Enhance Trial: Effects of NAD3® on Hallmarks of Aging and Clinical Endpoints of Health in Middle Aged Adults: A Subset Analysis Focused on Blood Cell NAD+ Concentrations and Lipid Metabolism
Abstract
:1. Introduction
2. Methods
2.1. Ethical Approval and Recruitment
2.2. Testing Sessions and Supplementation
2.3. Serum and Targeted PBMC NAD Metabolome Analyses
2.4. A priori Sample Size Calculations and Statistical Analysis
3. Results
3.1. General Outcomes
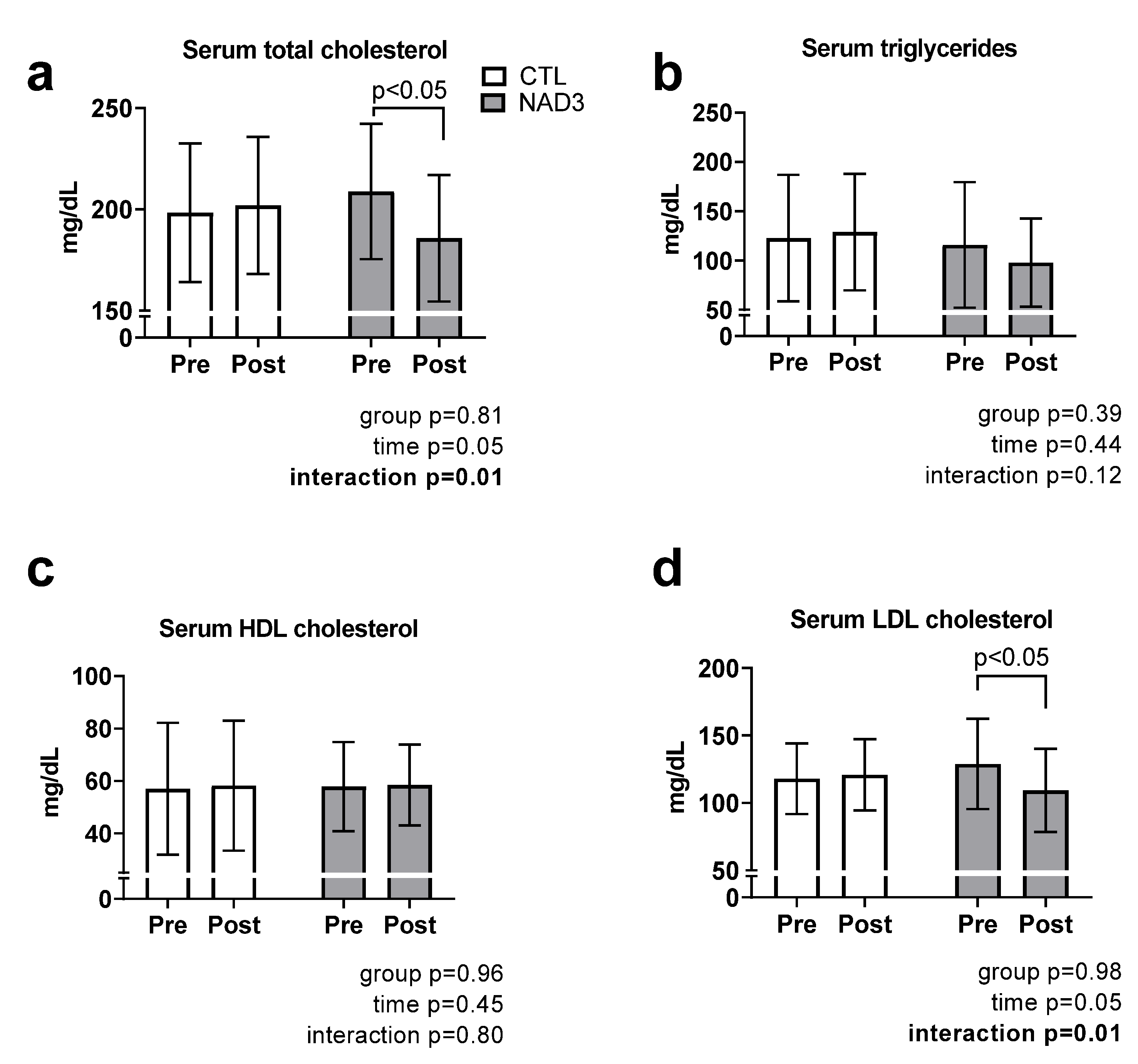
3.2. Effects of Supplementation on Serum Lipids
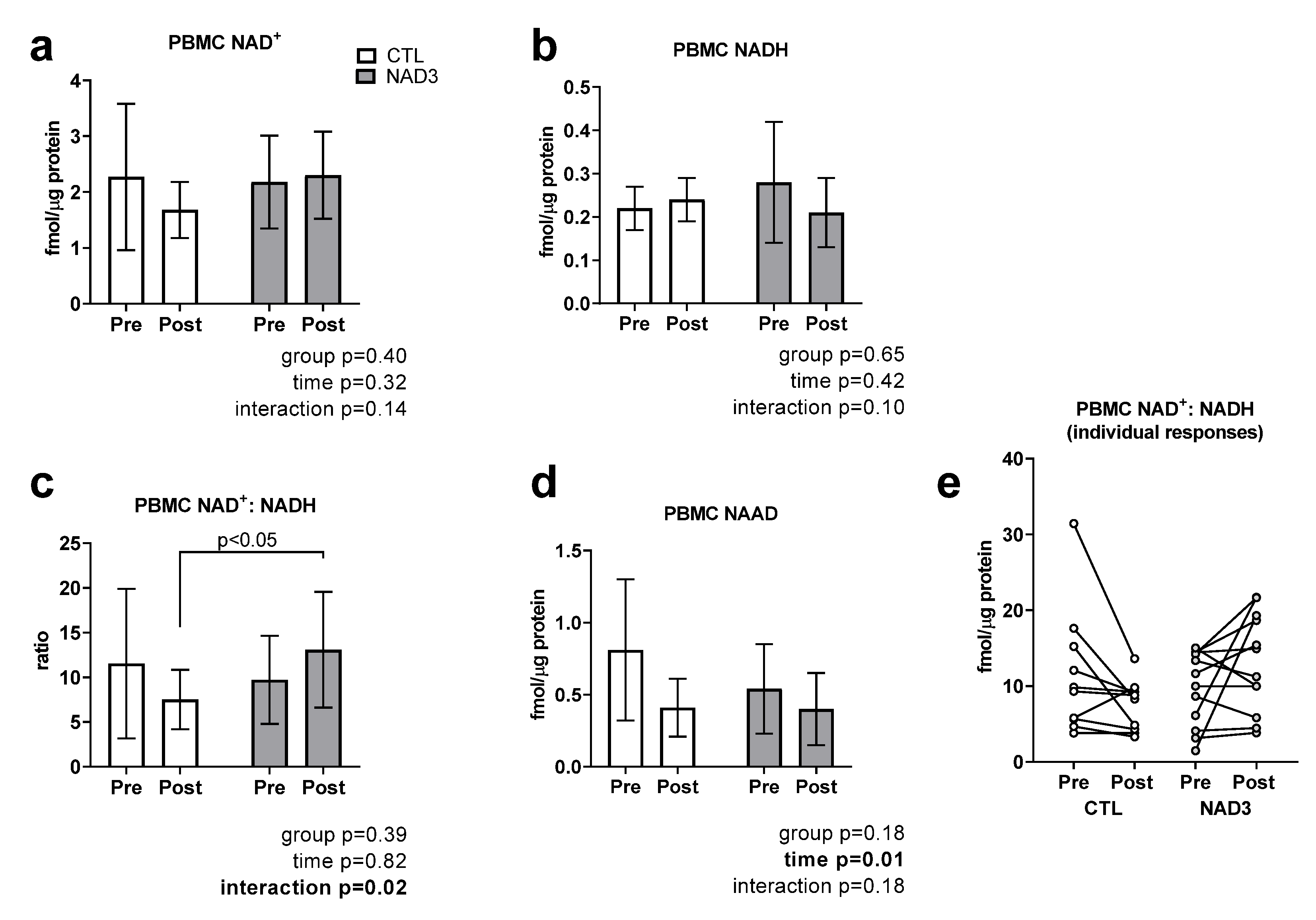
3.3. Effects of Supplementation on PBMC NAD+, NADH, NAD+/NADH, and NAAD
3.4. Effects of Supplementation on Blood Markers Indicative of Clinical Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef]
- Desai, A.; Grolleau-Julius, A.; Yung, R. Leukocyte function in the aging immune system. J. Leukoc. Biol. 2010, 87, 1001–1009. [Google Scholar] [CrossRef]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.Y.; Xiang, J.; Wang, Z.S.; Jin, J.; Wang, Y.Q.; Li, Q.S.; Li, D.; Fang, Z.T.; Lu, J.L.; Ye, J.H.; et al. Theacrine from Camellia kucha and Its Health Beneficial Effects. Front. Nutr. 2020, 7, 596823. [Google Scholar] [CrossRef]
- Gao, M.; Zheng, J.; Zheng, C.; Huang, Z.; Huang, Q. Theacrine alleviates chronic inflammation by enhancing TGF-beta-mediated shifts via TGF-beta/SMAD pathway in Freund’s incomplete adjuvant-induced rats. Biochem. Biophys. Res. Commun. 2020, 522, 743–748. [Google Scholar] [CrossRef]
- Wang, G.E.; Li, Y.F.; Zhai, Y.J.; Gong, L.; Tian, J.Y.; Hong, M.; Yao, N.; Wu, Y.P.; Kurihara, H.; He, R.R. Theacrine protects against nonalcoholic fatty liver disease by regulating acylcarnitine metabolism. Metabolism 2018, 85, 227–239. [Google Scholar] [CrossRef]
- Taylor, L.; Mumford, P.; Roberts, M.; Hayward, S.; Mullins, J.; Urbina, S.; Wilborn, C. Safety of TeaCrine(R), a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use. J. Int. Soc. Sports Nutr. 2016, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Mumford, P.W.; Osburn, S.C.; Fox, C.D.; Godwin, J.S.; Roberts, M.D. A Theacrine-Based Supplement Increases Cellular NAD(+) Levels and Affects Biomarkers Related to Sirtuin Activity in C2C12 Muscle Cells In Vitro. Nutrients 2020, 12, 3727. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Pirinen, E.; Auranen, M.; Khan, N.A.; Brilhante, V.; Urho, N.; Pessia, A.; Hakkarainen, A.; Kuula, J.; Heinonen, U.; Schmidt, M.S.; et al. Niacin Cures Systemic NAD(+) Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020, 31, 1078–1090.e1075. [Google Scholar] [CrossRef] [PubMed]
- Sauve, A.A. NAD+ and vitamin B3: From metabolism to therapies. J. Pharmacol. Exp. Ther. 2008, 324, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.W.; Ghimeray, A.K.; Park, C.H. Investigation of total phenolic, total flavonoid, antioxidant and allyl isothiocyanate content in the different organs of Wasabi japonica grown in an organic system. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Uto, T.; Hou, D.X.; Morinaga, O.; Shoyama, Y. Molecular Mechanisms Underlying Anti-Inflammatory Actions of 6-(Methylsulfinyl)hexyl Isothiocyanate Derived from Wasabi (Wasabia japonica). Adv. Pharm. Sci. 2012, 2012, 614046. [Google Scholar] [CrossRef] [Green Version]
- Greaney, A.J.; Maier, N.K.; Leppla, S.H.; Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 2016, 99, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Dose, J.; Schultheiss, G.; Rimbach, G. Anti-inflammatory potential of allyl-isothiocyanate--role of Nrf2, NF-(kappa) B and microRNA-155. J. Cell. Mol. Med. 2012, 16, 836–843. [Google Scholar] [CrossRef]
- Dashwood, R.H.; Ho, E. Dietary agents as histone deacetylase inhibitors: Sulforaphane and structurally related isothiocyanates. Nutr. Rev. 2008, 66 (Suppl. S1), S36–S38. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Proll, M.; Neuhoff, C.; Zhang, R.; Cinar, M.U.; Hossain, M.M.; Tesfaye, D.; Grosse-Brinkhaus, C.; Salilew-Wondim, D.; Tholen, E.; et al. Sulforaphane epigenetically regulates innate immune responses of porcine monocyte-derived dendritic cells induced with lipopolysaccharide. PLoS ONE 2015, 10, e0121574. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Munoz, P.; Mura, C.V.; Nunez, M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A.; McLelland, G.L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Freire, M.; de Cabo, R.; Bernier, M.; Sollott, S.J.; Fabbri, E.; Navas, P.; Ferrucci, L. Reconsidering the Role of Mitochondria in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1334–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Investig. 2018, 128, 3662–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Abe, Y.; Takayama, M.; Adachi, T.; Okano, H.; Hirose, N.; Arai, Y. Association among extracellular superoxide dismutase genotype, plasma concentration, and comorbidity in the very old and centenarians. Sci. Rep. 2021, 11, 8539. [Google Scholar] [CrossRef]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.A.; Moore, J.H.; Mesquita, P.H.C.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Fox, C.D.; Lopez, H.L.; Ziegenfuss, T.N.; Huggins, K.W.; et al. Resistance training increases muscle NAD(+) and NADH concentrations as well as NAMPT protein levels and global sirtuin activity in middle-aged, overweight, untrained individuals. Aging (Albany NY) 2020, 12, 9447–9460. [Google Scholar] [CrossRef]
- Feduccia, A.A.; Wang, Y.; Simms, J.A.; Yi, H.Y.; Li, R.; Bjeldanes, L.; Ye, C.; Bartlett, S.E. Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors. Pharm. Biochem. Behav. 2012, 102, 241–248. [Google Scholar] [CrossRef]
- Koupenova, M.; Johnston-Cox, H.; Vezeridis, A.; Gavras, H.; Yang, D.; Zannis, V.; Ravid, K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 2012, 125, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Yang, J.H.; Bae, M.J.; Yoo, W.K.; Ye, S.; Xue, C.C.; Li, C.G. Anti-oxidant and Anti-hypercholesterolemic Activities of Wasabia japonica. Evid. Based Complement. Altern. Med. 2010, 7, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwon, M.H.; Im, Y.S.; Seo, A.R.; Kim, K.Y.; Moon, H.R.; Yun, J.M. Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice. Nutrients 2020, 12, 3657. [Google Scholar] [CrossRef] [PubMed]
- Gwon, M.H.; Yun, J.M. Phenethyl Isothiocyanate Improves Lipid Metabolism and Inflammation via mTOR/PPARgamma/AMPK Signaling in the Adipose Tissue of Obese Mice. J. Med. Food 2021, 24, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.T.; Liu, Y.T.; Huang, C.S.; Lo, C.W.; Yao, H.T.; Chen, H.W.; Lii, C.K. Benzyl Isothiocyanate and Phenethyl Isothiocyanate Inhibit Adipogenesis and Hepatosteatosis in Mice with Obesity Induced by a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 7136–7146. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Li, Y.F.; Zhai, Y.J.; Chen, W.M.; Kurihara, H.; He, R.R. Theacrine, a purine alkaloid obtained from Camellia assamica var. kucha, attenuates restraint stress-provoked liver damage in mice. J. Agric. Food Chem. 2013, 61, 6328–6335. [Google Scholar] [CrossRef] [PubMed]
- Goody, M.F.; Henry, C.A. A need for NAD+ in muscle development, homeostasis, and aging. Skelet. Muscle 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rongvaux, A.; Andris, F.; Van Gool, F.; Leo, O. Reconstructing eukaryotic NAD metabolism. Bioessays 2003, 25, 683–690. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [Green Version]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Makarov, M.V.; Trammell, S.A.J.; Migaud, M.E. The chemistry of the vitamin B3 metabolome. Biochem. Soc. Trans. 2019, 47, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Conze, D.; Brenner, C.; Kruger, C.L. Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci. Rep. 2019, 9, 9772. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.K.; Sato, T.; Takanashi, Y.; Tamannaa, Z.; Kitamoto, T.; Odagiri, K.; Setou, M. A single oral supplementation of nicotinamide within the daily tolerable upper level increases blood NAD+ levels in healthy subjects. Transl. Med. Aging 2021, 5, 43–51. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Su, H.M.; Lin, Y.S.; Tsou, P.Y.; Chyuan, J.H.; Chao, P.M. A conjugated fatty acid present at high levels in bitter melon seed favorably affects lipid metabolism in hepatocytes by increasing NAD(+)/NADH ratio and activating PPARalpha, AMPK and SIRT1 signaling pathway. J. Nutr. Biochem. 2016, 33, 28–35. [Google Scholar] [CrossRef] [PubMed]
| Variable | CTL (n = 15) | NAD3 (n = 13) | p-Value |
|---|---|---|---|
| Gender | 6 M/9 F | 6 M/7 F | - |
| Age (years) | 51 ± 5 | 52 ± 6 | 0.57 |
| BMI (kg/m2) | 28.3 ± 3.9 | 29.0 ± 5 | 0.68 |
| Variable | Group | Pre | Post | 2 × 2 ANOVA p-Values | |
|---|---|---|---|---|---|
| Body Mass (kg) | CTL NAD3 | 80.0 ± 15.2 87.2 ± 20.9 | 80.5 ± 15.7 87.6 ± 21.9 | Group Time G × T | 0.70 0.13 0.77 |
| Systolic Blood Pressure (mm Hg) | CTL NAD3 | 128 ± 15 127 ± 11 | 127 ± 9 129 ± 8 | Group Time G × T | 0.88 0.83 0.52 |
| Diastolic Blood Pressure (mm Hg) | CTL NAD3 | 80 ± 7 78 ± 10 | 80 ± 8 80 ± 7 | Group Time G × T | 0.72 0.38 0.66 |
| Variable | Group | Pre | Post | 2 × 2 ANOVA | |
|---|---|---|---|---|---|
| WBCs (cells × 103/μL) | CTL | 5.46 ± 1.22 | 5.39 ± 1.17 | Group | 0.20 |
| NAD3 | 6.31 ± 2.69 | 6.51 ± 2.72 | Time | 0.86 | |
| G × T | 0.69 | ||||
| RBCs (cells × 103/μL) | CTL | 4.53 ± 0.54 | 4.57 ± 0.52 | Group | 0.78 |
| NAD3 | 4.44 ± 0.41 | 4.55 ± 0.39 | Time | 0.04 | |
| G × T | 0.28 | ||||
| Hemoglobin (g/dL) | CTL | 13.7 ± 1.9 | 13.9 ± 1.7 | Group | 0.66 |
| NAD3 | 14.0 ± 1.4 | 14.2 ± 1.3 | Time | 0.33 | |
| G × T | 0.80 | ||||
| Hematocrit (%) | CTL | 40.3 ± 4.6 | 41.5 ± 4.4 | Group | 0.82 |
| NAD3 | 40.6 ± 3.7 | 42.0 ± 2.9 | Time | 0.005 | |
| G × T | 0.82 | ||||
| Platelets (×103/uL) | CTL | 258 ± 83 | 247 ± 58 | Group | 0.23 |
| NAD3 | 282 ± 68 | 289 ± 62 | Time | 0.69 | |
| G × T | 0.18 | ||||
| Neutrophils (%) | CTL | 56.0 ± 6.9 | 55.5 ± 10.6 | Group | 0.61 |
| NAD3 | 52.6 ± 9.4 | 55.6 ± 10.6 | Time | 0.40 | |
| G × T | 0.25 | ||||
| Lymphocytes (%) | CTL | 32.3 ± 6.4 | 32.5 ± 7.0 | Group | 0.78 |
| NAD3 | 34.3 ± 8.4 | 32.1 ± 9.0 | Time | 0.40 | |
| G × T | 0.34 | ||||
| Monocytes (%) | CTL | 8.85 ± 1.68 | 9.08 ± 2.33 | Group | 0.36 |
| NAD3 | 8.25 ± 2.26 | 8.00 ± 3.49 | Time | 0.98 | |
| G × T | 0.61 | ||||
| Eosinophils (%) | CTL | 2.39 ± 1.04 | 2.39 ± 1.33 | Group | 0.11 |
| NAD3 | 4.00 ± 2.92 | 3.58 ± 2.91 | Time | 0.42 | |
| G × T | 0.42 | ||||
| Basophils (%) | CTL | 0.39 ± 0.65 | 0.46 ± 0.52 | Group | 0.17 |
| NAD3 | 0.75 ± 0.62 | 0.75 ± 0.75 | Time | 0.73 | |
| G × T | 0.73 | ||||
| Glucose (mg/dL) | CTL | 91.9 ± 8.5 | 97.2 ± 13.2 | Group | 0.20 |
| NAD3 | 96.3 ± 11.8 | 96.7 ± 14.2 | Time | 0.86 | |
| G × T | 0.69 | ||||
| BUN (mg/dL) | CTL | 12.8 ± 2.0 | 13.5 ± 2.6 | Group | 0.47 |
| NAD3 | 13.3 ± 2.2 | 14.2 ± 2.7 | Time | 0.18 | |
| G × T | 0.84 | ||||
| Creatinine (mg/dL) | CTL | 0.81 ± 0.19 | 0.84 ± 0.19 | Group | 0.33 |
| NAD3 | 0.86 ± 0.21 | 0.95 ± 0.22 § | Time | 0.001 | |
| G × T | 0.02 | ||||
| BUN:Creatinine | CTL | 16.6 ± 5.2 | 16.6 ± 4.8 | Group | 0.56 |
| NAD3 | 16.2 ± 4.1 | 15.2 ± 3.1 | Time | 0.47 | |
| G × T | 0.47 | ||||
| Sodium (mEq/mL) | CTL | 139 ± 3 | 139 ± 2 | Group | 0.69 |
| NAD3 | 139 ± 3 | 139 ± 3 | Time | 0.87 | |
| G × T | 0.63 | ||||
| Potassium (mEq/mL) | CTL | 4.25 ± 0.23 | 4.28 ± 0.24 | Group | 0.96 |
| NAD3 | 4.27 ± 0.18 | 4.26 ± 0.28 | Time | 0.82 | |
| G × T | 0.70 | ||||
| Chloride (mEq/mL) | CTL | 102 ± 3 | 103 ± 2 | Group | 0.49 |
| NAD3 | 104 ± 3 | 103 ± 3 | Time | 0.48 | |
| G × T | 0.33 | ||||
| Calcium (mg/dL) | CTL | 9.35 ± 0.28 | 9.36 ± 0.36 | Group | 0.33 |
| NAD3 | 9.42 ± 0.31 | 9.52 ± 0.32 | Time | 0.41 | |
| G × T | 0.48 | ||||
| Total Protein (g/dL) | CTL | 6.92 ± 0.44 | 6.89 ± 0.43 | Group | 0.52 |
| NAD3 | 6.75 ± 0.40 | 6.86 ± 0.40 | Time | 0.54 | |
| G × T | 0.35 | ||||
| Albumin (g/dL) | CTL | 4.37 ± 0.29 | 4.35 ± 0.28 | Group | 0.44 |
| NAD3 | 4.42 ± 0.18 | 4.44 ± 0.25 | Time | 0.92 | |
| G × T | 0.62 | ||||
| Bilirubin (g/dL) | CTL | 0.67 ± 0.25 | 0.58 ± 0.17 | Group | 1.00 |
| NAD3 | 0.59 ± 0.26 | 0.55 ± 0.19 | Time | 0.10 | |
| G × T | 0.67 | ||||
| Alkaline Phosphatase (U/L) | CTL | 75.5 ± 20.7 | 78.7 ± 22.5 | Group | 0.48 |
| NAD3 | 70.2 ± 16.5 | 72.6 ± 21.8 | Time | 0.08 | |
| G × T | 0.78 | ||||
| AST (U/L) | CTL | 26.4 ± 18.6 | 26.4 ± 16.8 | Group | 0.46 |
| NAD3 | 24.2 ± 9.0 | 20.6 ± 6.7 | Time | 0.11 | |
| G × T | 0.11 | ||||
| ALT (U/L) | CTL | 30.8 ± 24.2 | 31.0 ± 24.2 | Group | 0.42 |
| NAD3 | 27.2 ± 11.0 | 22.8 ± 8.7 | Time | 0.11 | |
| G × T | 0.07 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, M.D.; Osburn, S.C.; Godwin, J.S.; Ruple, B.A.; La Monica, M.B.; Raub, B.; Sandrock, J.E.; Ziegenfuss, T.N.; Lopez, H.L. Enhance Trial: Effects of NAD3® on Hallmarks of Aging and Clinical Endpoints of Health in Middle Aged Adults: A Subset Analysis Focused on Blood Cell NAD+ Concentrations and Lipid Metabolism. Physiologia 2022, 2, 20-31. https://doi.org/10.3390/physiologia2010002
Roberts MD, Osburn SC, Godwin JS, Ruple BA, La Monica MB, Raub B, Sandrock JE, Ziegenfuss TN, Lopez HL. Enhance Trial: Effects of NAD3® on Hallmarks of Aging and Clinical Endpoints of Health in Middle Aged Adults: A Subset Analysis Focused on Blood Cell NAD+ Concentrations and Lipid Metabolism. Physiologia. 2022; 2(1):20-31. https://doi.org/10.3390/physiologia2010002
Chicago/Turabian StyleRoberts, Michael D., Shelby C. Osburn, Joshua S. Godwin, Bradley A. Ruple, Michael B. La Monica, Betsy Raub, Jennifer E. Sandrock, Tim N. Ziegenfuss, and Hector L. Lopez. 2022. "Enhance Trial: Effects of NAD3® on Hallmarks of Aging and Clinical Endpoints of Health in Middle Aged Adults: A Subset Analysis Focused on Blood Cell NAD+ Concentrations and Lipid Metabolism" Physiologia 2, no. 1: 20-31. https://doi.org/10.3390/physiologia2010002
APA StyleRoberts, M. D., Osburn, S. C., Godwin, J. S., Ruple, B. A., La Monica, M. B., Raub, B., Sandrock, J. E., Ziegenfuss, T. N., & Lopez, H. L. (2022). Enhance Trial: Effects of NAD3® on Hallmarks of Aging and Clinical Endpoints of Health in Middle Aged Adults: A Subset Analysis Focused on Blood Cell NAD+ Concentrations and Lipid Metabolism. Physiologia, 2(1), 20-31. https://doi.org/10.3390/physiologia2010002







