The Difference between Male and Female Rats in Terms of Freezing and Aversive Ultrasonic Vocalization in an Active Avoidance Test
Abstract
1. Introduction
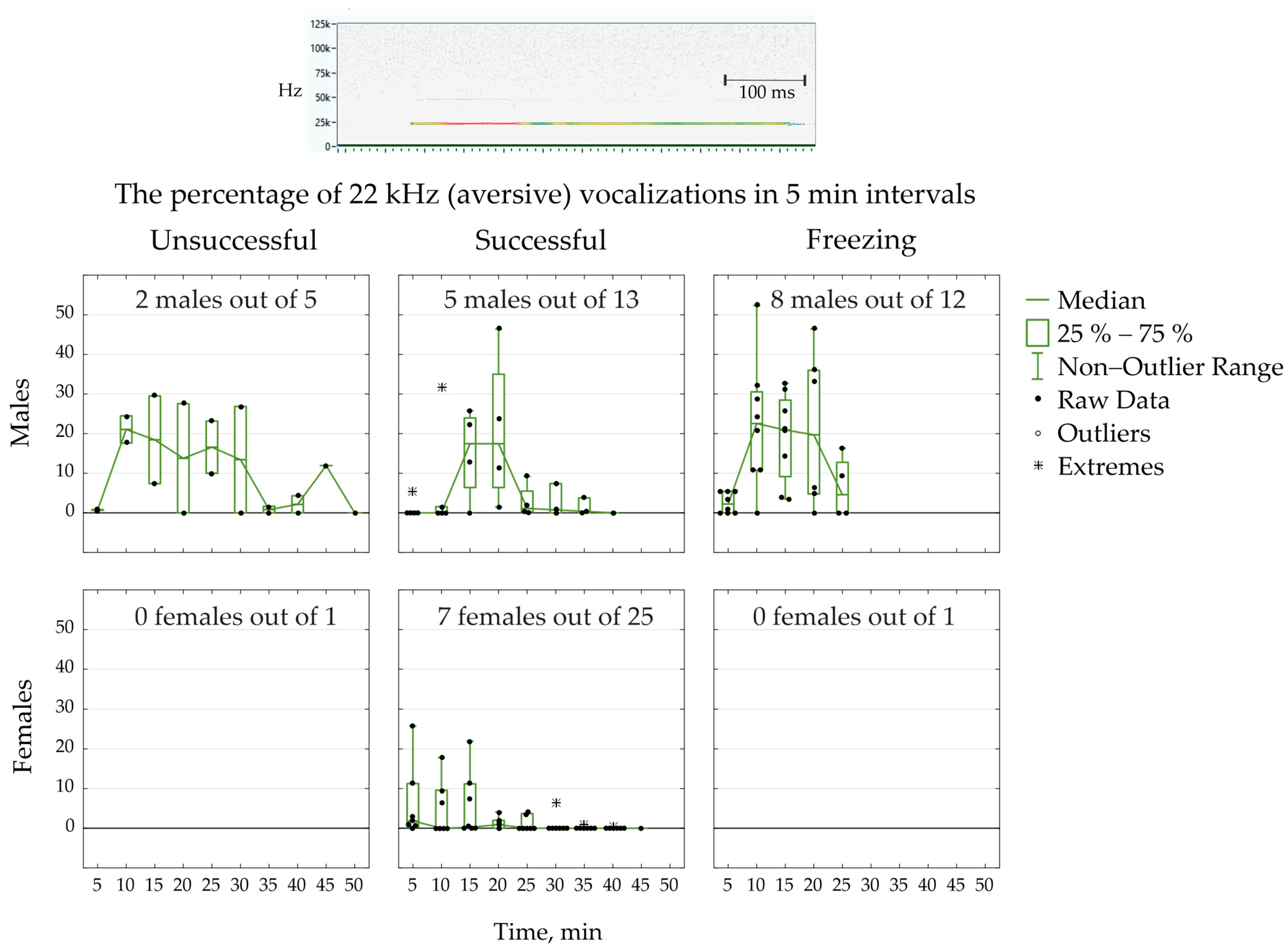
2. Results
- Successfully learned avoidance: The rat was trained to move to the “safe” chamber during tone stimulus (before electric stimulation) in five out of six trails.
- Unsuccessful learning: The rat showed less than five avoidances in a sequence of six trails.
- Freezing reaction: The rat stayed immobile, usually in a corner, and endured electrical stimulation. The test was stopped when the rat stayed immobile during 20 trails.
3. Discussion
3.1. Sex Differences in Performance of the Active Avoidance Test
- Males more likely experienced freezing (40% from males) than females (3.7% from females).
- Only 43.2% of males successfully learned the task, in contrast to females, who performed significantly better than males (93% of females successfully learned the task).
3.2. Ultrasonic Vocalization
3.3. Aversive Ultrasonic Vocalization and Freezing Behavior
3.4. Neural Substrates Underlying Active Avoidance
3.5. WAG/Rij and NEW Rats
3.6. Limitations and Further Directions
4. Materials and Methods
4.1. Active Avoidance Test
4.2. Ultrasound Recording and Analysis
4.3. The Estrous Cycle
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Boeke, E.A.; Moscarello, J.M.; LeDoux, J.E.; Phelps, E.A.; Hartley, C.A. Active Avoidance: Neural Mechanisms and Attenuation of Pavlovian Conditioned Responding. J. Neurosci. 2017, 37, 4808–4818. [Google Scholar] [CrossRef]
- Diehl, M.M.; Bravo-Rivera, C.; Quirk, G.J. The Study of Active Avoidance: A Platform for Discussion. Neurosci. Biobehav. Rev. 2019, 107, 229–237. [Google Scholar] [CrossRef]
- Ball, T.M.; Gunaydin, L.A. Measuring Maladaptive Avoidance: From Animal Models to Clinical Anxiety. Neuropsychopharmacology 2022, 47, 978–986. [Google Scholar] [CrossRef]
- Krypotos, A.-M.; Effting, M.; Kindt, M.; Beckers, T. Avoidance Learning: A Review of Theoretical Models and Recent Developments. Front. Behav. Neurosci. 2015, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Riccio, D.C.; Rohrbaugh, M.; Hodges, L.A. Developmental Aspects of Passive and Active Avoidance Learning in Rats. Dev. Psychobiol. 1968, 1, 108–111. [Google Scholar] [CrossRef]
- López-Moraga, A.; Beckers, T.; Luyten, L. The Effects of Stress on Avoidance in Rodents: An Unresolved Matter. Front. Behav. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Okawara, T.; Kawakami, K.; Ohta, R.; Kawaguchi, M. Alterations between High and Low-Avoidance Lines of Hatano Rats in Learning Behaviors, Ultrasonic Vocalizations, and Histological Characteristics in Hippocampus and Amygdala. Physiol. Behav. 2022, 245, 113670. [Google Scholar] [CrossRef]
- Brush, F.R.; Baron, S.; Froehlich, J.C.; Ison, J.R.; Pellegrino, L.J.; Phillips, D.S.; Sakellaris, P.C.; Williams, V.N. Genetic Differences in Avoidance Learning by Rattus Norvegicus: Escape/Avoidance Responding, Sensitivity to Electric Shock, Discrimination Learning, and Open-Field Behavior. J. Comp. Psychol. 1985, 99, 60–73. [Google Scholar] [CrossRef]
- Sangha, S.; Diehl, M.M.; Bergstrom, H.C.; Drew, M.R. Know Safety, No Fear. Neurosci. Biobehav. Rev. 2020, 108, 218–230. [Google Scholar] [CrossRef]
- Beckers, T.; Krypotos, A.M.; Boddez, Y.; Effting, M.; Kindt, M. What’s Wrong with Fear Conditioning? Biol. Psychol. 2013, 92, 90–96. [Google Scholar] [CrossRef]
- Louvart, H.; Maccari, S.; Lesage, J.; Léonhardt, M.; Dickes-Coopman, A.; Darnaudéry, M. Effects of a Single Footshock Followed by Situational Reminders on HPA Axis and Behaviour in the Aversive Context in Male and Female Rats. Psychoneuroendocrinology 2006, 31, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Cotton, M.M.; Benger, A.; Fyfe, L.; Moorehouse, K.; Russell, R. Learned Helplessness in Shuttlebox-Avoidance Behavior. Psychol. Rep. 1982, 51, 215–221. [Google Scholar] [CrossRef]
- Dalla, C.; Edgecomb, C.; Whetstone, A.S.; Shors, T.J. Females Do Not Express Learned Helplessness like Males Do. Neuropsychopharmacology 2008, 33, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Clinton, S.M.; Jackson, N.L.; Kerman, I.A. Learned Helplessness and Social Avoidance in the Wistar-Kyoto Rat. Front. Behav. Neurosci. 2014, 8, 109. [Google Scholar] [CrossRef]
- Ilango, A.; Shumake, J.; Wetzel, W.; Ohl, F.W. Contribution of Emotional and Motivational Neurocircuitry to Cue-Signaled Active Avoidance Learning. Front. Behav. Neurosci. 2014, 8, 107871. [Google Scholar] [CrossRef][Green Version]
- Ramirez, F.; Moscarello, J.M.; LeDoux, J.E.; Sears, R.M. Active Avoidance Requires a Serial Basal Amygdala to Nucleus Accumbens Shell Circuit. J. Neurosci. 2015, 35, 3470–3477. [Google Scholar] [CrossRef]
- Choi, J.-S.; Cain, C.K.; LeDoux, J.E. The Role of Amygdala Nuclei in the Expression of Auditory Signaled Two-Way Active Avoidance in Rats. Learn. Mem. 2010, 17, 139–147. [Google Scholar] [CrossRef]
- Moscarello, J.M.; LeDoux, J.E. Active Avoidance Learning Requires Prefrontal Suppression of Amygdala-Mediated Defensive Reactions. J. Neurosci. 2013, 33, 3815–3823. [Google Scholar] [CrossRef]
- Wang, J.; Bast, T.; Wang, Y.-C.; Zhang, W.-N. Hippocampus and Two-Way Active Avoidance Conditioning: Contrasting Effects of Cytotoxic Lesion and Temporary Inactivation. Hippocampus 2015, 25, 1517–1531. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Biological Functions of Rat Ultrasonic Vocalizations, Arousal Mechanisms, and Call Initiation. Brain Sci. 2021, 11, 605. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Communication of Adult Rats by Ultrasonic Vocalization: Biological, Sociobiological, and Neuroscience Approaches. ILAR J. 2009, 50, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Pniak, A. Social Contacts and Production of 50-KHz Short Ultrasonic Calls in Adult Rats. J. Comp. Psychol. 2002, 116, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J. Ultrasonic Vocalizations of Rats (Rattus norvegicus) during Mating, Play, and Aggression: Behavioral Concomitants, Relationship to Reward, and Self-Administration of Playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M. Principles of Rat Communication: Quantitative Parameters of Ultrasonic Calls in Rats. Behav. Genet. 2005, 35, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Fletcher, N.H. Rat Ultrasonic Vocalization: Short-Range Communication. In Handbook of Mammalian Vocalization; Brudzynski, S.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2010; pp. 69–76. ISBN 978-0-12-374593-4. [Google Scholar]
- Brudzynski, S. Pharmacology of Ultrasonic Vocalizations in Adult Rats: Significance, Call Classification and Neural Substrate. Curr. Neuropharmacol. 2015, 13, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M. Emission of 22 KHz Vocalizations in Rats as an Evolutionary Equivalent of Human Crying: Relationship to Depression. Behav. Brain Res. 2019, 363, 1–12. [Google Scholar] [CrossRef]
- Cuomo, V.; Cagiano, R.; De Salvia, M.A.; Mazzoccoli, M.; Persichella, M.; Renna, G. Ultrasonic Vocalization as an Indicator of Emotional State during Active Avoidance Learning in Rats. Life Sci. 1992, 50, 1049–1055. [Google Scholar] [CrossRef]
- Pupikina, M.; Sitnikova, E. Sex Differences in Behavior and Learning Abilities in Adult Rats. Life 2023, 13, 547. [Google Scholar] [CrossRef]
- Machado Figueiredo, R.; de Carvalho, M.C.; Brandão, M.L.; Lovick, T.A. Short-Term, Low-Dose Fluoxetine Prevents Oestrous Cycle-Linked Increase in Anxiety-like Behaviour in Female Rats. J. Psychopharmacol. 2019, 33, 548–557. [Google Scholar] [CrossRef]
- Coenen, A.M.L.; Van Luijtelaar, E.L.J.M. Genetic Animal Models for Absence Epilepsy: A Review of the WAG/Rij Strain of Rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- Coenen, A.M.L.; Van Luijtelaar, E.L.J.M. The WAG/Rij Rat Model for Absence Epilepsy: Age and Sex Factors. Epilepsy Res. 1987, 1, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, E.; Hramov, A.E.; Grubov, V.; Koronovsky, A.A.A.A. Rhythmic Activity in EEG and Sleep in Rats with Absence Epilepsy. Brain Res. Bull. 2016, 120, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N. Sex Still Matters: Has the Prevalence of Male-Only Studies of Drug Effects on Rodent Behaviour Changed during the Past Decade? Behav. Pharmacol. 2019, 30, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Borta, A.; Schwarting, R.K.W. Overt Behavior and Ultrasonic Vocalization in a Fear Conditioning Paradigm: A Dose–Response Study in the Rat. Neurobiol. Learn. Mem. 2005, 84, 228–240. [Google Scholar] [CrossRef]
- Graham, L.K.; Yoon, T.; Lee, H.J.; Kim, J.J. Strain and Sex Differences in Fear Conditioning: 22 KHz Ultrasonic Vocalizations and Freezing in Rats. Psychol. Neurosci. 2009, 2, 219–225. [Google Scholar] [CrossRef]
- Parsana, A.J.; Moran, E.E.; Brown, T.H. Rats Learn to Freeze to 22-KHz Ultrasonic Vocalizations through Autoconditioning. Behav. Brain Res. 2012, 232, 395–399. [Google Scholar] [CrossRef]
- Shanazz, K.; Dixon-Melvin, R.; Nalloor, R.; Thumar, R.; Vazdarjanova, A.I. Sex Differences In Avoidance Extinction After Contextual Fear Conditioning: Anxioescapic Behavior In Female Rats. Neuroscience 2022, 497, 146–156. [Google Scholar] [CrossRef]
- Gruene, T.M.; Flick, K.; Stefano, A.; Shea, S.D.; Shansky, R.M. Sexually Divergent Expression of Active and Passive Conditioned Fear Responses in Rats. eLife 2015, 4, e11352. [Google Scholar] [CrossRef]
- Dalla, C.; Shors, T.J. Sex Differences in Learning Processes of Classical and Operant Conditioning. Physiol. Behav. 2009, 97, 229–238. [Google Scholar] [CrossRef]
- Day, H.L.L.; Stevenson, C.W. The Neurobiological Basis of Sex Differences in Learned Fear and Its Inhibition. Eur. J. Neurosci. 2020, 52, 2466–2486. [Google Scholar] [CrossRef]
- Seffer, D.; Rippberger, H.; Schwarting, R.K.W.; Wöhr, M. Pro-Social 50-KHz Ultrasonic Communication in Rats: Post-Weaning but Not Post-Adolescent Social Isolation Leads to Social Impairments—Phenotypic Rescue by Re-Socialization. Front. Behav. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Houx, B.; Schwarting, R.K.W.; Spruijt, B. Effects of Experience and Context on 50-KHz Vocalizations in Rats. Physiol. Behav. 2008, 93, 766–776. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Anticipation of Play Elicits High-Frequency Ultrasonic Vocalizations in Young Rats. J. Comp. Psychol. 1998, 112, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bihari, A.; Hrycyshyn, A.W.; Brudzynski, S.M. Role of the Mesolimbic Cholinergic Projection to the Septum in the Production of 22 KHz Alarm Calls in Rats. Brain Res. Bull. 2003, 60, 263–274. [Google Scholar] [CrossRef]
- van der Zee, E.A.; Roozendaal, B.; Bohus, B.; Koolhaas, J.M.; Luiten, P.G. Muscarinic Acetylcholine Receptor Immunoreactivity in the Amygdala--I. Cellular Distribution Correlated with Fear-Induced Behavior. Neuroscience 1997, 76, 63–73. [Google Scholar] [CrossRef]
- Hagenaars, M.A.; Oitzl, M.; Roelofs, K. Updating Freeze: Aligning Animal and Human Research. Neurosci. Biobehav. Rev. 2014, 47, 165–176. [Google Scholar] [CrossRef]
- Kellis, D.M.; Kaigler, K.F.; Witherspoon, E.; Fadel, J.R.; Wilson, M.A. Cholinergic Neurotransmission in the Basolateral Amygdala during Cued Fear Extinction. Neurobiol. Stress 2020, 13, 100279. [Google Scholar] [CrossRef]
- Hoeller, A.A.; Costa, A.P.R.; Bicca, M.A.; Matheus, F.C.; Lach, G.; Spiga, F.; Lightman, S.L.; Walz, R.; Collingridge, G.L.; Bortolotto, Z.A.; et al. The Role of Hippocampal NMDA Receptors in Long-Term Emotional Responses Following Muscarinic Receptor Activation. PLoS ONE 2016, 11, e0147293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avissar, S.; Egozi, Y.; Sokolovsky, M. Studies on Muscarinic Receptors in Mouse and Rat Hypothalamus: A Comparison of Sex and Cyclical Differences. Neuroendocrinology 1981, 32, 295–302. [Google Scholar] [CrossRef]
- Cuomo, V.; De Salvia, M.A.; Lacomba, C.; Tattoli, M.; Cagiano, R. A New Experimental Approach for Detecting Emotional and Motivational Changes Produced by Neuroactive Compounds in Rodents. Ann. Ist. Super. Sanita 1990, 26, 89–94. [Google Scholar]
- Acquas, E.; Wilson, C.; Fibiger, H.C. Conditioned and Unconditioned Stimuli Increase Frontal Cortical and Hippocampal Acetylcholine Release: Effects of Novelty, Habituation, and Fear. J. Neurosci. 1996, 16, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C. Sex Differences in Dopaminergic and Cholinergic Activity and Function in the Nigro-Striatal System of the Rat. Psychoneuroendocrinology 1983, 8, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Pepeu, G. Sex and Gender Differences in the Brain Cholinergic System and in the Response to Therapy of Alzheimer Disease with Cholinesterase Inhibitors. Curr. Alzheimer Res. 2018, 15, 1077–1084. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-Induced Bradycardia in Mental Disorders: Foundations, Current Advances, Future Perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, J.M.J.; Barry, R.J.; Kuniecki, M.J.; van Luijtelaar, G. Fast, Transient Cardiac Accelerations and Decelerations during Fear Conditioning in Rats. Physiol. Behav. 2012, 105, 607–612. [Google Scholar] [CrossRef]
- Ogata, N.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Objective Measurement of Fear-Associated Learning in Dogs. J. Vet. Behav. 2006, 1, 55–61. [Google Scholar] [CrossRef]
- Godsil, B.P.; Quinn, J.J.; Fanselow, M.S. Body Temperature as a Conditional Response Measure for Pavlovian Fear Conditioning. Learn. Mem. 2000, 7, 353–356. [Google Scholar] [CrossRef]
- Marks, A.; Vianna, D.M.L.; Carrive, P. Nonshivering Thermogenesis without Interscapular Brown Adipose Tissue Involvement during Conditioned Fear in the Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1239–R1247. [Google Scholar] [CrossRef]
- Vianna, D.M.L.; Carrive, P. Changes in Cutaneous and Body Temperature during and after Conditioned Fear to Context in the Rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological Modulation of N-Methyl-D-Aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Rezvani, A.H.; Janowsky, D.S. Impaired Active Avoidance Responding in Rats Selectively Bred for Increased Cholinergic Function. Physiol. Behav. 1990, 47, 787–788. [Google Scholar] [CrossRef]
- Keeler, J.F.; Robbins, T.W. Translating Cognition from Animals to Humans. Biochem. Pharmacol. 2011, 81, 1356–1366. [Google Scholar] [CrossRef]
- Caldarone, B.J.; Zachariou, V.; King, S.L. Rodent Models of Treatment-Resistant Depression. Eur. J. Pharmacol. 2015, 753, 51–65. [Google Scholar] [CrossRef]
- Belzung, C.; Lemoine, M. Criteria of Validity for Animal Models of Psychiatric Disorders: Focus on Anxiety Disorders and Depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; Rey, C.E.; Monje, F.J. Rodent Models in Depression Research: Classical Strategies and New Directions. Ann. Med. 2010, 42, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical Modeling in Depression and Anxiety: Current Challenges and Future Research Directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Lazarini-Lopes, W.; Campos-Rodriguez, C.; Palmer, D.; N’Gouemo, P.; Garcia-Cairasco, N.; Forcelli, P.A. Absence Epilepsy in Male and Female WAG/Rij Rats: A Longitudinal EEG Analysis of Seizure Expression. Epilepsy Res. 2021, 176, 106693. [Google Scholar] [CrossRef]
- Sitnikova, E.; Smirnov, K. Active Avoidance Learning in WAG/Rij Rats with Genetic Predisposition to Absence Epilepsy. Brain Res. Bull. 2020, 165, 198–208. [Google Scholar] [CrossRef]
- Leo, A.; Citraro, R.; Tallarico, M.; Iannone, M.; Fedosova, E.; Nesci, V.; De Sarro, G.; Sarkisova, K.; Russo, E. Cognitive Impairment in the WAG/Rij Rat Absence Model Is Secondary to Absence Seizures and Depressive-like Behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109652. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; van Oijen, G. Establishing Drug Effects on Electrocorticographic Activity in a Genetic Absence Epilepsy Model: Advances and Pitfalls. Front. Pharmacol. 2020, 11, 395. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse Estrous Cycle Identification Tool and Images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T.A.; Zangrossi, H. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 1492. [Google Scholar] [CrossRef] [PubMed]



| Sex | Test Performance | N | Strain | N |
|---|---|---|---|---|
| Males | Successful | 14 | NEW | 12 |
| WAG/Rij | 1 ** | |||
| Unsuccessful | 4 | NEW | 2 | |
| WAG/Rij | 3 | |||
| Freezing | 12 * | NEW | 7 | |
| WAG/Rij | 5 | |||
| Total | 30 | |||
| Females | Successful | 25 | NEW | 11 |
| WAG/Rij | 14 | |||
| Unsuccessful | 1 | NEW | 0 | |
| WAG/Rij | 1 | |||
| Freezing | 1 | NEW | 0 | |
| WAG/Rij | 1 | |||
| Total | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrov, P.; Pupikina, M.; Adaeva, Z.; Sitnikova, E. The Difference between Male and Female Rats in Terms of Freezing and Aversive Ultrasonic Vocalization in an Active Avoidance Test. Physiologia 2023, 3, 406-420. https://doi.org/10.3390/physiologia3030028
Alexandrov P, Pupikina M, Adaeva Z, Sitnikova E. The Difference between Male and Female Rats in Terms of Freezing and Aversive Ultrasonic Vocalization in an Active Avoidance Test. Physiologia. 2023; 3(3):406-420. https://doi.org/10.3390/physiologia3030028
Chicago/Turabian StyleAlexandrov, Pavel, Maria Pupikina, Zabava Adaeva, and Evgenia Sitnikova. 2023. "The Difference between Male and Female Rats in Terms of Freezing and Aversive Ultrasonic Vocalization in an Active Avoidance Test" Physiologia 3, no. 3: 406-420. https://doi.org/10.3390/physiologia3030028
APA StyleAlexandrov, P., Pupikina, M., Adaeva, Z., & Sitnikova, E. (2023). The Difference between Male and Female Rats in Terms of Freezing and Aversive Ultrasonic Vocalization in an Active Avoidance Test. Physiologia, 3(3), 406-420. https://doi.org/10.3390/physiologia3030028







