Abstract
Background: Infections caused by multi-drug resistance (MDR) strains are potentially fatal public health issues worldwide that need pressing attention. Previous reports suggested using snake venom fractions as an effective alternative mechanism to the already available antibacterial drugs. In this study, we conducted a systematic review to analyze the bactericidal effects of snake venom phospholipases (PLA2s). Methods: From the beginning through 30 March 2022, we searched the PubMed and Embase databases in accordance with the most recent PRISMA recommendations. We also conducted a manual search to identify relevant reports to improve literature coverage. Results: A total of 24 studies were included based on the selection criteria to compile this review. Of them, 16 studies were obtained from the abovementioned databases and eight through manual searches. The other 8 studies were obtained through the references of the included studies. According to the review, we reported that some PLA2s showed more vigorous bactericidal activity on some Gram-negative and a moderate effect on Gram-negative and Gram-positive. Furthermore, we reported that the presence of p-bromophenacyl bromide (p-BPP) showed a significant decrease in enzymatic and associated antibacterial activities. Moreover, we observed that about 80% of the PLA2s reported in our systematic review study were those from the Viperidae family, whereas 20% came from the Elapidae family. Moreover, some variations were revealed in the current study regarding the mechanism of actions of the snake venom PLA2s (svPLA2s). Conclusion: This systematic review provides a comprehensive overview of the bactericidal effect of snake venom PLA2s and the analysis of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of PLA2s for bacterial strains. Varying bactericidal effects from various snake species and South American rattlesnakes were reported, presenting compelling concepts to the alternative search for therapies against bacterial resistance. Thus, further analysis of the bactericidal effects of other snake venoms PLA2s considering different strains is needed. Moreover, more data are needed to investigate other bacteria of public health priority using peptides and other purified snake toxins.
1. Introduction
Antimicrobial resistance is one of the most substantial risks to public health worldwide, including in developed countries in Europe [1]. According to the World Health Organization (WHO), infections caused by the multi-drug-resistant (MDR) strains are among the top ten reasons for mortality globally [2]. It is noteworthy that evidence about the development and spread of antibiotic-resistant bacteria is increasingly growing [3]. Furthermore, previous investigation has demonstrated that the improper use of antibiotics, such as vancomycin, can result in the development of vancomycin-intermediate (VISA) and vancomycin-resistant strains of bacteria such Enterococci [4]. Similarly, various bacteria, including Pseudomonas, Klebsiella, Enterobacter, Acinetobacter, and Salmonella, can resist several antibiotics [5]. Thus, laboratory- and clinical-based research geared toward discovering new and potent bactericidal candidates with unique mechanisms of action that could overcome antimicrobial resistance is warranted.
Studies on crude snake venoms and/or their fractions often result in potential therapeutic molecules against bacteria and other parasites [6]. Snake venoms are composed of a spectrum of protein-based constituents. These components could be categorized into four broad groups [6], namely: (1) The dominant group, which consists of the three-finger toxins (3FTx), phospholipases A2 (PLA2), snake venom metalloproteases (SVMP), and snake venom serine proteases (SVSP), (2) The second group consists of a small number of proteins, which includes Kunitz peptides (KUN), cysteine-rich secretory proteins (CRiSP), L-amino acid oxidases (LAAO), C-type lectins (CTL), disintegrins (DIS), and natriuretic peptides (NP), (3) The third group contains the rarely observed snake venom proteins, including venom nerve growth factor (VNGF), vascular endothelial growth factor (VEGF), acetylcholinesterases, hyaluronidases, 5′-nucleotidases, phosphodiesterases (PDE), and snake venom metalloprotease inhibitors, and (4) finally, the fourth group includes cobra venom factors (CVF), galactose-binding proteins, aminopeptidases, and waprins.
However, the proteins mentioned above may only be readily available in some venomous snakes. For instance, elapids snakes have Group I PLA2s and 3FTx, whereas the mambas are known to consist mainly of Kunitz peptides that are also known as dendrotoxins. The viperids consist of PLA2s and proteases as the most abundant protein groups with varying quantities of serine proteases and metalloproteases [7].
In this study, we present a thorough review based on the existing literature on the bactericidal effect of snake venom PLA2s, alongside an analysis of the given minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against the bacterial strains listed under the study, highlighting the potential of snake venom fraction as promising candidates against several species of antimicrobial resistant bacteria.
2. Methods
2.1. Search Strategy
Relevant studies that reported the bactericidal effects of PLA2s were searched from PubMed and Embase databases from inception until 30 March 2022. We utilized the search terms (“Bactericidal OR bacterial effect OR Antibacterial”) and (“Snake Venom PLA2s OR Venomous snake PLA2 OR Snake Venom Peptides OR Snake venom Components OR Fractions OR Enzymes OR Proteins”). The outcome of the searches from the two terms was then combined using the Boolean operator ‘AND’. We conducted a manual search of the literature to ensure more comprehensive coverage of the studies in the area of research. Reference lists of included articles that met eligibility criteria were also manually searched to identify any additional articles.
2.2. Eligibility Criteria
We included only original articles published in the English language that reported the bactericidal effect of snake venom PLA2s in our review. The study excluded reviews, articles on bactericidal effects of snake venoms’ fractions other than PLA2s, those published in a language other than English, and studies that employed the use of commercial venoms in which the snake specie information was not mentioned, as well as studies involving the effects of venoms on other parasites instead of bacteria.
2.3. Study Selection, Data Extraction and Data Synthesis
PRISMA systematic review procedure was utilized in the process of selecting the most relevant articles to be included in the study [8]. To avoid any potential bias in the search and/or inclusion of studies, two authors (ZUA and SSM) conducted the article screening processes at the title, abstract, and full-text phases. The same authors conducted the extraction of relevant data for the review. A customized excel sheet was used for the data extraction. Any disagreements during the article screening processes and the data extraction were resolved and discussed with all the other authors and then agreed upon by consensus. Data extracted included authors’ names, date of publication, snake species, PLA2s, bacterial isolates, minimum inhibitory concentration, minimum bactericidal concentration, and activities of PLA2s on the tested bacteria. The synthesis of the extracted data was guided by the “synthesis without meta-analysis” (SWIM) protocol [9].
3. Results
3.1. Search Results
We retrieved 223 reports from searching the databases, 91 and 124 were from PubMed and Embase, respectively. Furthermore, additional eight studies were identified manually through the search of the references of the included studies. After removing all duplicates and the screenings at the titles and abstract phases, the remaining articles were considered for further screening, which led to the exclusion of 163 unrelated articles as well as 17 duplicates. Furthermore, we examined the full texts of the remaining 43 articles, from which 19 studies were excluded (Figure 1). Finally, twenty-four studies met the eligibility criteria and were included. Furthermore, additional eight studies were identified manually through the search of the reference lists, totaling the included reports in this study 26. Table 1 lists the distinguishing features of the included studies, and Figure 1 shows the flowchart of the research search and screening procedures. Moreover, the summary data for MIC and MBC, as well as sequence data for the PLA2s extracted from the included studies, are provided in Table 2.
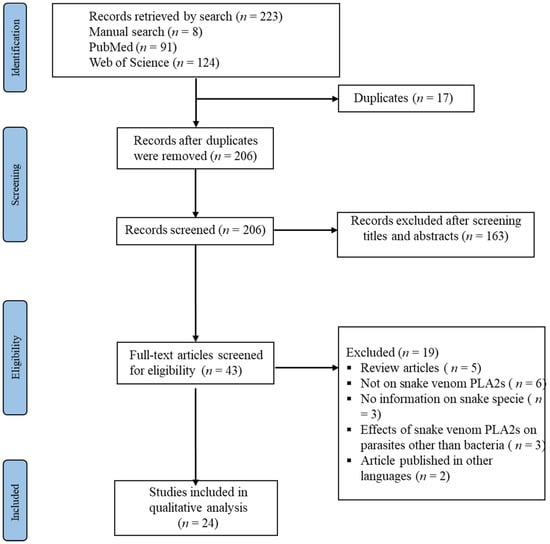
Figure 1.
PRISMA diagram for the study search and selection processes.

Table 1.
Features of the analyzed studies.

Table 2.
Summary table for MIC, MBC, and sequence data extracted from the included studies.
3.2. Bactericidal Effects of Snake Venom PLA2s
Snake venom PLA2s (svPLA2s) are enzymatic proteins with low molecular weights. They catalyze the hydrolysis of the 3-sn-phosphoglyceride-dependent calcium 2-acyl ester bond, yielding lysophospholipids and fatty acid products [6]. Snake venom PLA2s have similar toxicological profiles, including cytotoxicity, myotoxicity, oedema, inflammation, neurotoxicity, hypotension, anticoagulant activity, hemolysis, hyperalgesia, and microbicidal activity [5]. Some of the evaluated PLA2s (either acidic or basic) could present an IC50 against at least Gram-positive or Gram-negative bacteria, whereas others could not show any activity against any strain. Nunes et al. [1] reported that the acidic PLA2 from B. erythromela presented IC50 against Gram-positive bacteria but not negative bacteria. Torres et al. [15] reported that the basic PLA2 isolated from B. marajeonsis showed no inhibitory effect on neither P. aeruginosa or S. aureus.
Moreover, Jia et al. [17] reported that the PLA2 from Agkistrodon piscivorus leucostoma, AplAsp49, and AplLys49, presented no bactericidal effect against any of the bacterial strains. Moreover, Sudarshan et al. [10] reported that the basic PLA2 of D. russelliipulchella showed higher bactericidal activity against Gram-positive bacteria when compared with Gram-negative bacteria. It has been reported that the bactericidal activity of PLA2, especially the basic is related to the disturbances of bacteria membrane integrity [23]. Gram-negative bacteria have a cell wall that is made up of an inner membrane formed of phospholipids, an outer membrane made of asymmetric lipids, and a layer of peptidoglycans. This structure acts as a barrier to medications that have been established. A similar scenario exists for the PLA2, as the outer membrane naturally resists its activity [33]. On the other hand, Gram-positive bacteria possess just one layer of peptidoglycans and then an internal cell membrane, showing their prompt susceptibility to the action of PLA2. As such, the low bactericidal activity of some PLA2s in Gram-negative bacteria compared with Gram-positive bacteria could be due to the difference in the structure of their respective cell walls. Sudarshan et al. [10] reported that there was a strong, established relationship between the hemolytic and bactericidal activity of D. russelliipulchella PLA2.
Furthermore, it was reported that p-bromophenacyl bromide (p-BPP) presents a significant decrease in enzymatic activity and associated antibacterial activities, thereby destabilizing the membrane bilayer. Regarding PLA2 crotoxin A or B (PLA2- CA and PLA2- CB), Alves et al. [11] reported that both from C. durissus terrificus showed high bactericidal activity against R. solanacearum. Similarly, Samy et al. [19] reported that crotoxin B of C. durissus terrificus and daboiatoxin of Daboia russelli presented the most robust bactericidal activity against the two strains of B. pseudomallei (TES and KHW). Some PLA2s were reported to have shown bactericidal activity against both Gram-positive and Gram-negative bacteria [16,31]. Others presented a bactericidal effect on Gram-positive and not Gram-negative bacteria [12]. Of interest to note is that some PLA2 showed more vigorous bactericidal activity on some Gram-negative bacteria and moderate on other Gram-negative and Gram-positive bacteria [13].
3.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Bacterial Strains
Minimum inhibitory concentrations (MICs) are the lowest concentration of an antimicrobial that could inhibit the visible growth of a microorganism after overnight incubation. MICs are utilized by diagnostic laboratories mainly to confirm resistance but are most often used as a research tool to determine the in vitro activity of new antimicrobials [34]. In the works of Samy et al. [13] and Samy et al. [16], Vargas et al. [12], and Jia et al. [17], broth micro-dilution assay was employed. The procedure entails suspending fresh overnight bacterial cultures to a turbidity of 0.5 McFarland units and further subjecting them to a dilution in Mueller Hinton (MH) and tryptic soy (TS) broth, followed by a dilution to approximately 1.5 × 105–3.2 × 106 CFU/mL (CFU = colony forming unit). On the other hand, minimum bactericidal concentration (MBC) is referred to as the minimum bactericidal density required to kill bacteria; as such, it is opposed to mere bacteriostatic densities [35]. According to Samy et al. [13] and Samy et al. [16], the plating technique was carried out whereby the broth from the MICs well and those from above the wells were plated for each bacterial strain 3.2 × 106 CFU/mL onto approximate drug-free growth media MH and TS agar plates used for determining antibacterial activity. Surviving bacteria were then quantified using the dilution plate technique. On the starting inoculums, the quantitative colony counts were assessed. The lowest concentration that killed up to ≥99.9% of the starting inoculums was defined as the MBC. In the work of Jia et al. [17], the plating technique involved adding two hundred microliters of 1/1000 diluted overnight bacterial cultures to 1 mg/mL, 0.75 mg/mL, 0.1 mg/mL 50 µg/mL 25 µg/mL, 10 µg/mL PLA2 in the 96-well plates in parallel 50 µg/mL of the control (antibiotic), and PLA2 buffer as a negative control. Bacterial cultures that presented no growth were then plated on agar and incubated overnight to achieve colony-forming unit (CFU) enumeration.
4. Discussion
Overall, our systematic search identified 24 articles on the bactericidal effect of svPLA2s. The majority of the studies (80%) found through the search were on the PLA2s of snakes from the Viperidae family, whereas 20% were on the PLA2s from snakes of the Elapidae family. There are considerable variations in the composition of snake venoms recorded even from closely related species and within species [6]. Evidence of intra-genus or intra-specific variation in venom compositions has been documented in pit vipers and adders [36]. This variation was attributed to diet [37] or topography [38]. Repeated toxin-encoding genes, production processes, as well as functional and structural diversifications are other attributable factors [39]. For instance, the sea snake Laticauda Semifasciata S. venom has a simple composition, with only two prominent protein families, the 3FTxs and the PLA2s. However, about 50–100 peptides or proteins representing about 10–20 protein families are present in the venoms of rattlesnakes and mambas [7]. In general, cobra, kraits, and the hydrophids particularly have more negligible toxins such as 3FTxs and PLA2, whereas the venoms of vipers are composed of the more significant fractions with enzymatic roles such as snake venom metalloproteinase and snake venom serine protease [7]. For instance, amino acids, small peptides, carbohydrates, lipids, biogenic amines, and enzymes are contained in the venom of C. durissus terrificus, whereas that of B. jararaca is composed of peptides serine and metalloproteases [40]. As such, the activity of snake venoms varies due to differences in concentrations and compositions.
It has been reported that svPLA2s makes a considerable component of the venoms of the vipers and elapids [41] due to their biomedical importance over the other compositions of snake venoms [42]. They are proteins that belong to groups I and II. The group I PLA2s are those of snakes from the Elapidae family (Elapidae and Hydrophiinae) [7]. In contrast, those belonging to group II are from snakes of the Viperidae family (Viperinae and Catalina) [7]. The latter comprises two subgroups: the catalytically active Asp49-PLA2 and the catalytically inactive PLA2 homologs containing Lys49 residue [43]. The GIIA PLA2s were reported to have an essential role in the defense against bacteria. The ASP49- PLA2 and LYS49- PLA2 homologs were reported to have acted synergistically, increasing Ca2+ ions in the plasma membrane, resulting in the rapid death of myotubes [43].
Crotoxin is a non-covalent heterodimeric neurotoxin of two subunits; an active PLA2 and a chaperone peptide called crotaperone. The molecule has three peptide chains connected by seven disulfide bridges [44]. It is the main neurotoxin in the venom of the South American rattlesnake (C. durissus terrificus) and accounts for about 50% of its dry weight and it acts at the presynaptic membrane level. Pharmacologically, crotoxin promotes pre- and post-synaptic effects, indicating several interactions with excitable cells [29]. Crotoxin B, a basic neurotoxic phospholipase A, has three chain proteins that promote the lethal potency of crotoxins [19]. In contrast, crotoxin A (CA) is the acidic subunit of the crotoxin (Crtx) on which the essential subunit crotoxin B (CB) depend for the ability to bind specifically to the cell membrane [45].
There are variations in the mechanism of actions of svPLA2s. For instance, CaTx-II from C. adamanteus was reported to have inhibited the growth of E. aerogenes through the disintegration of its cell wall by generating pores in the membrane. Furthermore, the protein has been reported to promote the healing of wounds [16]. It is noteworthy that peptides produced by the breakdown of svPLA2 can interact with lipopolysaccharide (LPS), specifically, the lipid A component of S. aureus, causing membrane permeabilization and acting as a bactericide [16]. Various cationic peptides from B. asper’s svPLA2s exhibit bactericidal activity against K. pneumoniae, protect mice from S. enterica-induced peritonitis, and cause membrane permeabilization in S. aureus when they are derived from cationic peptides [31,46,47]. The derivatives of the carboxy terminus of svPLA2s found in these peptides, which range from ten to twenty-two amino acids, are crucial. Compared to the parent compounds, they are less toxic to eukaryotic cells and have greater bactericidal activity. Similarly, it has been documented that the C-terminal cationic/hydrophobic segment (residues 115–129) of svPLA2s has bactericidal potential. As such, identifying bactericidal positions in svPLA2s because of developing new therapeutics is promising [48,49,50]. Furthermore, there have been reports of varying MICs and MBCs against various bacterial strains, as well as sequence data of each PLA2.
4.1. Strength
Our systematic review has the following strengths; firstly, we conducted a thorough and extensive search of literature via PubMed and Embase databases, as well as a manual search of the literature, which enriched our search coverage. Secondly, we adopted appropriate quality rating tools for assessing the qualities of the included studies.
4.2. Limitations
The study is associated with some limitations that should be considered when interpreting the reported findings. Firstly, we excluded studies based on language (only those published in English), thereby limiting the ability to incorporate relevant data from studies in languages other than English. Moreover, most of the included studies did not include information on accession numbers. However, we have included the sequence information found from each study in our work to have representative information for at least each included study. Nevertheless, in our future research, we will deeply investigate the DNA sequence of PLA2 with nucleotide accession numbers when the data become available.
5. Conclusions
This systematic review provides a comprehensive overview of the bactericidal effect of snake venom PLA2s and analyses the minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs)of PLA2s for bacterial strains. Varying bactericidal effects of various PLA2s were reported, presenting compelling concepts to the alternative search for therapies against bacterial resistance. However, more data are needed to investigate the bactericidal effects of other snake venoms PLA2s using purified snake toxins. Thus, it is imperative to study other bacteria of public health importance using snake venoms and their associated purified snake toxins.
Author Contributions
Conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft, Z.U.A.; conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, validation, visualization, writing—original draft, writing—review and editing, S.S.M.; conceptualization, formal analysis, investigation, methodology, validation, writing—review and editing, supervision, H.A.-O.; formal analysis, investigation, methodology, validation, writing—review and editing, A.A.; formal analysis, investigation, methodology, validation, writing—review and editing, A.A.L.; Conceptualization, formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing, U.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this work are in the publicly domain.
Acknowledgments
The authors are appreciative to the handling editor and the unanimous reviewers for their insightful observations, which helped us to enhance the manuscript.
Conflicts of Interest
All authors declared no conflict of interest.
References
- Bocian, A.; Hus, K.K. Antibacterial properties of snake venom components. Chem. Pap. 2020, 74, 407–419. [Google Scholar] [CrossRef]
- World Health Organizations. Antimicrobial Resistance. Key Facts. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 11 March 2022).
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Cázares-Domínguez, V.; Cruz-Córdova, A.; Ochoa, S.A.; Escalona, G.; Arellano-Galindo, J.; Rodríguez-Leviz, A.; Hernández-Castro, R.; López-Villegas, E.O.; Xicohtencatl-Cortes, J. Vancomycin tolerant, methicillin-resistant Staphylococcus aureus reveals the effects of vancomycin on cell wall thickening. PLoS ONE 2015, 10, e0118791. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L.; et al. Antibiofilm activity of acidic phospholipase isoform isolated from Bothropserythromelas snake venom. Toxins 2020, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, Z.U.; Musa, S.S.; He, D.; Bello, U.M. Antiprotozoal effect of snake venoms and their fractions: A systematic review. Pathogens 2021, 10, 1632. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, S.; Dhananjaya, B.L. Antibacterial activity of an acidic phospholipase A2 (NN-XIb-PLA2) from the venom of Najanaja (Indian cobra). SpringerPlus 2016, 5, 112 . [Google Scholar] [CrossRef]
- Alves, R.d.C.; Vieira Júnior, J.R.; Freire, T.C.; Fonseca, A.S.; Sangi, S.C.; Barbieri, F.D.; Rocha, R.B.; Brito, L.G.; Pereira, S.D.; Luiz, M.B.; et al. Snake venoms and purified toxins as biotechnological tools to control Ralstonia solanacearum. Pesq. Agropec. Bras. 2020, 55, e01756. [Google Scholar] [CrossRef]
- Vargas, L.J.; Londoño, M.; Quintana, J.C.; Rua, C.; Segura, C.; Lomonte, B.; Núñez, V. An acidic phospholipase A2 with antibacterial activity from Porthidium nasutum snake venom. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Gopalakrishnakone, P.; Bow, H.; Puspharaj, P.N.; Chow, V.T. Identification and characterization of a phospholipase A2 from the venom of the Saw-scaled viper: Novel bactericidal and membrane damaging activities. Biochimie 2010, 92, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, S.; Dhananjaya, B.L. The antimicrobial activity of an acidic phospholipase A2 (NN-XIa-PLA2) from the venom of Najanajanaja (Indian Cobra). Appl. Biochem. 2015, 176, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.F.; Dantas, R.T.; Toyama, M.H.; Diz Filho, E.; Zara, F.J.; de Queiroz, M.G.; Nogueira, N.A.; de Oliveira, M.R.; de Oliveira Toyama, D.; Monteiro, H.S.; et al. Antibacterial and antiparasitic effects of Bothropsmarajoensis venom and its fractions: Phospholipase A2 and L-amino acid oxidase. Toxicon 2010, 55, 795–804. [Google Scholar] [CrossRef]
- Samy, R.P.; Kandasamy, M.; Gopalakrishnakone, P.; Stiles, B.G.; Rowan, E.G.; Becker, D.; Shanmugam, M.K.; Sethi, G. Chow VT. Wound healing activity and mechanisms of action of an antibacterial protein from the venom of the eastern diamondback rattlesnake (Crotalusadamanteus). PLoS ONE 2014, 9, e80199. [Google Scholar] [CrossRef]
- Jia, Y.; Villarreal, J. Phospholipases A2 purified from cottonmouth snake venoms display no antibacterial effect against four representative bacterial species. Toxicon 2018, 151, 1–4. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Ho, B.; Chow, V.T. Purification, characterization and bactericidal activities of basic phospholipase A2 from the venom of Agkistrodon halys (Chinese pallas). Biochimie 2008, 90, 1372–1388. [Google Scholar] [CrossRef]
- Samy, R.P.; Pachiappan, A.; Gopalakrishnakone, P.; Thwin, M.M.; Hian, Y.E.; Chow, V.T.; Bow, H.; Weng, J.T. In vitro antimicrobial activity of natural toxins and animal venoms tested against Burkholderia pseudomallei. BMC Infect. Dis. 2006, 6, 100. [Google Scholar]
- Samel, M.; Vija, H.; Kurvet, I.; Künnis-Beres, K.; Trummal, K.; Subbi, J.; Kahru, A.; Siigur, J. Interactions of PLA2-s from Viperalebetina, Viperaberusberus and Najanajaoxiana venom with platelets, bacterial and cancer cells. Toxins 2013, 5, 203–223. [Google Scholar] [CrossRef]
- Roberto, P.G.; Kashima, S.; Marcussi, S.; Pereira, J.O.; Astolfi-Filho, S.; Nomizo, A.; Giglio, J.R.; Fontes, M.R.; Soares, A.M.; França, S.C. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. Protein J. 2004, 23, 273–285. [Google Scholar] [CrossRef]
- Xu, C.; Ma, D.; Yu, H.; Li, Z.; Liang, J.; Lin, G.; Zhang, Y.; Lai, R. A bactericidal homodimeric phospholipases A2 from Bungarusfasciatus venom. Peptides 2007, 28, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, E.A.; Kayano, A.M.; Diniz-Sousa, R.; Setúbal, S.S.; Zanchi, F.B.; Zuliani, J.P.; Matos, N.B.; Almeida, J.R.; Resende, L.M.; Marangoni, S.; et al. Isolation, structural and functional characterization of a new Lys49 phospholipase A2 homologue from Bothropsneuwiediurutu with bactericidal potential. Toxicon 2016, 115, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Denegri, M.E.; Acosta, O.C.; Huancahuire-Vega, S.; Martins-de-Souza, D.; Marangoni, S.; Maruñak, S.L.; Teibler, G.P.; Leiva, L.C.; Ponce-Soto, L.A. Isolation and functional characterization of a new acidic PLA2 Ba SpII RP4 of the Bothropsalternatus snake venom from Argentina. Toxicon 2010, 56, 64–74. [Google Scholar] [CrossRef]
- Abid, I.; Jemel, I.; Alonazi, M.; Ben Bacha, A. A New Group II Phospholipase A2 from Walterinnesia aegyptia Venom with Antimicrobial, Antifungal, and Cytotoxic Potential. Processes 2020, 8, 1560. [Google Scholar] [CrossRef]
- Barbosa, P.S.; Martins, A.M.; Havt, A.; Toyama, D.O.; Evangelista, J.S.; Ferreira, D.P.; Joazeiro, P.P.; Beriam, L.O.; Toyama, M.H.; Fonteles, M.C.; et al. Renal and antibacterial effects induced by myotoxin I and II isolated from Bothrops jararacussu venom. Toxicon 2005, 46, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Shebl, R.I.; Mohamed, A.F.; Ali, A.E.; Amin, M.A. Antimicrobial profile of selected snake venoms and their associated enzymatic activities. Br. Microbiol Res. J. 2012, 2, 251–263. [Google Scholar] [CrossRef]
- Almeida, J.R.; Lancellotti, M.; Soares, A.M.; Calderón, L.A.; Ramírez, D.; González, W.; Marangoni, S.; Da Silva, S.L. CoaTx-II, a new dimeric Lys49 phospholipase A2 from Crotalus oreganus abyssus snake venom with bactericidal potential: Insights into its structure and biological roles. Toxicon 2016, 120, 147–158. [Google Scholar] [CrossRef]
- Toyama, M.H.; de Oliveira, D.G.; Beriam, L.O.; Novello, J.C.; Rodrigues-Simioni, L.; Marangoni, S. Structural, enzymatic and biological properties of new PLA2 isoform from Crotalusdurissusterrificus venom. Toxicon 2003, 41, 1033–1038. [Google Scholar] [CrossRef]
- Bacha, A.B.; Alonazi, M.A.; Elshikh, M.S.; Karray, A. A novel bactericidal homodimeric PLA2 group-I from Walterinnesiaaegyptia venom. Int. J. Biol. Macromol. 2018, 117, 1140–1146. [Google Scholar] [CrossRef]
- Santamaría, C.; Larios, S.; Quirós, S.; Pizarro-Cerda, J.; Gorvel, J.P.; Lomonte, B.; Moreno, E. Bactericidal and antiendotoxic properties of short cationic peptides derived from a snake venom Lys49 phospholipase A2. Antimicrob. Agents Chemother. 2005, 49, 1340–1345. [Google Scholar] [CrossRef]
- Costa, T.R.; Menaldo, D.L.; Oliveira, C.Z.; Santos-Filho, N.A.; Teixeira, S.S.; Nomizo, A.; Fuly, A.L.; Monteiro, M.C.; de Souza, B.M.; Palma, M.S.; et al. Myotoxic phospholipases A2 isolated from Bothropsbrazili snake venom and synthetic peptides derived from their C-terminal region: Cytotoxic effect on microorganism and tumor cells. Peptides 2008, 29, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Graham, G.G.; Scott, K.F. Antibacterial actions of secreted phospholipases A2. Review. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2008, 1781, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicr. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [PubMed]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef]
- Creer, S.; Malhotra, A.; Thorpe, R.S.; Stöcklin, R.S.; Favreau, P.S.; Hao Chou, W.S. Genetic and ecological correlates of intraspecific variation in pitviper venom composition detected using matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS) and isoelectric focusing. J. Mol. Evol. 2003, 56, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Sunagar, K.; Casewell, N.R.; Kochva, E.; Roelants, K.; Scheib, H.; Wüster, W.; Vidal, N.; Young, B.; Burbrink, F.; et al. The origin and evolution of the Toxicofera reptile venom system. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: Oxford, UK, 2015; Volume 2015, pp. 1–31. [Google Scholar]
- Miller, R.A.; Jian, J.; Beno, S.M.; Wiedmann, M.; Kovac, J. Intraclade variability in toxin production and cytotoxicity of Bacillus cereus group type strains and dairy-associated isolates. Appl. Environ. Microbiol. 2018, 84, e02479-17. [Google Scholar] [CrossRef] [PubMed]
- Nikpour, S.; Tabatabaie, F.; Sharifi, I.; Mostafavi, M.; Oliaee, R.T.; Sharifi, F.; Babaei, Z.; Jafari, E.; Salarkia, E.; Shahbazzadeh, D. The fraction of the snake venom, its leishmanicidal effect, and the stimulation of an anti-leishmania response in infected macrophages. Endocr. Metab. Immune Disord. -Drug Targets 2021, 21, 1115–1124. [Google Scholar] [CrossRef]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef]
- Deshwal, A.; Phan, P.; Datta, J.; Kannan, R.; Thallapuranam, S.K. A Meta-Analysis of the Protein Components in Rattlesnake Venom. Toxins 2021, 13, 372. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Fernandez, J.; Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Synergism between basic Asp49 and Lys49 phospholipase A2 myotoxins of viperid snake venom in vitro and in vivo. PLoS ONE 2014, 9, e109846. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.A.; Ferreira, R.S.; Barraviera, B.; Carvalho, F.C.; Barros, L.C.; Santos, L.D.; Pimenta, D.C. Crotalusdurissusterrificuscrotapotinnaturally displays preferred positions for aminoacid substitutions. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lomeo, R.; de Faria Gonçalves, A.P.; da Silva, C.N.; de Paula, A.T.; Santos, D.O.; Fortes-Dias, C.L.; Gomes, D.A.; de Lima, M.E. Crotoxin from Crotalusdurissusterrificus snake venom induces the release of glutamate from cerebrocorticalsynaptosomes via N and P/Q calcium channels. Toxicon 2014, 85, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Sudharshan, S.; Dhananjaya, B.L. Antibacterial potential of a basic phospholipase A2 (VRV-PL-VIIIa) from Daboia russeliipulchella (Russell’s viper) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus. Stat Pearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441868 (accessed on 18 July 2022).
- Samy, R.P.; Gopalakrishnakone, P.; Stiles, B.G.; Girish, K.S.; Swamy, S.N.; Hemshekhar, M.; Tan, K.S.; Rowan, E.G.; Sethi, G.; Chow, V.T.K. Snake venom phospholipases A2: A novel tool against bacterial diseases. Curr. Med. Chem. 2012, 19, 6150–6162. [Google Scholar] [CrossRef]
- Jami, A.A.; Fathi, B.; Jamshidi, A.; Zolfagharian, H.; Zare, M.A. Investigation of the antibacterial effect of venom of the Iranian snake Echiscarinatus. Iran. J. Vet. Sci Technol. 2010, 2, 93–100. [Google Scholar]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I.; et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).