Abstract
The roles of 17β estradiol (E2) and progesterone (P4), the primary female sex hormones, are pivotal in regulating various aspects of metabolism. E2 influences food intake, energy expenditure, adipose tissue distribution, and insulin sensitivity across multiple tissues. Meanwhile, P4 impacts energy expenditure, electrolyte balance, amino acid oxidation, muscle protein synthesis, and glucose metabolism. The interactions between these hormones affect macronutrient utilization, both at rest and during exercise. Acknowledging the need to incorporate sex hormone perturbations in research, this paper explores the current landscape of study design and menstrual cycle tracking for female-specific sport research. It emphasizes the importance of standardization in terminology, hormone phases, reference values, and affordable hormone detection methods to advance our understanding of how the menstrual cycle influences female athletes.
1. Introduction
The primary female sex hormones are 17B estradiol (E2), the predominant endogenous estrogen in humans, and progesterone (P4). E2 actions in hypothalamic nuclei differentially control food intake, energy expenditure, and white adipose tissue distribution [1,2,3,4]. E2 actions in skeletal muscle, liver, adipose tissue, and immune cells are involved in insulin sensitivity as well as the prevention of lipid accumulation and inflammation [4,5,6,7]. E2 actions in pancreatic islet β-cells also regulate insulin secretion, nutrient homeostasis, and survival [3,8,9]. Less is known about the specific mechanisms whereby progesterone exerts its metabolic influences; however, direct effects on energy expenditure through a progesterone-mediated increase in metabolic rate have been observed [10,11,12]. Additionally, progesterone alters serum electrolyte balance through P4-mediated increases in aldosterone [13,14,15,16], functions catabolically to increase amino acid oxidation and decrease muscle protein synthesis [17,18,19,20] and affects glucose metabolism through the upregulation of the facilitated diffusion glucose transporter 1 (GLUT1) to increase endometrial glycolytic metabolism, attenuating skeletal and hepatic glycolytic pathways [21,22,23,24]. Consequently, both hormones exert agonistic and antagonistic effects on metabolism and nutrient needs whereby the ratios and levels of E2 and P4 affect the proportions of macronutrients used as fuel, not only at rest but also during exercise [25].
Therefore, to effectively conduct research in pre-menopausal eumenorrheic female athletes, the perturbation of the sex hormones should be included in the methodology [26,27]. Yet due to the historical perspective and dogma around scientific design, as well as sociocultural discrepancies around funding, leadership positions, and the overall gender-data gap, female-specific research has not been performed or has not been executed well. Specifically, in sport and biomedical research, only 6% of human performance research focuses on women, often with methodology applied through the male lens, with female participation and outcomes investigated post hoc [28,29].
Early research using female participants, intentionally or unintentionally, often neglected to account for the effect of fluctuating hormone profiles (E2 and P4) [30] over the course of the menstrual cycle (MC) on exercise or substrate metabolism [31,32,33,34,35,36,37,38,39]. Sport performance studies would include females with males regardless of the female’s MC phase or combine eumenorrheic participants with hormonal contraceptive users [31,40]. In instances where MC phase validation was attempted, the method(s) such as counting cycle days, luteinising hormone testing, and body temperature, in retrospect, may potentially have been inadequate when used as the only method of verification.
A better awareness of the female hormone profile throughout the MC and a greater participation in high performance sports [41] has driven an emphasis to better understand how the menstrual phase influences biological females during exercise. As a result, an attempt to develop improved research methods for female athletes, including the validation and standardization of menstrual cycle tracking [42], has followed. The purpose of this review is to evaluate the current practices and obstacles of menstrual cycle tracking in sports research.
2. The Menstrual Cycle
The MC and its systemic effect on the body is a crucial area for research as it has been found that women frequently experience different adaptations and stress responses to their male counterparts [43,44]. For eumenorrheic women, the MC is characterized by fluctuations in several hormones, most notably the gonadal steroids, E2 and P4, and can be partitioned into the following six phases: early follicular, late follicular ovulation, early luteal, mid-luteal, and late luteal phases, Figure 1. Throughout each phase, fluctuations in hormones trigger not only changes in the reproductive system but also across a range of tissues throughout the body, which can have a direct effect on stress resilience, metabolism, and adaptations [45]. As a brief review, the length of a normal menstrual cycle is 21 to 40 days [46]. However, the length of a complete MC is not consistent. Its duration can be shortened or lengthened by a variety of factors such as energy balance, diet, exercise, disease state, pregnancy, stress, hormonal contraceptives, hormone therapies, and medication. The first half of the MC comprises the menstrual and follicular phases, during which time E2 levels are low (early follicular/menstrual), then they rise (mid follicular), and peak (late follicular), ending with the periovulatory phase in which follicular-stimulating hormone and luteinizing hormone reach peak concentrations. After ovulation, the second half of the cycle comprises the early luteal (during which time the E2 level drops and then rises, while P4 rises), the mid-luteal (during which time E2 and P4 levels peak), and finally, the late luteal phase (during which time E2 and P4 levels fall). These cyclic hormone changes can affect several physical and psychological attributes and may ultimately influence sports performance, although the effects are highly individual [45,47,48].
3. Female Hormone Impact on Sport Physiology
A complete review of endogenous hormonal effects on female physiology is beyond the scope of this article. Comprehensive reviews on the full scope of endogenous hormone effects are available [49,50]. However, their effect on key aspects of sport physiology is the primary assertion for menstrual cycle tracking and phase verification when conducting sport research in female participants.
Unfortunately, existing research in female applied sport and exercise sciences has often neglected to address the status of ovarian hormones on observed outcomes. These shortcomings are rooted in insufficient methodologies that fail to consider the impact of hormone fluctuations across distinct phases of the menstrual cycle. Such oversights can include but are not limited to the inclusion of participants based solely on the presence of a regularly occurring menstrual cycle, failure to document the menstrual phase, the use of only one method of menstrual phase verification and failing to control for participants with menstrual dysfunction. Furthermore, conducting large studies in elite female athletes, whose sport performance may be the most impacted by the variation in ovarian hormones across the MC, is difficult due to small study populations and the higher prevalence of menstrual dysfunction (up to 61%) in this specific population across many sporting disciplines [51]. To improve the quality of studies focused on female athletes, it is important to acknowledge the impact ovarian hormones have on physiological function, as well as provide a reasonable framework for future study design within lab and applied settings.
It has been suggested that most of the current research has been limited to comparisons of the distinct hormonal phases follicular (FOL) and luteal (LUT) without taking into consideration the moments of peak hormone levels found in the late FOL and mid LUT phases [30]. To improve the accuracy of the study design, an optimal testing strategy consisting of repeated serum assessments of E2 and P4 based on timings derived from the LH hormone and calendar tracking has been proposed [52]. Additionally, it is recommended that luteal phase testing take place 7–9 days post LH surge detection to capture adequate P4 levels to ensure the exclusion of anovulatory participants [52].
The introduction of this framework provides a gold standard for testing; however, its implementation in many settings is problematic. Here, we present a scoping review of existing phase verification techniques and their combined use to inform future methodologies in a wider variety of applied sporting environments.
3.1. Energy Metabolism
Energy metabolism during submaximal intensities when fasting is different between the sexes as evidenced by lower resting expiratory ratios (RER). Tarnopolsky and colleagues [31] observed a 7% difference in mean RER between males and females (0.94 and 0.87, respectively) during 15 km of running at 65% VO2max. Similar were the observations of Carter and colleagues [36] during 90 min of cycling at 60% peak O2. These differences are attributed to E2’s possible upregulation of fat breakdown through alterations in hormone and enzyme levels [53]. The exact mechanism is unknown; however, it is widely accepted that E2’s impact on liver function is directly responsible for females preferentially burning more fat (~7%) as a total percentage of energy expenditure than males at submaximal intensities (40–70%) of maximal aerobic capacity (VO2max) [31,34,36,38,54]. Further distinctions in energy metabolism exist among just females across the MC phases as evidenced by greater carbohydrate (CHO) utilization (~25%) during fasted submaximal efforts in the FOLL phase [35,37,38,55]. However, these differences in CHO oxidation between the phases do not appear to exist in a fed state and are negated when exogenous CHO is introduced [55].
Less clear is the role of P4 on energy metabolism. Observations in the literature allude to inconsistencies in the role of P4 in energy metabolism and how its influence is manifested singularly or in combination with E2 [54,56]. For example, Oosthuyse 2010 [25] asserted, citing rat model studies, that E2 co-administered with P4 suppressed the availability of glucose-impacted substrate metabolism during exercise. This observation was duplicated in human studies by Hackney and Devries who reported that muscle glycogen utilization was lower during exercise in the LUT phase despite P4 dominance in the hormone profile [35,38]. In contrast, D’Eon et al. [56] demonstrated via exogenous hormone manipulation in exercising females that high levels of P4 added to a high E2 environment reversed the glycogen sparing effect of E2, which may have resulted from the differences in hormone ratios created and/or the influence of synthetic exogenous hormones acting differently than naturally occurring endogenous forms. It is unclear whether the absolute level of E2 and P4 or the ratio of these hormone concentrations is the primary influencer of metabolism. Elevated levels of P4 are associated with the variability of luteal phase duration [46,57], while an increase in E2 to P4 ratio has been observed in some studies, but not all, to positively impact time to exhaustion during submaximal endurance exercise [25]. Both the concentration and ratio of these hormones are highly variable across the MC as well as between individuals [57], thus performance and study outcomes could be influenced by biological diversity.
While differences between male and female energy metabolism are well documented, a collection of studies shows no differences in energy metabolism between the MC phases. Casazza (2004) and Suh 2002 reported similar RER values in the FOL and LUT phases during submaximal 45 and 65% VO2max efforts. These reports independently concluded that substrate utilization is less determined by ovarian hormone profiles over the MC and is affected to a greater extent by exercise intensity and CHO availability [58,59]. Similarly, a comprehensive review of substrate metabolism by Boisseau et al. in 2021 reached a consensus that most studies do not detect a difference in energy metabolism between the menstrual phases; however, they did question the study environments from which the current literature is based [60]. Specifically, the authors of the review asserted that the literature was potentially biased to high-intensity efforts that negate fat oxidation benefits found in mid-LUT phases during protracted sporting disciplines (ultra-endurance) performed predominantly below <75% of VO2max.
3.2. Hydration and Fluid Balance
Fluctuating levels of E2 and P4 may exert an influence on the complex matrix of organ systems, hormone messengers, and neural triggers responsible for the management of fluid balance [61]. The exact impact these hormones is yet to be elucidated. Early studies were unable to isolate E2 or P4 to assess individual effects and relied upon oral contraceptives which provided hormone at levels 6–10-times that of regular endogenous production, but did observe lowered thirst stimulation and decreased osmotic threshold of the free water-regulating hormone arginine vasopressin when E2 was administered [62]. More recent studies utilizing gonadotropin-releasing hormone (GnRH) to manipulate hormone profiles have allowed MC sex hormones to be observed in isolation [62]. The results of these studies on fluid compartment distribution at rest indicate that plasma volume is increased by E2 and decreased by P4 [62] through oncotic (protein colloid concentrations) and hydrostatic (fluid diffusion via pressure gradients) regulatory mechanisms [63,64]. These mechanisms result in shifts of fluid between the intravascular and interstitial ECF compartments, but not an overall volume increase. These hormonally driven fluid shifts may be superseded by fluid balance mechanisms associated with exercise that drive fluid out of intravascular spaces into working muscle [65]. Interestingly, repeated observations by Stachenfeld et al. indicate MC hormones have only a small effect on total body water [62,66].
Not all studies detected changes in fluid balance. A blood lactate study conducted by McCracken, Ainsworth, and Hackney in 1994 indirectly observed no differences in hematocrit between mid-follicular and mid-luteal phases among nine eumenorrheic physically active females exercising between 20 and 60% VO2max during a continuous incremental treadmill challenge to exhaustion [67]. These data would indicate no significant changes in plasma volume associated with hormone profile and agrees with a larger review by Rodriguez-Giustiniani, Rodriguez-Sanchez, and Galloway [68]. More controlled testing and comparisons between distinct phases of the MC, such as late FOL phase where plasma volume is elevated [69], may be warranted due to the changing concentrations of protein colloids between phases and their relationship in human fluid dynamics [64,70].
Since most of the current sports hydration recommendations are derived from male studies, these hormonal influences may be relevant in a sport setting because women tend to have lower plasma volume, less extracellular fluid, and lower levels of absolute body water when compared with men [71].
3.3. Menstrual Phase Terminology
As illustrated early, leaders in the field of female research agree that verifying MC phase is critical to study design and the interpretation of results [26,27,72]. This verification becomes especially relevant when attempting to compare differences between the phases in any experimental study. One potential barrier to research lies in the varying interpretations of the distinctly different hormonal profiles by researchers. For example, three to seven phases have been used by various authors to differentiate distinct hormone profiles of the MC, Table 1. When compared, various phase nomenclatures can encompass one or more of the suggested hormone profiles causing inconsistencies in reporting and confusion in the literature, Figure 1.

Table 1.
Menstrual Phase Terminology and Alignment.
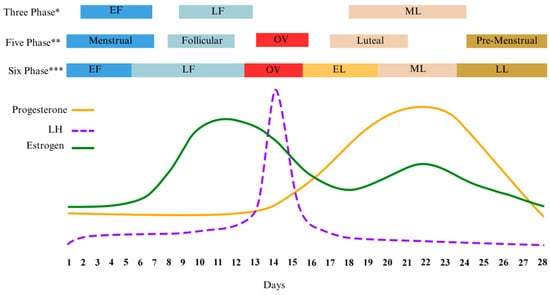
Figure 1.
Diagram of the different menstrual cycle phases found in the literature and overlaid with corresponding ovarian hormone levels. Adapted from McNulty [73]. * [27]; ** [18]; *** [73]. Early Follicular (EF); Late Follicular (LF); Early Luteal (EL); Mid Luteal (ML); Late Luteal (LL); Luteinizing Hormone (LH).
Figure 1.
Diagram of the different menstrual cycle phases found in the literature and overlaid with corresponding ovarian hormone levels. Adapted from McNulty [73]. * [27]; ** [18]; *** [73]. Early Follicular (EF); Late Follicular (LF); Early Luteal (EL); Mid Luteal (ML); Late Luteal (LL); Luteinizing Hormone (LH).
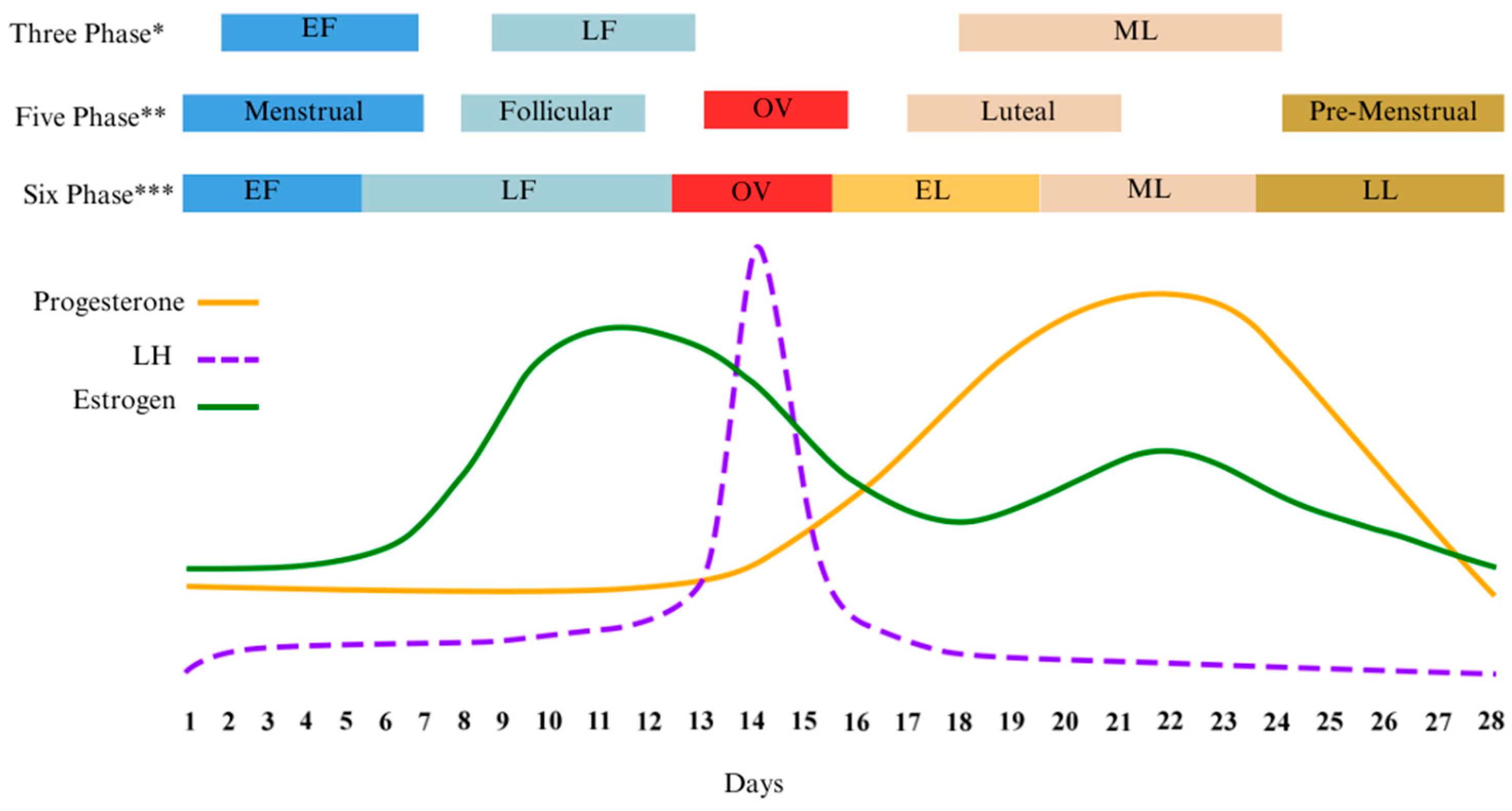
Low hormone or high hormone characterizations are also commonly used to differentiate the phases of the menstrual cycle. In comparative studies, these low and high phases are most often the target windows for study protocols as they represent the most noticeable differences in hormone profiles. Early FOL is associated with low E2 and P “low phase” while mid-LUT exhibits high E2 and P “high phase” [26,52]. More recently, Elliot-Sale and colleagues deviated from the conventional utilization of FOL and LUT for describing the menstrual cycle. Their aim was to move away from what the author characterizes as frequently ambiguous, wherein the phases are connected to indistinct or undefined ovarian steroid profiles. Instead, they recognized discrete phases within the menstrual cycle that correspond to average concentrations of E2 and P, designating these phases as 1–4 [26].
The alignment of specific MC phases to set days in a 28-day period of time is unlikely in the real world. This is partly due to the variability of the MC across individuals. Two extensive studies, observing 1060 and 612,613 total cycles, respectively, both concluded that the average MC lasts a total of 28 days. Furthermore, they identified the follicular phase to be the most volatile phase and responsible for the high degree of MC variability [46,74]. The average length of the FOL phase was 16.5 ± 3.4 and 16.9 ± 5.3, respectively, as compared with LUT 12.4 ± 2 and 12.4 ± 2.4 [46,74]. In both studies, the majority of all cycles were reported to last between 22 and 36 (28.9 ± 3.9) [46] or 25 and 30 (29.3 ± 5.2) [74] days in length.
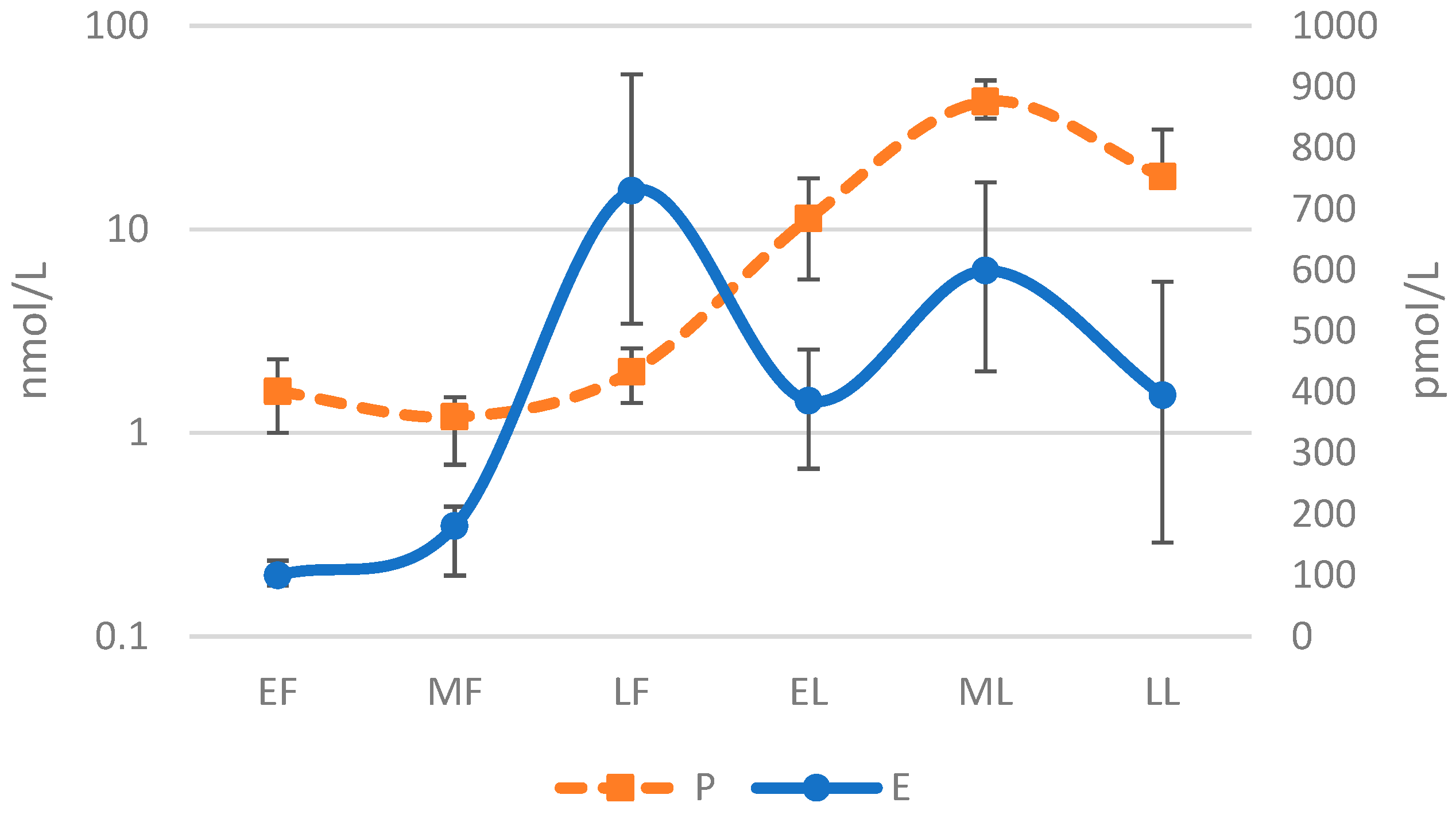
It should also be noted that ovarian hormone concentrations and their ratios also display high levels of inter-individual variability within each distinct phase. Dam and colleagues collected serum hormone levels in 40 females (n = 10 contraceptive users; n = 30 eumenorrheic) across six phases of the menstrual cycle. The median and interquartile data for E2 and P illustrate the considerable inter-individual differences in hormone levels within each distinct phase, Figure 2 [75]. Similar to the variation in hormone levels across the MC is the variability among individuals for which no clear set of reference value has been established at each phase of the MC for specific study populations, such as athletes who may have different hormone profiles than the average female. This variability is indicated by the observations of De Souza et al. that P values are lower and LUT phase length shorter in female runners when compared with sedentary populations [76].
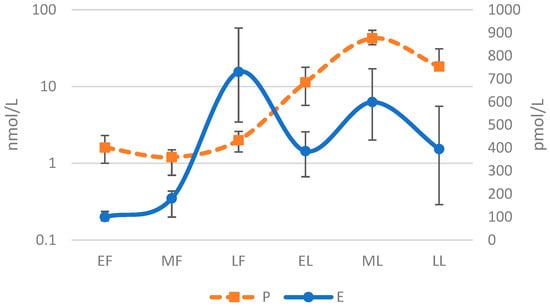
Figure 2.
Median and Interquartile Levels for Estrogen and Progesterone Across Six Phase Menstrual Cycle [75]. Early Follicular (EF); Mid Follicular (MF); Late Follicular (LF); Early Luteal (EL); Mid Luteal (ML); Late Luteal (LL); Progesterone (P); Estrogen (E). Log-10 applied to y-axis and demonstrates a sinusoidal wave pattern.
4. Methodologies Past and Present
The impact of the MC on sport performance is unclear. Inconsistencies in testing methodology, specifically MC phase verification, may be contributing to the lack of clarity [42]. Methods of validation can be divided into two group types (hormone profile or point of ovulation), providing a spectrum of effectiveness and practicality summarized in Table 2. Generally, methods with high utility, such as salivary ferning, cervical mucus, and basal body temperature, which are low cost and simple to use, tend to have lower levels of accuracy, except for urinary luteinizing hormone urine strips, rendering them unreliable individually. Furthermore, these methods are limited to determining the window of ovulation and provide no insight into ovarian hormone concentrations.

Table 2.
Summary of Menstrual Cycle Verification Methods.
4.1. Hormone Profile Validation
Venous hormone verification, the measure of blood serum E2 and P4, omits any predictive element and is the gold standard for determining hormone status by providing an exact snapshot in time [52,77]. Reference levels of E2 and P4 have been established for phases of the MC in a recent study by Stricker et al. [57] yet no consensus exists on minimum values for inclusion [42]. A wide range for the minimum level of P4 (2–16 nmol/L) has been recommended to properly exclude menstrual abnormalities such as LPD or anovulation [52].
4.2. Point of Ovulation Validation
In contrast, point of ovulation methods (PO) require some form of prediction or estimation by counting forwards of backwards from ovulation. Different methods of PO provide varying levels of accuracy, but all are subject to the variability of phase length.
4.2.1. Follicular Monitoring
The use of ultrasound to track the development of follicle size is a direct method of follicular monitoring, and with 80% accuracy is considered the gold standard for the detection of ovulation [78,79,80,81].
4.2.2. Salivary Ferning
The crystallization of NaCl in dried saliva occurs during the ovulation period creating a distinct pattern referred to as “ferning”. This pattern is created by the increase in E2 and directly mirrors ferning found with cervical smears. Less invasive and readily available, saliva samples present a low-cost detection method for ovulation but do require the use of a microscope [82]. An overall accuracy of 42–53% indicates the need to pair with other methods [79].
4.2.3. Counting Days
Establishes Day One of the MC and the beginning of the FOL phase with the appearance of bleeding. It retrospectively indicates the end of an MC with the start of the following cycle’s menses. Relies upon estimation to determine intra-phase lengths. Useful tool for determining total cycle length but varies widely in accuracy precluding the prediction of ovulation 18–59% [52,77].
4.2.4. Menstrual Cycle Tracking Apps
Most applications only provide a window of fertility with large variability between applications (4–12 days) and do predict an exact ovulation date. As expected, the probability of ovulation occurring in the fertility window increased with window length. Those applications providing ovulation dates did so at an accuracy of 21% or less [83].
4.2.5. Basal Body Temperature (BBT)
Temperature increases of 0.3 °C can occur during the LUT phase but do not happen in all females [52]. This rise in temperature can be used to determine the end of ovulation/fertility windows; however, menstrual irregularity can lower the accuracy of body temperature changes [79]. Additional confounding factors such as alcohol, stress, and climate can also influence BBT [79]. Due to a low cost with no technical expertise required, this method of PO is widely used. However, it is not viewed by all as a reliable predictor of ovulation, with a reported 22% rate of accuracy [84].
4.2.6. Urinary Luteinising Hormone (LH)
LH is a glycoprotein hormone that increases in the blood serum 35–44 h prior to ovulation with peak levels occurring 10–12 h prior to ovulation [79]. It can be measured in urine with low-cost strips, has high usability outside lab environments, and requires no technical knowledge. This method is highly accurate at predicting ovulation within a 48 h window [85]; however, there is potential for a LH surge to be detected without ovulation in infertile women [86]. Described as “the most validated method for estimating ovulation” [87], it has demonstrated to possess 100% congruence with ultrasonography, the gold standard [88].
4.2.7. Cervical Mucus
Cervical mucus can be used to determine ovulation with a 48.3–75.9% accuracy [79]. It involves the visual and tactile inspection of secreted vaginal fluid, from the lower part of the uterus, for consistency and color changes that occur near ovulation in response to changing levels of E2. Most notably, the rise in E2 before ovulation stimulates peak-type mucus characterized as clear, stretchy, and slippery indicating a strong probability of successful ovulation [89].
4.3. Combined Methods
Due to the variability of hormonal profiles across the female lifespan, no current single detection method, except for serum hormone testing, is adequate to verify where in the MC a female lies [26]. Due to the cost of serum hormone testing, strategies to minimize blood draws have been introduced. Schaumberg et al. have suggested a validation method for the “high hormone” mid-LUT phase with a reported accuracy of 90% in normally menstruating, physically active females [42]. This method utilized three different validation modalities. First, calendar tracking was implemented for three months to establish menstrual patterning i.e., average total MC length from which a day counting strategy could be structured. Second, LH hormone testing for seven consecutive days was completed using urinary strips to establish ovulation, which was approximated at two days after the LH surge. The mid-LUT phase was then estimated to start 6–8 days after ovulation. Third, a venous serum hormone assay was taken 6–12 days post confirmed LH surge. The Mid-LUT phase was validated through P serum levels of >6 ng/mL to exclude luteal phase deficiency [42].
Various studies have utilized combined methods of menstrual tracking which did not employ serum hormone testing as part of the protocol. Specific to research in female sports, De Jonge et al. has compiled a comprehensive list of these studies showing basal body temperature, urinary ovulation kits, and calendar-based counting as the most common [52]. Rogan and Black, while researching dietary intakes across the MC, similarly found several female studies verifying MC phase through a combination of methods that did not include hormone testing [90]. Both groups of authors, in line with others [26,77], concluded that the absence of serum hormone testing allowed for the inclusion of study participants with deficient levels of P in the luteal phase, which could skew results.
5. Obstacles to Phase Validation
The repeated venous blood sampling of endogenous E2 and P4, the costliest approach, appears to be the only method of pin-pointing the exact hormone profile of female study participants and at this time, it is also the only method that can be used independently. Therefore, the implementation of multiple repeated PO validation methods is required to limit the required blood draws, as validated by Schaumberg [42]. However, this method still requires costly serum hormone testing.
Testing procedures aside, existing inconsistencies in phase terminology can confuse study design and data comparisons across studies. Elliot-Sale and colleagues have recommended moving away from debating phase nomenclature to focus on the adoption of specific time points that represent hormone profiles that can be reliably studied and compared [26]. With the obvious variability of MC and, in particular, the early follicular phase length, it would seem prudent to focus on stability where it exists. Recent unpublished work by Francis et al. [91] alludes to such windows of hormone stability, identified as the late follicular, early luteal, and late luteal, which may provide optimal study opportunities. Briefly, the MC would be divided into two primary phases (FOL and LUT, differentiated by the signature LH peak and comprising three or more distinct hormone profiles characterized by physiologically relevant hormone levels with relative stability. However, it is important to note that the phases with more consistent lengths do not correspond to the periods of highest or lowest hormone levels, nor do they include the phases where the difference between hormones E2 and P4 is most pronounced. These phases, like the late follicular and mid-luteal phases, are the ones that may possess the most physiological significance.
Furthermore, beyond the > 6 ng/mL criteria for P4 used to confirm ovulation [42], there is an absence of similar relevant sports physiology reference values for E2 and P4 among the general population or sport-specific cohorts. This gap or lack of comprehensive benchmarks hinders the understanding of what hormone levels impart an influence on sports physiology. Additionally, as women experience physiological changes linked to training, the consequent effects on hormone levels remain challenging to monitor consistently across time without baseline reference values.
6. Future of Menstrual Cycle Tracking
The identification of gaps in study design and validation methodologies within the existing body of female performance research highlights the need for standardization. Further research would benefit from the creation of universal terminology as well as the standardization of distinct hormone phases, the establishment of hormone reference values, and the creation of low-cost, point-of-care detection devices for E2 and P4 which would greatly improve the validity of MC phase comparisons. A possible solution may come from the integration of the observations by Draper et al. [18] on hormone rhythmicity and the summary of hormone variability by Stricker et al. [57] along with the phase classification system presented by Elliot-Sale and colleagues [26]. The addition of this framework could aid researchers in navigating the hormone variability throughout an individual’s entire MC, including intra-phase durations. These suggestions could improve the reliability of research outcomes and cross study comparisons. However, they minimize the windows of testing availability and would rely on the obtainability of low-cost detection methods to expand research opportunity to large cohort and field studies. Until that time arrives, the requirement for ongoing female-focused research remains crucial. Thoughtfully constructed experiments that uphold rigorous population controls and inclusion/exclusion parameters, while utilizing a variety and/or combination of predictive outcome techniques such as basal body temperature tracking, urine LH strips, and calendar monitoring, ought to be acknowledged, especially in field studies: an area lacking adequate representation in the literature. However, it is advisable to approach their findings with consideration.
Author Contributions
Conceptualization, A.D. and S.T.S.; writing—original draft preparation, A.D.; writing—review and editing, S.T.S. and M.B.; supervision, S.T.S. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hevener, A.L.; Ribas, V.; Moore, T.M.; Zhou, Z. The Impact of Skeletal Muscle ERα on Mitochondrial Function and Metabolic Health. Endocrinology 2020, 161, bqz017. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Seeley, R.J.; Clegg, D.J. Sexual Differences in the Control of Energy Homeostasis. Front. Neuroendocrinol. 2009, 30, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The Role of Estrogen in Adipose Tissue Metabolism: Insights into Glucose Homeostasis Regulation. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Brown, L.M.; Clegg, D.J. The Role of Hypothalamic Estrogen Receptors in Metabolic Regulation. Front. Neuroendocrinol. 2014, 35, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.L.; Zhou, Z.; Drew, B.G.; Ribas, V. The Role of Skeletal Muscle Estrogen Receptors in Metabolic Homeostasis and Insulin Sensitivity. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1043, pp. 257–284. [Google Scholar]
- Hevener, A.L.; Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V. The Impact of ERα Action on Muscle Metabolism and Insulin Sensitivity—Strong Enough for a Man, Made for a Woman. Mol. Metab. 2018, 15, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ropero, A.B.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A. The Role of Estrogen Receptors in the Control of Energy and Glucose Homeostasis. Steroids 2008, 73, 874–879. [Google Scholar] [CrossRef]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired Estrogen Receptor Action in the Pathogenesis of the Metabolic Syndrome. Mol. Cell. Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef]
- Chen, J.Q.; Brown, T.R.; Russo, J. Regulation of Energy Metabolism Pathways by Estrogens and Estrogenic Chemicals and Potential Implications in Obesity Associated with Increased Exposure to Endocrine Disruptors. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 1128–1143. [Google Scholar] [CrossRef]
- Howe, J.C.; Rumpler, W.V.; Seale, J.L. Energy Expenditure by Indirect Calorimetry in Premenopausal Women: Variation within One Menstrual Cycle. J. Nutr. Biochem. 1993, 4, 268–273. [Google Scholar] [CrossRef]
- Zhang, S.; Osumi, H.; Uchizawa, A.; Hamada, H.; Park, I.; Suzuki, Y.; Tanaka, Y.; Ishihara, A.; Yajima, K.; Seol, J.; et al. Changes in Sleeping Energy Metabolism and Thermoregulation during Menstrual Cycle. Physiol. Rep. 2020, 8, e14353. [Google Scholar] [CrossRef]
- Benton, M.J.; Hutchins, A.M.; Dawes, J.J. Effect of Menstrual Cycle on Resting Metabolism: A Systematic Review and Metaanalysis. PLoS ONE 2020, 15, e0236025. [Google Scholar] [CrossRef]
- Chapman, A.B.; Zamudio, S.; Woodmansee, W.; Merouani, A.; Osorio, F.; Johnson, A.; Moore, L.G.; Dahms, T.; Coffin, C.; Abraham, W.T.; et al. Systemic and Renal Hemodynamic Changes in the Luteal Phase of the Menstrual Cycle Mimic Early Pregnancy. Am. J. Physiol. Ren. Physiol. 1997, 273, F777–F782. [Google Scholar] [CrossRef]
- Szmuilowicz, E.D.; Adler, G.K.; Williams, J.S.; Green, D.E.; Yao, T.M.; Hopkins, P.N.; Seely, E.W. Relationship between Aldosterone and Progesterone in the Human Menstrual Cycle. J. Clin. Endocrinol. Metab. 2006, 91, 3981–3987. [Google Scholar] [CrossRef]
- Pechère-Bertschi, A.; Maillard, M.; Stalder, H.; Brunner, H.R.; Burnier, M. Renal Segmental Tubular Response to Salt during the Normal Menstrual Cycle. Kidney Int. 2002, 61, 425–431. [Google Scholar] [CrossRef]
- Olson, B.R.; Forman, M.R.; Lanza, E.; McAdam, P.A.; Beecher, G.; Kimzey, L.M.; Campbell, W.S.; Raymond, E.G.; Brentzel, S.L.; Güttsches-Ebeling, B. Relation between Sodium Balance and Menstrual Cycle Symptoms in Normal Women. Ann. Intern. Med. 1996, 125, 564–567. [Google Scholar] [CrossRef]
- Landau, R.L.; Lugibihl, K. The Effect of Progesterone on Amino Acid Metabolism. J. Clin. Endocrinol. Metab. 1961, 21, 1355–1363. [Google Scholar] [CrossRef]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual Cycle Rhythmicity: Metabolic Patterns in Healthy Women. Sci. Rep. 2018, 8, e14568. [Google Scholar] [CrossRef]
- Faustmann, G.; Meinitzer, A.; Magnes, C.; Tiran, B.; Obermayer-Pietsch, B.; Gruber, H.J.; Ribalta, J.; Rock, E.; Roob, J.M.; Winklhofer-Roob, B.M. Progesterone-Associated Arginine Decline at Luteal Phase of Menstrual Cycle and Associations with Related Amino Acids and Nuclear Factor KB Activation. PLoS ONE 2018, 13, e0200489. [Google Scholar] [CrossRef] [PubMed]
- Kriengsinyos, W.; Wykes, L.J.; Goonewardene, L.A.; Ball, R.O.; Pencharz, P.B. Phase of Menstrual Cycle Affects Lysine Requirement in Healthy Women. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E489–E496. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Febbraio, M.A. Effect of the Ovarian Hormones on GLUT4 Expression and Contraction-Stimulated Glucose Uptake. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1139–E1146. [Google Scholar] [CrossRef] [PubMed]
- Flannery, C.A.; Choe, G.H.; Cooke, K.M.; Fleming, A.G.; Radford, C.C.; Kodaman, P.H.; Jurczak, M.J.; Kibbey, R.G.; Taylor, H.S. Insulin Regulates Glycogen Synthesis in Human Endometrial Glands through Increased GYS2. J. Clin. Endocrinol. Metab. 2018, 103, 2843–2850. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, J.; Wang, Y.; Sun, J.; Li, Z.; Sui, L.; Fan, J.; Liu, C.; Shang, Y.; Kong, L.; et al. Progesterone Regulates Glucose Metabolism Through Glucose Transporter 1 to Promote Endometrial Receptivity. Front. Physiol. 2020, 11, 543148. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of Glucose Metabolism from a Liver-Centric Perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism: Implications for Exercise Performance in Eumenorrhoeic Women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Women as Participants: A Working Guide for Standards of Practice for Research on Women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef]
- Janse De Jonge, X.A.K. Effects of the Menstrual Cycle on Exercise Performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- Paul, R.W.; Sonnier, J.H.; Johnson, E.E.; Hall, A.T.; Osman, A.; Connors, G.M.; Freedman, K.B.; Bishop, M.E. Inequalities in the Evaluation of Male Versus Female Athletes in Sports Medicine Research: A Systematic Review. Am. J. Sports Med. 2023, 51, 3335–3342. [Google Scholar] [CrossRef]
- Deldicque, L. Editorial: Women in Elite Sports and Performance Enhancement: 2021. Front. Sports Act. Living 2022, 4, 999969. [Google Scholar] [CrossRef]
- Sims, S.T.; Heather, A.K. Myths and Methodologies: Reducing Scientific Design Ambiguity in Studies Comparing Sexes and/or Menstrual Cycle Phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, L.J.; MacDougall, J.D.; Atkinson, S.A.; Tarnopolsky, M.A.; Sutton, J.R. Gender Differences in Substrate for Endurance Exercise. J. Appl. Physiol. 1990, 68, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; McCracken-Compton, M.A.; Ainsworth, B. Substrate Responses to Submaximal Exercise in the Midfollicular and Midluteal Phases of the Menstrual Cycle. Int. J. Sport Nutr. 1994, 4, 299–308. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Atkinson, S.A.; Phillips, S.M.; MacDougall, J.D. Carbohydrate Loading and Metabolism during Exercise in Men and Women. J. Appl. Physiol. 1995, 78, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.J.; Pagliassotti, M.J.; Hobbs, K.; Hill, J.O. Fuel Metabolism in Men and Women during and after Long-Duration Exercise. J. Appl. Physiol. 1998, 85, 1823–1832. [Google Scholar] [CrossRef]
- Hackney, A.C. Influence of Oestrogen on Muscle Glycogen Utilization during Exercise. Acta Physiol. Scand. 1999, 167, 273–274. [Google Scholar] [CrossRef]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate Utilization during Endurance Exercise in Men and Women after Endurance Training. Am. J. Physiol. Endocrinol. Metab. 2001, 280, 898–907. [Google Scholar] [CrossRef]
- Zderic, T.W.; Coggan, A.R.; Ruby, B.C. Glucose Kinetics and Substrate Oxidation during Exercise in the Follicular and Luteal Phases. J. Appl. Physiol. 2001, 90, 447–453. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual Cycle Phase and Sex Influence Muscle Glycogen Utilization and Glucose Turnover during Moderate-Intensity Endurance Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 1120–1128. [Google Scholar] [CrossRef]
- Stannard, S.R.; Buckley, A.J.; Edge, J.A.; Thompson, M.W. Adaptations to Skeletal Muscle with Endurance Exercise Training in the Acutely Fed versus Overnight-Fasted State. J. Sci. Med. Sport 2010, 13, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Miotto, P.M.; McGlory, C.; Holloway, T.M.; Phillips, S.M.; Holloway, G.P. Sex Differences in Mitochondrial Respiratory Function in Human Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R909–R915. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.S. Female Athletes, Women’s Sport, and the Sport Media Commercial Complex: Have We Really “Come a Long Way, Baby”? Sport Manag. Rev. 2015, 18, 331–342. [Google Scholar] [CrossRef]
- Schaumberg, M.A.; Jenkins, D.G.; Janse de Jonge, X.A.K.; Emmerton, L.M.; Skinner, T.L. Three-Step Method for Menstrual and Oral Contraceptive Cycle Verification. J. Sci. Med. Sport 2017, 20, 965–969. [Google Scholar] [CrossRef]
- Ansdell, P.; Thomas, K.; Hicks, K.M.; Hunter, S.K.; Howatson, G.; Goodall, S. Physiological Sex Differences Affect the Integrative Response to Exercise: Acute and Chronic Implications. Exp. Physiol. 2020, 105, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Oydanich, M.; Babici, D.; Zhang, J.; Rynecki, N.; Vatner, D.E.; Vatner, S.F. Mechanisms of Sex Differences in Exercise Capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R832–R838. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Ware, L.; Capodilupo, E.R. Patterns of Endogenous and Exogenous Ovarian Hormone Modulation on Recovery Metrics across the Menstrual Cycle. BMJ Open Sport Exerc. Med. 2021, 7, e001047. [Google Scholar] [CrossRef]
- Fehring, R.J.; Schneider, M.; Raviele, K. Variability in the Phases of the Menstrual Cycle. JOGNN—J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Bruinvels, G.; Hackney, A.C.; Pedlar, C.R. Menstrual Cycle: The Importance of Both the Phases and the Transitions Between Phases on Training and Performance. Sports Med. 2022, 52, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Bruinvels, G.; Goldsmith, E.; Blagrove, R.; Simpkin, A.; Lewis, N.; Morton, K.; Suppiah, A.; Rogers, J.P.; Ackerman, K.E.; Newell, J.; et al. Prevalence and Frequency of Menstrual Cycle Symptoms Are Associated with Availability to Train and Compete: A Study of 6812 Exercising Women Recruited Using the Strava Exercise App. Br. J. Sports Med. 2021, 55, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, T.K.; Braunstein, G.D. Physiological Effects of Androgens in Women. In Androgen Excess Disorders in Women; Humana Press: Totowa, NJ, USA, 2007; pp. 49–62. [Google Scholar]
- Farage, M.A.; Neill, S.; MacLean, A.B. Physiological Changes Associated with the Menstrual Cycle a Review. Obstet. Gynecol. Surv. 2009, 64, 58–72. [Google Scholar] [CrossRef]
- Gimunová, M.; Paulínyová, A.; Bernaciková, M.; Paludo, A.C. The Prevalence of Menstrual Cycle Disorders in Female Athletes from Different Sports Disciplines: A Rapid Review. Int. J. Environ. Res. Public Health 2022, 19, 14243. [Google Scholar] [CrossRef]
- de Jonge, X.J.; Thompson, B.; Ahreum, H.A.N. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef]
- Kendrick, Z.V.; Ellis, G.S. Effect of Estradiol on Tissue Glycogen Metabolism and Lipid Availability in Exercised Male Rats. J. Appl. Physiol. 1991, 71, 1694–1699. [Google Scholar] [CrossRef]
- Devries, M.C. Sex-Based Differences in Endurance Exercise Muscle Metabolism: Impact on Exercise and Nutritional Strategies to Optimize Health and Performance in Women. Exp. Physiol. 2016, 101, 243–249. [Google Scholar] [CrossRef]
- Campbell, S.E.; Angus, D.J.; Febbraio, M.A. Glucose Kinetics and Exercise Performance during Phases of the Menstrual Cycle: Effect of Glucose Ingestion. Am. J. Physiol. Endocrinol. Metab. 2001, 281, 817–825. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Sharoff, C.; Chipkin, S.R.; Grow, D.; Ruby, B.C.; Braun, B. Regulation of Exercise Carbohydrate Metabolism by Estrogen and Progesterone in Women. Am. J. Physiol. Endocrinol. Metab. 2002, 283, 1046–1055. [Google Scholar] [CrossRef]
- Stricker, R.; Eberhart, R.; Chevailler, M.C.; Quinn, F.A.; Bischof, P.; Stricker, R. Establishment of Detailed Reference Values for Luteinizing Hormone, Follicle Stimulating Hormone, Estradiol, and Progesterone during Different Phases of the Menstrual Cycle on the Abbott ARCHITECT® Analyzer. Clin. Chem. Lab. Med. 2006, 44, 883–887. [Google Scholar] [CrossRef]
- Casazza, G.A.; Jacobs, K.A.; Suh, S.H.; Miller, B.F.; Horning, M.A.; Brooks, G.A. Menstrual Cycle Phase and Oral Contraceptive Effects on Triglyceride Mobilization during Exercise. J. Appl. Physiol. 2004, 97, 302–309. [Google Scholar] [CrossRef]
- Suh, S.H.; Casazza, G.A.; Horning, M.A.; Miller, B.F.; Brooks, G.A. Luteal and Follicular Glucose Fluxes during Rest and Exercise in 3-h Postabsorptive Women. J. Appl. Physiol. 2002, 93, 42–50. [Google Scholar] [CrossRef]
- Boisseau, N.; Isacco, L. Substrate Metabolism during Exercise: Sexual Dimorphism and Women’s Specificities. Eur. J. Sport Sci. 2022, 22, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Wenner, M.M.; Stachenfeld, N.S. Sex Hormones and Environmental Factors Affecting Exercise. In Sex Hormones, Exercise and Women: Scientific and Clinical Aspects; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 151–170. ISBN 9783319445588. [Google Scholar]
- Stachenfeld, N.S. Sex Hormone Effects on Body Fluid Regulation. Exerc. Sport Sci. Rev. 2008, 36, 152. [Google Scholar] [CrossRef] [PubMed]
- Tollan, A.; Kvenild, K.; Strand, H.; Øian, P.; Maltau, J.M. Increased Capillary Permeability for Plasma Proteins in Oral Contraceptive Users. Contraception 1992, 45, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Øian, P.; Tollan, A.; Fadnes, H.O.; Noddeland, H.; Maltau, J.M. Transcapillary Fluid Dynamics during the Menstrual Cycle. Am. J. Obstet. Gynecol. 1987, 156, 952–955. [Google Scholar] [CrossRef]
- Steinach, M.; Lichti, J.; Maggioni, M.A.; Fähling, M. A Fluid Shift for Endurance Exercise-Why Hydration Matters. Acta Physiol. 2019, 227, e13347. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; Keefe, D.L. Estrogen Effects on Osmotic Regulation of AVP and Fluid Balance. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E711–E721. [Google Scholar] [CrossRef]
- McCracken, M.; Ainsworth, B.; Hackney, A.C. Effects of the Menstrual Cycle Phase on the Blood Lactate Responses to Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Giustiniani, P.; Rodriguez-Sanchez, N.; Galloway, S.D.R. Fluid and Electrolyte Balance Considerations for Female Athletes. Eur. J. Sport Sci. 2021, 22, 697–708. [Google Scholar] [CrossRef]
- Sims, S.T.; Rehrer, N.J.; Bell, M.L.; Cotter, J.D. Preexercise Sodium Loading Aids Fluid Balance and Endurance for Women Exercising in the Heat. J. Appl. Physiol. 2007, 103, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.; Lui, F. Physiology, Colloid Osmotic Pressure. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Retzlaff, J.A.; Tauxe, W.N.; Kiely, J.M.; Stroebel, C.F. Erythrocyte Volume, Plasma Volume, and Lean Body Mass in Adult Men and Women. Blood 1969, 33, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Cable, N.T.; Elliott, K.J. The Influence of Reproductive Hormones on Muscle Strength. Biol. Rhythm Res. 2010, 35, 235–244. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Bull, J.R.; Rowland, S.P.; Scherwitzl, E.B.; Scherwitzl, R.; Danielsson, K.G.; Harper, J. Real-World Menstrual Cycle Characteristics of More than 600,000 Menstrual Cycles. NPJ Digit. Med. 2019, 2, 83. [Google Scholar] [CrossRef]
- Dam, T.V.; Dalgaard, L.B.; Sevdalis, V.; Bibby, B.M.; Janse De Jonge, X.; Gravholt, C.H.; Hansen, M. Muscle Performance during the Menstrual Cycle Correlates with Psychological Well-Being, but Not Fluctuations in Sex Hormones. Med. Sci. Sports Exerc. 2022, 54, 1678–1689. [Google Scholar] [CrossRef]
- De Souza, M.J.; Miller, B.E.; Loucks, A.B.; Luciano, A.A.; Pescatello, L.S.; Campbell, C.G.; Lasley, B.L. High Frequency of Luteal Phase Deficiency and Anovulation in Recreational Women Runners: Blunted Elevation in Follicle-Stimulating Hormone Observed during Luteal-Follicular Transition1. J. Clin. Endocrinol. Metab. 1998, 83, 4220–4232. [Google Scholar] [CrossRef]
- Wideman, L.; Montgomery, M.M.; Levine, B.J.; Beynnon, B.D.; Shultz, S.J. Accuracy of Calendar-Based Methods for Assigning Menstrual Cycle Phase in Women. Sports Health 2013, 5, 143–149. [Google Scholar] [CrossRef]
- Kyei-Mensah, A.; Maconochie, N.; Zaidi, J.; Pittrof, R.; Campbell, S.; Tan, S.L. Transvaginal Three-Dimensional Ultrasound: Reproducibility of Ovarian and Endometrial Volume Measurements. Fertil. Steril. 1996, 66, 718–722. [Google Scholar] [CrossRef]
- Su, H.; Yi, Y.; Wei, T.; Chang, T.; Cheng, C. Detection of Ovulation, a Review of Currently Available Methods. Bioeng. Transl. Med. 2017, 2, 238–246. [Google Scholar] [CrossRef]
- Ecochard, R.; Boehringer, H.; Rabilloud, M.; Marret, H. Chronological Aspects of Ultrasonic, Hormonal, and Other Indirect Indices of Ovulation. BJOG 2001, 108, 822–829. [Google Scholar] [CrossRef]
- Queenan, J.T.; O’Brien, G.D.; Bains, L.M.; Simpson, J.; Collins, W.P.; Campbell, S. Ultrasound Scanning of Ovaries to Detect Ovulation in Women. Fertil. Steril. 1980, 34, 99–105. [Google Scholar] [CrossRef]
- Sruthi Priya, B.; Pushpaja, M.; Siva Kumar, A.V.; Maruthy, K.N. Does the Salivary Fern Pattern Determine Fertile Period in Reproductive Female? Clin. Epidemiol. Glob. Health 2020, 8, 698–701. [Google Scholar] [CrossRef]
- Johnson, S.; Marriott, L.; Zinaman, M. Can Apps and Calendar Methods Predict Ovulation with Accuracy? Curr. Med. Res. Opin. 2018, 34, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.L.; Fehring, R.J. Basal Body Temperature Assessment: Is It Useful to Couples Seeking Pregnancy? MCN Am. J. Matern./Child Nurs. 2005, 30, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Berglund Scherwitzl, E.; Lindén Hirschberg, A.; Scherwitzl, R. Identification and Prediction of the Fertile Window Using NaturalCycles. Eur. J. Contracept. Reprod. Health Care 2015, 20, 403–408. [Google Scholar] [CrossRef]
- Killick, S.; Elstein, M. Pharmacologic Production of Luteinized Unruptured Follicles by Prostaglandin Synthetase Inhibitors. Fertil. Steril. 1987, 47, 773–777. [Google Scholar] [CrossRef]
- Blake, K.R.; Dixson, B.J.W.; O’Dean, S.M.; Denson, T.F. Standardized Protocols for Characterizing Women’s Fertility: A Data-Driven Approach. Horm. Behav. 2016, 81, 74–83. [Google Scholar] [CrossRef]
- Guida, M.; Tommaselli, G.A.; Palomba, S.; Pellicano, M.; Moccia, G.; Di Carlo, C.; Nappi, C. Efficacy of Methods for Determining Ovulation in a Natural Family Planning Program. Fertil. Steril. 1999, 72, 900–904. [Google Scholar] [CrossRef]
- Najmabadi, S.; Schliep, K.C.; Simonsen, S.E.; Porucznik, C.A.; Egger, M.J.; Stanford, J.B. Cervical Mucus Patterns and the Fertile Window in Women without Known Subfertility: A Pooled Analysis of Three Cohorts. Hum. Reprod. 2021, 36, 1784–1795. [Google Scholar] [CrossRef]
- Rogan, M.M.; Black, K.E. Dietary Energy Intake across the Menstrual Cycle: A Narrative Review. Nutr. Rev. 2023, 81, 869–886. [Google Scholar] [CrossRef]
- Francis, G.; Keay, N. Analysis of the Variability in the Timing of Physiological Events in the Menstrual Cycle. Researchgate 2023, 1–13, in press. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).