Abstract
Prediabetes, a pivotal phase in glucose metabolism between normalcy and diabetes, exerts a profound influence on the aging process and the risk of age-related diseases. This comprehensive review delves into the intricate web of blood-based biomarkers that collectively expedite senescence, marking the transition from a state of health to age-related complications. Key findings underscore the significance of diverse biomarkers, such as telomere length, p16INK4a, senescence-associated secretory phenotype (SASP) factors, DNA methylation clocks, advanced glycation end products (AGEs), inflammatory and oxidative stress markers, circulating hormones, and additional factors such as folate, B12, and osteocalcin. Not only do these biomarkers serve as indicators of senescence but they also actively fuel chronic inflammation, oxidative stress, and metabolic dysregulation, all of which contribute to accelerated aging. The implications of this understanding are profound, as prediabetes emerges as a critical period in an individual’s life, influencing various physiological systems, including the vascular and neural systems, metabolic functions, hormonal regulation, and bone health. Recognizing the profound influence of prediabetes on senescence provides a foundation for personalized intervention strategies to mitigate age-related complications and promote healthy aging. Future research directions call for a more diverse array of biomarkers, the in-depth exploration of their roles, and the development of tailored precision medicine strategies to ensure a holistic understanding and effective management of prediabetes-induced senescence and its implications for aging. This knowledge has far-reaching implications for public health and clinical practice, emphasizing the need for early detection and intervention in prediabetic individuals to enhance the quality of life in an aging population with diverse needs.
1. Introduction
The global prevalence of prediabetes, a metabolic state characterized by insulin resistance and elevated blood glucose levels below the threshold for diabetes diagnosis, has emerged as a significant public health concern [1]. Prediabetes is a critical precursor to type 2 diabetes (T2D); however, it often remains undiagnosed, and individuals with prediabetes are at a heightened risk of transitioning to T2D [2]. It is also associated with a range of adverse health outcomes and age-related complications, including cardiovascular diseases, neurodegenerative disorders, and frailty [3]. Given the growing aging population worldwide, a comprehensive understanding of how prediabetes impacts senescence is of great significance.
The relationship between aging and chronic illnesses such as diabetes has drawn significant attention in the field of public health as the world’s population ages [4]. Between normoglycemia and overt diabetes, prediabetes, which is defined by increased blood glucose levels, is a critical stage in the continuum of glucose dysregulation [5]. It is critical to comprehend the many mechanisms by which prediabetes affects the aging process. The realization that prediabetes contributes to an accelerated aging phenotype and predisposes people to diabetes is the driving force behind this extensive research [6]. The evaluation of blood-based biomarkers, which act as markers of the underlying physiological and molecular processes connecting prediabetes and senescence, is a crucial component of this study [7].
Assessing blood-based biomarkers within the framework of prediabetes-induced senescence provides a comprehensive viewpoint on the complex interplay between metabolic dysregulation and aging [8]. This is especially important because aging-related diseases and prediabetes share many pathophysiological pathways [9]. This review is important because it could provide researchers and physicians with the resources, they need to identify people who are at risk of developing prediabetes, as well as premature aging. By facilitating early intervention and preventive steps to lessen age-related issues, it provides a proactive approach to healthcare [10]. Furthermore, identifying the biomarkers linked to senescence brought on by prediabetes is crucial for deciphering the molecular causes of aging, and presents opportunities for focused interventions that can lessen the negative effects of prediabetes on the health of older persons [11].
In addition to improving our understanding of the aging process, the scientific investigation of blood-based biomarkers in prediabetes-induced senescence holds enormous promise for enhancing the health and well-being of prediabetic individuals [12]. We can identify new treatment targets and preventive measures by dissecting the relationships between particular biomarkers and accelerated aging [13]. With a deeper understanding of the relationship between metabolic health and senescence, and, ultimately, develop guidance for the development of interventions to promote healthy aging and lessen the burden of age-related diseases among prediabetic individuals, these insights could have far-reaching implications for the fields of gerontology and diabetes care [14].
This comprehensive review investigates the relationship between prediabetes and age-related changes, with a specific focus on blood-based biomarkers. This study will delve into the impact of prediabetes on telomere length, p16INK4a expression, senescence-associated secretory phenotype (SASP) factors, DNA methylation clocks, advanced glycation end products (AGEs), inflammatory markers, and oxidative stress markers. This study will further explore the influence of prediabetes on circulating hormones, growth factors, and metabolic markers. This study provides a thorough exploration of these various blood-based biomarkers that are associated with both prediabetes and senescence by synthesizing existing knowledge on these biomarkers. Understanding this connection is of paramount importance in the context of contemporary healthcare, as it has the potential to shed light on the mechanisms underlying accelerated biological aging in prediabetic individuals and provide valuable insights into the pathogenesis of age-related chronic diseases. This analysis will contribute to our understanding of the complex relationship between prediabetes and the aging process, ultimately facilitating the development of targeted interventions and strategies for mitigating the adverse effects of prediabetes-induced senescence.
2. Age-Related Changes in Plasma Biochemistry and Vascular Dynamics in Prediabetes
2.1. Prediabetes as a Precursor of Age-Related Vascular Changes
A key prelude to age-related vascular alterations is prediabetes, which is defined by high blood glucose levels that do not fulfil the criteria for diabetes [15]. The circulatory system, which includes the arteries and veins, undergoes structural and functional changes because of the complex and diverse process of vascular aging [16]. It is essential for the emergence of hypertension and associated problems, and age-related cardiovascular illnesses [17]. Prediabetes can dramatically accelerate vascular aging, increasing the risk of cardiovascular morbidity and mortality in patients with it. Prediabetes affects approximately one in three persons in many countries [18].
Oxidative stress is one of the main ways that prediabetes affects the aging of blood vessels [19]. Increased oxidative stress, which is defined as an imbalance between reactive oxygen species (ROS) and antioxidant defences, is common in prediabetic patients [19]. This oxidative load may eventually contribute to vascular aging by causing oxidative damage to the walls of blood vessels [20]. A chronic rise in blood glucose and variations in insulin resistance in prediabetes intensify oxidative stress and foster an environment favorable for the oxidative alteration of lipids and proteins in the vascular system [21]. This accelerates these vascular alterations and increases the risk of age-related cardiovascular problems such as endothelial dysfunction and atherosclerosis [21].
Furthermore, prediabetes is closely associated with chronic low-grade inflammation, often referred to as “meta-inflammation” [22]. Prediabetes intensifies inflammatory processes, which primarily cause vascular aging [19]. The pro-inflammatory condition of prediabetes can encourage immune cells within the vascular wall to become activated, triggering an inflammatory response that hastens the aging process of the vessels [23]. As a result, blood vessel stiffening, atherosclerotic plaque development, and decreased vascular reactivity occur [23]. The significance of prediabetes as a prelude to vascular alterations associated with aging is noteworthy, emphasizing the need to comprehend these mechanisms and determine pertinent blood-based indicators to oversee and address this metabolic disorder [24]. This review explores the role of such biomarkers in tracking the progression of vascular aging in prediabetes and their potential for mitigating the adverse outcomes associated with this accelerated aging process.
2.2. Altered Plasma Biochemistry and Implications for Senescence
Prediabetes significantly impacts the biochemical makeup of blood, creating conditions for accelerated senescence [25]. A vital factor in determining general health, plasma biochemistry affects many physiological functions [26]. Blood plasma composition is altered in prediabetic individuals because of the dysregulated glucose and lipid metabolism in the systemic environment [27]. Elevated levels of circulating inflammatory markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6), are one of the major biochemical alterations linked to prediabetes (see Figure 1) [28]. Pro-inflammatory signals play a role in the persistent low-grade inflammation that is frequently observed in prediabetes, a condition that is sometimes called “Inflammaging” [29]. Since inflammation is a major factor in senescence, a persistently inflammatory environment speeds up the aging of many organ systems [30].
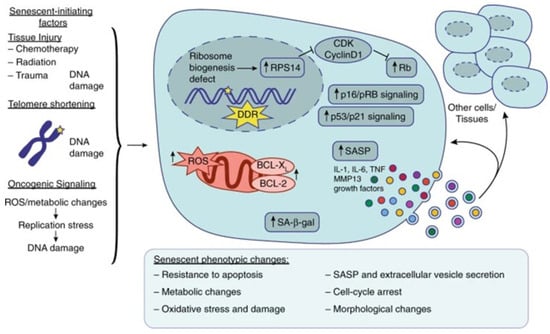
Figure 1.
Overview of prediabetes-induced vascular senescence as adapted from source and redrawn from Bio Render. Several factors can induce senescence in different tissues, such as tissue injury, telomere shortening, and oncogenic signaling, which all lead to DNA damage, the DNA damage response (DDR), and consequent cell cycle arrest by the activation of p16/pRB signaling and/or p53/p21 signaling. Nucleolar stress and ribosome biogenesis defects can also induce RPS14 accumulation in the nucleus and activate Rb by inhibiting the CDK4/cyclin D1 complex, leading to cell cycle arrest. Senescent cells also exhibit increased senescence-associated β-galactosidase (SA-β-gal) production, reactive oxygen species (ROS) accumulation, and anti-apoptotic factors such as BCL-XL and BCL-2. Senescent cells exhibit several phenotypic changes such as resistance to apoptosis, oxidative stress and damage, metabolic changes, morphological changes, cell cycle arrest, and extracellular vesicle secretion containing SASP factors such as IL-1, IL-6, TNF, MMP13, and various growth factors. This SASP can either feedback in an autocrine manner to the senescent cell, or, in a paracrine manner, influence and promote senescence and inflammation in the surrounding cells and tissues [31].
Moreover, abnormal lipid profiles, such as elevated triglyceride and decreased high-density lipoprotein (HDL) cholesterol levels, are linked to prediabetes [32]. Due to its pro-atherogenic nature, dyslipidemia encourages the build-up of cholesterol in blood vessel walls and the onset of atherosclerosis, a disease generally associated with advanced age [33]. Atherosclerosis raises the risk of cardiovascular events such as heart attacks and strokes by limiting blood flow and encouraging the formation of blood clots [34]. The combined effect of these changes in parameters related to plasma biochemistry speeds up the aging process of organ and vascular systems [7].
Prediabetes causes hyperglycemia by upsetting glucose homeostasis and going beyond inflammation and dyslipidemia [35]. Elevated blood glucose levels promote the glycation of proteins, including those essential for vascular health [36]. This process results in the formation of advanced glycation end products (AGEs), which promote oxidative stress and arterial wall stiffening [37]. AGEs have broad implications for age-related complications such as renal dysfunction and neurodegenerative diseases, in addition to their association with vascular senescence [38]. Examining the interaction between these biochemical alterations and the aging process in prediabetes is essential because the altered plasma biochemistry in prediabetes is a critical link in the chain of events that accelerates senescence.
2.3. Role of Biomarkers in Plasma Biochemistry Changes
As a metabolic state that lies between normoglycemia and diabetes, prediabetes is characterized by a complex interplay of biochemical factors, many of which are important biomarkers for changes in plasma biochemistry [39]. These biomarkers are essential for understanding the pathophysiological changes linked to prediabetes, and how they affect senescence [40]. Several important biomarkers have emerged in the context of changes in plasma biochemistry, providing insight into the biochemical dysregulations that underlie accelerated aging and age-related vascular dynamics [41].
2.3.1. Telomere Length
Genomic stability depends on telomeres, which are protective caps that sit at the ends of chromosomes [42]. Since telomere shortening is a sign of cellular aging and senescence, their length is a crucial factor in determining cellular lifespan [43]. Telomere shortening occurs at a significantly faster rate in prediabetes, a disorder characterized by oxidative stress, low-grade inflammation, and chronic metabolic disruptions [44]. These environmental factors, frequently associated with prediabetes, hasten the attrition of telomeres [45]. Telomere length thus functions as a biomarker reflecting the accelerated aging of cells in prediabetic subjects. This phenomenon has broader implications because, in addition to indicating cellular aging, short telomeres also contribute to the general senescence observed in prediabetic people [44]. Deciphering the complex interplay between prediabetes and accelerated senescence, which will illuminate the molecular mechanisms underlying this condition’s effects on aging and age-related illnesses, requires an understanding of the dynamics of telomere length.
2.3.2. p16INK4a
In the context of cellular senescence, p16INK4a functions as a critical regulator. This biomarker regulates senescence-related irreversible cell growth arrest, functioning as a sentinel [46]. The expression of p16INK4a is generally higher and more prominent in prediabetic than in non-prediabetic individuals [47]. This upregulation, which is a result of the inflammatory and metabolic environment linked to prediabetes, indicates a higher frequency of senescent cells [48]. The high frequency of p16INK4a expression in prediabetics highlights the harmful effects of this metabolic disorder on the integrity of cells [49]. A state of cellular stress and dysfunction, indicated by elevated p16INK4a levels, is linked to prediabetic individuals’ age-related health conditions, as well as the general aging process [50]. The function of p16INK4a is crucial for unravelling the connection between prediabetes and cellular senescence, providing insights into the molecular processes that underpin the condition’s impact on the aging process [51].
2.3.3. Senescence-Associated Secretory Phenotype (SASP) Factors
SASP is a broad category of substances secreted by senescent cells, which includes growth factors chemokine and proinflammatory cytokines [52]. These elements have a significant impact on nearby cells and tissues and the microenvironment in which senescent cells are located [52]. Chronic low-grade inflammation, a characteristic feature of prediabetes, primarily drives the production of SASP factors [53]. Consequently, increased blood levels of SASP factors are linked to prediabetes [54]. These biomarkers demonstrate how senescent cells actively promote inflammation and modify plasma biochemistry [55]. Elevated SASP factor levels in prediabetes serve as indicators of the role of senescence in systemic changes in plasma biochemistry and the overall inflammatory milieu [56]. The senescence process in prediabetes and its consequences for age-related health conditions are further complicated by the interaction between senescence and inflammation, which is reflected by SASP factors [57]. The functions of SASP factors in modifications to plasma biochemistry are essential for clarifying how prediabetes hastens senescence and influences aging [58].
2.3.4. DNA Methylation Clocks
Epigenetic modifications, specifically DNA methylation, are essential for controlling gene expression and are crucial in the aging process [59]. DNA methylation clocks have become useful biomarkers for determining the rate of aging because they quantify the epigenetic age of cells or tissues [60]. These epigenetic clocks frequently exhibit an accelerated aging pattern in the context of prediabetes [60]. Metabolic and oxidative stress linked to prediabetes disrupts DNA methylation patterns, explaining the acceleration of this process [61]. As a result, prediabetic individuals show changes in their epigenetic landscape that correspond to an older biological age [60]. These biomarkers provide important clues about the epigenetic modifications that accelerate senescence in prediabetes, and shed light on the underlying mechanisms and consequences of age-related disorders [62].
2.3.5. Advanced Glycation End Products (AGEs)
Advanced glycation end products, commonly known as AGEs, serve as critical biomarkers in prediabetes-induced senescence [63]. Prediabetes is often associated with elevated levels of AGEs, which stem from the persistent hyperglycemia and oxidative stress characteristic of the condition [19]. AGEs are formed through a non-enzymatic reaction between sugars and proteins, and their accumulation is indicative of glycation and oxidative damage [64]. In prediabetes, the heightened levels of AGEs underscore the accelerated aging of tissues and systems, with profound implications for age-related complications [65]. These biomarkers reflect the complex biochemical changes that drive senescence, and contribute to age-related alterations in vascular dynamics and other physiological processes [66]. The functions of DNA methylation clocks are crucial for clarifying the ways in which prediabetes affects the epigenetic control of aging and its wider consequences for health conditions associated with aging [67].
3. Circulating Hormones and Growth Factors Associated with Aging in Prediabetes
3.1. Hormonal Shifts in Prediabetes and Their Influence on Senescence
A complex interplay of hormonal changes with significant implications for the senescence process characterizes prediabetes [25]. An interruption in the insulin-like growth factor (IGF) axis is one of the primary hormonal changes linked to prediabetes [68]. Changes in the levels and bioavailability of insulin-like growth factor 1 (IGF-1), a hormone essential for tissue repair, cell growth, and differentiation, are frequently observed in prediabetic individuals [69]. Insulin resistance, a defining feature of prediabetes, can hinder IGF-1 signaling and exacerbate systemic metabolic abnormalities [70]. This disturbance of the IGF axis causes the accelerated aging of cells and is associated with the senescence of different tissues, which affects the ability of tissues to regenerate and their overall physiological function [71]. Furthermore, prediabetics have different hormonal profiles for other growth factors, such as brain-derived neurotrophic factor (BDNF), which is important for neuronal health and cognitive function [72]. Particularly in older adults, altered BDNF levels in prediabetes patients can have an impact on cognitive health [73]. The complex relationships between hormonal changes in prediabetes and their effects on aging are highlighted by disruptions in BDNF signaling, which can cause cognitive decline and intensify the senescence process [74].
Moreover, changes in leptin and adiponectin, which are secreted by adipose tissue, are part of the hormonal landscape of prediabetes [75]. Lower levels of adiponectin, a hormone that has anti-inflammatory and insulin-sensitizing qualities, are frequently observed in prediabetic people [76]. Decreased levels of adiponectin can exacerbate aging-related metabolic alterations and insulin resistance [77]. However, because of modifications in adipose tissue, prediabetes is also associated with changes in leptin, a hormone involved in appetite regulation and energy expenditure [21]. These changes in hormones can affect body weight, metabolism, and general health, which can further accelerate the aging process [78]. Deciphering the complex hormonal changes associated with prediabetes and how they affect senescence is essential to understand the wider effects of metabolic conditions on aging and age-related health issues [79].
3.2. Growth Factors and Their Role in Age-Associated Processes
Growth factors play a particularly important role in prediabetes-induced senescence [63]. Growth factors are essential for the coordination of several physiological processes. Examples of these include vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding proteins (IGFBPs) [80]. The hormonal milieu in prediabetic individuals frequently involves changes in the IGF-1 and IGFBP system [81]. These alterations affect tissue healing, cell division, and growth, and are intimately related to insulin resistance and metabolic dysregulation [82]. The IGF-1 signaling axis is disrupted, which speeds up senescence and reduces the ability of cells and tissues to regenerate [83]. Moreover, prediabetes is associated with alterations in VEGF levels, which are crucial regulators of angiogenesis and vascular homeostasis [84]. Changes in VEGF levels can affect tissue perfusion and vascular dynamics, which can lead to age-related vascular alterations that are frequently observed in people with prediabetes [85].
Clarifying the intricate interactions between hormonal changes and senescence requires an understanding of the roles played by these growth factors in age-related processes [86]. Changes in growth factors, such as bone morphogenetic proteins (BMPs) and brain-derived neurotrophic factor (BDNF), are accompanied by modifications in IGF-1, IGFBPs, and VEGF, which in turn affect different physiological systems [87]. The regulation of cellular growth, tissue repair, angiogenesis, and neuroprotection depends on these growth factors, and their disruption in prediabetes highlights the complex effects of this metabolic disorder on the aging process [88]. Gaining insight into how growth factor dysregulation in prediabetes affects age-related physiological changes and the emergence of age-related health conditions requires an understanding of the complexities of this condition (see Table 1) [89].

Table 1.
Circulating hormones and growth factors associated with aging in prediabetes.
3.3. Role of Biomarkers in Hormonal Changes
3.3.1. Circulating Growth Hormone (GH) and Insulin-like Growth Factor 1 (IGF-1)
Variations in GH and IGF-1 levels are common in prediabetic individuals because of insulin resistance and metabolic changes, which are characteristics of prediabetes [70]. The insulin resistance seen in prediabetes is closely associated with these hormonal alterations, which indicate the endocrine system’s influence on the disease and its function in mediating senescence [109].
3.3.2. Dehydroepiandrosterone Sulfate (DHEA-S)
A significant decrease in the levels of the steroid hormone dehydroepiandrosterone sulfate (DHEA-S), which is generated by the adrenal glands, is frequently observed in prediabetic individuals [110]. This hormonal change plays a crucial role in the context of senescence caused by prediabetes because it adds to the range of hormonal aging changes [57]. The synthesis of sex hormones, such as estrogen and testosterone, which are essential for many physiological functions, begins with the production of DHEA-S [111]. The complex relationship between prediabetes and senescence is further highlighted by the decrease in DHEA-S levels in prediabetes, which indicates a significant hormonal shift that affects the aging process [112].
3.3.3. Testosterone (In Men)
Changes in testosterone levels are frequently noted in male prediabetic individuals, affecting various aspects of the aging process [98]. These alterations may significantly affect bone density, muscle mass, and other aging-related factors [98]. The essential male sex hormone testosterone is essential for preserving bone health, muscle mass, and general vigour [113]. The hormonal changes associated with prediabetes underscore the impact of the illness on the endocrine system and its role in determining the course of aging in men.
3.3.4. Oestrogen (In Women)
Changes in estrogen levels in prediabetic women can have a substantial effect on different aspects of aging [114]. These hormonal shifts may have an impact on cardiovascular health, bone density, and many other aging-related factors [114]. The main female sex hormone estrogen has a significant impact on preserving cardiovascular health, bone health, and general well-being [115]. Changes in estrogen levels in people with prediabetes highlight the impact of the disease on the endocrine system and how it affects how women age [116].
4. Age-Associated Inflammatory Factors in Prediabetes
4.1. Inflammatory Mediators in Prediabetes-Induced Senescence
Chronic low-grade inflammation, a hallmark of prediabetes, significantly influences age-associated senescence [117]. A range of inflammatory mediators are prominent in this context. Notably, prediabetic individuals frequently have elevated levels of proinflammatory cytokines such as interleukin-6 (IL-6), C-reactive protein (CRP), and others [19]. These mediators of inflammation play a pivotal role as biomarkers reflecting the chronic inflammatory state associated with prediabetes [19]. For example, CRP is a sensitive indicator of systemic inflammation, and IL-6 is essential for controlling inflammation and immune responses [118]. Their increased risk of prediabetes is an essential component of the disease’s pathophysiology, rather than just a side effect [118]. The existence of these inflammatory mediators indicates how chronic inflammation contributes to senescence and how it speeds up aging in prediabetic individuals [119].
Furthermore, there is a strong correlation between prediabetes and elevated oxidative stress, which amplifies the influence of inflammatory mediators on senescence [19]. Reactive oxygen species (ROS) and oxidative damage are caused by the proinflammatory state associated with prediabetes, which is characterized by the release of factors such as tumour necrosis factor-alpha (TNF-α) [120]. These molecular events highlight the complex interplay between oxidative stress and inflammation in prediabetes-induced senescence [121]. Understanding how inflammatory mediators contribute to prediabetes-induced senescence offers important new understandings of the processes that underlie the condition’s acceleration of aging and its consequences for age-related medical disorders (see Table 2).

Table 2.
Age-associated inflammatory mediators in prediabetes-induced senescence.
4.2. Chronic Inflammation and Its Implications for Senescence
In prediabetes, chronic inflammation is at the forefront of age-associated senescence and significantly affects multiple physiological systems [19]. Chronic low-grade inflammation, a feature of prediabetes, largely promotes senescence [117]. In this context, prediabetic individuals frequently have elevated levels of various inflammatory mediators, including interleukin-1 beta (IL-1β), interleukin-18 (IL-18), and high-sensitivity C-reactive protein (hs-CRP) [131]. These inflammatory factors are largely responsible for the systemic inflammation that accelerates aging [132]. Pro-inflammatory cytokines that can worsen immune responses and inflammatory pathways include IL-1β and IL-18 [133]. The persistent inflammatory state in prediabetes is reflected in hs-CRP, a sensitive marker of systemic inflammation [134]. The increased levels of these mediators indicate the critical role that chronic inflammation plays in the senescence brought on by prediabetes, which impacts multiple physiological systems and hastens age-related health issues [135].
Moreover, oxidative stress and the inflammatory milieu in prediabetes are tightly linked, which further complicates the senescence process [136]. Tumor necrosis factor-alpha (TNF-α) and other inflammatory mediators in prediabetes frequently contribute to the production of reactive oxygen species (ROS) and oxidative damage [120]. These molecular interactions increase the level of oxidative stress in prediabetic individuals, which strengthens the influence of chronic inflammation on aging [19]. The interaction between oxidative stress and inflammation highlights the complex processes underlying the senescence observed in prediabetes [137]. It also emphasizes the necessity of investigating therapeutic approaches that address oxidative stress and inflammation to lessen the effects of prediabetes on age-related health issues and premature aging [138].
4.3. Role of Biomarkers in Inflammatory Factors
4.3.1. Senescence-Associated Secretory Phenotype (SASP) Factors
Higher than normal levels of SASP factors, a set of secreted factors linked to senescent cells, are frequently observed in prediabetic individuals [139]. The chronic inflammation frequently seen in prediabetes is the cause of this increase in SASP factors [140]. SASP factors are important indicators that highlight the proactive function of senescent cells in promoting inflammation and modifying the inflammatory environment in people with prediabetes [141].
4.3.2. Inflammatory Markers
Elevated levels of well-known inflammatory markers, such as interleukin-6 (IL-6) and C-reactive protein (CRP), are frequently used to diagnose prediabetes [142]. These markers indicate the presence of the low-grade chronic inflammation commonly observed in prediabetic patients [19]. A sensitive indicator of systemic inflammation, CRP, captures the persistent proinflammatory milieu linked to prediabetes [143]. Similarly, IL-6, an important modulator of inflammation and immune responses, is crucial in mediating the inflammatory processes associated with prediabetes [144]. These inflammatory markers demonstrate the active role that chronic inflammation plays in determining the course of senescence and its role in the accelerated aging observed in prediabetic individuals. The mechanisms underlying senescence in prediabetes and its implications for age-related health conditions are clarified by these markers, which also serve as important indicators of inflammation.
5. Vascular and Neural System Aging in Patients with Prediabetes
5.1. Prediabetes-Induced Vascular Changes and Senescence
Prediabetes has a major impact on the vascular system’s aging process, initiating a series of alterations that greatly accelerate senescence [145]. The accelerated progression of atherosclerosis and endothelial dysfunction are two of the main characteristics of vascular aging in prediabetes [146]. Specifically, endothelial dysfunction is a major factor in aging because it indicates a decline in the endothelium’s ability to control vascular tone, preserve homeostasis, and avoid blood clot formation [147]. Chronic hyperglycemia and inflammation cause endothelial dysfunction in prediabetic patients, which speeds up vascular senescence [19]. The existence of these vascular alterations highlights the complex interplay among prediabetes, endothelial dysfunction, and senescence, underscoring the diverse effects of prediabetes on the aging process of the vascular system [148].
Moreover, age-related changes in prediabetes can also affect the neural system, which is intricately linked to the vascular system [85]. The illness affects overall neurological health and cognitive function by accelerating neural senescence [85]. A common feature of aging is cognitive decline, which is more likely to occur in people with prediabetes [149]. Vascular alterations associated with prediabetes, such as decreased cerebral blood flow and microvascular dysfunction, contribute to neurodegenerative processes. Together, these elements cause the aging of neural tissue to occur more quickly (see Table 3) [150]. Deciphering the connection between vascular alterations caused by prediabetes and their consequences for the aging of the neural system is essential to understand the larger influence of this metabolic disorder on aging and its consequences for neurological and cognitive health [151].

Table 3.
Biomarkers of prediabetes-induced vascular and neural system aging.
5.2. Neural System Aging and Cognitive Implications in Prediabetes
Prediabetes affects not only the aging of the vascular system, but also the aging of the neural system, which has important implications for cognition [172]. An inevitable aspect of aging is cognitive decline, which is more likely to occur early in prediabetic people [173]. Vascular changes linked to prediabetes are intimately linked to neurodegenerative changes that are observed in the condition [174]. Through processes such as decreased cerebral blood flow and microvascular dysfunction, chronic hyperglycemia and inflammation in prediabetes directly affect neural tissue. Together, these elements cause the aging of neural tissue to occur more quickly [175]. Prediabetic individuals exhibit more severe cognitive deficits, especially in areas linked to memory, executive function, and information processing speed [172]. Gaining an appreciation of the full impact of this metabolic condition on aging and cognitive health requires an understanding of the relationship between prediabetes-induced neural system aging and its cognitive implications [176].
Furthermore, prediabetes can impact neural senescence through pathways involving oxidative stress, inflammation, and metabolic alterations [137]. Neuroinflammation, a defining feature of neurodegenerative diseases such as Alzheimer’s disease, can result from these processes [137]. Proinflammatory mediators in the central nervous system, activated by chronic inflammation and oxidative stress in prediabetes, exacerbate neural senescence [177]. The complex interactions among these variables highlight the significance of treating cognitive health in people with prediabetes and creating plans to lessen the effects of neural system aging on cognition [178].
5.3. Role of Biomarkers in Vascular and Neural Aging
5.3.1. Advanced Glycation End Products (AGEs)
Advanced glycation end products (AGEs) are important biomarkers in the context of vascular and neural system aging in prediabetes [179]. Elevated levels of AGEs are a common feature of prediabetes and have significant implications for the development of age-related complications and the acceleration of aging [85]. A class of molecules known as AGEs is produced when proteins and lipids undergo non-enzymatic glycation and oxidation. The increased production and accumulation of AGEs is a result of prediabetes’ chronic hyperglycemia and oxidative stress [179]. These molecules actively contribute to the vascular and neural system aging observed in prediabetic individuals, in addition to reflecting the biochemical changes that drive senescence [7]. The increased levels of AGEs in prediabetes indicate that this biomarker plays a crucial role in determining the course of aging, emphasizing the significance of addressing AGEs-related mechanisms so as to lessen the effects of prediabetes on the brain and vascular systems.
5.3.2. Endothelial Dysfunction
The vascular system ages significantly because of prediabetes, and endothelial dysfunction is emerging as a critical biomarker [180]. A common feature of vascular aging is endothelial dysfunction, which is worsened by this condition [180]. Endothelial cells are essential for controlling blood flow and preserving vascular homeostasis [181]. Chronic inflammation and hyperglycemia aggravate endothelial dysfunction in prediabetic individuals, indicating a crucial phase of vascular senescence [19]. A more noticeable effect on endothelial markers, such as von Willebrand factor (vWF), a vital mediator of blood clotting and vascular health, is linked to endothelial dysfunction [182]. To lessen the effects of aging on prediabetic people, it is important to address endothelial markers, as the existence of endothelial dysfunction in the condition highlights the complex relationship between vascular aging and prediabetes [183].
6. Systemic Inflammation in Prediabetes
6.1. Link between Prediabetes and Systemic Inflammation
The long-term, low-grade inflammation associated with aging is markedly worsened in prediabetic patients, resulting in a complex interaction between the metabolic disorder and aging [56]. Prediabetes, via various interrelated mechanisms, plays a major role in the promotion of inflammation [19]. The enduring proinflammatory condition associated with prediabetes, which is fuelled by oxidative stress and chronic hyperglycemia, is crucial to this connection [184]. Systemic inflammation is facilitated by these factors, which also activate proinflammatory pathways and release proinflammatory cytokines. Interestingly, elevated levels of C-reactive protein (CRP), interleukin-6 (IL-6), and other inflammatory markers are frequently observed in prediabetic individuals, supporting the link between prediabetes and systemic inflammation (see Figure 2) [185]. These inflammation biomarkers show the complex relationship between prediabetes and the accelerated aging process, which ultimately results in the systemic inflammation seen in this population. They also serve as indicators of the elevated inflammatory state in prediabetic individuals [186].
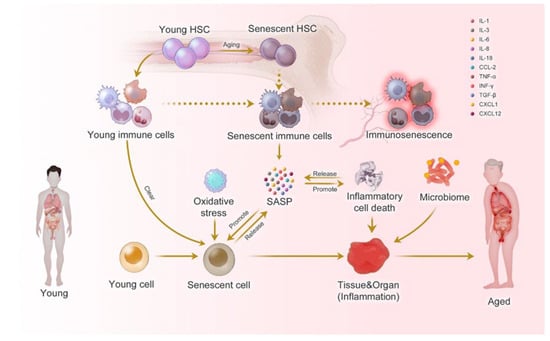
Figure 2.
Prediabetes-induced systemic Inflammaging (focusing on immunosenescence, which is immune system aging) as adapted from source and BioRender: Inflammaging at the molecular, cellular, and organ levels. During the aging process, almost all cells in the body undergo senescence, a state characterized by dysfunction and a senescence-associated secretory phenotype (SASP). SASP affects immune cells, impairing their ability to recognize and eliminate senescent cells, thereby contributing to immunosenescence. Immunosenescence can impair the immune response to infections and diseases, making the organism more vulnerable to illnesses. Moreover, the accumulation of senescent cells can trigger inflammation in organs, leading to organ damage and an increased risk of age-related diseases. This process is intensified by positive feedback loops that drive the accumulation of inflammation and organ damage, leading to further inflammation and an even higher risk of aging-related diseases [30].
Prediabetes also affects the endocrine system, causing hormonal changes that feed inflammation, which further contributes to systemic inflammation [78]. For example, prediabetes is frequently associated with altered levels of adipokines, such as adiponectin and leptin, which promote a proinflammatory milieu [187]. This hormonal imbalance also intensifies inflammation by affecting the delicate balance between pro- and anti-inflammatory components of the body [188]. The complex interaction of inflammatory markers, hormonal changes, and prediabetes highlights the complexity of systemic inflammation in this metabolic condition [189]. The connection between prediabetes and systemic inflammation is crucial in understanding the wider influence of this illness on the aging process and its consequences for health issues associated with aging.
6.2. Inflammatory Factors and Their Contribution to Senescence
Chronic inflammation, a hallmark of systemic inflammation in prediabetes, largely contributes to the aging of various physiological systems [190]. Several important inflammatory markers, including interleukin-6 (IL-6) and C-reactive protein (CRP), are frequently elevated in prediabetics. These markers indicate a chronic proinflammatory condition that accelerates aging by substantially contributing to senescence [19]. A sensitive measure of systemic inflammation, CRP is a good way to determine whether prediabetes is still linked to a proinflammatory state [28]. Similarly, IL-6 plays a major role in coordinating the inflammatory processes associated with prediabetes. It is a central regulator of immune responses and inflammation [191]. The existence of these inflammatory markers emphasizes the crucial role they play in encouraging senescence and the faster aging seen in prediabetic people.
Moreover, oxidative stress, which increases the effect of inflammatory factors on senescence, is intimately linked to chronic inflammation in prediabetes [119]. Reactive oxygen species (ROS) and oxidative damage are fostered by the proinflammatory state associated with prediabetes, which is marked by the release of factors such as Tumor necrosis factor-alpha (TNF-α) [192]. These molecular events highlight the complex interplay between oxidative stress and inflammation in driving senescence induced by prediabetes [192]. The coexistence of oxidative stress and chronic inflammation in prediabetes, as well as their combined effect on senescence, provide a thorough understanding of the mechanisms through which the condition speeds up aging, and emphasize the significance of treating inflammation in prediabetic individuals to manage age-related health conditions.
6.3. Role of Biomarkers in Systemic Inflammation
6.3.1. Inflammatory Markers (Continued)
Interleukin-6 (IL-6) and C-reactive protein (CRP) are two inflammatory markers that play a significant role in the context of systemic inflammation in prediabetes [193]. Elevated levels of these inflammatory markers are often associated with prediabetes, indicating a persistent proinflammatory state that is commonly seen in prediabetes [19]. As a sensitive measure of systemic inflammation, CRP is an important window into the persistent proinflammatory environment linked to prediabetes [194]. Similarly, IL-6, an important modulator of inflammation and immune responses, is crucial in mediating the inflammatory processes associated with prediabetes [19]. These inflammatory markers are important markers of inflammation and play a major role in the chronic inflammation that occurs with aging in people with prediabetes [137]. Understanding the mechanisms by which prediabetes accelerates systemic inflammation and its implications for age-related health conditions depends on the identification of these biomarkers.
6.3.2. Oxidative Stress Markers
The role of elevated oxidative stress in the context of systemic inflammation in prediabetes cannot be underestimated [195]. Elevated oxidative stress is frequently linked to prediabetes, which can worsen the age-related rise in reactive oxygen species (ROS) and oxidative damage [137]. Oxidative stress is closely associated with the chronic proinflammatory state that characterizes prediabetes, which fosters the production of reactive oxygen species (ROS) and subsequent oxidative damage [196]. The intricate relationship between prediabetes, inflammation, and oxidative stress is highlighted by the presence of oxidative stress markers, which further highlights the complex web of factors that contribute to systemic inflammation [197]. Understanding the wider effects of prediabetes on aging and its consequences for age-related health issues, especially considering systemic inflammation, requires an understanding of the role of oxidative stress markers in this metabolic condition [198].
Furthermore, the immune system’s inflammatory reactions are triggered by the interaction of advanced glycation end products (AGE) and their associated Receptor for AGEs (RAGE) [199,200]. The non-enzymatic glycation of proteins, lipids, and nucleic acids produces AGEs, which attach to RAGE and start signaling cascades, which lead to inflammation and oxidative stress [200]. Tissue damage and dysfunction are the consequences of this interaction, which induces the release of pro-inflammatory cytokines and chemokines. With potential benefits for reducing age-related disorders and fostering healthy aging, targeting the AGE–RAGE axis has emerged as a viable therapeutic approach for preventing senescence and inflammation carried on by AGEs [201]. A particular strategy for tackling the harmful consequences of aging and age-related diseases is the use of therapeutic interventions targeted at AGE–RAGE signaling disruption [202]. These interventions have the potential to reduce inflammation and maintain tissue homeostasis.
7. Regeneration and Metabolic Disorders in Prediabetes
7.1. Impaired Regeneration Mechanisms in Patients with Prediabetes
The body’s ability to regenerate itself is greatly impacted by prediabetes, and this ability is crucial for preserving tissue integrity and fending off the consequences of aging [203]. Several variables, such as oxidative stress, metabolic dysregulation, and chronic inflammation, are associated with impaired regeneration mechanisms in prediabetes [136]. Chronic inflammation, a hallmark of prediabetes, hampers the body’s ability to initiate and sustain regenerative processes [204]. Inflammatory mediators that are activated during a persistent proinflammatory state obstruct the body’s regenerative processes, especially regarding tissue repair and cellular turnover [205]. Prediabetic individuals may encounter delayed wound healing and compromised tissue regeneration, particularly in tissues with a high cellular turnover rate, such as the skin and the lining of the gastrointestinal tract. This hindrance to regeneration predominantly affects these tissues [206]. Understanding prediabetes’ effects on aging and its consequences for age-related health conditions requires an understanding of the interaction between inflammation and regeneration brought on by the condition [204].
Metabolic dysregulation in prediabetes further compounds the impairment of regeneration mechanisms. A defining feature of prediabetes is insulin resistance, which impairs the body’s capacity to use glucose effectively for cellular upkeep and energy production. Because glucose is a necessary fuel for many regenerative mechanisms, such as tissue repair and cell proliferation, this metabolic disruption has a negative impact on the regenerative processes [204,205,206]. Cellular energy deficiencies impair the body’s capacity to mount a strong regenerative response in prediabetes, which is one of the factors contributing to the impaired regeneration seen in prediabetic patients [206]. Gaining an appreciation of the complete influence of prediabetes on the aging process and its consequences for age-related health complications requires an understanding of the connection between impaired regeneration, prediabetes, and related metabolic disorders.
7.2. Metabolic Disorders and Their Impact on Senescence
Prediabetes, a metabolic condition characterized by insulin resistance and dysregulated glucose metabolism, significantly impacts senescence and the body’s ability to regenerate itself [119]. One characteristic that sets prediabetes apart is insulin resistance, which impairs the cells’ abilities to absorb glucose [136]. The main energy source for cellular processes, including regeneration, is glucose [207]. Metabolic abnormalities in prediabetic people make it difficult for glucose to be used efficiently, upsetting the energy balance required for regenerative processes [208]. This metabolic dysregulation impairs the body’s capacity for effective tissue regeneration and repair, which in turn adds to the general senescence observed in prediabetic patients [89]. The complex relationship between senescence and metabolic disorders highlights how critical it is to address metabolic factors in order to lessen the effects of prediabetes on aging.
Moreover, mitochondrial dysfunction, a major contributor to aging, is closely linked to metabolic disorders in prediabetes [209]. Impaired insulin signaling and elevated oxidative stress have a deleterious effect on prediabetic mitochondria, which are the cellular powerhouses in charge of producing energy [196]. Because these mitochondrial disruptions reduce the cell’s ability to produce energy for regenerative processes, they worsen cellular aging and senescence [196]. The interplay between metabolic disorders, mitochondrial dysfunction, and senescence highlights the complex mechanisms by which prediabetes accelerates the aging process (see Figure 3) [210]. This underscores the importance of addressing metabolic factors to manage age-related health conditions in prediabetic individuals [210]. Comprehending how metabolic disorders contribute to prediabetes-induced senescence is essential for understanding how this metabolic condition affects aging in general, and what that means for age-related health complications.
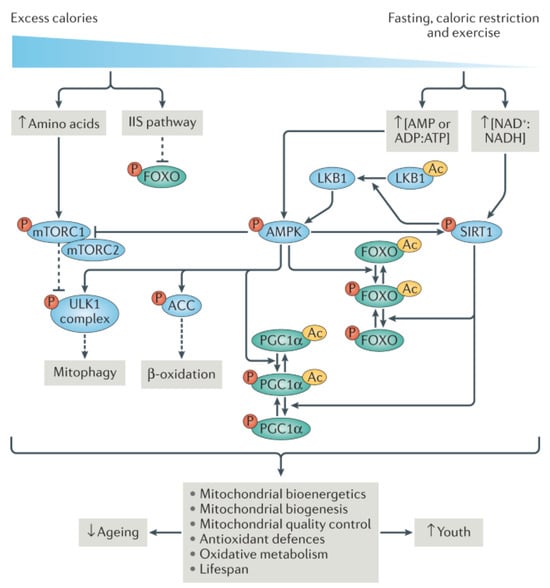
Figure 3.
Prediabetes-induced metabolic aging as adapted from source: Signaling pathways linked to longevity govern the maintenance of mitochondrial health. Restricting caloric intake lowers the levels of glucose, amino acids, and lipids, while simultaneously increasing the concentrations of crucial metabolites like NAD+ and AMP. These metabolites play pivotal roles in regulating metabolic sensors such as SIRTs, AMPK, TOR, and insulin–IGF1 signaling pathways. Subsequently, downstream of these metabolic sensors, transcription factors like FOXO and PGC1α coordinate mitochondrial function and equilibrium. Disruption of this intricate regulatory network compromises mitochondrial homeostasis, leading to susceptibility to frailty and various diseases. Noteworthy components of this system include acetyl groups (Ac), acetyl-CoA carboxylase (ACC), liver kinase B1 (LKB1), and UNC51-like kinase 1 (ULK1) [130].
7.2.1. Mitochondrial Dysfunction
Mitochondria are cellular powerhouses responsible for energy production, and their proper functioning is essential for various cellular processes, including regeneration [211]. However, in prediabetes, insulin resistance and metabolic alterations lead to disturbances in mitochondrial function, which manifest as decreased mitochondrial efficiency and increased oxidative stress [212]. The compromised mitochondrial function associated with prediabetes not only affects cellular energy production and exacerbates aging and senescence in prediabetic individuals [213]; mitochondrial dysfunction is also a critical biomarker associated with metabolic changes in prediabetes.
7.2.2. Red Blood Cell Distribution Width (RDW)
Increased red blood cell distribution width (RDW) is a common biomarker of prediabetes, reflecting the condition’s associated inflammation and metabolic abnormalities [214]. Red blood cell width variation is a measure of underlying inflammation and metabolic disturbances. These conditions are suggested by an increase in RDW [215]. When elevated RDW is observed in prediabetic patients, it indicates systemic inflammation and metabolic changes that impact aging [216]. The association between RDW, inflammation, and metabolic alterations highlights the necessity of addressing these factors to reduce the risk of age-related health complications in prediabetic individuals [217].
7.2.3. Hemoglobin A1c (HbA1c)
Elevated HbA1c levels in prediabetes can significantly affect how people age [218]. Higher HbA1c values indicate chronic hyperglycemia, whereas lower levels indicate average blood glucose levels over an extended period [219]. In prediabetic people, long-term exposure to high blood sugar levels can hasten the aging process by impacting multiple organs and systems [88]. Advanced glycation end products (AGEs), which can harm proteins and lipids and result in tissue dysfunction, are formed in part by chronic hyperglycemia [88,219]. Because of the critical role that glycation-induced damage plays in the aging of organs and tissues, HbA1c is an important biomarker for understanding how prediabetes affects age-related health conditions, especially regarding diabetes-related aging.
7.2.4. Serum Albumin
Serum albumin levels, a biomarker of nutritional status, may be lower in prediabetic individuals [220]. A drop in serum albumin levels may impact frailty and nutritional deficits [221]. The protein albumin is necessary to keep the colloid osmotic pressure constant and to carry vital nutrients throughout the bloodstream [222]. Reduced serum albumin levels in prediabetes patients may indicate insufficient dietary intake or poor nutrient absorption, which can lead to nutritional deficiencies [223]. These dietary deficits, especially in important vitamins and minerals, can worsen age-related health problems [224]. In addition, decreased serum albumin levels have been linked to frailty in the elderly, which makes this biomarker an important one for assessing the possibility of frailty in people with prediabetes [221]. To effectively manage age-related health complications in this population, it is essential to comprehend the relationship between prediabetes, lower serum albumin levels, and their implications for nutritional status and frailty [225].
7.2.5. Folate and B12
Deficits in certain nutrients, especially folate and vitamin B12, can have a major impact on the metabolic alterations linked to prediabetes [226]. These deficiencies directly impact the processes of DNA methylation and repair, which are crucial for preserving genomic stability and controlling aging [227]. The prevalence of these nutritional deficiencies can be higher in prediabetic individuals, which can accelerate aging [228]. The correct operation of enzymes involved in DNA methylation, a process that regulates gene expression and controls cellular functions, depends on folate and vitamin B12 [225]. Dysregulated DNA methylation can impact aging-related gene expression patterns when it is insufficient [229,230]. Deducing the complete effects of this metabolic condition on aging and its implications for age-related health complications requires an understanding of the role that folate and vitamin B12 deficiencies play in prediabetes-induced metabolic changes and their impact on senescence.
7.2.6. Osteocalcin
Changes in osteocalcin levels are a sign of prediabetes and a biomarker of bone health [231]. Osteoblasts, the cells that form bones, produce a protein called osteocalcin [232]. These changes in osteocalcin levels in prediabetic people have consequences for bone health, which is important for aging people [233]. Osteocalcin is essential for preserving the strength and density of bones [234]. Variations in its concentrations can affect bone turnover and, in turn, bone quality. This change in osteocalcin levels may result in decreased bone mineralization and density, which could put older people at higher risk for fractures and other bone-related problems [235]. It is critical to comprehend the relationship between osteocalcin levels and prediabetes to develop risk-reduction strategies and to comprehend the possible effects on bone health as we age [236].
7.2.7. Adiponectin
Reduced levels of adiponectin, an adipokine with important metabolic and anti-inflammatory properties, can aggravate insulin resistance and age-related metabolic changes in prediabetes [237]. The main function of the protein hormone adiponectin, which is secreted by adipose tissue, is to control the metabolism of fats and carbohydrates [238]. These metabolic processes can be disrupted by decreased adiponectin levels in prediabetes, especially regarding the body’s inability to properly use insulin, which eventually results in insulin resistance [70]. One of the main causes of aging-related metabolic alterations is deteriorating insulin resistance [239]. Therefore, comprehending the mechanisms underlying metabolic alterations and their implications for age-related health conditions requires an understanding of the relationship between prediabetes and adiponectin levels [240].
7.2.8. Leptin
Because of changes in adipose tissue, prediabetics may have altered leptin levels, which can ultimately impact metabolism and aging [241]. The hormone leptin, which is mostly produced by adipocytes, is essential for controlling metabolism and hunger [242]. These changes in leptin levels in prediabetes can have far-reaching effects [243]. Leptin resistance or decreased sensitivity to leptin may arise from abnormalities in leptin signaling caused by alterations in adipose tissue [244]. Overeating, weight gain, and an unbalanced energy intake are all associated with leptin resistance, and can aggravate metabolic changes and hasten the aging process [78]. Addressing the underlying mechanisms of age-related health issues in prediabetic individuals requires an understanding of the complex relationship between prediabetes, altered leptin levels, and their impact on metabolism and aging [245].
7.2.9. Brain-Derived Neurotrophic Factor (BDNF)
In older adults, altered BDNF levels in prediabetes can have a major impact on cognitive health [246]. A protein called BDNF is essential for the development, upkeep, and plasticity of brain neurons [247]. Changes in BDNF levels in prediabetes patients may impact cognitive function [248]. A higher risk of neurodegenerative diseases and cognitive decline has been linked to decreased BDNF levels [249]. A key component of aging is cognitive health, and abnormalities in BDNF levels can affect memory, learning, and other cognitive processes [250]. Understanding the possible effects on cognitive health in the context of aging and creating strategies to support cognitive well-being in prediabetic individuals require an understanding of the relationship between prediabetes and altered BDNF levels [251].
7.2.10. IGF-Binding Proteins
Metabolic and aging-related effects can be attributed to changes in IGF-binding proteins (IGFBPs) in prediabetes, which can have a major effect on the bioavailability of insulin-like growth factor 1 (IGF-1) [252]. IGFBPs play a crucial role in controlling the availability of IGF-1, a growth factor that is important for metabolism, cell division, and growth. Changes in IGFBPs in prediabetes can affect IGF-1 binding and release, which may affect its signaling pathways [253]. Variations in IGF-1 bioavailability can affect metabolic regulation and tissue maintenance, among other physiological processes [254]. Understanding the relationship between prediabetes and IGFBPs is crucial to understanding the mechanisms underlying the effects related to metabolism and aging in this population [88]. Understanding IGFBPs’ function in the context of prediabetes offers valuable information about possible treatment targets for reducing the age-related effects of this illness.
7.2.11. Homocysteine
An amino acid called homocysteine, which is involved in many metabolic processes, has been linked to an increased risk of cardiovascular disease, and may be more common in prediabetic individuals [255]. It is well recognized that homocysteine contributes to the development of atherosclerosis and other cardiovascular diseases [256]. Metabolic abnormalities and changes in folate and vitamin B12 levels can cause elevated homocysteine levels in prediabetes [255]. This homocysteine increase can intensify oxidative stress and endothelial dysfunction, two conditions that hasten the vascular system’s aging process [256]. Recognizing the age-related implications of prediabetes, especially regarding the vascular system, requires an understanding of the relationship between elevated homocysteine levels, cardiovascular risk, and the condition [257].
7.2.12. Insulin Resistance Markers
One of the hallmarks of prediabetes is elevated insulin resistance markers, which can worsen with age [258]. One such marker is the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) [258]. One of the main features of prediabetes is insulin resistance, which is typified by the body’s decreased sensitivity to the insulin hormone. Insulin resistance markers are frequently elevated in prediabetic individuals, indicating a compromised ability of cells to effectively absorb glucose [259]. Insulin resistance can progress more quickly in older people [260]. The deterioration of insulin resistance is a major factor in the age- and metabolic-related consequences of prediabetes [261]. Inferring the complete impact of prediabetes on the aging process requires an understanding of the role of insulin resistance markers and their potential to intensify age-related metabolic changes.
7.2.13. N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP)
NT-proBNP is produced in the body as a byproduct of BNP breakdown, and functions as a precursor to BNP [262]. The level of NT-proBNP found in the blood is a good indicator of how heart-related diseases, such as heart failure and increased cardiac strain, are developing [262,263]. In clinical contexts, this biomarker is crucial since it provides insightful information for prognostic and diagnostic assessments of cardiovascular diseases [263]. NT-proBNP is a vital tool that helps with the early diagnosis and treatment of heart failure, especially in high-risk populations including the elderly and those with diabetes [264]. It should be noted that decreased renal function and the natural aging process can also cause elevated NT-proBNP levels. The steady increase in NT-proBNP levels in the bloodstream throughout life is acknowledged as a major biological characteristic linked to longer lifespans, emphasizing its potential as a biomarker for evaluating age-related cardiovascular health [265,266]. The breakdown of BNP yields NT-proBNP, which is a dependable marker of increased cardiac strain and the advancement of heart disorders, including heart failure. Its usefulness extends to diagnostic and prognostic assessments, where it is extremely beneficial for the early diagnosis and efficient management of cardiovascular diseases, particularly in susceptible groups such as the elderly and those with diabetes [267]. Additionally, the correlation between increased levels of NT-proBNP and aging and reduced renal function highlights its wider significance in evaluating cardiovascular health in a variety of demographic contexts. The slow increase in circulation levels of NT-proBNP over time indicates a significant biological trait associated with longer life expectancy, highlighting its utility as a biomarker for evaluating cardiovascular health related to aging [268].
7.3. The Negative Effects of Senescence and Pre-Diabetes on Renal Function and Association with Early Stages of Chronic Kidney Disease
Senescence, the physiological aging process, and pre-diabetes, a metabolic condition that occurs before the onset of type 2 diabetes mellitus (T2DM), have deleterious impacts on renal function, and may be involved in the development of chronic kidney disease (CKD) in its early stages [269,270]. Senescence is a broad term for a variety of molecular and cellular changes that upset the homeostatic equilibrium in the renal microenvironment and cause damage to the kidneys’ structure and function. These alterations include extracellular matrix remodeling and cellular senescence, which is defined by irreversible cell cycle arrest, and the secretion of pro-inflammatory cytokines like interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-alpha (TNF-α) [269,270,271]. These changes ultimately promote renal fibrosis and impaired renal function. Moreover, senescent cells accumulate in the renal parenchyma over time, exacerbating renal inflammation, oxidative stress, and DNA damage, further compromising renal integrity and function [272].
Additionally, the slight impairment of glucose metabolism in prediabetes sets off a series of pathophysiological pathways that have a deleterious effect on renal function [273]. Renal endothelial dysfunction, glomerular hypertrophy, and the increased renal tubular reabsorption of glucose are caused by chronic hyperglycemia and insulin resistance linked to pre-diabetes [273]. These factors promote a pro-inflammatory and pro-fibrotic environment in the kidneys. These changes not only compromise renal hemodynamics, but also facilitate the onset of tubular and glomerular damage, which establishes the foundation for the initial phases of chronic kidney disease [274]. Moreover, renal injury and dysfunction are further aggravated by prediabetes-induced dyslipidemia, oxidative stress, and the activation of the renin–angiotensin–aldosterone system (RAAS), which increases the likelihood of developing overt type 2 diabetes and severe CKD [275]. To slow the progression of CKD and related comorbidities, early intervention strategies targeting metabolic and aging-related pathways, as well as biomarkers like transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and fibroblast growth factor-23 (FGF-23), are crucial. Together, these factors create an environment that is favorable for renal pathology [276].
8. Perspectives for Future Research
8.1. Gaps in Current Understanding and the Need for Further Research
Although this thorough review clarifies the complex interactions among prediabetes, senescence, and blood-based biomarkers, there are still some unanswered questions that underscore the need for a more in-depth study in the future [277]. First, investigating the temporal correlations between these biomarkers and prediabetes-induced senescence is imperative [12]. Examining whether biomarkers are early markers of prediabetes or if the condition only manifests them as it advances could yield important information for prompt diagnosis and treatment [278]. Furthermore, additional investigation is required to clarify the interplay and possible synergies between these biomarkers [278]. It is crucial to understand how various biomarkers may work together to accelerate age-related diseases and senescence in prediabetic patients. This is an area that requires more research [279].
Furthermore, more research is needed to determine how pharmacological and lifestyle interventions affect blood-based biomarkers of prediabetes-induced senescence [280]. It is crucial to find interventions that can adjust these biomarker levels, delay aging and lower the chance of age-related problems in prediabetic patients [60]. Furthermore, it is an exciting direction for future research to investigate the potential of these biomarkers as therapeutic targets [281]. Creating therapies that directly target the identified biomarkers could provide fresh approaches to improving health outcomes and delaying prediabetes-related senescence [282]. All things considered, filling in these knowledge gaps and conducting additional research are essential to improving our comprehension of prediabetes-induced senescence and creating practical plans to lessen its effects on aging and age-related illnesses [283].
8.2. Potential Blood-Based Biomarkers and Intervention Strategies
Future studies should concentrate on finding more blood-based biomarkers that can function as early warning systems for age-related complications linked to prediabetes-induced senescence [6]. The identification of new biomarkers would improve our capacity to identify prediabetes early and to take preventive action [284]. Furthermore, studies should focus on creating customized intervention plans according to the unique biomarker profiles of prediabetic individuals [285]. Customized therapies that focus on each patient’s distinct biomarker patterns could offer more accurate and successful methods for slowing aging and averting age-related illnesses [286]. It is crucial to investigate how pharmacological treatments, dietary changes, and exercise routines might affect these biomarkers [286]. These intervention strategies may improve the general health and longevity of prediabetic individuals, while also delaying the onset of senescence.
Subsequent investigations should delve into the complex functions of supplementary blood-based indicators, such as folate and B12, concerning prediabetes-induced senescence [287]. It is crucial to investigate how dietary deficiencies, particularly regarding important nutrients like folate and B12, affect senescence and the effects of aging in prediabetic individuals [288]. Gaining knowledge about how these biomarkers affect DNA methylation and repair—both essential for good aging—can help in identifying the underlying processes.
Furthermore, future studies should concentrate on the function of biomarkers such as osteocalcin in bone health and aging [289]. The importance of osteocalcin in preserving bone density and general skeletal health is widely established, and in the context of prediabetes-induced senescence, it can provide information about strategies for protecting musculoskeletal integrity in older prediabetic patients [233].
For a more comprehensive understanding of the condition’s impact on aging, a thorough assessment of these additional biomarkers in prediabetes-induced senescence is imperative. It can reveal novel approaches to addressing nutritional inadequacies and bone health, which are two critical facets of aging well. Future studies examining these biomarkers may lead to better therapeutic and preventive strategies for prediabetic patients to support healthy aging.
9. Conclusions and Insights
9.1. Summarizing the Key Findings on Blood-Based Biomarkers in Prediabetes-Induced Senescence
Several important findings have been highlighted in this thorough review of blood-based biomarkers in prediabetes-induced senescence. Prediabetes is linked to a complex web of biomarkers that interact to speed up aging, increase the chance of developing age-related illnesses, and impact different physiological systems [290]. Telomere length, p16INK4a, advanced glycation end products (AGEs), senescence-associated secretory phenotype (SASP) factors, DNA methylation clocks, inflammatory markers, oxidative stress markers, circulating hormones, growth factors, and other biomarkers such as folate and B12, osteocalcin, and others, are some of these biomarkers [291]. The review highlights the fact that these biomarkers are active contributors to chronic inflammation, oxidative stress, and metabolic dysregulation—all of which accelerate aging—in addition to acting as markers of senescence. With the potential to improve quality of life and encourage healthy aging, a thorough understanding of these biomarkers and their complex relationships with prediabetes-induced senescence serves as a basis for the development of customized intervention strategies aimed at mitigating age-related complications in prediabetic individuals.
9.2. Implications for Understanding Senescence and Aging-related Disorders in Prediabetes
It is critical to comprehend the effects of aging and disorders related to senescence in the context of prediabetes. This thorough analysis emphasizes that prediabetes is a crucial stage that profoundly affects age-related disorders and the aging process; it is not just a stage between normal glucose metabolism and diabetes. In this case, the complex interactions among blood-based biomarkers accelerate senescence, making it essential. The ramifications are extensive and touch on many physiological systems, such as the nervous and circulatory systems, metabolism, inflammation, and hormone control [292]. In addition, the review indicates that the influences of prediabetes on senescence can vary greatly among individuals, emphasizing the necessity of tailored intervention approaches to successfully lessen the effects of aging-related illnesses [293]. These findings highlight the significance of early detection and intervention in prediabetic individuals to promote healthy aging and lower the burden of age-related diseases, with significant implications for public health and clinical practice. In the end, gaining an understanding of the consequences of senescence in prediabetes is essential for improving the well-being and quality of life of an aging population with a variety of needs.
9.3. Future Directions for Research Incorporating Diverse Biomarkers in Prediabetes-Induced Senescence
Future studies on prediabetes-induced senescence should focus on incorporating an even wider range of biomarkers to obtain a thorough grasp of the complex mechanisms underlying this condition. This strategy may entail both the discovery of novel blood-based biomarkers and a closer examination of their functions. Studies should investigate the interactions among these biomarkers and evaluate how they all contribute to the aging process of physiological systems, which in turn affects age-related illnesses. Future research should also place high priority on creating novel diagnostic instruments and intervention plans that use these various biomarkers for precision medicine. This will enable prediabetic patients to receive individualized care that will delay senescence and encourage healthy aging. A more nuanced understanding of the variability of prediabetes and the complex nature of senescence will be possible through the integration of various biomarkers, thereby opening the door to more efficient and individualized treatments of this illness and its effects on aging.
Author Contributions
Conceptualization, N.A.M., A.G.-A.M. and A.K.; writing—original draft preparation, A.G.-A.M. and N.A.M.; writing—review and editing, A.G.-A.M., N.A.M. and A.K.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the College of Health Sciences of the University of Kwazulu-Natal for the support. The authors thank Nonjabulo Ntombikhona Magwaza for assisting in editing the format of the manuscript and her inputs.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Echouffo-Tcheugui, J.B.; Selvin, E. Prediabetes and What It Means: The Epidemiological Evidence. Annu. Rev. Public Health 2021, 42, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, M.E.; Zapico, S.C. Frailty, cognitive decline, neurodegenerative diseases, and nutrition interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X.; Zhang, X.; Wang, S. Spatiotemporal evolution of global population aging from 1960 to 2017. BMC Public Health 2019, 19, 127. [Google Scholar]
- Rizos, E.C.; Kanellopoulou, A.; Filis, P.; Markozannes, G.; Chaliasos, K.; Ntzani, E.E.; Tzamouranou, A.; Tentolouris, N. and Tsilidis, K.K. Difference on glucose profile from continuous glucose monitoring in people with prediabetes vs. normoglycemic individuals: A matched-pair analysis. J. diabetes Sci. Technol. 2024, 18, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From pre-diabetes to diabetes: Diagnosis, treatments and translational research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Rybtsova, N.; Berezina, T.; Kagansky, A.; Rybtsov, S. Can blood-circulating factors unveil and delay your biological aging? Biomedicines 2020, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. 2017, 10, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- De Biasi, A.; Wolfe, M.; Carmody, J.; Fulmer, T.; Auerbach, J. Creating an age-friendly public health system. Innov. Aging 2020, 4, igz044. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Y.; Luo, Y.; Song, Y.; Xiong, G.; Ma, Y.; Sun, X.; Kan, C. Metabolic diseases and healthy aging: Identifying environmental and behavioral risk factors and promoting public health. Front. Public Health 2023, 11, 1253506. [Google Scholar] [CrossRef] [PubMed]
- Bahour, N.; Cortez, B.; Pan, H.; Shah, H.; Doria, A.; Aguayo-Mazzucato, C. Diabetes mellitus correlates with increased biological age as indicated by clinical biomarkers. Geroscience 2022, 44, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Aging Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Mota, R.I.; Morgan, S.E.; Bahnson, E.M. Diabetic vasculopathy: Macro and microvascular injury. Curr. Pathobiol. Rep. 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fhayli, W.; Boëté, Q.; Harki, O.; Briançon-Marjollet, A.; Jacob, M.-P.; Faury, G. Rise and fall of elastic fibers from development to aging. Consequences on arterial structure-function and therapeutical perspectives. Matrix Biol. 2019, 84, 41–56. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, Y.; Zheng, M.; Pan, A.; Wang, M.; Zhao, M.; Li, Y.; Yao, S.; Chen, S.; Wu, S. Association of age of onset of hypertension with cardiovascular diseases and mortality. J. Am. Coll. Cardiol. 2020, 75, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Brannick, B.; Dagogo-Jack, S. Prediabetes and Cardiovascular Disease: Pathophysiology and Interventions for Prevention and Risk Reduction. Endocrinol. Metab. Clin. N. Am. 2018, 47, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.; Mikolajczyk, T. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Baetta, R.; Mallia, A.; Banfi, C.; Tremoli, E. Platelets in healthy and disease states: From biomarkers discovery to drug targets identification by proteomics. Int. J. Mol. Sci. 2020, 21, 4541. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Andle, J.; Lee, T.B.; Midha, A.; Talemal, L.; Chipashvili, V.; Hollister-Lock, J.; Van Deursen, J.; Weir, G.; Bonner-Weir, S. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019, 30, 129–142.e124. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.-G. Role of insulin in health and disease: An update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of dietary red raspberry consumption on pre-diabetes and type 2 diabetes mellitus parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Samouda, H. Clinical and biological risk factors associated with inflammation in patients with type 2 diabetes mellitus. BMC Endocr. Disord. 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Barbu, E.; Popescu, M.-R.; Popescu, A.-C.; Balanescu, S.-M. Inflammation as a precursor of atherothrombosis, diabetes and early vascular aging. Int. J. Mol. Sci. 2022, 23, 963. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.J.; Jairoun, A.A.; Farajallah, A.; Shanabli, S. Prevalence of dyslipidemia and factors affecting lipid profile in patients with type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Fernando, S.; Schwarz, N.; Tan, J.T.; Bursill, C.A.; Psaltis, P.J. Inflammation as a therapeutic target in atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef]
- Honda, M. Reverse Heart Disease Naturally: Cures for High Cholesterol, Hypertension, Arteriosclerosis, Blood Clots, Aneurysms, Myocardial Infarcts and More; Hatherleigh Press: Hobart, NY, USA, 2019. [Google Scholar]
- Gilon, P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. J. Mol. Biol. 2020, 432, 1367–1394. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Massaccesi, L.; Corsi Romanelli, M.M. Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers. J. Clin. Med. 2021, 10, 4792. [Google Scholar] [CrossRef]
- Kosmopoulos, M.; Drekolias, D.; Zavras, P.D.; Piperi, C.; Papavassiliou, A.G. Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Nobili, F.; Teunissen, C.E.; Simó, R.; Scheltens, P. Understanding multifactorial brain changes in type 2 diabetes: A biomarker perspective. Lancet Neurol. 2020, 19, 699–710. [Google Scholar] [CrossRef]
- Kudryashova, K.S.; Burka, K.; Kulaga, A.Y.; Vorobyeva, N.S.; Kennedy, B.K. Aging biomarkers: From functional tests to multi-omics approaches. Proteomics 2020, 20, 1900408. [Google Scholar] [CrossRef]
- Zheng, L.; Meng, Y.; Campbell, J.L.; Shen, B. Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases. Nucleic Acids Res. 2020, 48, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.S.M.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere length and oxidative stress and its relation with metabolic syndrome components in the aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and aging: Two sides of the same coin. Obes. Rev. 2020, 21, e12991. [Google Scholar] [CrossRef] [PubMed]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Mbatha, N.A.; Mzimela, N.C.; Mushebenge, A.G.-A.; Khathi, A. Investigating the Impact of Prediabetes on the Aging Process through Longitudinal Analysis of Blood-Based Biomarkers: A Systematic Review and Meta-Analysis Protocol. medRxiv 2023. [Google Scholar] [CrossRef]
- Yi, H.-S.; Kim, S.Y.; Kim, J.T.; Lee, Y.-S.; Moon, J.S.; Kim, M.; Kang, Y.E.; Joung, K.H.; Lee, J.H.; Kim, H.J. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019, 10, 249. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, L. The signaling of cellular senescence in diabetic nephropathy. Oxidative Med. Cell. Longev. 2019, 2019, 7495629. [Google Scholar] [CrossRef]
- Li, N.; Liu, F.; Yang, P.; Xiong, F.; Yu, Q.; Li, J.; Zhou, Z.; Zhang, S.; Wang, C.-Y. Aging and stress induced β cell senescence and its implication in diabetes development. Aging 2019, 11, 9947. [Google Scholar] [CrossRef]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Samakkarnthai, P.; Monroe, D.G.; Farr, J.N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Macrì, F.; Castiglione, S.; Badi, I.; Vinci, M.C.; Zuccolo, E. MicroRNA-34a: The bad guy in age-related vascular diseases. Cell. Mol. Life Sci. 2021, 78, 7355–7378. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between inflammaging and immunosenescence during aging. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in aging and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Tracy, E.P.; Hughes, W.; Beare, J.E.; Rowe, G.; Beyer, A.; LeBlanc, A.J. Aging-induced impairment of vascular function: Mitochondrial redox contributions and physiological/clinical implications. Antioxid. Redox Signal. 2021, 35, 974–1015. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, A.; Freeman, W.M.; Jackson, J.; Wren, J.D.; Porter, H.; Richardson, A. The role of DNA methylation in epigenetics of aging. Pharmacol. Ther. 2019, 195, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Salameh, Y.; Bejaoui, Y.; El Hajj, N. DNA methylation biomarkers in aging and age-related diseases. Front. Genet. 2020, 11, 171. [Google Scholar] [CrossRef]
- Samblas, M.; Milagro, F.I.; Martínez, A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics 2019, 14, 421–444. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Barkabi-Zanjani, S.; Ghorbanzadeh, V.; Aslani, M.; Ghalibafsabbaghi, A.; Chodari, L. Diabetes mellitus and the impairment of male reproductive function: Possible signaling pathways. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Koffi, K.A.; Doublier, S.; Ricort, J.-M.; Babajko, S.; Nassif, A.; Isaac, J. The role of GH/IGF axis in dento-alveolar complex from development to aging and therapeutics: A narrative review. Cells 2021, 10, 1181. [Google Scholar] [CrossRef]
- Ehtewish, H.; Arredouani, A.; El-Agnaf, O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int. J. Mol. Sci. 2022, 23, 6144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Parihar, A.; Talaiya, T.; Dubey, K.; Porwal, B.; Parihar, M.S. Cognitive impairments in type 2 diabetes, risk factors and preventive strategies. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190105. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Kiss, T.; Tarantini, S.; Nyúl-Tóth, Á.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Tabak, A.; Institoris, A. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H740–H761. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Koźniewski, K. Anti-inflammatory strategies targeting metaflammation in type 2 diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.-Y.; Zhang, H.; Tan, P.-C.; Zhou, S.-B.; Li, Q.-F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, V.; Sell, C.; Lorenzini, A. Obesity may accelerate the aging process. Front. Endocrinol. 2019, 10, 266. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; De Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Aging Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.; Haywood, N.J.; Matthews, C.; Cheema, H.; Wheatcroft, S.B. Insulin-like growth factor binding proteins and angiogenesis: From cancer to cardiovascular disease. Cytokine Growth Factor Rev. 2019, 46, 28–35. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radic. Biol. Med. 2021, 176, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Scicchitano, B.M.; Musarò, A. Signals from the niche: Insights into the role of IGF-1 and IL-6 in modulating skeletal muscle fibrosis. Cells 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Albiero, M.; Bonora, B.M.; Avogaro, A. Angiogenic abnormalities in diabetes mellitus: Mechanistic and clinical aspects. J. Clin. Endocrinol. Metab. 2019, 104, 5431–5444. [Google Scholar] [CrossRef]
- Schalkwijk, C.; Stehouwer, C. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; D’Adda di Fagagna, F. Cellular senescence in aging: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological aspects of selected myokines in skeletal muscle: Focus on aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Holly, J.M.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef] [PubMed]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, L.E.; Cordoba-Chacon, J.; Kineman, R.D. Growth Hormone and Insulin-Like Growth Factor 1 Regulation of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, 1812–1824. [Google Scholar] [CrossRef]
- Biadgo, B.; Tamir, W.; Ambachew, S. Insulin-like Growth Factor and its Therapeutic Potential for Diabetes Complications—Mechanisms and Metabolic Links: A Review. Rev. Diabet. Stud. 2020, 16, 24–34. [Google Scholar] [CrossRef]
- Elpers, A.-L.; Steptoe, A. Associations between dehydroepiandrosterone sulphate (DHEAS) and cognitive function in 5,061 older men and women in the English Longitudinal Study of Aging. Psychoneuroendocrinology 2020, 117, 104702. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Maiti, A.; Sinha, A.; Basu, A.; Das, T.C. Study of serum testosterone level in males with Prediabetes, before and after 3 months of metformin therapy, in patients attending a tertiary care hospital in West Bengal, Eastern India. J. Med. Sci. Clin. Res. 2019, 7, 32–43. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Van den Beld, A.W.; Kaufman, J.M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; Van der Lely, A.J. The physiology of endocrine systems with aging. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Hsieh, S.T.; Chen, C.L.; Yang, W.S.; Lee, P.C.; Lin, M.T.; Chen, C.N.; Yang, P.J. Circulating Diabetic Candidate Neurotrophic Factors, Brain-Derived Neurotrophic Factor and Fibroblast Growth Factor 21, in Sleeve Gastrectomy. Sci. Rep. 2020, 10, 5341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Aleksic, S.; Gao, T.; Weiss, E.F.; Demetriou, E.; Verghese, J.; Holtzer, R.; Barzilai, N.; Milman, S. Insulin-like Growth Factor-1 and IGF Binding Proteins Predict All-Cause Mortality and Morbidity in Older Adults. Cells 2020, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Betriu, À.; López-Cano, C.; Hernández, M.; Fernández, E.; Purroy, F.; Bermúdez-López, M.; Farràs-Sallés, C.; Barril, S.; Pamplona, R. Characteristics of atheromatosis in the prediabetes stage: A cross-sectional investigation of the ILERVAS project. Cardiovasc. Diabetol. 2019, 18, 154. [Google Scholar] [CrossRef]
- Mangge, H.; Herrmann, M.; Almer, G.; Zelzer, S.; Moeller, R.; Horejsi, R.; Renner, W. Telomere shortening associates with elevated insulin and nuchal fat accumulation. Sci. Rep. 2020, 10, 6863. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef]
- Reale, A.; Tagliatesta, S.; Zardo, G.; Zampieri, M. Counteracting aged DNA methylation states to combat aging and age-related diseases. Mech. Aging Dev. 2022, 206, 111695. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of Circulating Sex Hormones with Inflammation and Disease Severity in Patients with COVID-19. JAMA Netw. Open 2021, 4, e2111398. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification—A Narrative Review. Diabetes Ther. 2020, 11, 1271–1291. [Google Scholar] [CrossRef] [PubMed]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, anti-inflammatory, anti-obesogenic, and antidiabetic properties of tea polyphenols—The positive impact of regular tea consumption as an element of prophylaxis and pharmacotherapy support in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, S.; Wang, L.; Pan, T.; Zhong, X. The relationship between red blood cell distribution and islet β-cell function indexes in patients with type 2 diabetes. BMC Endocr. Disord. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Torres-Zegarra, C.; Sundararajan, D.; Benson, J.; Seagle, H.; Witten, M.; Walders-Abramson, N.; Simon, S.; Huguelet, P.; Nokoff, N.; Cree-Green, M. Care for adolescents with polycystic ovary syndrome: Development and prescribing patterns of a multidisciplinary clinic. J. Pediatr. Adolesc. Gynecol. 2021, 34, 617–625. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, X.; Zhou, J.; Qiu, K.; Deng, M.; Lin, Z.; Mosha, S.S.; Li, W. The association of serum cortisol level with microalbuminuria in patients with type 2 diabetes and prediabetes. Int. J. Med. Sci. 2020, 17, 2998. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.J.; Veras, K.; De Oliveira Carvalho, C.R. Dehydroepiandrosterone on metabolism and the cardiovascular system in the postmenopausal period. J. Mol. Med. 2020, 98, 39–57. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.-R.; Chen, K.-H. The Utilization of dehydroepiandrosterone as a sexual hormone precursor in premenopausal and postmenopausal women: An overview. Pharmaceuticals 2021, 15, 46. [Google Scholar] [CrossRef]
- Cappola, A.R.; Auchus, R.J.; El-Hajj Fuleihan, G.; Handelsman, D.J.; Kalyani, R.R.; McClung, M.; Stuenkel, C.A.; Thorner, M.O.; Verbalis, J.G. Hormones and Aging: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1835–1874. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstrom, W.J.; Palmert, M.R.; Corona, G.; Dohle, G.R. Paediatric and adult-onset male hypogonadism. Nat. Rev. Dis. Primers 2019, 5, 38. [Google Scholar] [CrossRef]
- Hur, M.-H.; Hong, J.H.; Yeo, S. Effects of aromatherapy on stress, fructosamine, fatigue, and sleep quality in prediabetic middle-aged women: A randomised controlled trial. Eur. J. Integr. Med. 2019, 31, 100978. [Google Scholar] [CrossRef]
- Bartz, D.; Chitnis, T.; Kaiser, U.B.; Rich-Edwards, J.W.; Rexrode, K.M.; Pennell, P.B.; Goldstein, J.M.; O’Neal, M.A.; LeBoff, M.; Behn, M. Clinical advances in sex-and gender-informed medicine to improve the health of all: A review. JAMA Intern. Med. 2020, 180, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, R.; Parrillo, L.; Longo, M.; Florese, P.; Desiderio, A.; Zatterale, F.; Miele, C.; Raciti, G.A.; Beguinot, F. Molecular basis of aging in chronic metabolic diseases. J. Endocrinol. Investig. 2020, 43, 1373–1389. [Google Scholar] [CrossRef] [PubMed]
- Lavillegrand, J.-R.; Garnier, M.; Spaeth, A.; Mario, N.; Hariri, G.; Pilon, A.; Berti, E.; Fieux, F.; Thietart, S.; Urbina, T. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: Inflammatory response of SARS-CoV-2 patients. Ann. Intensive Care 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.A.; Guillén-Nieto, G.E.; Rodríguez-Rodríguez, N.; Mendoza-Mari, Y.; Bringas-Vega, M.L.; Berlanga-Saez, J.O.; García del Barco Herrera, D.; Martinez-Jimenez, I.; Hernandez-Gutierrez, S.; Valdés-Sosa, P.A. Cellular senescence as the pathogenic hub of diabetes-related wound chronicity. Front. Endocrinol. 2020, 11, 573032. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Szűcs, G.; Sója, A.; Péter, M.; Sárközy, M.; Bruszel, B.; Siska, A.; Földesi, I.; Szabó, Z.; Janáky, T.; Vígh, L. Prediabetes induced by fructose-enriched diet influences cardiac lipidome and proteome and leads to deterioration of cardiac function prior to the development of excessive oxidative stress and cell damage. Oxidative Med. Cell. Longev. 2019, 2019, 3218275. [Google Scholar] [CrossRef]
- Peña, A.; Olson, M.L.; Ayers, S.L.; Sears, D.D.; Vega-López, S.; Colburn, A.T.; Shaibi, G.Q. Inflammatory Mediators and Type 2 Diabetes Risk Factors before and in Response to Lifestyle Intervention among Latino Adolescents with Obesity. Nutrients 2023, 15, 2442. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M. and Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. JAMDA 2013, 14, 877–882. [Google Scholar]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Liu, A. Chemokines in Prediabetes and Type 2 Diabetes: A Meta-Analysis. Front. Immunol. 2021, 12, 622438. [Google Scholar] [CrossRef] [PubMed]
- Avramovic, D.; Archaimbault, S.A.; Kemble, A.M.; Gruener, S.; Lazendic, M.; Westenskow, P.D. TGFβ1 Induces Senescence and Attenuated VEGF Production in Retinal Pericytes. Biomedicines 2022, 10, 1404. [Google Scholar] [CrossRef]
- Tan, Q.; Liang, N.; Zhang, X.; Li, J. Dynamic Aging: Channeled Through Microenvironment. Front. Physiol. 2021, 12, 702276. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, B.-t.; Wan, S.-r.; Ma, X.-m.; Long, Y.; Xu, Y.; Jiang, Z.-z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef]
- Khalil, R.; Diab-Assaf, M.; Lemaitre, J.-M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023, 12, 915. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in aging and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Drake, A.M.; Coughlan, M.T.; Christophersen, C.T.; Snelson, M. Resistant starch as a dietary intervention to limit the progression of diabetic kidney disease. Nutrients 2022, 14, 4547. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Tardif, J.-C.; Libby, P.; Camici, G.G. Inflamm-aging: The role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Elimam, H.; Abdulla, A.M.; Taha, I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Narasimhan, A.; Flores, R.R.; Robbins, P.D.; Niedernhofer, L.J. Role of cellular senescence in type II diabetes. Endocrinology 2021, 162, bqab136. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Tsoupras, A. The Impact of Nutrition and Statins on Cardiovascular Diseases; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Lainampetch, J.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Prangthip, P.; Soonthornworasiri, N.; Puduang, S.; Wechjakwen, N.; Kwanbunjan, K. Association of tumor necrosis factor alpha, interleukin 6, and C-reactive protein with the risk of developing type 2 diabetes: A retrospective cohort study of rural Thais. J. Diabetes Res. 2019, 2019, 9051929. [Google Scholar] [CrossRef]
- Randeria, S.N.; Thomson, G.J.; Nell, T.A.; Roberts, T.; Pretorius, E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Moshapa, F.T.; Riches-Suman, K.; Palmer, T.M. Therapeutic targeting of the proinflammatory IL-6-JAK/STAT signalling pathways responsible for vascular restenosis in type 2 diabetes mellitus. Cardiol. Res. Pract. 2019, 2019, 9846312. [Google Scholar] [CrossRef]
- Chi, C.; Li, D.-J.; Jiang, Y.-J.; Tong, J.; Fu, H.; Wu, Y.-H.; Shen, F.-M. Vascular smooth muscle cell senescence and age-related diseases: State of the art. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Ares, S.P.; Cardelo, M.M.; Gutiérrez-Mariscal, F.D.; Torres-Peña, J.; García-Rio, A.; Katsiki, N.M.; Malagón, M.; López-Miranda, J.; Pérez-Martínez, P.M.; Yubero-Serrano, E. Endothelial dysfunction and advanced glycation end products in patients with newly diagnosed versus established diabetes: From the CORDIOPREV study. Nutrients 2020, 12, 238. [Google Scholar] [PubMed]
- Biswas, I.; Khan, G.A. Endothelial dysfunction in cardiovascular diseases. In Basic and Clinical Understanding of Microcirculation; IntechOpen: Vienna, Austria, 2020; Volume 10. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial dysfunction: Is there a hyperglycemia-induced imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef]
- Marseglia, A.; Fratiglioni, L.; Kalpouzos, G.; Wang, R.; Bäckman, L.; Xu, W. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: A population-based cohort study. Alzheimer’s Dement. 2019, 15, 25–33. [Google Scholar] [CrossRef]
- Yan, C.; Zhou, Y.; Chen, Q.; Luo, Y.; Zhang, J.H.; Huang, H.; Shao, A. Dysfunction of the neurovascular unit in diabetes-related neurodegeneration. Biomed. Pharmacother. 2020, 131, 110656. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Borghini, A.; Andreassi, M.G. The molecular biomarkers of vascular aging and atherosclerosis: Telomere length and mitochondrial DNA4977 common deletion. Mutat. Res./Rev. Mutat. Res. 2020, 784, 108309. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; De Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Aging Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Prašnikar, E.; Borišek, J.; Perdih, A. Senescent cells as promising targets to tackle age-related diseases. Aging Res. Rev. 2021, 66, 101251. [Google Scholar] [CrossRef]
- Yeh, S.H.-H.; Shie, F.-S.; Liu, H.-K.; Yao, H.-H.; Kao, P.-C.; Lee, Y.-H.; Chen, L.-M.; Hsu, S.-M.; Chao, L.-J.; Wu, K.-W. A high-sucrose diet aggravates Alzheimer’s disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice. Neurobiol. Aging 2020, 90, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Song, J. The importance of BDNF and RAGE in diabetes-induced dementia. Pharmacol. Res. 2020, 160, 105083. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.B.; Infante-Garcia, C.; Weinstock, L.D.; Ramos-Rodriguez, J.J.; Hierro-Bujalance, C.; Fernandez-Ponce, C.; Wood, L.B.; Garcia-Alloza, M. Amyloid beta and diabetic pathology cooperatively stimulate cytokine expression in an Alzheimer’s mouse model. J. Neuroinflamm. 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Infante-Garcia, C.; Garcia-Alloza, M. Review of the effect of natural compounds and extracts on neurodegeneration in animal models of diabetes mellitus. Int. J. Mol. Sci. 2019, 20, 2533. [Google Scholar] [CrossRef] [PubMed]
- Maneechote, C.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Chronic Pharmacological Modulation of Mitochondrial Dynamics Alleviates Prediabetes-Induced Myocardial Ischemia–Reperfusion Injury by Preventing Mitochondrial Dysfunction and Programmed Apoptosis. Cardiovasc. Drugs Ther. 2023, 37, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Pisano, C.; Bertoldo, F.; Massoud, R.; Dolci, S.; Ruvolo, G. Red Blood Cell Distribution Width, Vascular Aging Biomarkers, and Endothelial Progenitor Cells for Predicting Vascular Aging and Diagnosing/Prognosing Age-Related Degenerative Arterial Diseases. Rejuvenation Res. 2019, 22, 399–408. [Google Scholar] [CrossRef]
- Pinna, A.; Carlino, P.; Serra, R.; Boscia, F.; Dore, S.; Carru, C.; Zinellu, A. Red Cell Distribution Width (RDW) and Complete Blood Cell Count-Derived Measures in Non-Arteritic Anterior Ischemic Optic Neuropathy. Int. J. Med. Sci. 2021, 18, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Joly-Amado, A.; Buee, L.; Vieau, D.; Blum, D. Overview on Tau and Tauopathies. Tau Biol. 2020, 1184, 259. [Google Scholar]
- Powrie, Y.S.L.; Smith, C. Central intracrine DHEA synthesis in aging-related neuroinflammation and neurodegeneration: Therapeutic potential? J. Neuroinflamm. 2018, 15, 289. [Google Scholar] [CrossRef]
- Thonusin, C.; Pantiya, P.; Sumneang, N.; Chunchai, T.; Nawara, W.; Arunsak, B.; Siri-Angkul, N.; Sriwichaiin, S.; Chattipakorn, S.C.; Chattipakorn, N. Effectiveness of high cardiorespiratory fitness in cardiometabolic protection in prediabetic rats. Mol. Med. 2022, 28, 31. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocr. 2014, 35, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yan, F.; Tian, J.; Qiao, A.; Yan, D. Potential clinical biomarkers and perspectives in diabetic cardiomyopathy. Diabetol. Metab. Syndr. 2023, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Naval, M.J.; Vargas-Soria, M.; Hierro-Bujalance, C.; Baena-Nieto, G.; Garcia-Alloza, M.; Infante-Garcia, C.; Del Marco, A. Alzheimer’s disease and diabetes: Role of diet, microbiota and inflammation in preclinical models. Biomolecules 2021, 11, 262. [Google Scholar] [CrossRef]
- Meng, J.-M.; Cao, S.-Y.; Wei, X.-L.; Gan, R.-Y.; Wang, Y.-F.; Cai, S.-X.; Xu, X.-Y.; Zhang, P.-Z.; Li, H.-B. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: An updated review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Carrageta, D.F.; Dias, T.R.; Alves, M.G.; Oliveira, P.F.; Monteiro, M.P.; Silva, B.M. Anti-obesity potential of natural methylxanthines. J. Funct. Foods 2018, 43, 84–94. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Lee, J.H.; Jahrling, J.B.; Denner, L.; Dineley, K.T. Targeting insulin for Alzheimer’s disease: Mechanisms, status and potential directions. J. Alzheimer’s Dis. 2018, 64, S427–S453. [Google Scholar] [CrossRef] [PubMed]
- Van Duinkerken, E.; Ryan, C.M. Diabetes mellitus in the young and the old: Effects on cognitive functioning across the life span. Neurobiol. Dis. 2020, 134, 104608. [Google Scholar] [CrossRef]
- Lee, S.Y.; Wang, J.; Chao, C.T.; Chien, K.L.; Huang, J.W. Frailty is associated with a higher risk of developing delirium and cognitive impairment among patients with diabetic kidney disease: A longitudinal population-based cohort study. Diabet. Med. 2021, 38, e14566. [Google Scholar] [CrossRef]
- Hayden, M.R. Type 2 diabetes mellitus increases the risk of late-onset Alzheimer’s disease: Ultrastructural remodeling of the neurovascular unit and diabetic gliopathy. Brain Sci. 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C.; Dias, J.P.; Santos, V.M.; Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, B.M. The action of polyphenols in diabetes mellitus and Alzheimer’s disease: A common agent for overlapping pathologies. Curr. Neuropharmacol. 2019, 17, 590–613. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.H.; Hegde, V. Obesity and diabetes mediated chronic inflammation: A potential biomarker in Alzheimer’s disease. J. Pers. Med. 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, T.J.; Gale, S.A.; McFeeley, B.M.; Nicastri, C.M.; Daffner, K.R. Promoting successful cognitive aging: A ten-year update. J. Alzheimer’s Dis. 2021, 81, 871–920. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.W.; Mack, J.J. Endothelial control of cerebral blood flow. Am. J. Pathol. 2021, 191, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Coco, C.; Sgarra, L.; Potenza, M.A.; Nacci, C.; Pasculli, B.; Barbano, R.; Parrella, P.; Montagnani, M. Can epigenetics of endothelial dysfunction represent the key to precision medicine in type 2 diabetes mellitus? Int. J. Mol. Sci. 2019, 20, 2949. [Google Scholar] [CrossRef]
- Johnson, J.; Jaggers, R.M.; Gopalkrishna, S.; Dahdah, A.; Murphy, A.J.; Hanssen, N.M.; Nagareddy, P.R. Oxidative stress in neutrophils: Implications for diabetic cardiovascular complications. Antioxid. Redox Signal. 2022, 36, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-reactive protein in diabetic inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The role of inflammation in diabetic retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; De la Higuera, M.; Frühbeck, G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [PubMed]
- Placha, D.; Jampilek, J. Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of obesity-induced inflammation on cardiovascular diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Bashir, H.; Bhat, S.A.; Majid, S.; Hamid, R.; Koul, R.K.; Rehman, M.U.; Din, I.; Bhat, J.A.; Qadir, J.; Masood, A. Role of inflammatory mediators (TNF-α, IL-6, CRP), biochemical and hematological parameters in type 2 diabetes mellitus patients of Kashmir, India. Med. J. Islam. Repub. Iran 2020, 34, 5. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble urokinase plasminogen activator receptor (suPAR) as a biomarker of systemic chronic inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging Dis. 2019, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, A.e.; Furman, C.; Landrieu, I.; Cantrelle, F.X.; Mortelecque, J.; Grolaux, G.; Gillery, P.; Tessier, F.; Lipka, E.; Billamboz, M. Development of Receptor for Advanced Glycation End Products (RAGE) ligands through target directed dynamic combinatorial chemistry: A novel class of possible antagonists. Chem.–A Eur. J. 2024, 20, e202303255. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Gao, Y.; Zeng, C.; Hua, R.; Guo, Y.; Wang, Y.; Wang, Z. Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Sci. Hum. Wellness 2024, 13, 1118–1134. [Google Scholar] [CrossRef]
- Soraci, L.; Corsonello, A.; Paparazzo, E.; Montesanto, A.; Piacenza, F.; Olivieri, F.; Gambuzza, M.E.; Savedra, E.V.; Marino, S.; Lattanzio, F. Neuroinflammaging: A Tight Line between Normal Aging and Age-Related Neurodegenerative Disorders. Aging Dis. 2024, 15, 2. [Google Scholar]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and human aging: Probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Moura, J.; Madureira, P.; Leal, E.; Fonseca, A.; Carvalho, E. Immune aging in diabetes and its implications in wound healing. Clin. Immunol. 2019, 200, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Wosinski, P.; Salazar-Noratto, G.E.; Bensidhoum, M.; Bizios, R.; Marashi, S.-A.; Potier, E.; Sheng, P.; Petite, H. Glucose Metabolism: Optimizing Regenerative Functionalities of Mesenchymal Stromal Cells Postimplantation. Tissue Eng. Part B Rev. 2023, 29, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, K.K.; Jiang, X.; Xu, A.; Cheng, K.K. The role of adipose tissue senescence in obesity-and aging-related metabolic disorders. Clin. Sci. 2020, 134, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Uttekar, B.; Chowdhary, S.; Rikhy, R. Mitochondria lead the way: Mitochondrial dynamics and function in cellular movements in development and disease. Front. Cell Dev. Biol. 2022, 9, 781933. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Nunes, S.; Rolo, A.P.; Reis, F.; Palmeira, C.M. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front. Physiol. 2019, 9, 1857. [Google Scholar] [CrossRef]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: An organ-based analysis. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Lamiral, Z.; Bakris, G.; Mehta, C.; White, W.B.; Zannad, F. Red cell distribution width in patients with diabetes and myocardial infarction: An analysis from the EXAMINE trial. Diabetes Obes. Metab. 2021, 23, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Arkew, M.; Gemechu, K.; Haile, K.; Asmerom, H. Red blood cell distribution width as novel biomarker in cardiovascular diseases: A literature review. J. Blood Med. 2022, 13, 413–424. [Google Scholar] [CrossRef]
- Kavvasoglu, B.; Akdemir, S.; Kurt, M. A routine but overlooked parameter for impaired glucose control: Red cell distribution width. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2431–2436. [Google Scholar] [PubMed]
- Zhou, W.; Sailani, M.R.; Contrepois, K.; Zhou, Y.; Ahadi, S.; Leopold, S.R.; Zhang, M.J.; Rao, V.; Avina, M.; Mishra, T. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 2019, 569, 663–671. [Google Scholar] [CrossRef]
- Gonzalez, A.; Deng, Y.; Lane, A.; Benkeser, D.; Cui, X.; Staimez, L.; Ford, C.; Khan, F.; Markley Webster, S.; Leong, A. Impact of mismatches in HbA1c vs glucose values on the diagnostic classification of diabetes and prediabetes. Diabet. Med. 2020, 37, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Bergenstal, R.M.; Cheng, P.; Kollman, C.; Carlson, A.L.; Johnson, M.L.; Rodbard, D. The relationships between time in range, hyperglycemia metrics, and HbA1c. J. Diabetes Sci. Technol. 2019, 13, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Sivamaruthi, B.S.; Chaiyasut, C. Do probiotics improve the health status of individuals with diabetes mellitus? A review on outcomes of clinical trials. BioMed Res. Int. 2019, 2019, 1531567. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, I.; Fujihara, Y.; Iwaya, C.; Kitajima, Y.; Tajima, M.; Honda, M.; Teruya, Y.; Asakawa, H.; Ito, T.; Eda, T. Low serum albumin, aspartate aminotransferase, and body mass are risk factors for frailty in elderly people with diabetes–a cross-sectional study. BMC Geriatr. 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.T.; Elbaih, A.H. Principles of intravenous fluids therapy. EC Emerg. Med. Crit. Care 2020, 4, 24–46. [Google Scholar]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Sasdelli, A.S.; Caraceni, P.; Marchesini, G.; Ravaioli, F. Nutrition in patients with type 2 diabetes: Present knowledge and remaining challenges. Nutrients 2021, 13, 2748. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in aging, health and diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Wang, J.; Maxwell, C.A.; Yu, F. Biological processes and biomarkers related to frailty in older adults: A state-of-the-science literature review. Biol. Res. Nurs. 2019, 21, 80–106. [Google Scholar] [CrossRef]
- Boachie, J.; Adaikalakoteswari, A.; Samavat, J.; Saravanan, P. Low vitamin B12 and lipid metabolism: Evidence from pre-clinical and clinical studies. Nutrients 2020, 12, 1925. [Google Scholar] [CrossRef]
- Tiwari, V.; Wilson, D.M. DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef]
- Dhar, G.A.; Saha, S.; Mitra, P.; Nag Chaudhuri, R. DNA methylation and regulation of gene expression: Guardian of our health. Nucl. 2021, 64, 259–270. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Wang, L.; Gao, Y.; Feng, G.; Li, G.; Zou, J.; Yu, M.; Li, Y.F.; Liu, C. Lipid metabolism dysfunction induced by age-dependent DNA methylation accelerates aging. Signal Transduct. Target. Ther. 2022, 7, 162. [Google Scholar] [CrossRef]
- Zhao, H.; Qi, C.; Zheng, C.; Gan, K.; Ren, L.; Song, G. Effects of glycated hemoglobin level on bone metabolism biomarkers in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 1785–1791. [Google Scholar] [CrossRef]
- Bellido, T.; Plotkin, L.I.; Bruzzaniti, A. Bone cells. In Basic and Applied Bone Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–55. [Google Scholar]
- Costantini, S.; Conte, C. Bone health in diabetes and prediabetes. World J. Diabetes 2019, 10, 421. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-osteoclast communication and bone homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Anabtawi, A.; Le, T.; Putman, M.; Tangpricha, V.; Bianchi, M.L. Cystic fibrosis bone disease: Pathophysiology, assessment and prognostic implications. J. Cyst. Fibros. 2019, 18, S48–S55. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Scott, D.; Ebeling, P.R. Exploring the links between common diseases of aging—Osteoporosis, sarcopenia and vascular calcification. Clin. Rev. Bone Miner. Metab. 2019, 17, 1–23. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Bleve, A.; Motta, F.; Durante, B.; Pandolfo, C.; Selmi, C.; Sica, A. Immunosenescence, inflammaging, and frailty: Role of myeloid cells in age-related diseases. Clin. Rev. Allergy Immunol. 2022, 64, 1–22. [Google Scholar] [CrossRef]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Loreni, F.; Mureddu, S.; Signoriello, G.; Scisciola, L.; Barbieri, M.; Rizzo, M.R. Pericoronary fat inflammation and Major Adverse Cardiac Events (MACE) in prediabetic patients with acute myocardial infarction: Effects of metformin. Cardiovasc. Diabetol. 2019, 18, 126. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, D.; Xiu, M.; Li, J.; Zhang, X.Y. Diabetes mellitus, cognitive deficits and serum BDNF levels in chronic patients with schizophrenia: A case-control study. J. Psychiatr. Res. 2021, 134, 39–47. [Google Scholar] [CrossRef] [PubMed]
- De Vincenti, A.P.; Ríos, A.S.; Paratcha, G.; Ledda, F. Mechanisms that modulate and diversify BDNF functions: Implications for hippocampal synaptic plasticity. Front. Cell. Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Sumbul-Sekerci, B.; Sekerci, A.; Pasin, O.; Durmus, E.; Yuksel-Salduz, Z.I. Cognition and BDNF levels in prediabetes and diabetes: A mediation analysis of a cross-sectional study. Front. Endocrinol. 2023, 14, 1120127. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Recent advances on the role of brain-derived neurotrophic factor (BDNF) in neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.J.; Khan, S.K.; Pappachan, J.M.; Jeeyavudeen, M.S. Diabetes and cognitive function: An evidence-based current perspective. World J. Diabetes 2023, 14, 92–109. [Google Scholar] [CrossRef]
- Amiri, N.; Fathei, M.; Mosaferi Ziaaldini, M. Effects of resistance training on muscle strength, insulin-like growth factor-1, and insulin-like growth factor–binding protein-3 in healthy elderly subjects: A systematic review and meta-analysis of randomized controlled trials. Hormones 2021, 20, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bailes, J.; Soloviev, M. Insulin-like growth factor-1 (IGF-1) and its monitoring in medical diagnostic and in sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. Role of IGF-1 system in the modulation of longevity: Controversies and new insights from a centenarians’ perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Mutavdzin, S.S.; Djuric, D.M. Homocysteine and Related B Vitamins in Pre-diabetes and Diabetes Mellitus. In Biochemistry of Cardiovascular Dysfunction in Obesity; Springer: Berlin/Heidelberg, Germany, 2020; pp. 329–351. [Google Scholar]
- Moretti, R.; Giuffre, M.; Caruso, P.; Gazzin, S.; Tiribelli, C. Homocysteine in neurology: A possible contributing factor to small vessel disease. Int. J. Mol. Sci. 2021, 22, 2051. [Google Scholar] [CrossRef]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef]
- Sondrup, N.; Termannsen, A.-D.; Eriksen, J.N.; Hjorth, M.F.; Færch, K.; Klingenberg, L.; Quist, J.S. Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2022, 62, 101594. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A. Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and type 2 diabetes: Role in inflammation, oxidative stress, and mitochondrial function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Thupakula, S.; Nimmala, S.S.R.; Ravula, H.; Chekuri, S.; Padiya, R. Emerging biomarkers for the detection of cardiovascular diseases. Egypt. Heart J. 2022, 74, 77. [Google Scholar] [CrossRef]
- Mariappan, V.; Srinivasan, R.; Pratheesh, R.; Jujjuvarapu, M.R.; Pillai, A.B. Predictive biomarkers for the early detection and management of heart failure. Heart Fail. Rev. 2023, 29, 331–353. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Ceriello, A.; Catrinoiu, D.; Chandramouli, C.; Cosentino, F.; Dombrowsky, A.C.; Itzhak, B.; Lalic, N.M.; Prattichizzo, F.; Schnell, O.; Seferović, P.M. Heart failure in type 2 diabetes: Current perspectives on screening, diagnosis and management. Cardiovasc. Diabetol. 2021, 20, 218. [Google Scholar] [CrossRef]
- Saraiva, R.M.M.d.S.d.S. Aging Biomarkers-A Global View. Doctoral Thesis, PhD- University of Coimbra, Coimbra, Portugal, 2021. Available online: https://hdl.handle.net/10316/98330 (accessed on 4 April 2024).
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as diagnostic biomarkers for cardiac dysfunction in both clinical and forensic medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, H.; Mügge, A.; Trampisch, H.J.; Scharnagl, H.; März, W.; Kara, K. NT-proBNP for risk prediction of cardiovascular events and all-cause mortality: The getABI-study. IJC Heart Vasc. 2020, 29, 100553. [Google Scholar] [CrossRef]
- Gao, P.; Zou, X.; Sun, X.; Zhang, C. Cellular senescence in metabolic-associated kidney disease: An update. Cells 2022, 11, 3443. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Komers, R. Pathophysiology of the diabetic kidney. Compr. Physiol. 2011, 1, 1175. [Google Scholar] [PubMed]
- Docherty, M.H.; Baird, D.P.; Hughes, J.; Ferenbach, D.A. Cellular senescence and senotherapies in the kidney: Current evidence and future directions. Front. Pharmacol. 2020, 11, 543955. [Google Scholar] [CrossRef]
- Chen, Y.; Kanwar, Y.S.; Chen, X.; Zhan, M. Aging and diabetic kidney disease: Emerging pathogenetic mechanisms and clinical implications. Curr. Med. Chem. 2024, 31, 697–725. [Google Scholar] [CrossRef]
- Baranowska-Jurkun, A.; Matuszewski, W.; Bandurska-Stankiewicz, E. Chronic microvascular complications in prediabetic states—An overview. J. Clin. Med. 2020, 9, 3289. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ha, X.; Yang, S.; Tian, X.; Jiang, H. Advances in understanding and treating diabetic kidney disease: Focus on tubulointerstitial inflammation mechanisms. Front. Endocrinol. 2023, 14, 1232790. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Ngubane, P.S.; Khathi, A. Investigating the Effects of Diet-Induced Pre-Diabetes on the Functioning of Calcium-Regulating Organs in Male Sprague Dawley Rats: Effects on Selected Markers. Front. Endocrinol 2022, 11, 13:914189. [Google Scholar] [CrossRef]
- Ma, H.; Liu, S.; Li, S.; Xia, Y. Targeting growth factor and cytokine pathways to treat idiopathic pulmonary fibrosis. Front. Pharmacol. 2022, 13, 918771. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N. Artificial intelligence and early detection of pancreatic cancer: 2020 summative review. Pancreas 2021, 50, 251. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, J.T.; Sun, P.; Wang, L.L.; Sun, L.Z.; Pang, S.G. Effects of lifestyle interventions on glucose regulation and diabetes risk in adults with impaired glucose tolerance or prediabetes: A meta-analysis. Arch. Endocrinol. Metab. 2022, 66, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef]
- Han, H. Clinical-community linkage strategies. In Applied Population Health Approaches for Asian American Communities, 1st ed.; John Wiley & Sons: Hoboken, NJ, Canada, 2022; p. 151. [Google Scholar]
- Justice, J.N.; Leng, I.; LeBrasseur, N.K.; Tchkonia, T.; Kirkland, J.L.; Mitin, N.; Liu, Y.; Kritchevsky, S.B.; Nicklas, B.J.; Ding, J. Caloric restriction intervention alters specific circulating biomarkers of the senescence-associated secretome in middle-aged and older adults with obesity and prediabetes in an 18-week randomized controlled trial. J. Gerontol. Ser. A 2024, 79, glad214. [Google Scholar] [CrossRef]
- Bönhof, G.J.; Herder, C.; Strom, A.; Papanas, N.; Roden, M.; Ziegler, D. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr. Rev. 2019, 40, 153–192. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, M.; González-González, M.; Martagón, A.J.; Hlavinka, V.; Willson, R.C.; Rito-Palomares, M. Recent developments in biomarkers for diagnosis and screening of type 2 diabetes mellitus. Curr. Diabetes Rep. 2022, 22, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Hillersdal, L. Aging biomarkers and the measurement of health and risk. Hist. Philos. Life Sci. 2021, 43, 28. [Google Scholar] [CrossRef]
- Akbulut, S. An assessment of serum vitamin B12 and folate in patients with Crohn’s disease. Medicine 2022, 101, e31892. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Z.-J.; Yu, X.; Wang, P. Dietary regulation in health and disease. Signal Transduct. Target. Ther. 2022, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Bottani, M.; Banfi, G.; Lombardi, G. The clinical potential of circulating miRNAs as biomarkers: Present and future applications for diagnosis and prognosis of age-associated bone diseases. Biomolecules 2020, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Paton, B.; Suarez, M.; Herrero, P.; Canela, N. Glycosylation biomarkers associated with age-related diseases and current methods for glycan analysis. Int. J. Mol. Sci. 2021, 22, 5788. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, N.; Krupa, R. Molecular Aspects of Senescence and Organismal Aging-DNA Damage Response, Telomeres, Inflammation and Chromatin. Int. J. Mol. Sci. 2021, 22, 590. [Google Scholar] [CrossRef]
- Jänig, W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).