In Vitro Disinfection Efficacy Assay on Giardia duodenalis Cysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Axenic Culture of Giardia Trophozoites
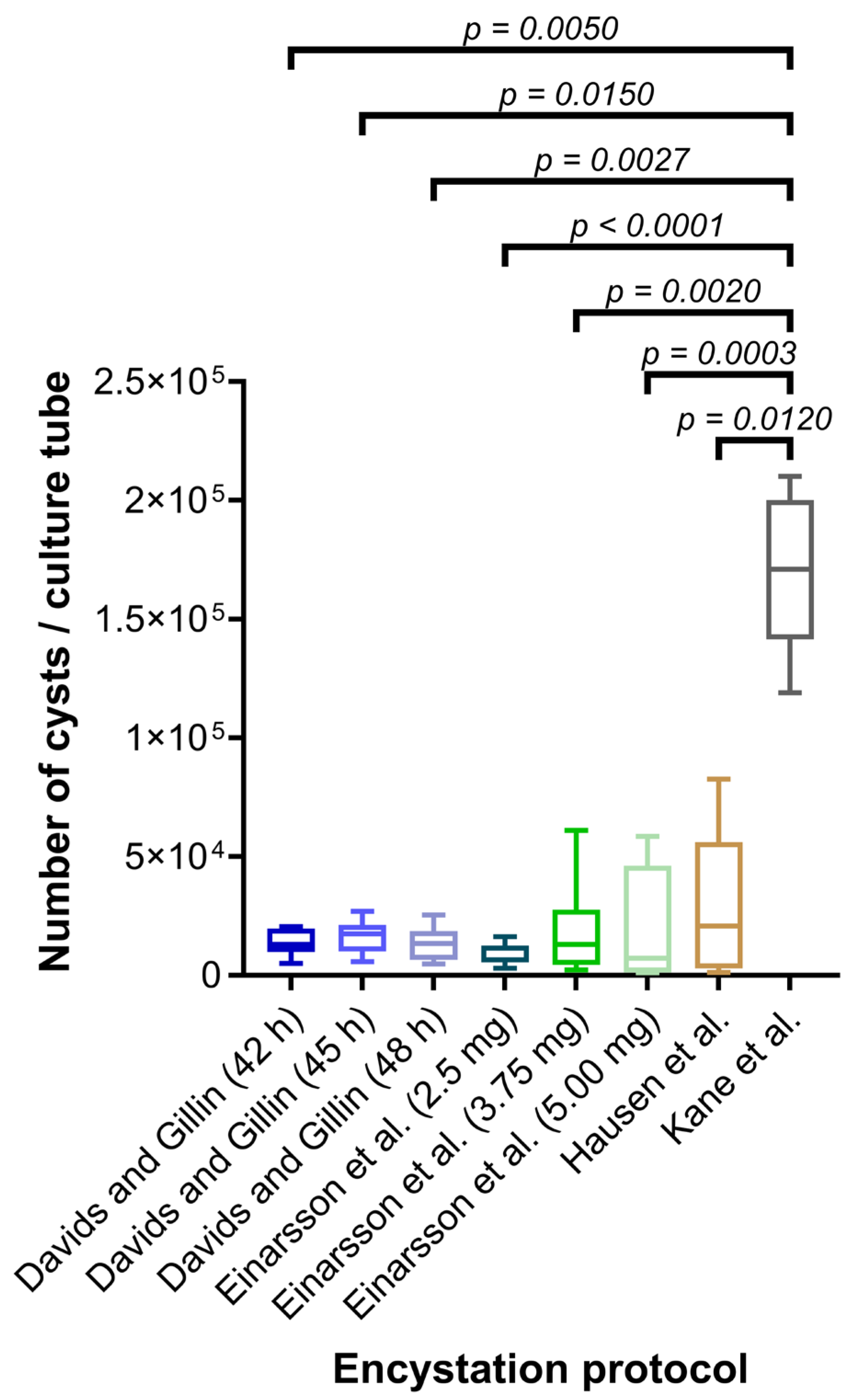
2.2. Comparison of Protocols for Encystation
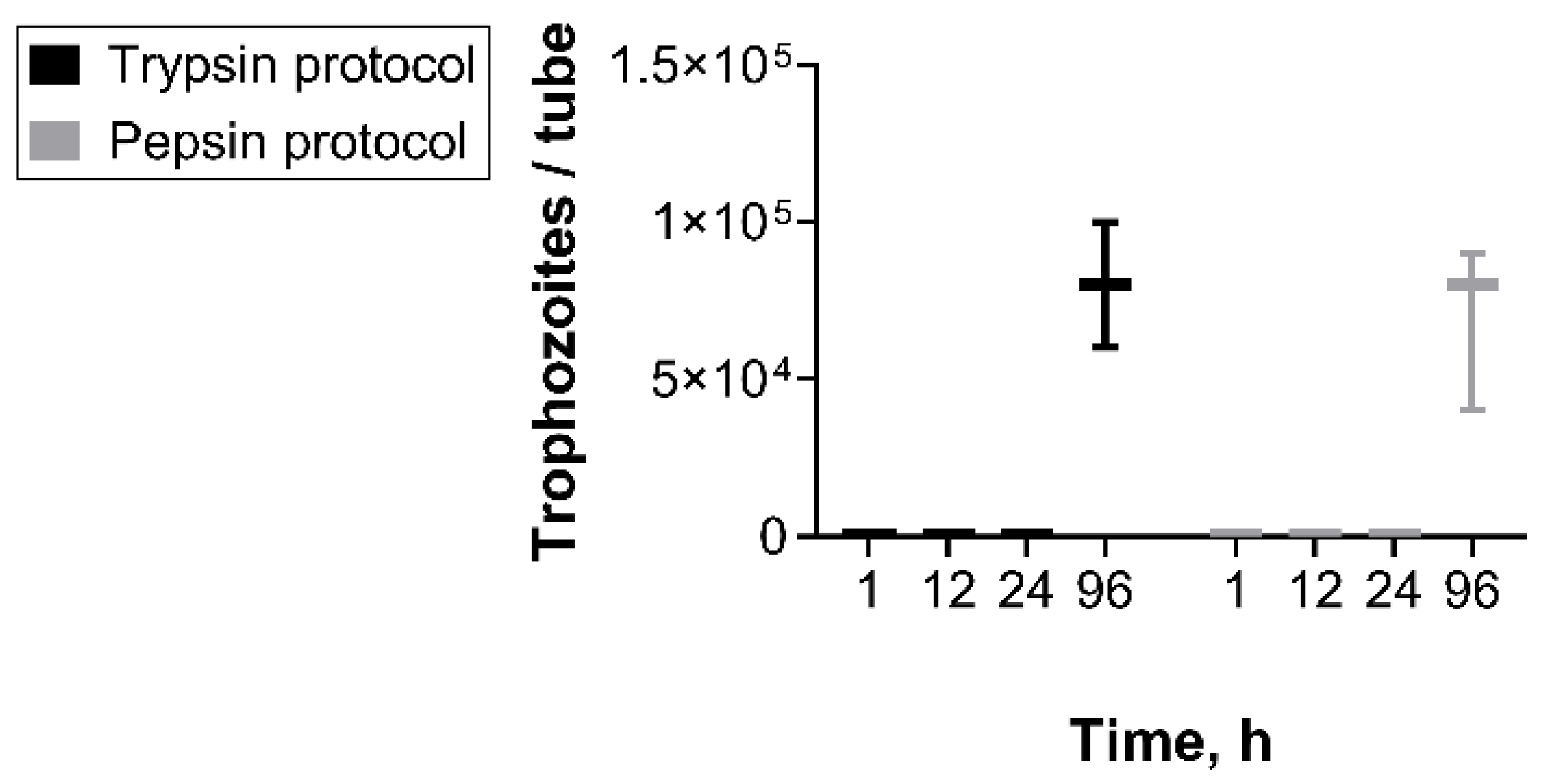
2.3. Comparison of Protocols for Excystation
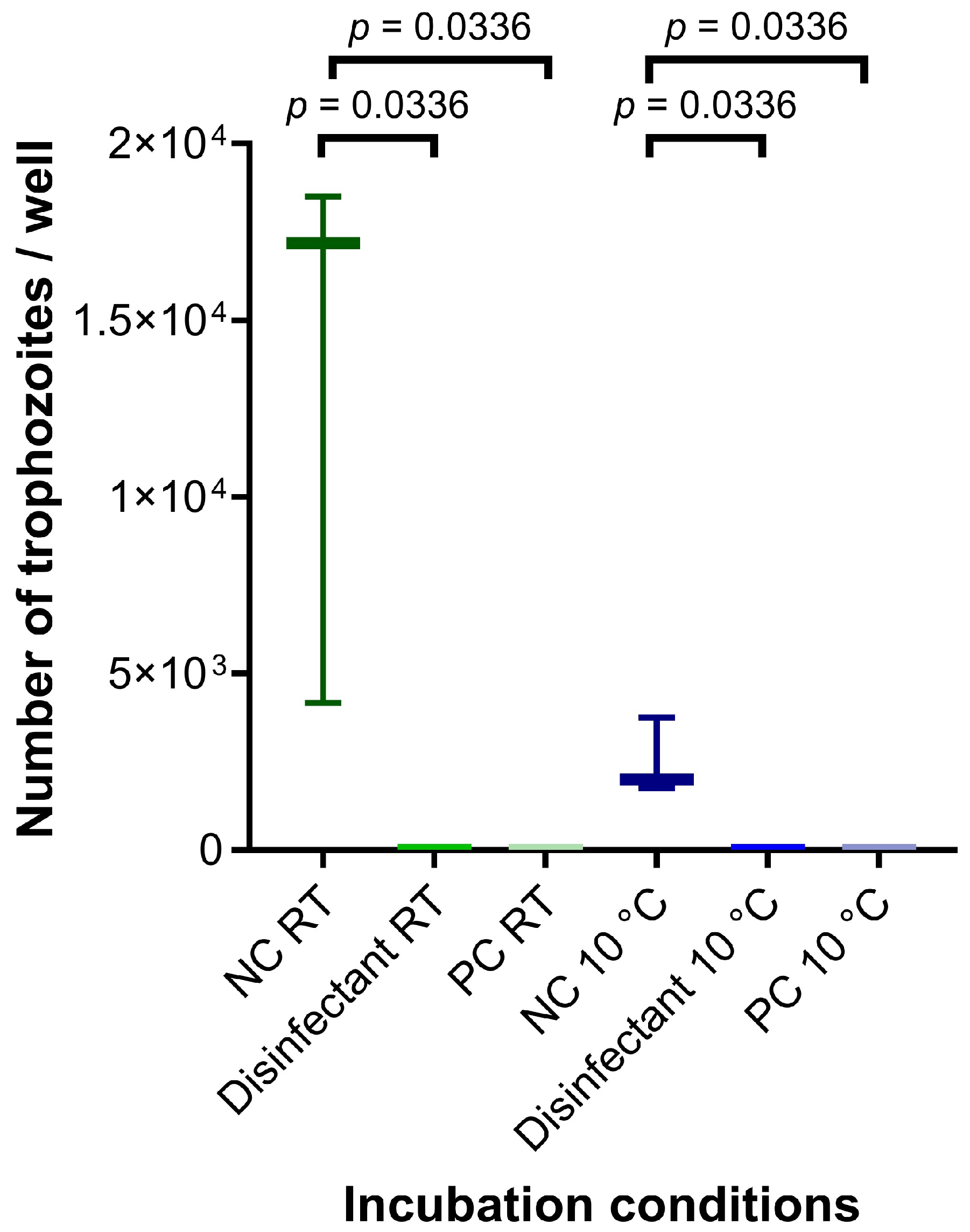
2.4. Assessment of Inactivation by Disinfection of In Vitro Obtained Cysts
2.5. Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | L(+)-ascorbic acid |
| AEC | ammonium iron(III) citrate |
| BB | bovine bile |
| BS | heat-inactivated donor bovine serum |
| cysteine | L-Cysteine hydrochloride monohydrate |
| d | days |
| ddH2O | double-distilled water |
| FCS | heat-inactivated fetal calf serum |
| glucose | D(+)-Glucose |
| GM | modified TYI-S-33 medium |
| H2O2 | 30% hydrogen peroxide solution |
| KH2PO4 | potassium di-hydrogen phosphate |
| K2HPO4 | di-potassium hydrogen phosphate |
| LA | L(+)-Lactic acid calcium salt hydrate |
| NaCl | sodium chloride |
| NaDCC | sodium dichloroisocyanurate solution |
| NaOH | sodium hydroxide |
| NC | negative control |
| PB | bile extract porcine |
| PBS | phosphate-buffered saline |
| PC | positive control |
| RT | room temperature |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| WSH | water of standardized hardness |
Appendix A
| Ingredients | Medium#1 | Medium#2 | Medium#3 | Medium#4 | ||
|---|---|---|---|---|---|---|
| Casein peptone, g | 1.8 | 1.8 | 1.8 | 1.8 | ||
| D(+)-Glucose, g | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Yeast extract, g | 0.9 | 0.9 | 0.9 | 0.9 | ||
| NaCl, mg | 200 | 200 | 200 | 200 | ||
| Cysteine, mg | 200 | 200 | 200 | 200 | ||
| K2HPO4, mg | 100 | 100 | 100 | 100 | ||
| KH2PO4, mg | 60 | 60 | 60 | 60 | ||
| AA, mg | 20 | 20 | 20 | 20 | ||
| AEC, mg | 2.28 | 2.28 | 2.28 | 2.28 | ||
| FCS, mL | 10 | - | 10 | 10 | ||
| BS, mL | - | 10 | - | - | ||
| BB, mg | - | 250 | 375 | 500 | 500 | 1000 |
| PB, mg | 250 | - | - | - | ||
| LA, mg | 55 | - | 0.55 | - | ||
| pH value | 7.8 | 7.8 | 7.8 | 7.8 | ||
References
- Adam, R.D. Giardia duodenalis: Biology and Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e0002419. [Google Scholar] [CrossRef]
- Fink, M.Y.; Singer, S.M. The Intersection of Immune Responses, Microbiota and Pathogenesis in Giardiasis. Trends Parasitol. 2017, 33, 901–913. [Google Scholar] [CrossRef]
- Robuffo, I.; Barassi, G.; Cordas, D. Inflammatory protein caspase-1 plays a crucial role in the immune response during microbial infections. Int. J. Infect. 2025, 9, 45–49. [Google Scholar]
- Sardinha-Silva, A.; Alves-Ferreira, E.V.C.; Grigg, M.E. Intestinal immune responses to commensal and pathogenic protozoa. Front. Immunol. 2022, 13, 963723. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Goh, J.; Phillips, M.; Guselle, N.; McAllister, T.A. Giardia Cyst and Cryptosporidium Oocyst Survival in Water, Soil, and Cattle Feces. J. Environ. Qual. 1999, 28, 1991–1996. [Google Scholar] [CrossRef]
- Xu, H.; Jin, Y.; Wu, W.; Li, P.; Wang, L.; Li, N.; Feng, Y.; Xiao, L. Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasit. Vectors 2016, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Bandini, G.; Motari, E.; Samuelson, J. Ethanol and isopropanol in concentrations present in hand sanitizers sharply reduce excystation of Giardia and Entamoeba and eliminate oral infectivity of Giardia cysts in gerbils. Antimicrob. Agents Chemother. 2015, 59, 6749–6754. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.O.; Thomaz-Soccol, V.; Paulino, R.C.; Alcântara de Castro, E. Effect of vinegar on the viability of Giardia duodenalis cysts. Int. J. Food Microbiol. 2009, 128, 510–512. [Google Scholar] [CrossRef]
- El Zawawy, L.A.; El-Said, D.; Ali, S.M.; Fathy, F.M. Disinfection efficacy of sodium dichloroisocyanurate (NADCC) against common food-borne intestinal protozoa. J. Egypt. Soc. Parasitol. 2010, 40, 165–185. [Google Scholar]
- Jarroll, E.L.; Bingham, A.K.; Meyer, E.A. Effect of chlorine on Giardia lamblia cyst viability. Appl. Environ. Microbiol. 1981, 41, 483–487. [Google Scholar] [CrossRef]
- Adeyemo, F.E.; Singh, G.; Reddy, P.; Bux, F.; Stenström, T.A. Efficiency of chlorine and UV in the inactivation of Cryptosporidium and Giardia in wastewater. PLoS ONE 2019, 14, e0216040. [Google Scholar] [CrossRef] [PubMed]
- Kondo Nakada, L.Y.; Urbano Dos Santos, L.; Guimarães, J.R. Pre-ozonation of surface water: An effective water treatment process to reduce the risk of infection by Giardia in drinking water. Environ. Pollut. 2020, 266, 115144. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J.C.; Rice, E.W.; Schaefer, F.W. Comparison of animal infectivity and excystation as measures of Giardia muris cyst inactivation by chlorine. Appl. Environ. Microbiol. 1985, 50, 1115–1117. [Google Scholar] [CrossRef]
- Marchin, G.L.; Fina, L.R.; Lambert, J.L.; Fina, G.T. Effect of resin disinfectants-I3 and -I5 on Giardia muris and Giardia lamblia. Appl. Environ. Microbiol. 1983, 46, 965–969. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Ito, S.; Tsunoda, K.; Shimada, K.; Taki, T.; Matsui, T. Disinfectant effects of several chemicals against Toxoplasma oocysts. Nihon Juigaku Zasshi 1975, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Dyachenko, V.; Keidel, J.; Schmäschke, R.; Daugschies, A. Combination of cell culture and quantitative PCR (cc-qPCR) to assess disinfectants efficacy on Cryptosporidium oocysts under standardized conditions. Vet. Parasitol. 2010, 167, 43–49. [Google Scholar] [CrossRef]
- Dresely, I.; Daugschies, A.; Lendner, M. Establishment of a germ carrier assay to assess disinfectant efficacy against oocysts of coccidian parasites. Parasitol. Res. 2015, 114, 273–281. [Google Scholar] [CrossRef]
- Delling, C.; Lendner, M.; Müller, U.; Daugschies, A. Improvement of in vitro evaluation of chemical disinfectants for efficacy on Cryptosporidium parvum oocysts. Vet. Parasitol. 2017, 245, 5–13. [Google Scholar] [CrossRef]
- Kane, A.V.; Ward, H.D.; Keusch, G.T.; Pereira, M.E. In vitro encystation of Giardia lamblia: Large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J. Parasitol. 1991, 77, 974–981. [Google Scholar] [CrossRef]
- Hausen, M.A.; Freitas, J.C.M.; Monteiro-Leal, L.H. The effects of metronidazole and furazolidone during Giardia differentiation into cysts. Exp. Parasitol. 2006, 113, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Davids, B.J.; Gillin, F.D. Methods for Giardia Culture, Cryopreservation, Encystation, and Excystation In Vitro. In Giardia; Luján, H.D., Svärd, S., Eds.; Springer Vienna: Vienna, Austria, 2011; pp. 381–394. ISBN 978-3-7091-0197-1. [Google Scholar]
- Einarsson, E.; Troell, K.; Hoeppner, M.P.; Grabherr, M.; Ribacke, U.; Svärd, S.G. Coordinated Changes in Gene Expression Throughout Encystation of Giardia intestinalis. PLoS Negl. Trop. Dis. 2016, 10, e0004571. [Google Scholar] [CrossRef] [PubMed]
- Hausen, M.A.; Oliveira, R.P.d.; Gadelha, A.P.R.; Campanati, L.; de Carvalho, J.J.; de Carvalho, L.; Barbosa, H.S. Giardia lamblia: A report of drug effects under cell differentiation. Parasitol. Res. 2009, 105, 789–796. [Google Scholar] [CrossRef]
- Bingham, A.K.; Meyer, E.A. Giardia excystation can be induced in vitro in acidic solutions. Nature 1979, 277, 301–302. [Google Scholar] [CrossRef]
- Keister, D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 487–488. [Google Scholar] [CrossRef]
- Touz, M.C.; Nores, M.J.; Slavin, I.; Piacenza, L.; Acosta, D.; Carmona, C.; Luján, H.D. Membrane-associated dipeptidyl peptidase IV is involved in encystation-specific gene expression during Giardia differentiation. Biochem. J. 2002, 364, 703–710. [Google Scholar] [CrossRef]
- Faso, C.; Bischof, S.; Hehl, A.B. The proteome landscape of Giardia lamblia encystation. PLoS ONE 2013, 8, e83207. [Google Scholar] [CrossRef]
- Hehl, A.B.; Marti, M.; Köhler, P. Stage-Specific Expression and Targeting of Cyst Wall Protein–Green Fluorescent Protein Chimeras in Giardia. Mol. Biol. Cell 2000, 11, 1789–1800. [Google Scholar] [CrossRef]
- Gillin, F.D.; Boucher, S.E.; Rossi, S.S.; Reiner, D.S. Giardia lamblia: The roles of bile, lactic acid, and pH in the completion of the life cycle in vitro. Exp. Parasitol. 1989, 69, 164–174. [Google Scholar] [CrossRef]
- Boucher, S.E.; Gillin, F.D. Excystation of in vitro-derived Giardia lamblia cysts. Infect. Immun. 1990, 58, 3516–3522. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Schaefer, F.W. Improved in vitro excystation procedure for Giardia lamblia cysts. J. Clin. Microbiol. 1981, 14, 709–710. [Google Scholar] [CrossRef]
- Feely, D.E.; Gardner, M.D.; Hardin, E.L. Excystation of Giardia muris induced by a phosphate-bicarbonate medium: Localization of acid phosphatase. J. Parasitol. 1991, 77, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, I.; Maux, M.; Helmi, K.; Hoffmann, L.; Schwartzbrod, J.; Cauchie, H.-M. Quantification of Giardia transcripts during in vitro excystation: Interest for the estimation of cyst viability. Water Res. 2009, 43, 2728–2738. [Google Scholar] [CrossRef]
- Bingham, A.K.; Jarroll, E.L.; Meyer, E.A.; Radulescu, S. Giardia sp.: Physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp. Parasitol. 1979, 47, 284–291. [Google Scholar] [CrossRef]
- Hautus, M.A.; Kortbeek, L.M.; Vetter, J.C.; Laarman, J.J. In vitro excystation and subsequent axenic growth of Giardia lamblia. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 858–861. [Google Scholar] [CrossRef]
- Buchel, L.-A.; Gorenflot, A.; Chochillon, C.; Savel, J.; Gobert, J.-G. In vitro Excystation of Giardia from Humans: A Scanning Electron Microscopy Study. J. Parasitol. 1987, 73, 487. [Google Scholar] [CrossRef]
- Smith, A.J.; Lauwaet, T.; Davids, B.J.; Gillin, F.D. Giardia lamblia Nek1 and Nek2 kinases affect mitosis and excystation. Int. J. Parasitol. 2012, 42, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, W.Q.; Mena, K.D. Assessment of waterborne protozoan passage through conventional drinking water treatment process in Venezuela. J. Water Health 2012, 10, 324–336. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; González-Warleta, M.; Mezo, M. Cryptosporidium spp. and Giardia duodenalis as pathogenic contaminants of water in Galicia, Spain: The need for safe drinking water. Int. J. Hyg. Environ. Health 2015, 218, 132–138. [Google Scholar] [CrossRef]
- Ramo, A.; Del Cacho, E.; Sánchez-Acedo, C.; Quílez, J. Occurrence of Cryptosporidium and Giardia in raw and finished drinking water in north-eastern Spain. Sci. Total Environ. 2017, 580, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.-A.; Linden, K.G.; Faubert, G. Inactivation of Giardia lamblia cysts by polychromatic UV. Lett. Appl. Microbiol. 2009, 48, 790–792. [Google Scholar] [CrossRef]
- Linden, K.G.; Shin, G.-A.; Faubert, G.; Cairns, W.; Sobsey, M.D. UV disinfection of Giardia lamblia cysts in water. Environ. Sci. Technol. 2002, 36, 2519–2522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Smith, D.W.; Belosevic, M. Morphological changes of Giardia lamblia cysts after treatment with ozone and chlorine. J. Environ. Eng. Sci. 2004, 3, 495–506. [Google Scholar] [CrossRef]
- Sammarro Silva, K.J.; Sabogal-Paz, L.P. Cryptosporidium spp. and Giardia spp. (oo)cysts as target-organisms in sanitation and environmental monitoring: A review in microscopy-based viability assays. Water Res. 2021, 189, 116590. [Google Scholar] [CrossRef]
- Rousseau, A.; La Carbona, S.; Dumètre, A.; Robertson, L.J.; Gargala, G.; Escotte-Binet, S.; Favennec, L.; Villena, I.; Gérard, C.; Aubert, D. Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: A review of methods. Parasite 2018, 25, 14. [Google Scholar] [CrossRef] [PubMed]
- Delling, C.; Holzhausen, I.; Daugschies, A.; Lendner, M. Inactivation of Cryptosporidium parvum under laboratory conditions. Parasitol. Res. 2016, 115, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef] [PubMed]
- You, M.-J. Suppression of Eimeria tenella sporulation by disinfectants. Korean J. Parasitol. 2014, 52, 435–438. [Google Scholar] [CrossRef]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirchner, M.; Delling, C.; Daugschies, A. In Vitro Disinfection Efficacy Assay on Giardia duodenalis Cysts. Hygiene 2025, 5, 54. https://doi.org/10.3390/hygiene5040054
Kirchner M, Delling C, Daugschies A. In Vitro Disinfection Efficacy Assay on Giardia duodenalis Cysts. Hygiene. 2025; 5(4):54. https://doi.org/10.3390/hygiene5040054
Chicago/Turabian StyleKirchner, Manuela, Cora Delling, and Arwid Daugschies. 2025. "In Vitro Disinfection Efficacy Assay on Giardia duodenalis Cysts" Hygiene 5, no. 4: 54. https://doi.org/10.3390/hygiene5040054
APA StyleKirchner, M., Delling, C., & Daugschies, A. (2025). In Vitro Disinfection Efficacy Assay on Giardia duodenalis Cysts. Hygiene, 5(4), 54. https://doi.org/10.3390/hygiene5040054






