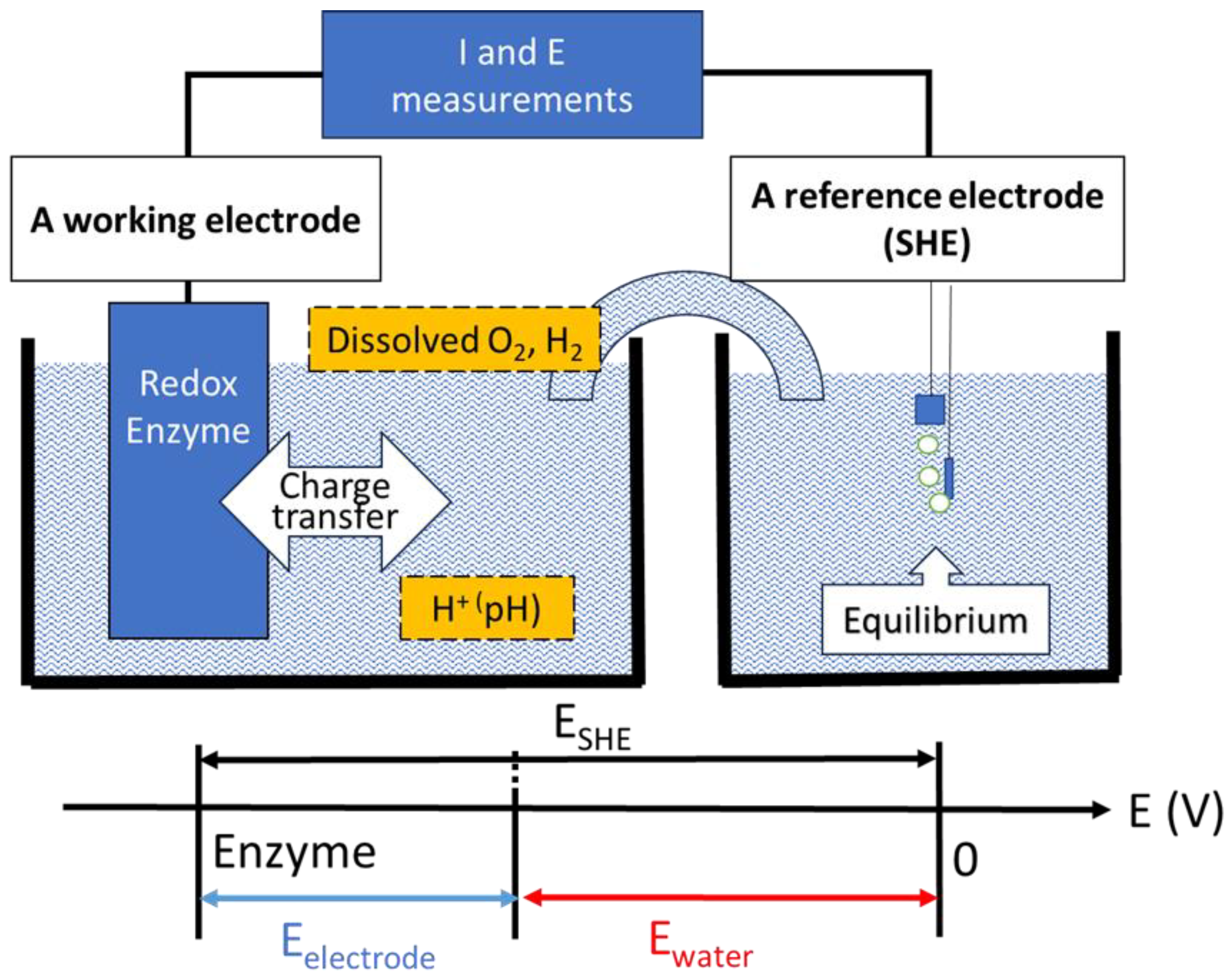
2. Redox Potential of Water as the Environment of the Redox Enzyme
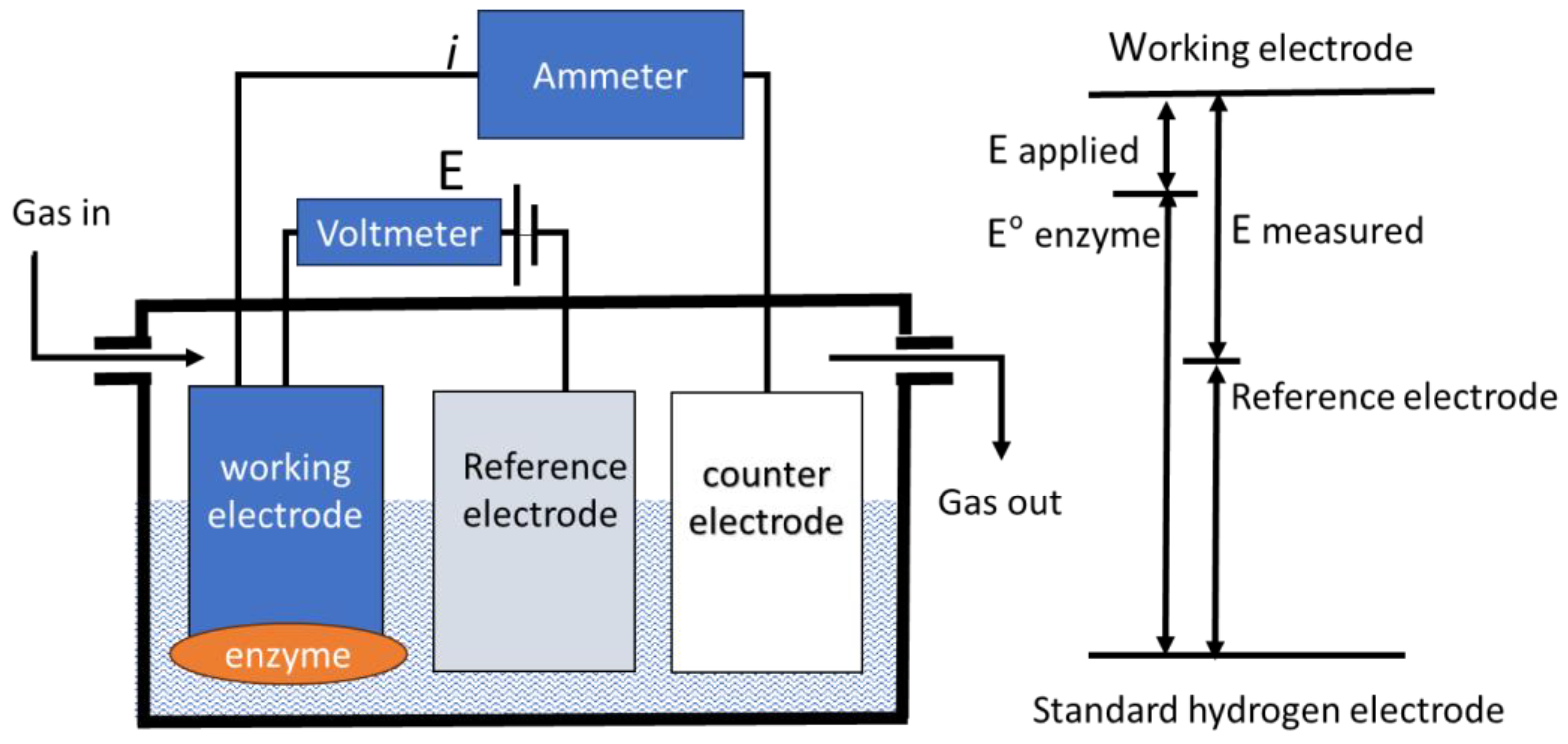
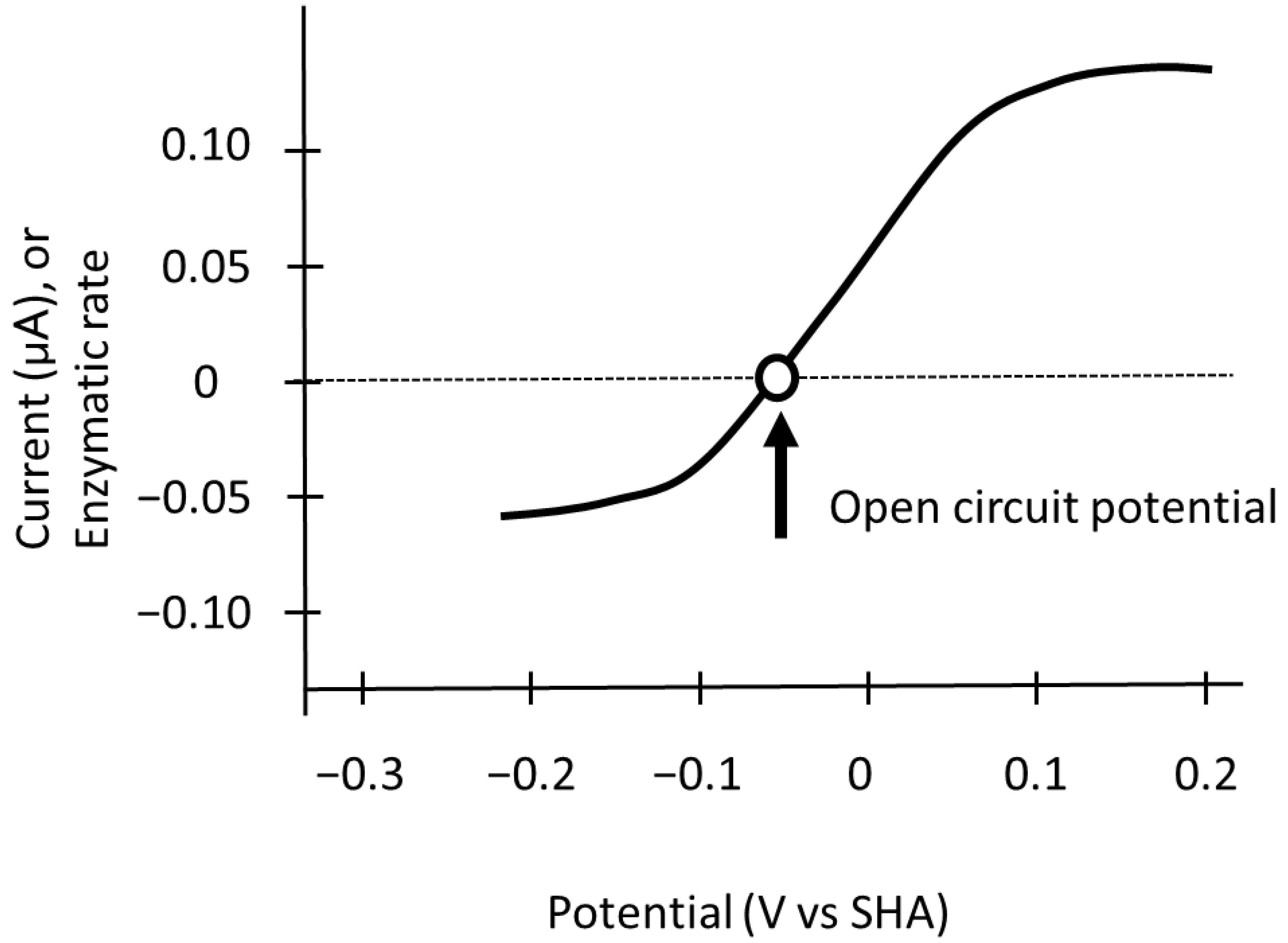
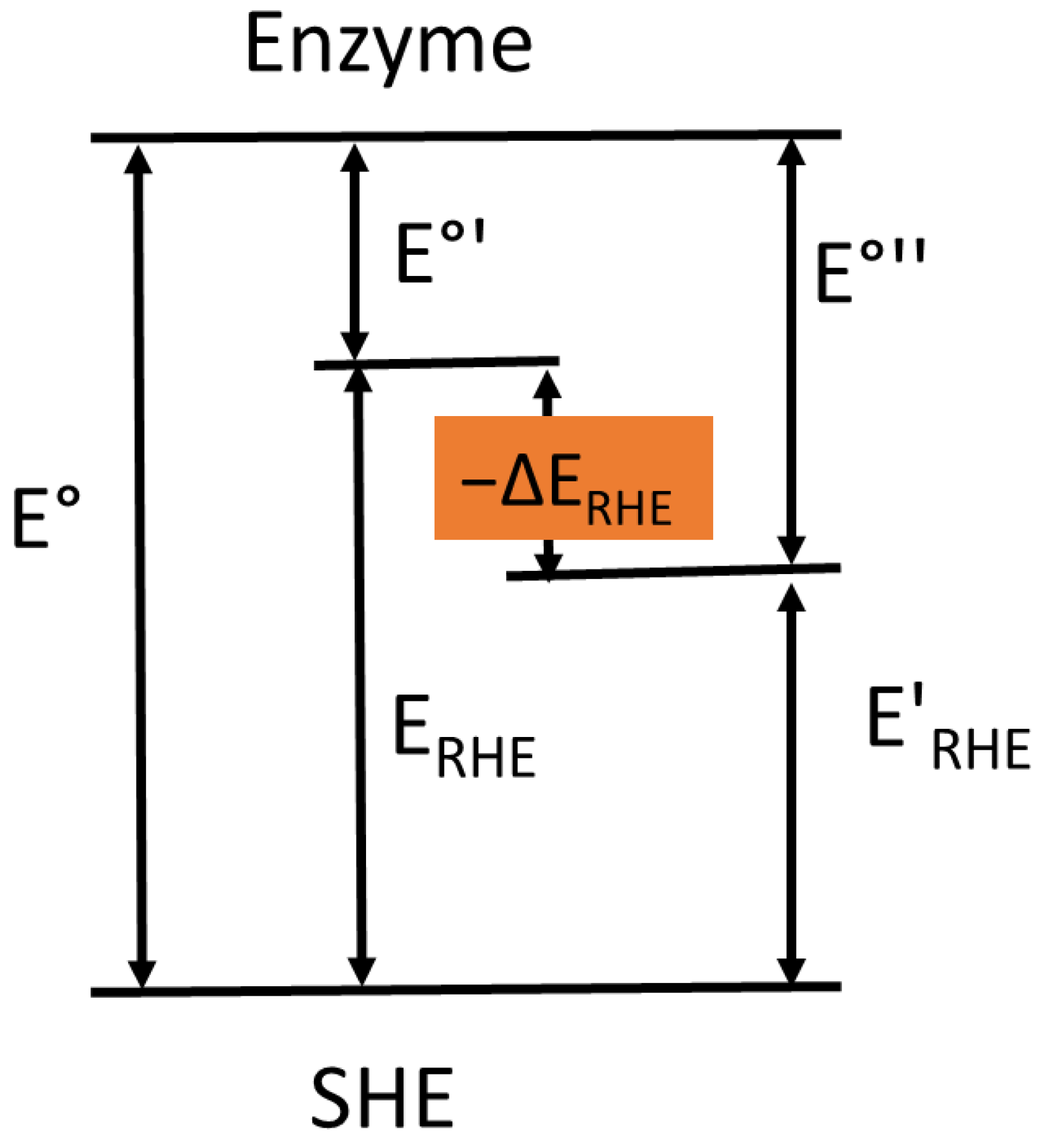
3. Measurement of the Reversible Hydrogen Electrode Potential as a Reference Electrode
4. Alterations in the Electrode Potential of the Active Site of Oxidoreductases
5. Environmental O2 and/or H2 Levels Change the Direction of Redox Reactions and Reactive Oxygen Species (ROS) Generation
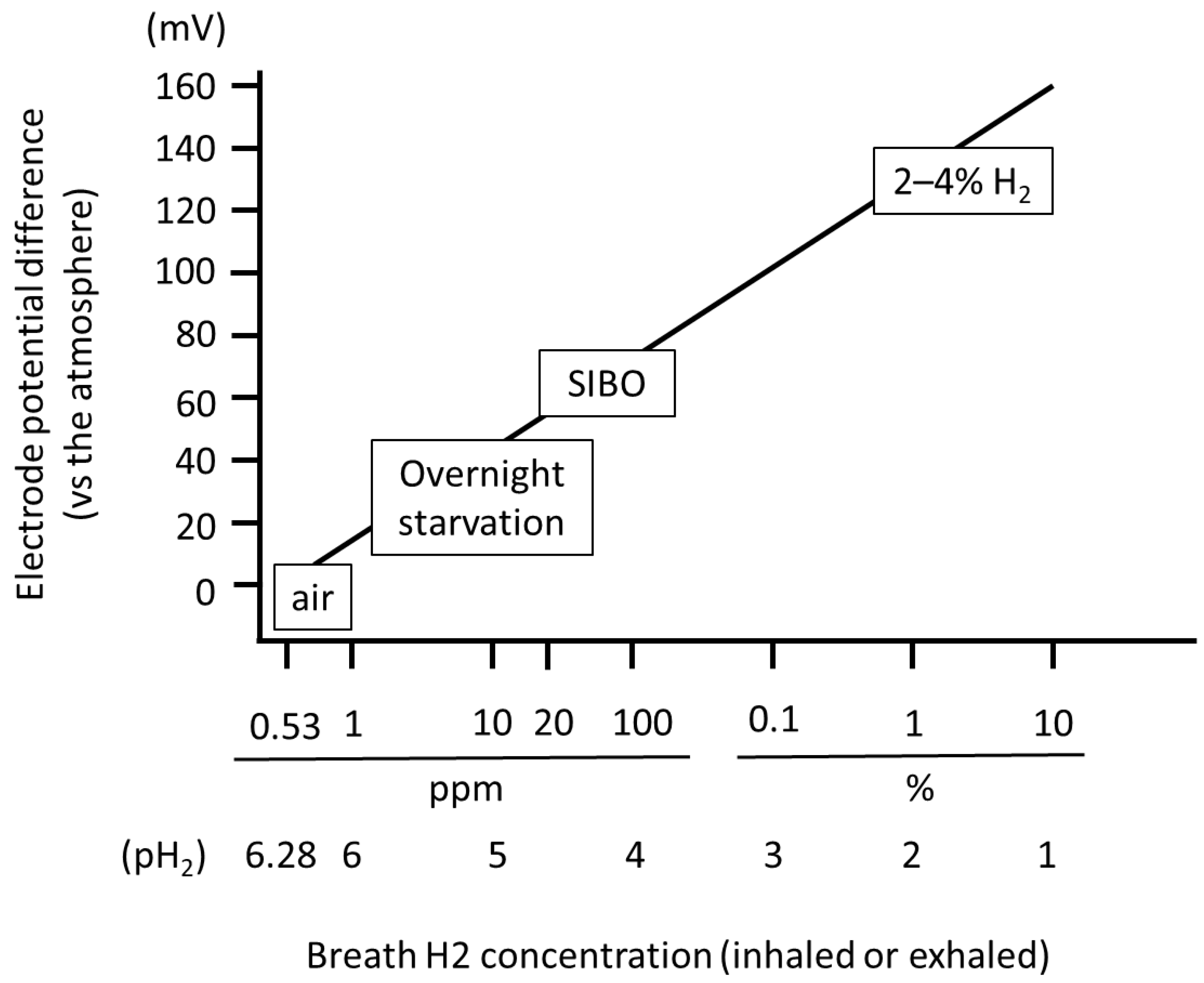
6. Fluctuations in Environmental H2 Concentration and Metabolism
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| O2 | Oxygen |
| H2O | Water |
| H+ | Hydrogen ion |
| H2 | Hydrogen |
| OCP | Open Circuit Potential |
| SHE | Standard Hydrogen Electrode |
| SDH | Succinate dehydrogenase |
| DOT | Dissolved Oxygen Tension |
| RHE | Reversible Hydrogen Electrode |
| ROS | Reactive Oxygen Species |
| RBC | Red blood cell |
| ATA | Atmosphere absolute |
| SIBO | Small intestinal bacterial overgrowth |
| CO2 | Carbon dioxide |
| G6PD | Glucose 6 phosphate dehydrogenase |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| GSH | glutathione |
| GSSG | Glutathione disulfide |
| GR | Glutathione reductase |
References
- McGuinness, K.N.; Fehon, N.; Feehan, R.; Miller, M.; Mutter, A.C.; Rybak, L.A.; Nam, J.; AbuSalim, J.E.; Atkinson, J.T.; Heidari, H.; et al. The energetics and evolution of oxidoreductases in deep time. Proteins 2024, 92, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.W.; Shorten, P.R.; Altermann, E.H.; Roy, N.C.; McNabb, W.C. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes 2018, 10, 270–288. [Google Scholar] [CrossRef]
- Elliott, S.J.; Léger, C.; Pershad, H.R.; Hirst, J.; Heffron, K.; Ginet, N.; Blasco, F.; Rothery, R.A.; Weiner, J.H.; Armstrong, F.A. Detection and interpretation of redox potential optima in the catalytic activity of enzymes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2002, 1555, 54–59. [Google Scholar] [CrossRef]
- Adamson, H.; Bond, A.M.; Parkin, A. Probing biological redox chemistry with large amplitude Fourier transformed ac voltammetry. Chem Commun. (Camb.) 2017, 53, 9519–9533. [Google Scholar] [CrossRef]
- Léger, C.; Bertrand, P. Direct Electrochemistry of Redox Enzymes as a Tool for Mechanistic Studies. Chem. Rev. 2008, 108, 2379–2438. [Google Scholar] [CrossRef]
- Gates, A.J.; Kemp, G.L.; To, C.Y.; Mann, J.; Marritt, S.J.; Mayes, A.G.; Richardoson, D.J.; Butt, J.N. The relationship between redox enzyme activity and electrochemical potential-cellular and mechanistic implications from protein film electrochemistry. Phys. Chem. Chem. Phys. 2011, 13, 7720–7731. [Google Scholar] [CrossRef]
- Pershad, H.R.; Hirst, J.; Cochran, B.; Ackrell, B.A.C.; Armstrong, F.A. Voltammetric studies of bidirectional catalytic electron transport in Escherichia coli succinate dehydrogenase: Comparison with the enzyme from beef heart mitochondria. Biochim. Biophys. Acta-Bioenerg. 1999, 1412, 262–272. [Google Scholar] [CrossRef]
- Hirst, J.; Sucheta, A.; Ackrell, B.A.C.; Armstrong, F. Electrocatalytic Voltammetry of Succinate Dehydrogenase: Direct Quantification of the Catalytic Properties of a Complex Electron-Transport Enzyme. J. Am. Chem. Soc. 1996, 118, 5031–5038. [Google Scholar] [CrossRef]
- Chaplin, M. Water Structure and Science. Available online: https://water.lsbu.ac.uk/water (accessed on 17 September 2025).
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. Bioessays 2015, 37, 921–928. [Google Scholar] [CrossRef]
- Pluschkell, S.B.; Flickinger, M.C. Improved methods for investigating the external redox potential in hybridoma cell culture. Cytotechnology 1995, 19, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Acosta, A.; Gómez, A.; Ramírez, O.T. Control of redox potential in hybridoma cultures: Effects on MAb production, metabolism, and apoptosis. J. Ind. Microbiol. Biotechnol. 2012, 39, 1189–1198. [Google Scholar] [CrossRef]
- Milo, R.; Phillips, R. What is the redox potential of a cell? In Cell Biology by the Numbers, 1st ed.; Garland Science: New York, NY, USA, 2015; pp. 189–196. [Google Scholar]
- Buck, R.P.; Rondinini, S.; Covington, A.; Baucke, F.; Brett, C.; Camoes, M.; Milton, M.; Mussini, T.; Naumann, R.; Pratt, K.; et al. Measurement of pH definition, standards, and procedures. Pure Appl. Chem. 2002, 74, 2169–2200. [Google Scholar] [CrossRef]
- Scholz, F. From the Leiden jar to the discovery of the glass electrode by Max Cremer. J. Solid. State Electrochem. 2011, 15, 5–14. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Pittman, R.N. Oxygen transport. In Regulation of Tissue Oxygenation, 2nd ed.; Morgan & Claypool: San Rafael, CA, USA, 2016; pp. 1–99. [Google Scholar] [CrossRef]
- Martineau, F.; Fourel, F.; Bodergat, A.M.; Lecuyer, C. D/H equilibrium fractionation between H2O and H2 as a function of the salinity of aqueous solutions. Chem. Geol. 2012, 291, 236–240. [Google Scholar] [CrossRef]
- Rosen, M.; Schuldiner, S. The H+/H2 equilibrium potential dependence on H2 partial pressure on gold electrodes. Electrochim. Acta 1973, 18, 687–690. [Google Scholar] [CrossRef]
- Jerkiewicz, G. Standard and Reversible Hydrogen Electrodes: Theory, Design, Operation, and Applications. ACS Catal. 2020, 10, 8409–8417. [Google Scholar] [CrossRef]
- Novelli, P.C.; Lang, P.M.; Masarie, K.A.; Hurst, D.F.; Myers, R.; Elkins, J.W. Molecular hydrogen in the troposphere: Global distribution and budget. J. Geophys. Res. 1999, 104, 30427–30444. [Google Scholar] [CrossRef]
- Lappan, R.; Shelley, G.; Islam, Z.F.; Leung, P.M.; Lockwood, S.; Nauer, P.A.; Jirapanjawat, T.; Ni, G.; Chen, Y.J.; Kessler, A.J.; et al. Molecular hydrogen in seawater supports growth of diverse marine bacteria. Nat. Microbiol. 2023, 8, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.; Gaskins, H. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 504–518. [Google Scholar] [CrossRef]
- Yamamoto, R.; Homma, K.; Suzuki, S.; Sano, M.; Sasaki, J. Hydrogen gas distribution in organs after inhalation: Real-time monitoring of tissue hydrogen concentration in rat. Sci. Rep. 2019, 9, 1255. [Google Scholar] [CrossRef]
- Kiyama, T.; Tokunaga, A.; Naji, A.; Barbul, A. Changes in the negative logarithm of end-tidal hydrogen partial pressure indicate the variation of electrode potential in healthy Japanese subjects. Sci. Rep. 2023, 13, 15473. [Google Scholar] [CrossRef]
- Roscher, J.; Holze, R. Reference Electrodes. Encyclopedia 2023, 3, 478–489. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins containing cytochrome, iron-sulfur, or copper redox centers. Chem. Rev. 2014, 23, 4366–4469. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Krebs, H.A. The redox state of nicotinamide adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Adv. Enzym. Regul. 1967, 5, 409–434. [Google Scholar] [CrossRef]
- Kirlin, W.G.; Kirlin, W.G.; Cai, J.; Thompson, S.; Díaz, D.; Kavanagh, T.J.; Jones, D.P. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic. Biol. Med. 1999, 27, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Miyazaki, K.; Hwang, J.; Yamamoto, T.; Sakuda, A. Electrode Potentials Part 1: Fundamentals and Aqueous Systems. Electrochemistry 2022, 90, 102001. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [CrossRef]
- Boettcher, S.W.; Oener, S.Z.; Lonergan, M.C.; Surendranath, Y.; Ardo, S.; Brozek, C.; Kempler, P.A. Potentially Confusing: Potentials in Electrochemistry. ACS Energy Lett. 2021, 6, 261–266. [Google Scholar] [CrossRef]
- Ratnaraj, J.; Kabon, B.; Talcott, M.R.; Sessler, D.I.; Kurz, A. Supplemental oxygen and carbon dioxide each increase subcutaneous and intestinal intramural oxygenation. Anesth. Analg. 2004, 99, 207–211. [Google Scholar] [CrossRef]
- Muir, E.R.; Cardenas, D.P.; Duong, T.Q. MRI of brain tissue oxygen tension under hyperbaric conditions. Neuroimage 2016, 133, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, C.J.; Dough, R.H.; Cooper, D.Y.; Emmel, G.L.; Loeschcke, H.H.; Schmidt, C.F. Oxygen toxicity; effects in man of oxygen inhalation at 1 and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolism. J. Appl. Physiol. 1953, 5, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.; Secor, J.T.; Eckmann, D.M. Hyperbaric oxygen rapidly produces intracellular bioenergetics dysfunction in human pulmonary cells. Chem. Biol. Interact. 2024, 404, 111266. [Google Scholar] [CrossRef]
- Wiese, A.K.; Prior, S.; Chambers, T.C.; Higuchi, M. Intracellular Oxygen Concentration Determined By Mitochondrial Respiration Regulates Production of Reactive Oxygen Species. Integr. Cancer Biol. Res. 2017, 1, 6. [Google Scholar]
- Mik, E.G.; Johannes, T.; Zuurbier, C.J.; Heinen, A.; Houben-Weerts, J.H.; Balestra, G.M.; Stap, J.; Beek, J.F.; Ince, C. In vivo mitochondrial oxygen tension measured by a delayed fluorescence lifetime technique. Biophys. J. 2008, 95, 3977–3990. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Talukder, M.A.H. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef]
- Stub, D.; Smith, K.; Bernard, S.; Nehme, Z.; Stephenson, M.; Bray, J.E.; Cameron, P.; Barger, B.; Ellims, A.H.; Taylor, A.J.; et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 2015, 131, 2143–2150. [Google Scholar] [CrossRef]
- Tabata Fukushima, C.; Dancil, I.S.; Clary, H.; Shah, N.; Nadtochiy, S.M.; Brookes, P.S. Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions. Redox Biol. 2024, 70, 103047. [Google Scholar] [CrossRef]
- Higareda, A.E.; Possani, L.D.; Ramírez, O.T. The use of culture redox potential and oxygen uptake rate for assessing glucose and glutamine depletion in hybridoma cultures. Biotechnol. Bioeng. 1997, 56, 555–563. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef]
- Ishihara, G.; Kawamoto, K.; Komori, N.; Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem. Biophys. Res. Commun. 2020, 522, 965–970. [Google Scholar] [CrossRef]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, M.; Iwata, M.; Toda, Y.; Nakae, Y.; Kondo, T. Circadian rhythm of breath hydrogen in young women. J. Gastroenterol. 1998, 33, 472–476. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar]
- Avallone, E.V.; De Carolis, A.; Loizos, P.; Corrado, C.; Vernia, P. Hydrogen breath test–diet and basal H2 excretion: A technical note. Digestion 2010, 82, 39–41. [Google Scholar] [CrossRef]
- van Munster, I.P.; de Boer, H.M.; Jansen, M.C.; de Haan, A.F.; Katan, M.B.; van Amelsvoort, J.M.; Nagengast, F.M. Effect of resistant starch on breath-hydrogen and methane excretion in healthy volunteers. Am. J. Clin. Nutr. 1994, 59, 626–630. [Google Scholar] [CrossRef]
- Nagano, A.; Ohge, H.; Tanaka, T.; Takahashi, S.; Uemura, K.; Murakami, Y.; Sueda, T. Effects of different types of dietary fibers on fermentation by intestinal flora. Hiroshima J. Med. Sci. 2018, 67, 1–5. [Google Scholar]
- Yao, C.K.; Tuck, C.J.; Barrett, J.S.; Canale, K.E.; Philpott, H.L.; Gibson, P.R. Poor reproducibility of breath hydrogen testing: Implications for its application in functional bowel disorders. United Eur. Gastroenterol. J. 2017, 5, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Uetsuki, K.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Iida, T.; Yamamoto, K.; Furukawa, K.; Nakamura, M.; Honda, T.; Ishigami, M.; et al. Measurement of fasting breath hydrogen concentration as a simple diagnostic method for pancreatic exocrine insufficiency. BMC Gastroenterol. 2021, 21, 211. [Google Scholar] [CrossRef]
- Jung, S.E.; Joo, N.S.; Han, K.S.; Kim, K.N. Obesity Is Inversely Related to Hydrogen-Producing Small Intestinal Bacterial Overgrowth in Non-Constipation Irritable Bowel Syndrome. J. Korean Med. Sci. 2017, 32, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Adzavon, Y.M.; Xie, F.; Yi, Y.; Jiang, X.; Zhang, X.; He, J.; Zhao, P.; Liu, M.; Ma, S.; Ma, X. Long-term and daily use of molecular hydrogen induces reprogramming of liver metabolism in rats by modulating NADP/NADPH redox pathways. Sci. Rep. 2022, 12, 3904. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, O.; Yano, T. Electrochemical bioreactor with immobilized glucose-6-phosphate dehydrogenase on the rotating graphite disc electrode modified with phenazine methosulfate. Enzym. Microb. Technol. 1993, 15, 525–529. [Google Scholar] [CrossRef]
- Pannala, V.R.; Bazil, J.N.; Camara, A.K.S.; Dash, R.K. A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radic. Biol. Med. 2013, 65, 1385–1397. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiyama, T. Environmental Hydrogen Concentration as a Novel Factor Determining Changes in Redox Potential. Physiologia 2025, 5, 36. https://doi.org/10.3390/physiologia5040036
Kiyama T. Environmental Hydrogen Concentration as a Novel Factor Determining Changes in Redox Potential. Physiologia. 2025; 5(4):36. https://doi.org/10.3390/physiologia5040036
Chicago/Turabian StyleKiyama, Teruo. 2025. "Environmental Hydrogen Concentration as a Novel Factor Determining Changes in Redox Potential" Physiologia 5, no. 4: 36. https://doi.org/10.3390/physiologia5040036
APA StyleKiyama, T. (2025). Environmental Hydrogen Concentration as a Novel Factor Determining Changes in Redox Potential. Physiologia, 5(4), 36. https://doi.org/10.3390/physiologia5040036





