Hematological Parameters and Iron Status in Adult Men and Women Using Altitude Adjusted and Unadjusted Hemoglobin Values for Anemia Diagnosis in Cusco, Peru (3400 MASL)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Design
2.2. Enrolment and Variables Measured
2.3. Sampling and Laboratory Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassebaum, N.J. The Global Burden of Anemia. Hematol. Oncol. Clin. N. Am. 2016, 30, 247–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, K.d.S.; Yamaji, N.; Rahman, M.O.; Suto, M.; Takemoto, Y.; Garcia-Casal, M.N.; Ota, E. Nutrition-specific interventions for preventing and controlling anaemia throughout the life cycle: An overview of systematic reviews. Cochrane Database Syst Rev. 2021, 26, CD013092. [Google Scholar] [CrossRef]
- Toki, Y.; Ikuta, K.; Kawahara, Y.; Niizeki, N.; Kon, M.; Enomoto, M.; Tada, Y.; Hatayama, M.; Yamamoto, M.; Ito, S.; et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron deficiency. Int. J. Hematol. 2017, 106, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 12 February 2021).
- Addo, O.Y.; Yu, E.X.; Williams, A.M.; Young, M.F.; Sharma, A.J.; Mei, Z.; Kassebaum, N.J.; Jefferds, M.E.D.; Suchdev, P.S. Evaluation of Hemoglobin Cutoff Levels to Define Anemia Among Healthy Individuals. JAMA Netw. Open 2021, 4, e2119123. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Tapia, V.; Vásquez-Velásquez, C. Changes in hemoglobin levels with age and altitude in preschool-aged children in Peru: The assessment of two individual-based national databases. Ann. N. Y. Acad. Sci. 2021, 1488, 67–82. [Google Scholar] [CrossRef]
- Mellors, P.W.; Kourelis, T.; Go, R.S.; Muchtar, E.; Gertz, M.A.; Kumar, S.K.; Buadi, F.K.; Kapoor, P.; Lacy, M.Q.; Dingli, D.; et al. Characteristics and risk factors for thrombosis in POEMS syndrome: A retrospective evaluation of 230 patients. Am. J. Hematol. 2021, 11, 26422. [Google Scholar] [CrossRef]
- Mangin, O. High oxygen affinity hemoglobins. Rev. Med. Interne 2017, 38, 106–112. [Google Scholar] [CrossRef]
- Tremblay, J.C.; Hoiland, R.L.; Howe, C.A.; Coombs, G.B.; Vizcardo-Galindo, G.A.; Figueroa-Mujíca, R.J.; Bermudez, D.; Gibbons, T.D.; Stacey, B.S.; Bailey, D.M.; et al. Global REACH 2018: High Blood Viscosity and Hemoglobin Concentration Contribute to Reduced Flow-Mediated Dilation in High-Altitude Excessive Erythrocytosis. Hypertension 2019, 73, 1327–1335. [Google Scholar] [CrossRef]
- Wu, T.Y.; Wang, X.Q.; Wei, C.Y.; Cheng, H.; Wang, X.; Li, Y.; Dong, G.; Zhao, H.; Young, P.; Li, G.; et al. Hemoglobin levels in Tibet: Different effect of age and gender for Tibetans vs Han. Comp. Clin. Path. 2005, 14, 25–35. [Google Scholar] [CrossRef]
- Gonzales, G.F. Peruvian contributions to the study on human reproduction at high altitude: From the chronicles of the Spanish conquest to the present. Respir. Physiol. Neurobiol. 2007, 158, 172–179. [Google Scholar] [CrossRef]
- Stuber, T.; Scherrer, U. Circulatory Adaptation to Long-Term High Altitude Exposure in Aymaras and Caucasians. Prog. Cardiovasc. Dis. 2010, 52, 534–539. [Google Scholar] [CrossRef]
- Instituto de Estadistica e Informática I. Indicadores de Resultados de Programas Presupuestales 2015–2020. Encuesta Demográfica y de Salud Familiar; Lima, Peru, 2021. Available online: https://proyectos.inei.gob.pe/endes/2020/ppr/Indicadores_de_Resultados_de_los_Programas_Presupuestales_ENDES_2020.pdf (accessed on 29 December 2021).
- Gonzales, G.F.; Tapia, V.; Gasco, M.; Carrillo, C.E.; Fort, A.L. Association of hemoglobin values at booking with adverse maternal outcomes among Peruvian populations living at different altitudes. Int. J. Gynecol. Obstet. 2012, 117, 134–139. [Google Scholar] [CrossRef]
- Beall, C.M.; Brittenham, G.M.; Strohl, K.P.; Blangero, J.; Williams-Blangero, S.; Goldstein, M.C.; Decker, M.J.; Vargas, E.; Villena, M.; Soria, R.; et al. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am. J. Phys. Anthropol 1998, 106, 385–400. [Google Scholar] [CrossRef]
- Rothhammer, F.; Fuentes-Guajardo, M.; Chakraborty, R.; Lorenzo Bermejo, J.; Dittmar, M. Neonatal Variables, Altitude of Residence and Aymara Ancestry in Northern Chile. PLoS ONE 2015, 10, e0121834. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.D.; Boy, E.; Flowers, C.; Mdel, C.D. The influence of high-altitude living on body iron. Blood 2005, 106, 1441–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado, A.; Gonzales, G.F. Pulse oxygen saturation in healthy newborns at term in Cusco, Peru. Int. J. Gynecol. Obstet. 2006, 95, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Salirrosas, A. Arterial oxygen saturation in healthy newborns delivered at term in Cerro de Pasco (4340 m) and Lima (150 m). Reprod. Biol. Endocrinol. 2005, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.M.; Callacondo, D.; Rojas-Camayo, J.; Quesada-Olarte, J.; Wang, X.; Uchida, N.; Maric, I.; Remaley, A.T.; Leon-Velarde, F.; Villafuerte, F.C.; et al. SENP1, but not fetal hemoglobin, differentiates Andean highlanders with chronic mountain sickness from healthy individuals among Andean highlanders. Exp. Hematol. 2016, 44, 483–490.e2. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Yaquetto, D.E.; Caballero, L.; Gonzales, G.F. Association between Plasma N-Acylethanolamides and High Hemoglobin Concentration in Southern Peruvian Highlanders. High. Alt. Med. Biol. 2017, 18, 322–329. [Google Scholar] [CrossRef]
- Sarna, K.; Gebremedin, A.; Brittenham, G.M.; Beall, C.M. WHO hemoglobin thresholds for altitude increase the prevalence of anemia among Ethiopian highlanders. Am. J. Hematol. 2018, 93, E229–E231. [Google Scholar] [CrossRef] [Green Version]
- Sarna, K.; Brittenham, G.M.; Beall, C.M. Current WHO hemoglobin thresholds for altitude and misdiagnosis of anemia among Tibetan highlanders. Am. J. Hematol. 2020, 95, E134–E136. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Begazo, J.; Alarcón-Yaquetto, D.E. Suitability of Haemoglobin Adjustment to Define Anaemia at High Altitudes. Acta Haematol. 2020, 143, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Choque-Quispe, B.M.; Alarcón-Yaquetto, D.E.; Paredes-Ugarte, W.; Zaira, A.; Ochoa, A.; Gonzales, G.F. Is the prevalence of anemia in children living at high altitudes real? An observational study in Peru. Ann. N. Y. Acad. Sci. 2020, 1473, 35–47. [Google Scholar] [CrossRef]
- Choque-Quispe, B.M.; Paz, V.; Gonzales, G.F. Proportion of anemia attributable to iron deficiency in high-altitude infant populations. Ann. Hematol. 2019, 98, 2601–2603. [Google Scholar] [CrossRef] [PubMed]
- Rujeerapaiboon, N.; Tantiworawit, A.; Piriyakhuntorn, P.; Rattanathammethee, T.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Norasetthada, L.; Fanhchaksai, K.; Charoenkwan, P. Correlation Between Serum Ferritin and Viral Hepatitis in Thalassemia Patients. Hemoglobin 2021, 45, 175–179. [Google Scholar] [CrossRef]
- Beguin, Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin. Chim. Acta 2003, 329, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Koistinen, P.O.; Rusko, H.; Irjala, K.; Rajamäki, A.; Penttinen, K.; Sarparanta, V.P.; Karpakka, J.; Leppäluoto, J. EPO, red cells, and serum transferrin receptor in continuous and intermittent hypoxia. Med. Sci. Sports Exerc. 2000, 32, 800–804. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Looker, A.C. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017, 106, 1606S–1614S. [Google Scholar] [CrossRef] [Green Version]
- Hanif, N.; Anwer, F. Chronic Iron Deficiency. 2021. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32809711 (accessed on 29 December 2021).
- Gonzales, G.F.; Rubín de Celis, V.; Begazo, J.; Del Rosario Hinojosa, M.; Yucra, S.; Zevallos-Concha, A.; Tapia, V. Correcting the cut-off point of hemoglobin at high altitude favors misclassification of anemia, erythrocytosis and excessive erythrocytosis. Am. J. Hematol. 2018, 93, E12–E16. [Google Scholar] [CrossRef] [PubMed]
- Manckoundia, P.; Barben, J.; Asgassou, S.; Putot, A.; Konaté, A. Iron deficiency in adults: To understand what biological evaluation should be carried out. Rev. Med. Liege 2020, 75, 791–796. [Google Scholar]
- Manckoundia, P.; Konaté, A.; Hacquin, A.; Nuss, V.; Mihai, A.M.; Vovelle, J.; Dipanda, M.; Putot, S.; Barben, J.; Putot, A. Iron in the General Population and Specificities in Older Adults: Metabolism, Causes and Consequences of Decrease or Overload, and Biological Assessment. Clin. Interv. Aging 2020, 15, 1927–1938. [Google Scholar] [CrossRef]
- Donker, A.E.; van der Staaij, H.; Swinkels, D.W. The critical roles of iron during the journey from fetus to adolescent: Developmental aspects of iron homeostasis. Blood Rev. 2021, 50, 100866. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyszyn, M.; Comín-Colet, J.; Voors, A.A.; van Veldhuisen, D.J.; Enjuanes, C.; Moliner-Borja, P.; Rozentryt, P.; Poloński, L.; Banasiak, W.; Ponikowski, P.; et al. Iron deficiency and red cell indices in patients with heart failure. Eur. J. Heart Fail. 2018, 20, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.N.; Wang, S.J.; Chen, C.; Rausch, V.; Elshaarawy, O.; Mueller, S. Direct modulation of hepatocyte hepcidin signaling by iron. World J. Hepatol. 2021, 13, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Bardou-Jacquet, E. Revisiting hemochromatosis: Genetic vs. phenotypic manifestations. Ann. Transl. Med. 2021, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- León-Velarde, F.; Maggiorini, M.; Reeves, J.T.; Aldashev, A.; Asmus, I.; Bernardi, L.; Ge, R.L.; Hackett, P.; Kobayashi, T.; Moore, L.G.; et al. Consensus Statement on Chronic and Subacute High Altitude Diseases. High. Alt. Med. Biol. 2005, 6, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.J.; Addo, O.Y.; Mei, Z.; Suchdev, P.S. Reexamination of hemoglobin adjustments to define anemia: Altitude and smoking. Ann. N. Y. Acad. Sci. 2019, 1450, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Abitbol, V.; Borderie, D.; Polin, V.; Maksimovic, F.; Sarfati, G.; Esch, A.; Tabouret, T.; Dhooge, M.; Dreanic, J.; Perkins, G.; et al. Diagnosis of iron deficiency in inflammatory bowel disease by transferrin receptor-ferritin index. Medicine 2015, 94, e1011. [Google Scholar] [CrossRef]
- Suega, K.; Kandarini, Y.; Tubung, J. Role of Soluble Transferrin Receptor and Transferrin Receptor-Ferritin Index to Detect Iron Deficiency Anemia in Regular Hemodialysis Patients. Open Access Maced. J. Med. Sci. 2019, 7, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallianpur, A.R.; Wen, W.; Erwin, A.L.; Clifford, D.B.; Hulgan, T.; Robbins, G.K. Higher iron stores and the HFE 187C> G variant delay onset of peripheral neuropathy during combination antiretroviral therapy. PLoS ONE. 2020, 15, e0239758. [Google Scholar] [CrossRef]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Alende-Castro, V.; Alonso-Sampedro, M.; Gude, F.; Gonzalez-Quintela, A. Serum Concentrations of Interleukin 6 in the General Adult Population: Possible Implications for Anti-IL-6 Therapy in SARS-Cov-2 Infection and IL-6-Related Diseases. J. Investig. Allergol. Clin. Immunol. 2021, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.R. Red Cell Indices. Butterworths; 1990. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21250103 (accessed on 10 March 2021).
- Gonzales, G.F.; Steenland, K.; Tapia, V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R1477–R1485. [Google Scholar] [CrossRef] [Green Version]
- Aparco Balboa, J.P.; Bullón, L.; Cusirramos, S. Impact of micronutrient powder on anemia in children aged 10-35 months in apurimac, Peru. Rev. Peru Med. Exp. Salud Publica 2019, 36, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Richalet, J.-P. Adaptation à l’hypoxie chronique des populations de haute altitude. Rev. Mal. Respir. 2021, 38, 395–403. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Breenfeldt Andersen, A.; Bonne, T.C.; Bejder, J.; Jung, G.; Ganz, T.; Nemeth, E.; Olsen, N.V.; Huertas, J.R.; Nordsborg, N.B. Effects of altitude and recombinant human erythropoietin on iron metabolism: A randomized controlled trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R152–R161. [Google Scholar] [CrossRef] [PubMed]
- Emrich, I.E.; Scheuer, A.; Wagenpfeil, S.; Ganz, T.; Heine, G.H. Increase of plasma erythroferrone levels during high-altitude exposure: A sub-analysis of the TOP OF HOMe study. Am. J. Hematol. 2021, 96, E179–E181. [Google Scholar] [CrossRef]
- Lundgrin, E.L.; Janocha, A.J.; Koch, C.D.; Gebremedhin, A.; Di Rienzo, A.; Alkorta-Aranburu, G.; Brittenham, G.M.; Erzurum, S.C.; Beall, C.M. Plasma hepcidin of Ethiopian highlanders with steady-state hypoxia. Blood 2013, 122, 1989–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theurl, I.; Mattle, V.; Seifert, M.; Mariani, M.; Marth, C.; Weiss, G. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood 2006, 107, 4142–4148. [Google Scholar] [CrossRef] [Green Version]
- Belo, L.; Rocha, S.; Valente, M.J.; Coimbra, S.; Catarino, C.; Bronze-da-Rocha, E.; Rocha-Pereira, P.; do Sameiro-Faria, M.; Oliveira, J.G.; Madureira, J.; et al. Hepcidin and diabetes are independently related with soluble transferrin receptor levels in chronic dialysis patients. Ren. Fail. 2019, 41, 662–672. [Google Scholar] [CrossRef] [Green Version]
- Krawiec, P.; Pac-Kożuchowska, E. Soluble transferrin receptor and soluble transferrin receptor/log ferritin index in diagnosis of iron deficiency anemia in pediatric inflammatory bowel disease. Dig. Liver. Dis. 2019, 51, 352–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, B.; McNeil, Y.; Brewster, D.R. Soluble transferrin receptor in Aboriginal children with a high prevalence of iron deficiency and infection. Trop. Med. Int. Health. 2004, 9, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Findik, D.D. Gender-related variations in iron metabolism and liver diseases. World J. Hepatol 2010, 2, 302. [Google Scholar] [CrossRef]
- Kong, W.-N.; Niu, Q.-M.; Ge, L.; Zhang, N.; Yan, S.F.; Chen, W.B.; Chang, Y.Z.; Zhao, S.E. Sex Differences in Iron Status and Hepcidin Expression in Rats. Biol. Trace Elem. Res. 2014, 160, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3363. [Google Scholar] [CrossRef] [Green Version]
- Gromadzka, G.; Wierzbicka, D.; Litwin, T.; Przybyłkowski, A. Difference in iron metabolism may partly explain sex-related variability in the manifestation of Wilson’s disease. J. Trace Elem. Med. Biol. 2020, 62, 126637. [Google Scholar] [CrossRef]
- Rauber, M.R.; Pilger, D.A.; Cecconello, D.K.; Falcetta, F.S.; Marcondes, N.A.; Faulhaber, G.A.M. Hepcidin is a useful biomarker to evaluate hyperferritinemia associated with metabolic syndrome. An. Acad. Bras. Cienc. 2019, 91, e20180286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rametta, R.; Dongiovanni, P.; Pelusi, S.; Francione, P.; Iuculano, F.; Borroni, V.; Fatta, E.; Castagna, A.; Girelli, D.; Fargion, S.; et al. Hepcidin resistance in dysmetabolic iron overload. Liver Int. 2016, 36, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Rametta, R.; Fracanzani, A.L.; Fargion, S.; Dongiovanni, P. Dysmetabolic Hyperferritinemia and Dysmetabolic Iron Overload Syndrome (DIOS): Two Related Conditions or Different Entities? Curr. Pharm. Des. 2020, 26, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, J.; Saxena, R.; Choudhry, V.P.; Dwivedi, S.N.; Bhargava, M. Erythrocyte indices for discriminating thalassaemic and non-thalassaemic microcytosis in Indians. Natl. Med. J. India 1999, 12, 266–267. [Google Scholar]
- Needs, T.; Gonzalez-Mosquera, L.F.; Lynch, D.T. Beta Thalassemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 18 July 2021. [Google Scholar]
- Bajwa, H.; Basit, H. Thalassemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 11 August 2021. [Google Scholar]
- Castillo, J. Hemoglobinas anormales en el Perú, su importancia genética y antropológica en nuestro mestizaje [Abnormal hemoglobins in Peru, their genetic and anthropological importance in our miscegenation]. An. Acad. Nac. Med. 2002, 1–15. Available online: https://anmperu.org.pe/anales/2002/VI_HEMOGLOBINASANORMALESENELPERU.pdf (accessed on 29 December 2021).
- Schoorl, M.; Schoorl, M.; van der Gaag, D.; Bartels, P.C. Effects of iron supplementation on red blood cell hemoglobin content in pregnancy. Hematol. Rep. 2012, 4, e24. [Google Scholar] [CrossRef]
- Gonzales, E.; Huamán-Espino, L.; Gutiérrez, C.; Aparco, J.P.; Pillaca, J. Caracterización de la anemia en niños menores de cinco años de zonas urbanas de Huancavelica y Ucayali en el Perú [Characterization of anemia in children under five years of age from urban areas of Huancavelica and Ucayali, Peru]. Rev. Peru. Med. Exp. Salud Publica 2015, 32, 431–439. [Google Scholar] [CrossRef] [PubMed]
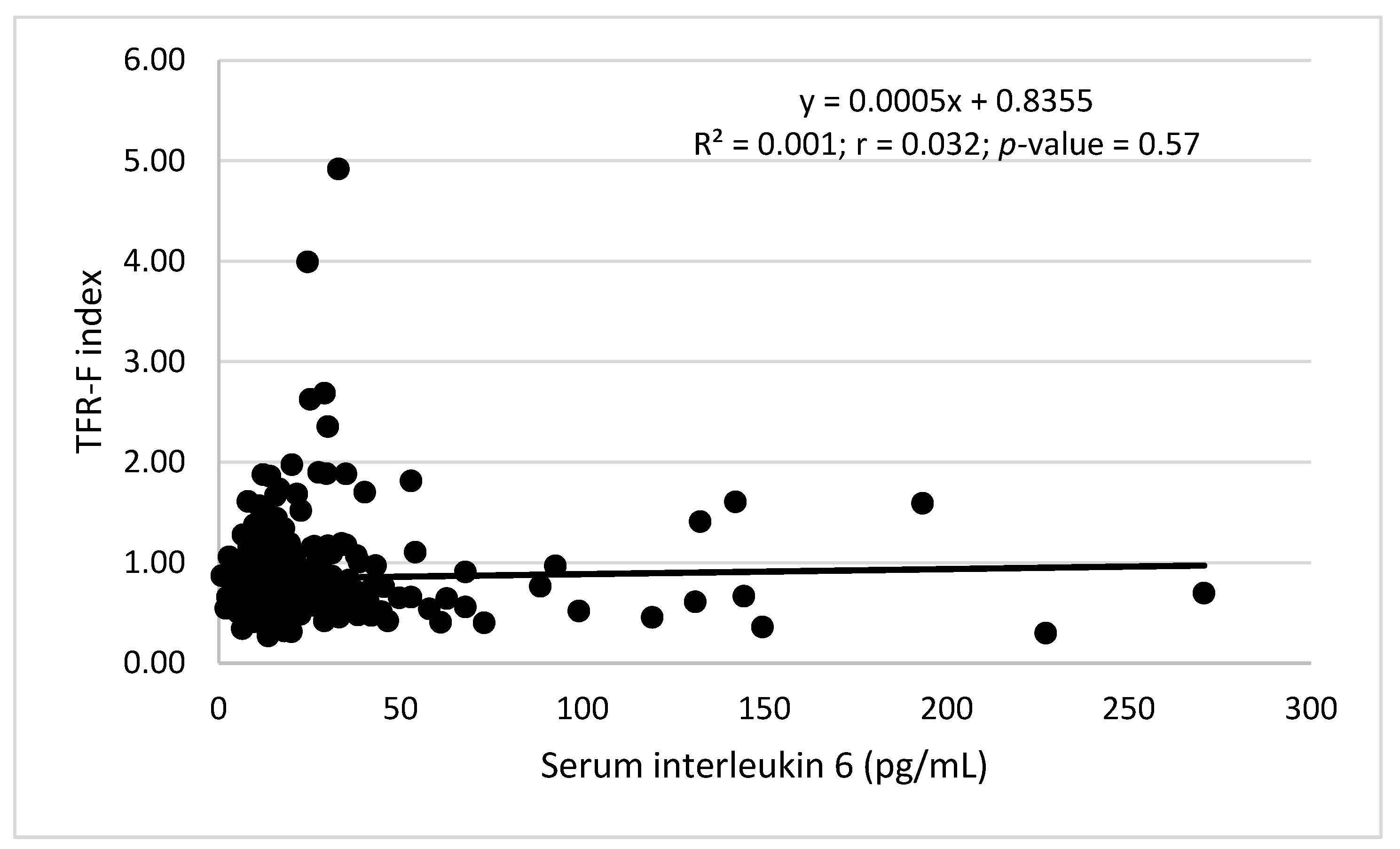




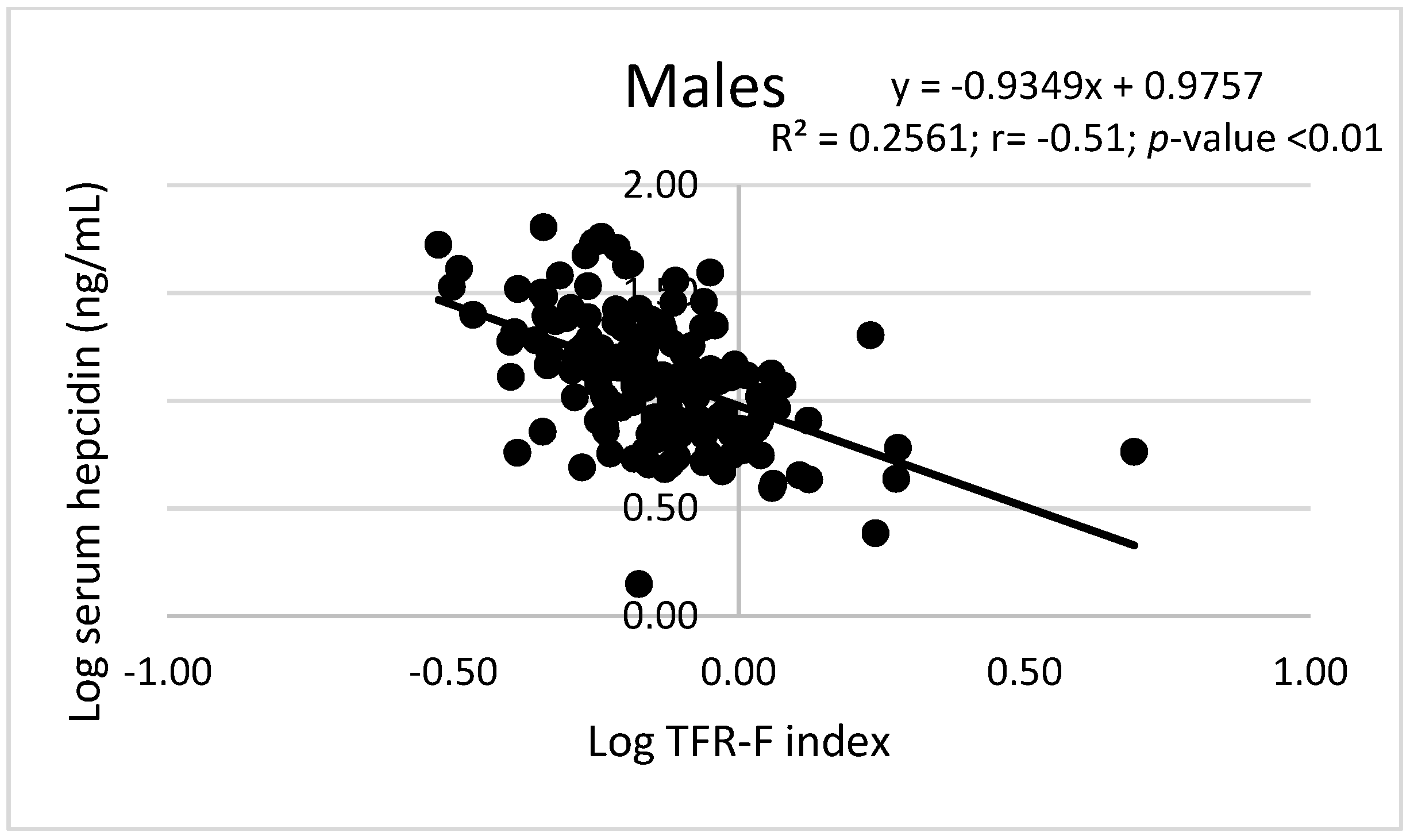

| Characteristics | Men (156) | Women (189) | Overall (345) |
|---|---|---|---|
| Age (years) | 46.23 [16.86] | 44.80 [14.42] | 45.42 [15.59] |
| BMI (kg/m2) | 26.27 [4.12] | 27.37 [4.95] | 26.91 [4.64] |
| SBP (mm Hg) | 118.40 [14.36] | 117.65 [14.97] | 117.97 [14.67] |
| DBP (mm Hg) | 75.76 [9.49] | 75.20 [12.09] | 75.44 [10.96] |
| SpO2 (%) | 91.51 [3.12] | 91.06 [3.16] | 91.25 [3.16] |
| Hb (g/dL) | 17.29 [0.99] | 15.35 [1.09] | 16.23 [1.48] |
| Adjusted Hb (g/dL) | 14.79 [0.99] | 12.85 [1.09] | 13.73 [1.48] |
| Hematocrit (%) | 50.21 [3.24] | 45.14 [2.88] | 47.42 [3.89] |
| RBC (*106) | 5.458 [0.362] | 4.948 [0.316] | 5.177 [0.427] |
| MCV (fL) | 91.93 [3.24] | 90.85 [6.32] | 91.34 [5.19] |
| MCH (pg) | 31.72 [1.15] | 31.14 [2.06] | 31.41 [1.67] |
| MCHC (g/dL) | 34.47 [0.62] | 34.29 [2.75] | 34.38 [2.04] |
| RDW-CV (%) | 12.09 [0.49] | 12.10 [0.69] | 12.09 [0.74] |
| Reticulocytes | 1.28 [0.37] | 1.30 [0.27] | 1.29 [0.37] |
| CMS score | 2.57 [2.62] | 3.84 [3.29] | 3.28 [2.97] |
| Biological Variable | Men (156) | Women (189) | Overall (345) |
|---|---|---|---|
| Ferritin (ng/mL) | 50.0 [45.71] | 24.43 [21.82] | 36.48 [37.32] |
| sTfR (ug/mL) | 1.13 [0.37] | 1.10 [0.41] | 1.11 [0.37] |
| TFR-F index | 0.77 [0.37] | 0.91 [0.41] | 0.85 [0.55] |
| Total Body Iron (mg/Kg) | 11.19 [2.99] | 9.05 [2.74] | 10.06 [2.97] |
| Hepcidin (ng/mL) | 16.41 [12.36] | 11.20 [8.65] | 13.65 [10.77] |
| Erythropoietin (mIU/mL) | 9.39 [5.87] | 10.53 [7.14] | 9.99 [6.49] |
| Men | Women | Total | |
|---|---|---|---|
| Anemia | 0/103 (0%) | 2/126 (1.55%) | 2/229 (0.87%) |
| Adjusted Anemia | 4/103 (3.9%) | 17/126 (13.5%) * | 21/229 (9.17%) |
| TFR-F index | |||
| >2 | 1 (0.68%) | 4 (2.39%) | 5 (1.58%) |
| 1–2 | 22 (14.86%) | 41 (24.55%) ** | 63 (20%) |
| <1 | 125 (84.45%) | 122 (73.05%) ** | 247 (78.41%) |
| Low MCV (<80 fL) | 0/103 (0%) | 4/126 (3.17%) | 4/229 (1.75%) |
| Low MCH (<27 pg/mL) | 0/103 (0%) | 4/126 (3.17%) | 4/229 (1.75%) |
| Low MCV and MCH | 0/103 (0%) | 3/126 (2.38%) | 3/229 (1.31%) |
| Low Hb, MCV and MCH | 0/103 (0%) | 2/126 (1.58%) | 2/229 (0.87%) |
| Elevated IL-6 (>50 pg/mL) | 9/148 (0%) | 12/167 (7.18%) | 21/315 (8.25%) |
| Anemia (2) | Adjusted Anemia (21) | |
|---|---|---|
| IDA | 0/2 (0%) | 0/21 (0%) |
| TFR-F Index | ||
| <1 | 1/2 (50%) | 13/21 (61.9%) |
| 1–2 | 1/2 (50%) | 5/21 (23.8%) |
| >2 | 0/2 (0%) | 3/21 (14.3%) * |
| Low MCV (<80 fL) | 2/2 (100%) | 3/21 (14.3%) * |
| Low MCH (<37 pg/mL) | 2/2 (100%) | 4/21 (19.0%) * |
| Low MCV and MCH | 2/2 (100%) | 3/21 (14.3%) * |
| Elevated IL-6 (>50 pg/mL) | 0/2 (0%) | 2/21 (9.5%) |
| Healthy Subjects | Adjusted Anemia TRF-F ≥ 1 | Adjusted Anemia TRF-F < 1 | Size Effect Cohen’s d | |
|---|---|---|---|---|
| Male/female | 116/115 | 0/8 | 4/9 | |
| Age | 45.85 [15.50] | 36.12 [10.32] * | 40.82 [11.34] | 0.43 |
| BMI | 26.94 [5.77] | 26.97 [6.03] | 25.47 [2.73] | 0.32 |
| ASP mm Hg | 117.24 [13.52] | 119.62 [19.03] | 110.41 [7.23] | 0.64 |
| ADP mm Hg | 74.86 [11.09] | 76.12 [10.86] | 72.58 [6.93] | 0.39 |
| SpO2 | 91.32 [3.19] | 92.12 [2.82] | 92.25 [1.59] | 0.05 |
| CMS score | 3.35 [3.19] | 3.37 [3.46] | 2.69 [2.46] | 0.23 |
| Hb | 16.38 [1.36 ] | 13.23 [0.98] * | 14.14 [1.29] * | 0.79 |
| Corrected Hb | 13.88 [1.36] | 10.73 [0.98] * | 11.63 [1.29] * | 0.79 |
| RBC count * 106 | 5.18 [0.45] | 4.97 [0.39] | 4.68 [0.3] * | 0.83 |
| MCV | 91.81 [5.31] | 81.58 [6.37] * | 90.0 [6.96] # | 1.26 |
| MCH | 31.65 [1.36] | 26.81 [3.04] * | 30.2 [2.73] ## | 1.17 |
| MCHC | 34.34 [0.75] | 32.75 [1.35] * | 33.49 [0.99] ** | 0.62 |
| RDW | 12.00 [0.61] | 13.47 [1.24] * | 12.62 [1.53] | 0.61 |
| Hepcidin | 15.21 [11.09] | 4.31 [2.25] * | 13.22 [6.42] # | 1.84 |
| Log hepcidin | 1.08 [0.30] | 0.59 [0.19] * | 1.03 [0.27] # | 1.88 |
| TBI (mg/Kg) | 11.0 [16.86] | 4.54 [1.63] * | 10.56 [1.41] # | 3.95 |
| TFR-F Index | 0.68 [0.15] | 1.90 [0.59] * | 0.62 [0.12] # | 3.00 |
| TFR-F Index < 1 | 231/231 (100%) | 0/8 (0%) * | 13/13 (100%) # | |
| TFR-F Index > 2 | 0/231 (0%) | 3/8 (37.5%) | 0/13 (0%) | |
| EPO | 8.92 [4.86] | 20.14 [10.85] * | 9.31 [6.18] ## | 1.22 |
| IL-6 | 16.50 [9.27] | 22.12 [9.02] | 28.88 [27.18] | 0.33 |
| Hemoglobin (g/dL) | Men Pearson Correlation, p | Women Pearson Correlation, p |
|---|---|---|
| Serum Ferritin | 0.05, >0.05 | 0.21, <0.05 |
| TBI | −0.01, >0.05 | 0.28, <0.01 |
| Log. Hepcidin | 0.07, >0.05 | 0.24, <0.01 |
| Serum EPO | −0.17, >0.05 | −0.32, <0.01 |
| TFR-F Index | Odds Ratio ± Std. Error | z | P | 95% | C.I |
|---|---|---|---|---|---|
| Sex | 1.33 ± 0.49 | 0.77 | 0.44 | 0.64 | 2.75 |
| Age (years) | 1.00 ± 0.01 | 0.22 | 0.83 | 0.98 | 1.02 |
| RBC count | 1.68 ± 0.76 | 1.15 | 0.25 | 0.69 | 4.06 |
| MCV (fL) | 1.01 ± 0.03 | 0.35 | 0.73 | 0.95 | 1.08 |
| RDW-CV | 2.04 ± 0.58 | 2.49 | 0.01 | 1.16 | 3.56 |
| Constant | 4.63 × 10 −6 ± 2.68 × 10−5 | −2.12 | 0.03 | 5.45 × 10 −11 | 0.39 |
| Adjusted Anemia (Men) | Odds Ratio ± Std. Error | z | P | 95% | C.I |
| RBC count | 0.0004 ± 0.002 | −2.01 | 0.045 | 1.87 × 10 −7 | 0.834 |
| MCV (fL) | 0.56 ± 0.154 | −2.10 | 0.035 | 0.330 | 0.961 |
| RDW-CV | 6.77 ± 13.197 | 0.98 | 0.327 | 0.148 | 309.32 |
| TFR-F Index | 0.068 ± 0.324 | −0.57 | 0.569 | 6.86 × 10 −6 | 691.21 |
| Constant | 3.04 × 10 29 ± 1.36 × 10 31 | 1.52 | 0.129 | 2.86 × 10−9 | 3.23 × 10 67 |
| Adjusted anemia (Women) | Odds Ratio ± Std. Error | z | P | 95% | C.I |
| RBC count | 0.0004 ± 0.0009 | −3.54 | 0.001 | 5.86 × 10−6 | 0.031 |
| MCV (fL) | 0.96 ± 0.04 | −0.84 | 0.401 | 0.891 | 1.047 |
| RDW-CV | 9.31 ± 6.58 | 3.16 | 0.002 | 2.331 | 37.214 |
| TFR-F Index | 2.60 ± 2.41 | 1.03 | 0.302 | 0.423 | 15.988 |
| Constant | 29,111.11 ± 272,116.3 | 1.10 | 0.271 | 0.0003 | 2.63 × 1012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón-Yaquetto, D.E.; Figueroa-Mujica, R.; Valverde-Bruffau, V.; Vásquez-Velásquez, C.; Sánchez-Huamán, J.J.; Jimenez-Troncoso, L.; Rozas-Gamarra, R.; Gonzales, G.F. Hematological Parameters and Iron Status in Adult Men and Women Using Altitude Adjusted and Unadjusted Hemoglobin Values for Anemia Diagnosis in Cusco, Peru (3400 MASL). Physiologia 2022, 2, 1-19. https://doi.org/10.3390/physiologia2010001
Alarcón-Yaquetto DE, Figueroa-Mujica R, Valverde-Bruffau V, Vásquez-Velásquez C, Sánchez-Huamán JJ, Jimenez-Troncoso L, Rozas-Gamarra R, Gonzales GF. Hematological Parameters and Iron Status in Adult Men and Women Using Altitude Adjusted and Unadjusted Hemoglobin Values for Anemia Diagnosis in Cusco, Peru (3400 MASL). Physiologia. 2022; 2(1):1-19. https://doi.org/10.3390/physiologia2010001
Chicago/Turabian StyleAlarcón-Yaquetto, Dulce E., Ramón Figueroa-Mujica, Valeria Valverde-Bruffau, Cinthya Vásquez-Velásquez, Juan José Sánchez-Huamán, Luis Jimenez-Troncoso, Rodrigo Rozas-Gamarra, and Gustavo F. Gonzales. 2022. "Hematological Parameters and Iron Status in Adult Men and Women Using Altitude Adjusted and Unadjusted Hemoglobin Values for Anemia Diagnosis in Cusco, Peru (3400 MASL)" Physiologia 2, no. 1: 1-19. https://doi.org/10.3390/physiologia2010001
APA StyleAlarcón-Yaquetto, D. E., Figueroa-Mujica, R., Valverde-Bruffau, V., Vásquez-Velásquez, C., Sánchez-Huamán, J. J., Jimenez-Troncoso, L., Rozas-Gamarra, R., & Gonzales, G. F. (2022). Hematological Parameters and Iron Status in Adult Men and Women Using Altitude Adjusted and Unadjusted Hemoglobin Values for Anemia Diagnosis in Cusco, Peru (3400 MASL). Physiologia, 2(1), 1-19. https://doi.org/10.3390/physiologia2010001







