Eco-Friendly vs. Traditional Cleaning in Healthcare Settings: Microbial Safety and Environmental Footprint
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Plan
2.2. Microbiological Evaluation
2.3. Microbial Identification
2.4. Cleaning Procedures
- Cleaning Products:
- Textiles (cloths and mops):
- Tools and Equipment:
- Sanitary Cleaning and Descaling:
- Textile Reconditioning (washing mops and cloths):
2.5. Methodology for Comparative LCA Analysis
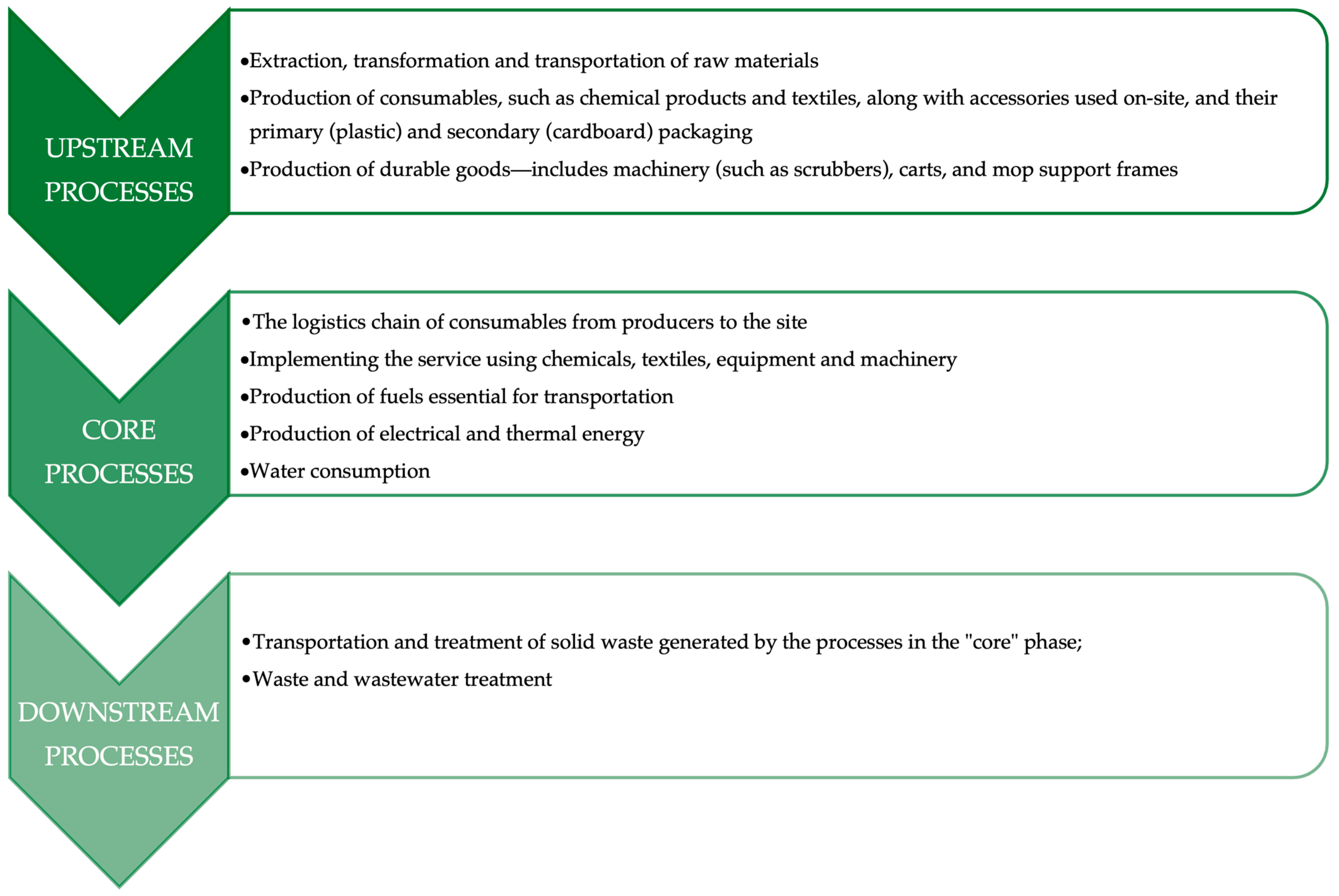
2.5.1. Upstream Phase
- The extraction and initial processing of raw materials;
- The transportation of raw materials and semi-finished goods to manufacturing or supply facilities;
- The production of consumable items—such as cleaning agents (detergents and disinfectants), textile components (e.g., mops, cloths), and ancillary tools—along with their primary (typically plastic) and secondary (e.g., cardboard) packaging;
- The manufacture of durable equipment, defined as products with a service life exceeding three years. This category includes floor-cleaning machines (e.g., scrubbers), cleaning carts, and mop handle frames.
2.5.2. Core Phase
- The distribution and delivery of consumable products (e.g., detergents, textiles) from production facilities to the operational site;
- The actual performance of cleaning activities, involving the use of chemical agents, textile materials, equipment, and mechanized tools;
- The generation of fuels required for transport related to service implementation;
- The provision and consumption of electrical and thermal energy on site, necessary for powering cleaning machinery and equipment;
- The use of water, both for diluting cleaning chemicals and for laundering cleaning textiles.
2.5.3. Downstream Phase
- The collection, transportation, and disposal of solid waste produced during cleaning activities performed in the core phase;
- The management and treatment of wastewater, originating from operations such as textile laundering and chemical product dilution.
2.6. Methodological Assumptions of the Comparative Study
2.7. Statistical Analysis
3. Results
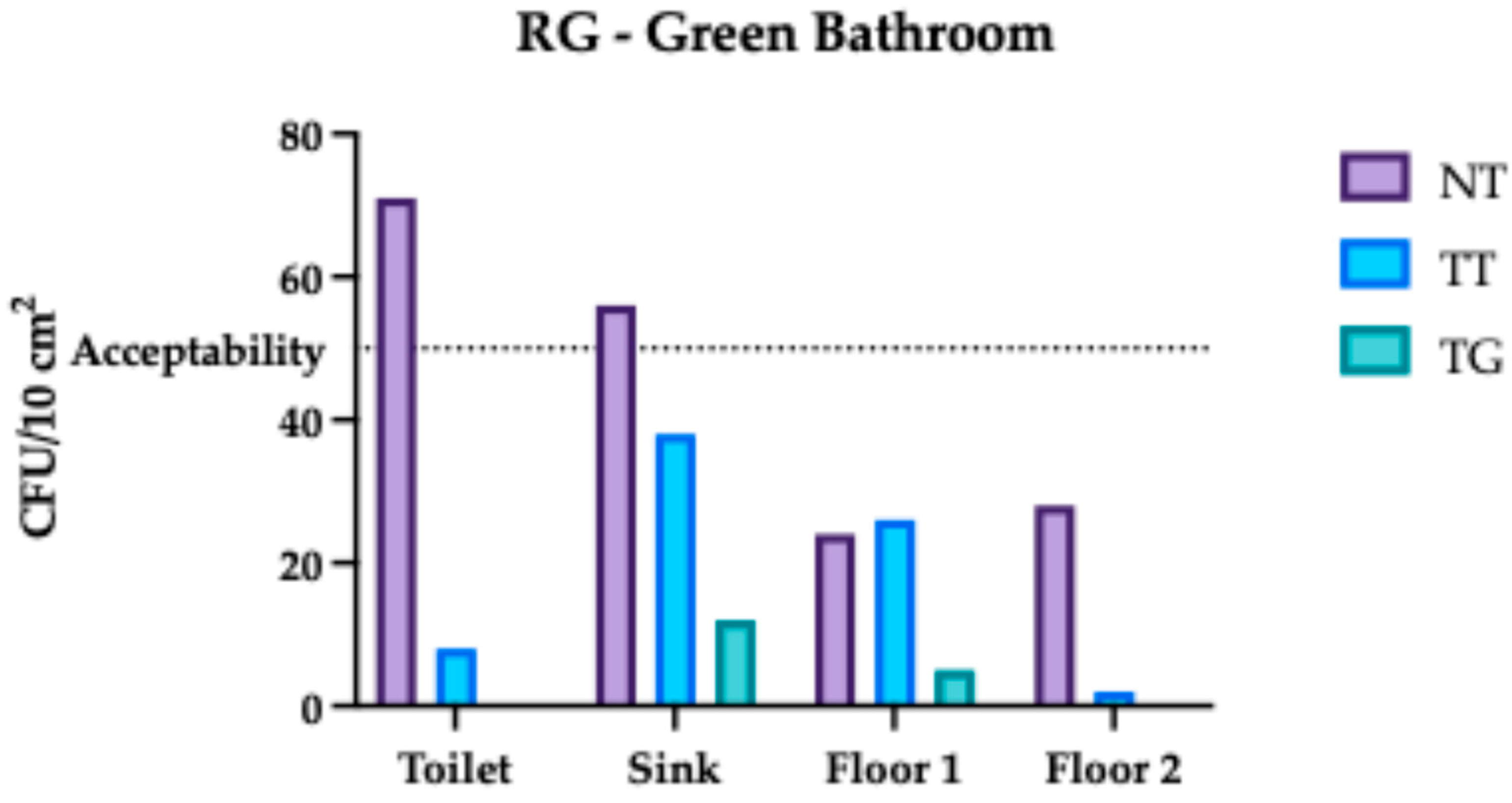
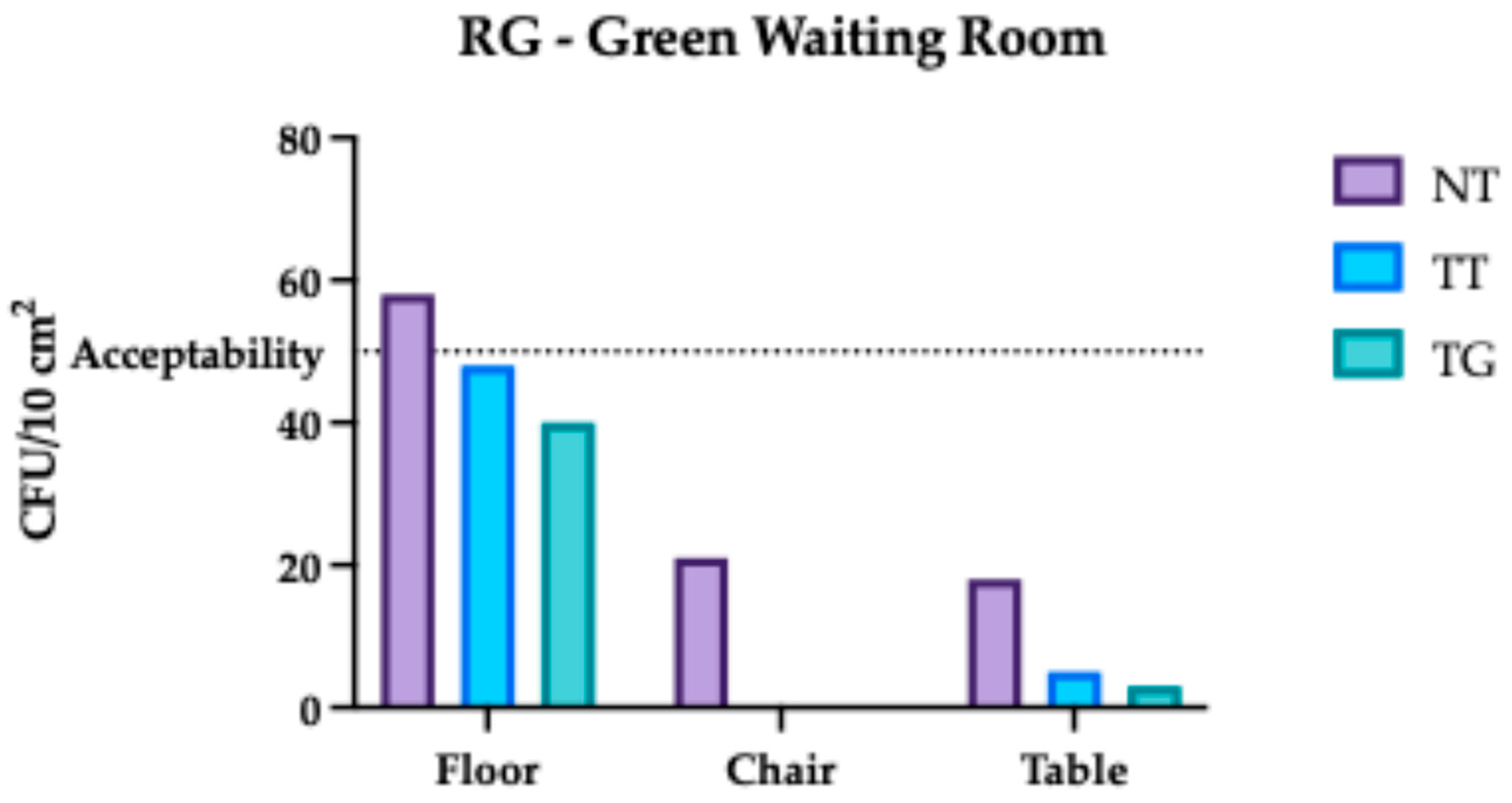
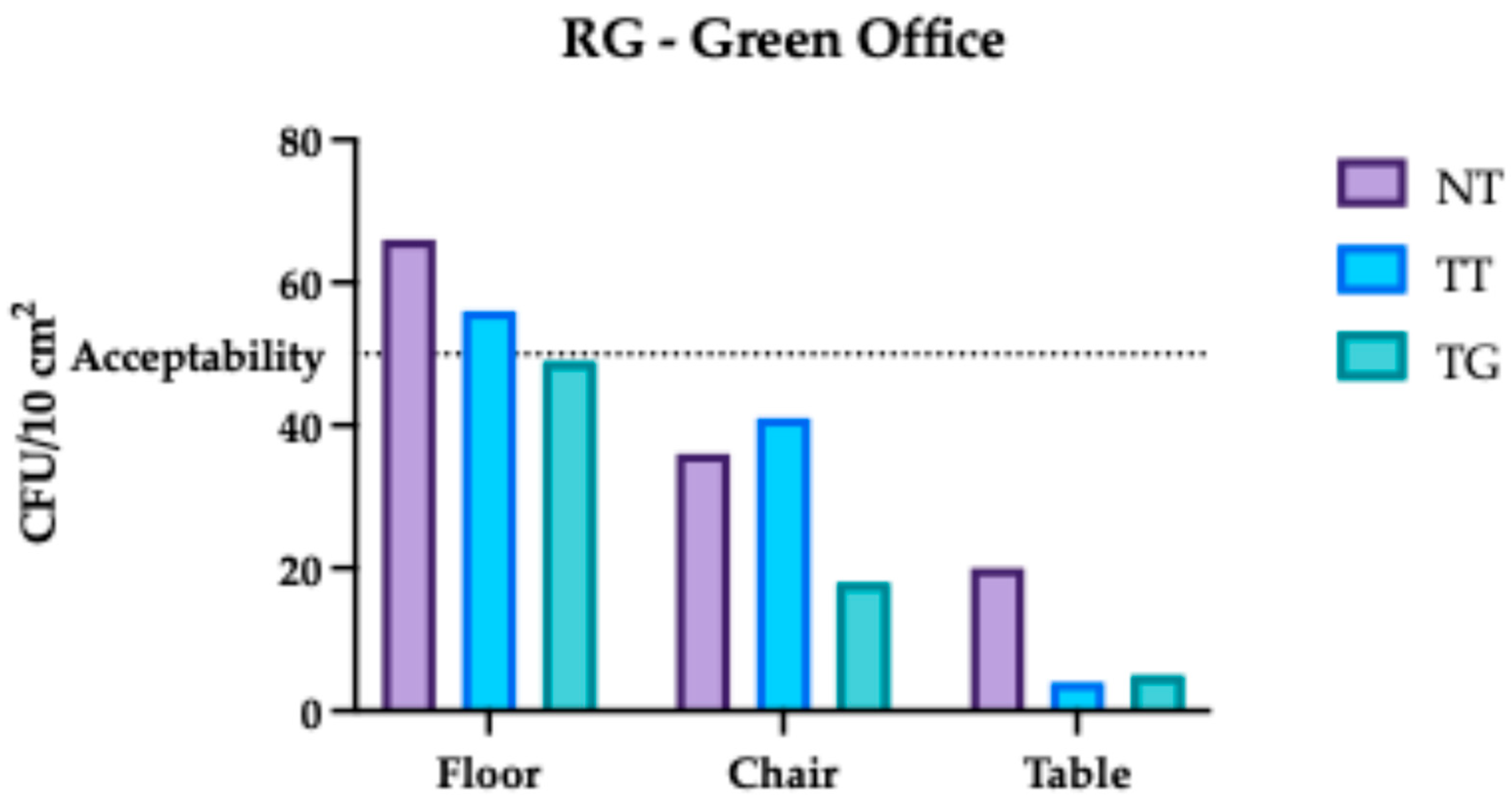
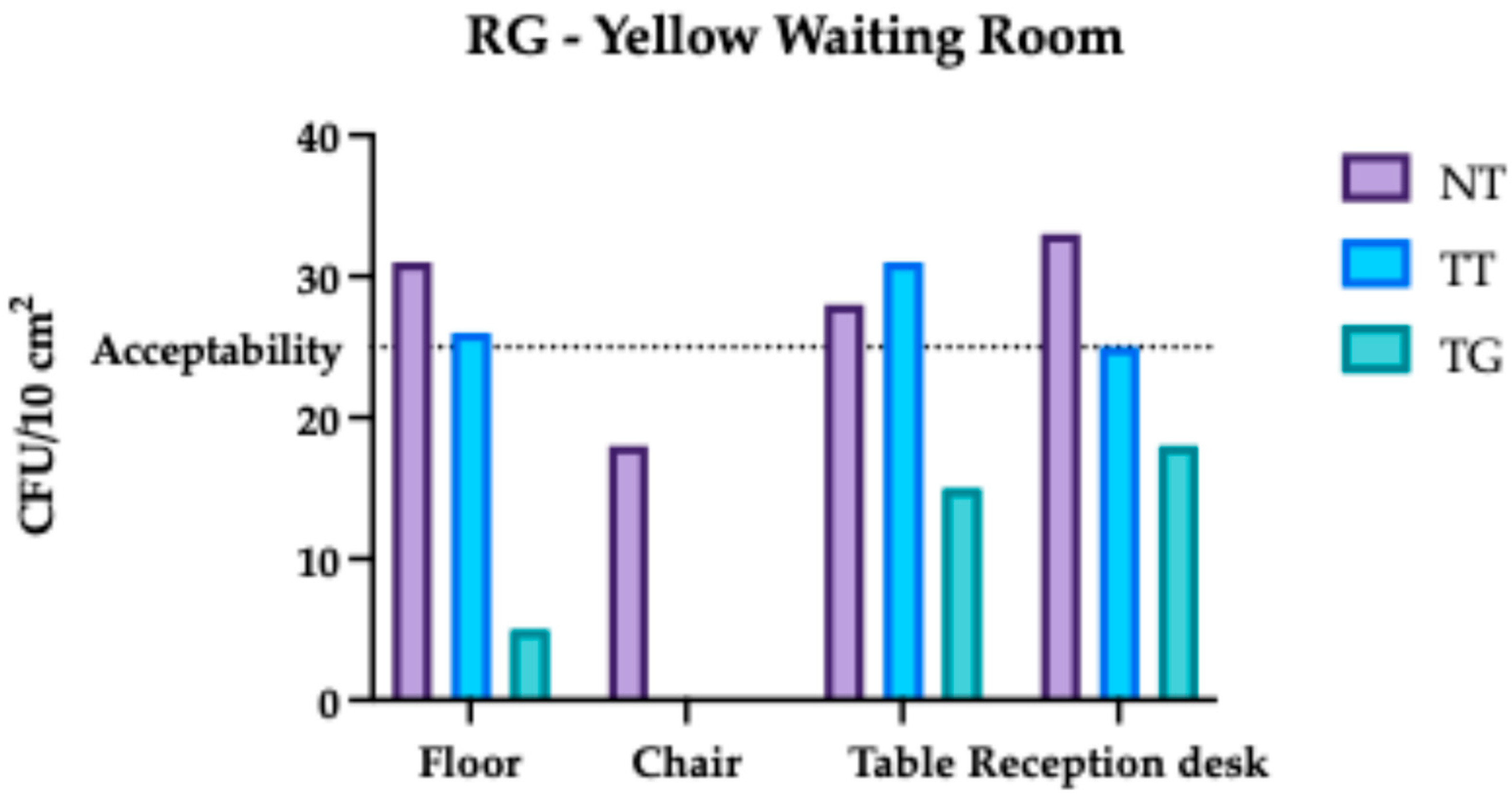
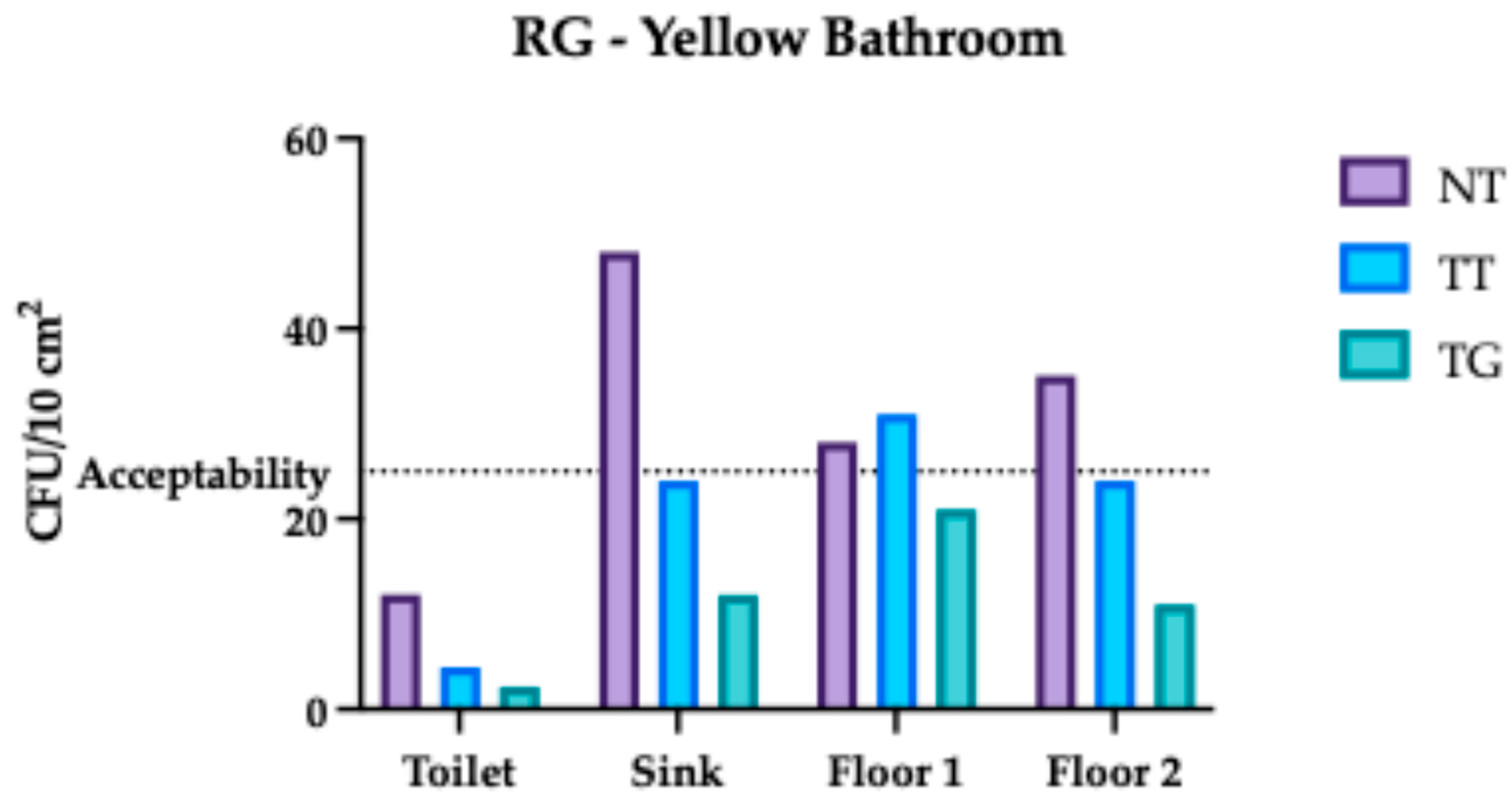
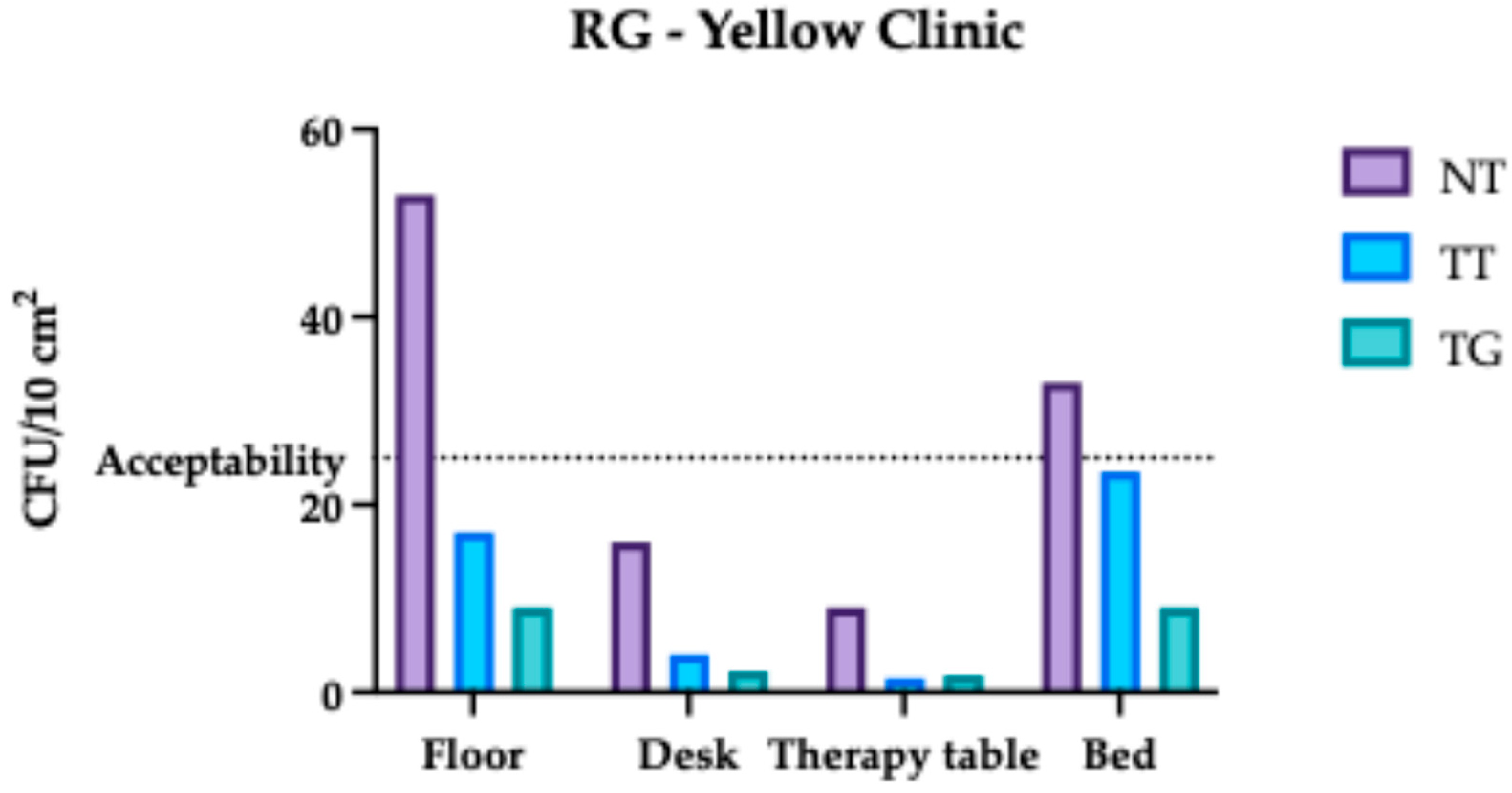
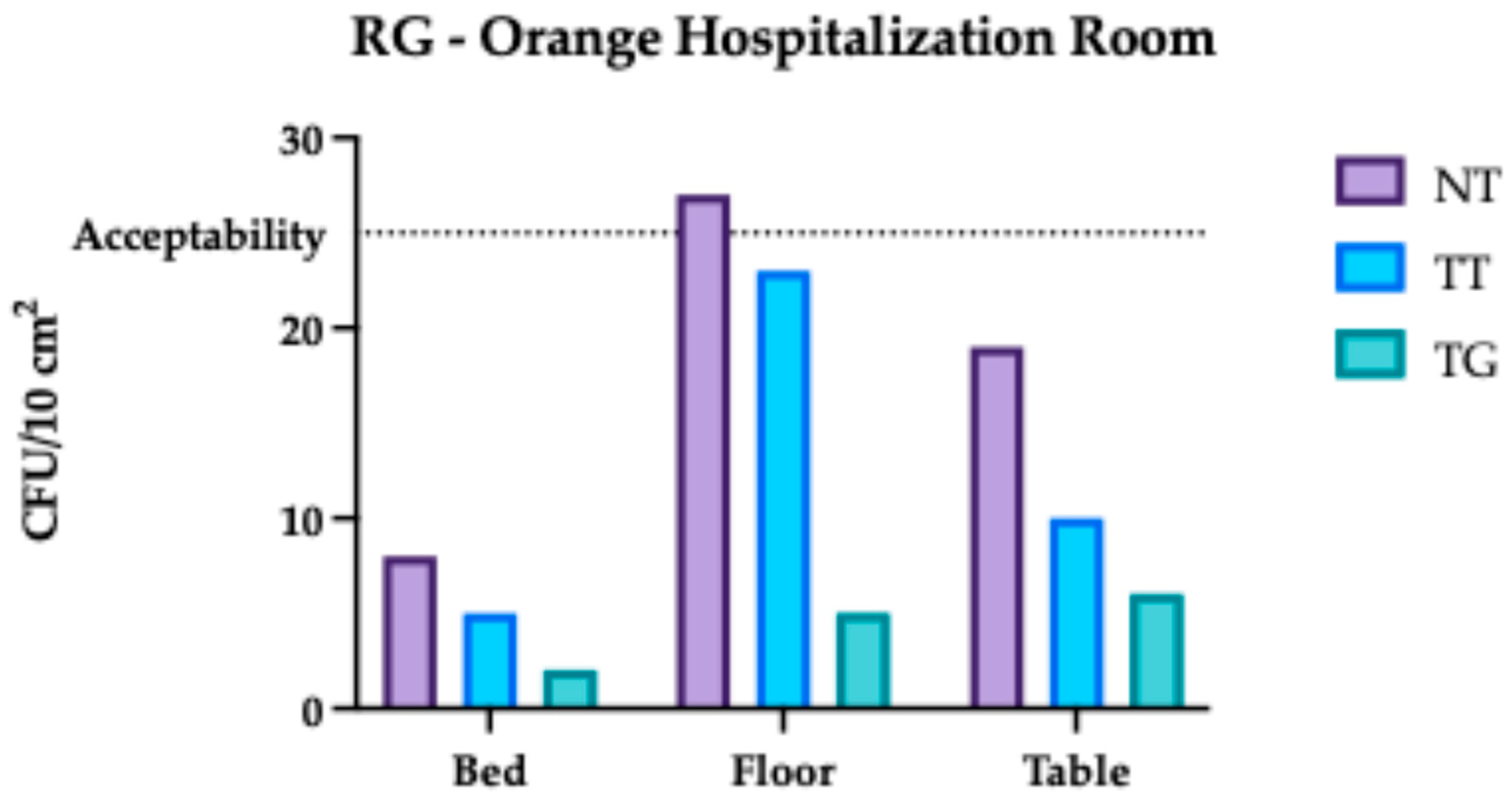
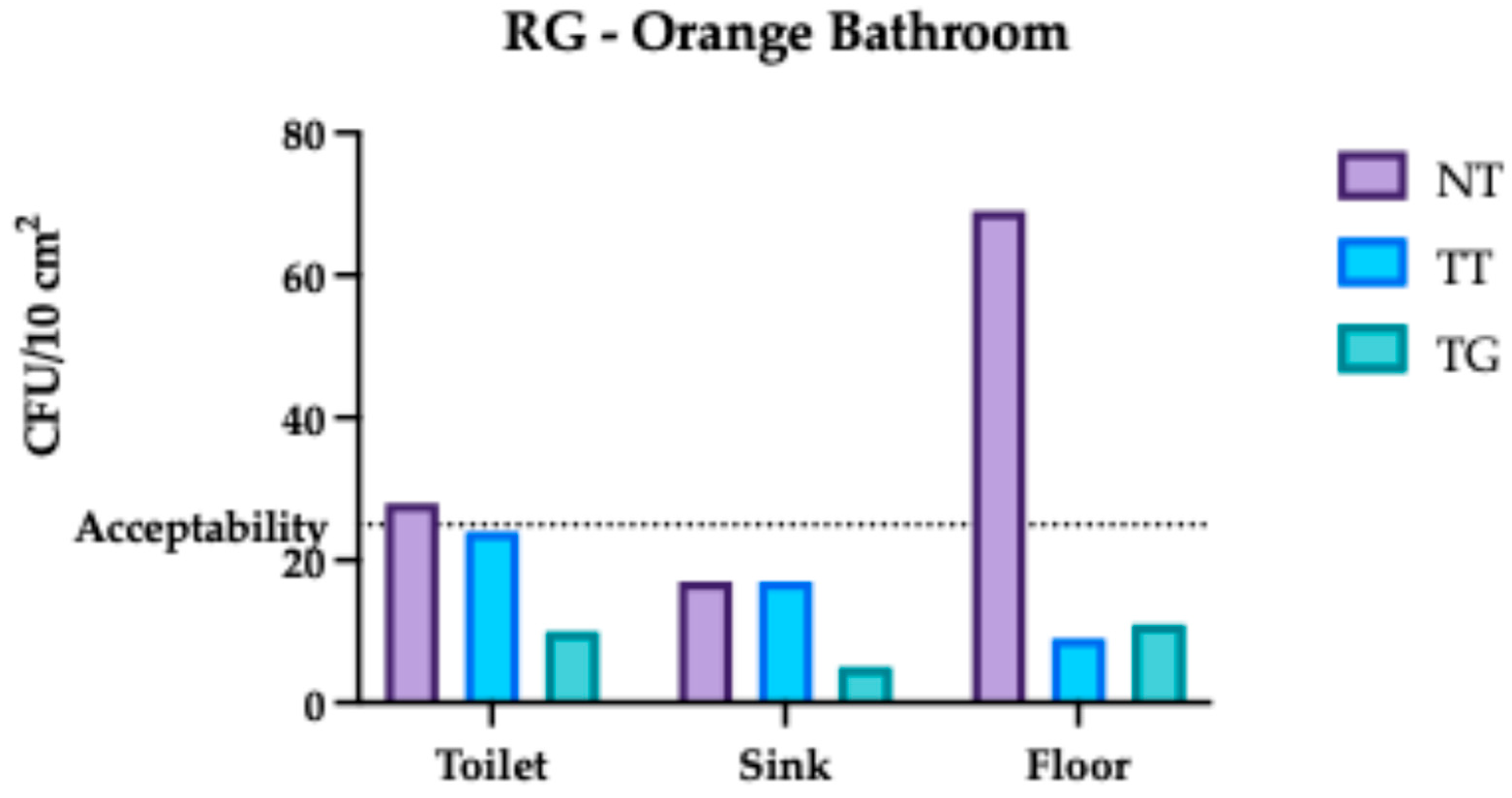
3.1. Regina Margherita Microbiological Assessment
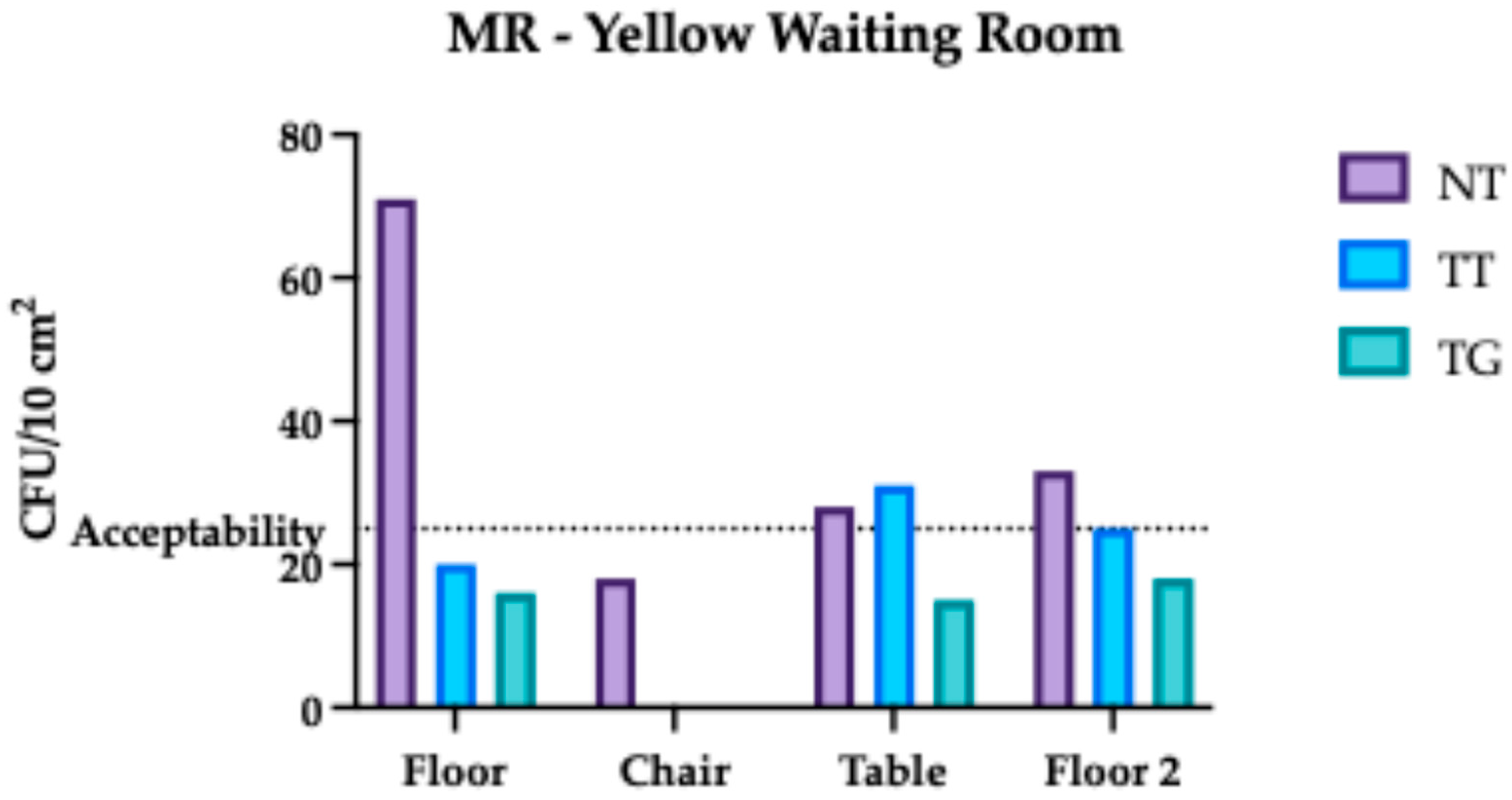
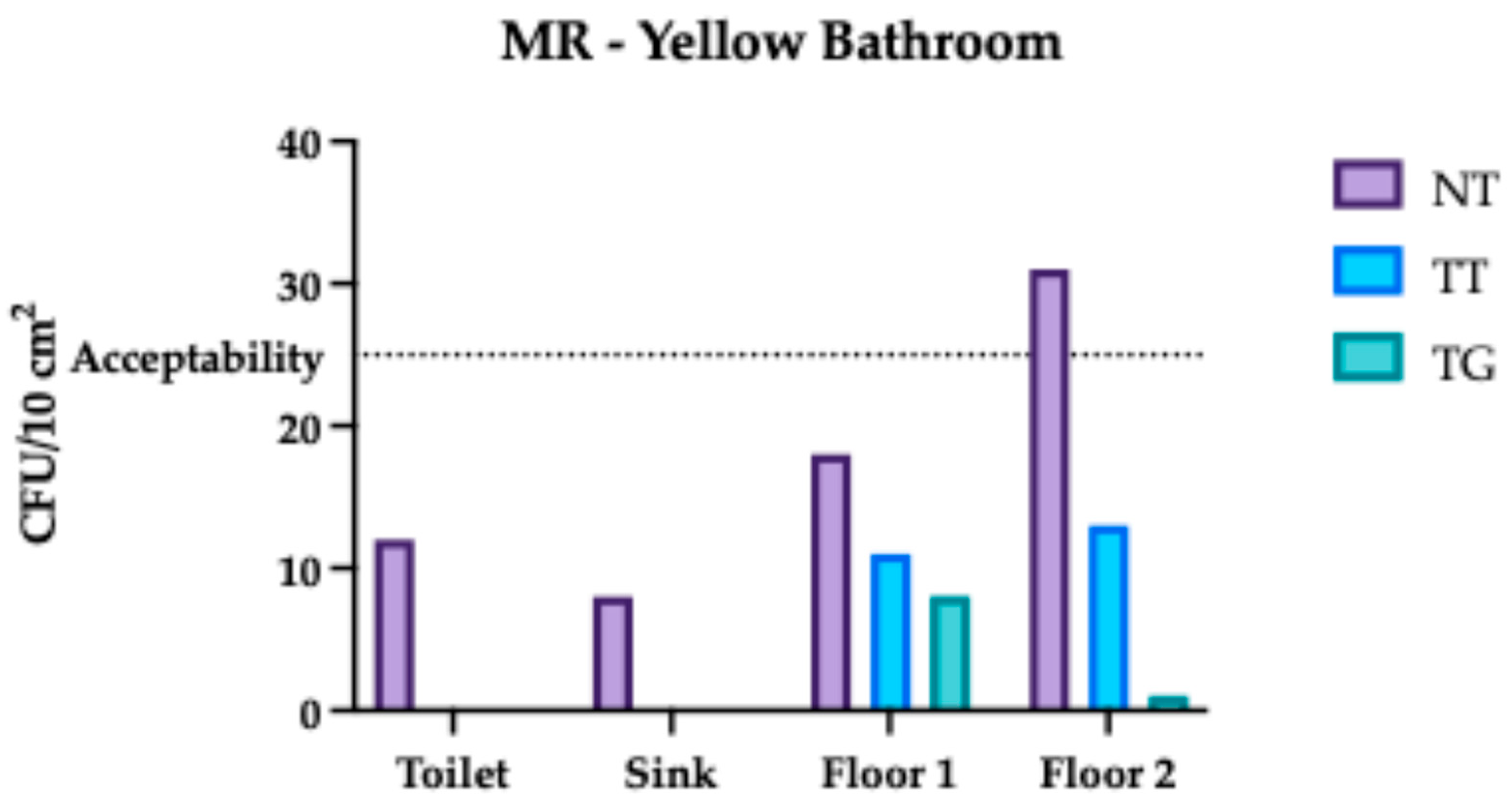
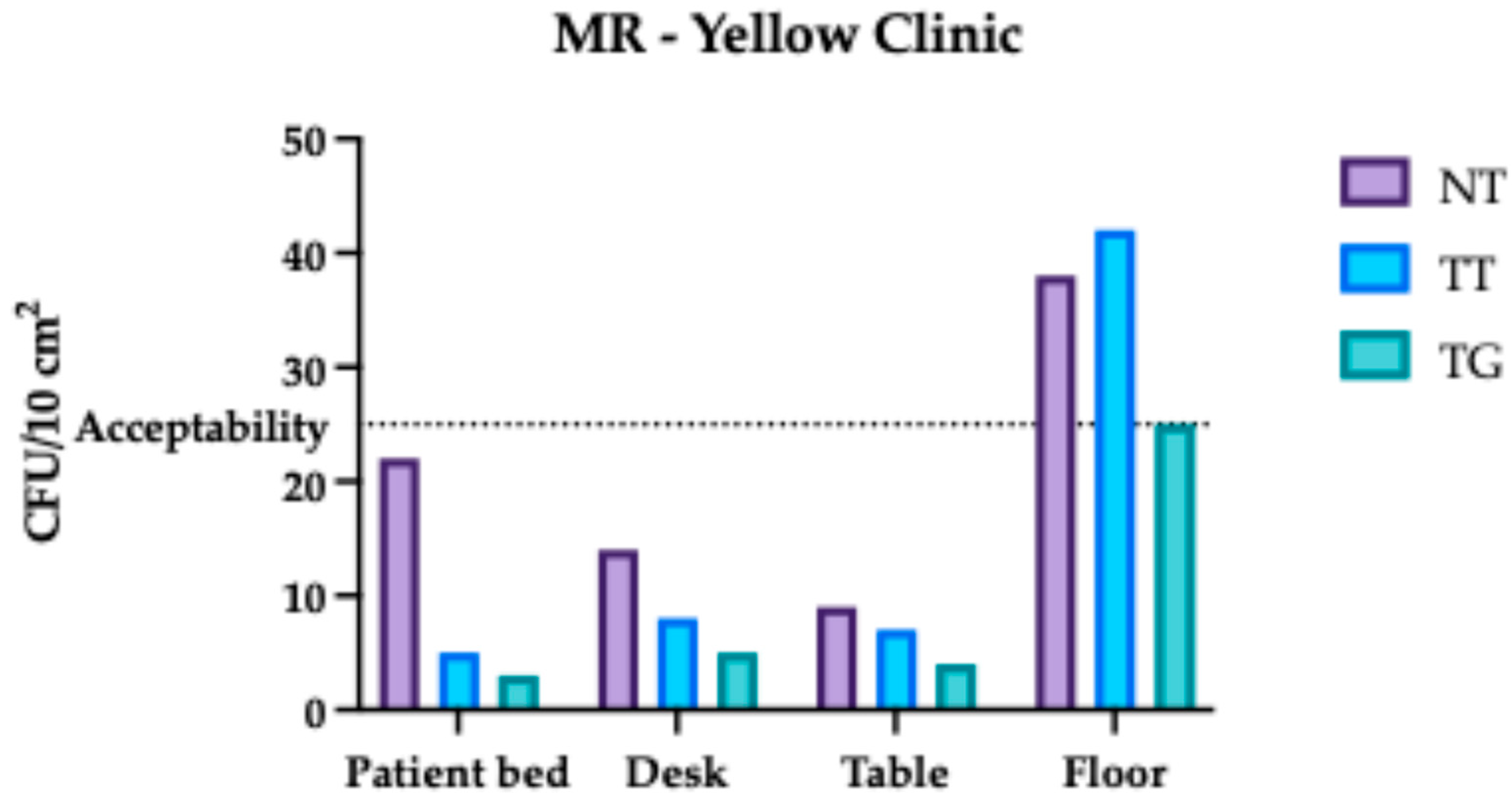
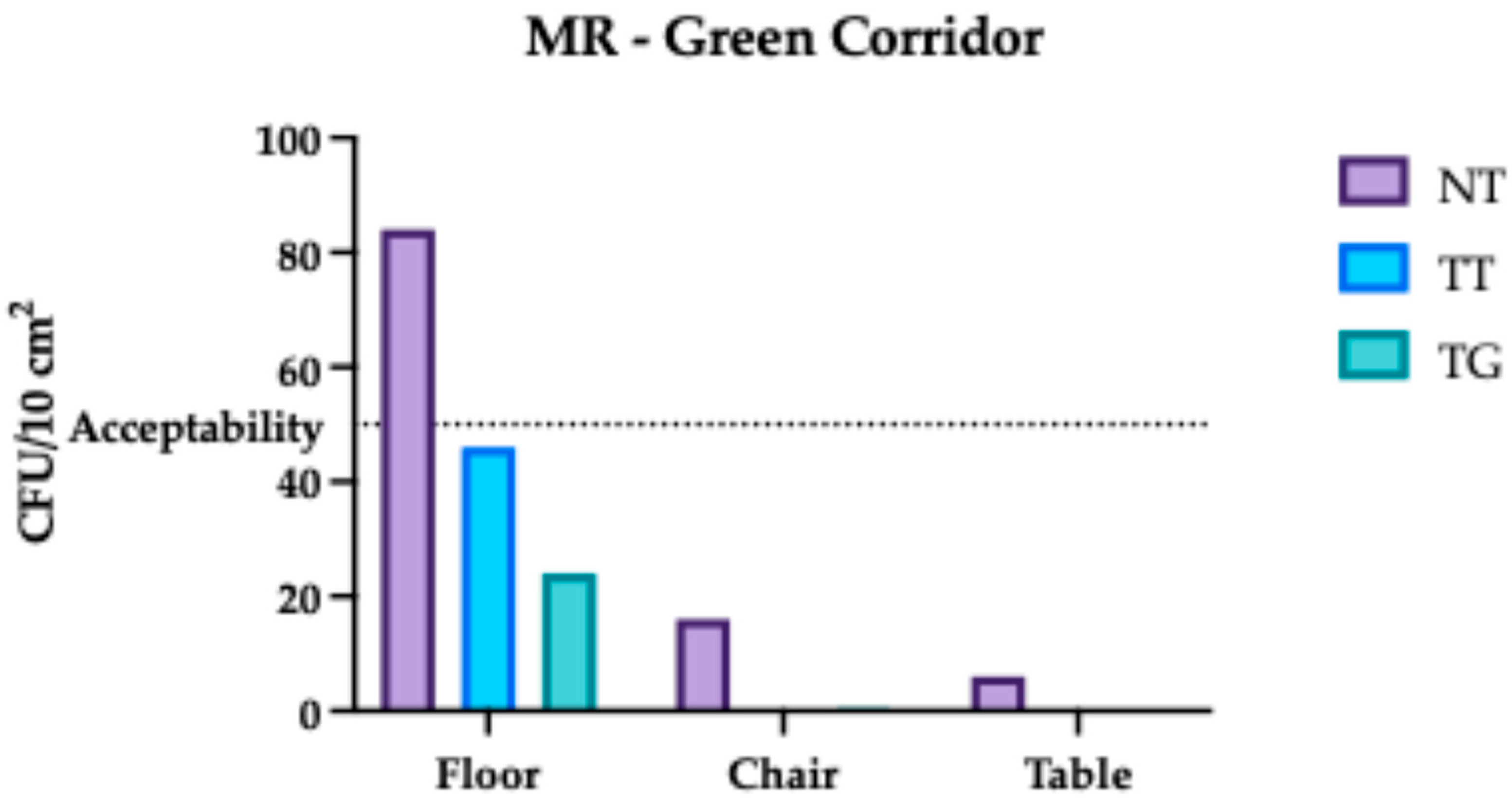
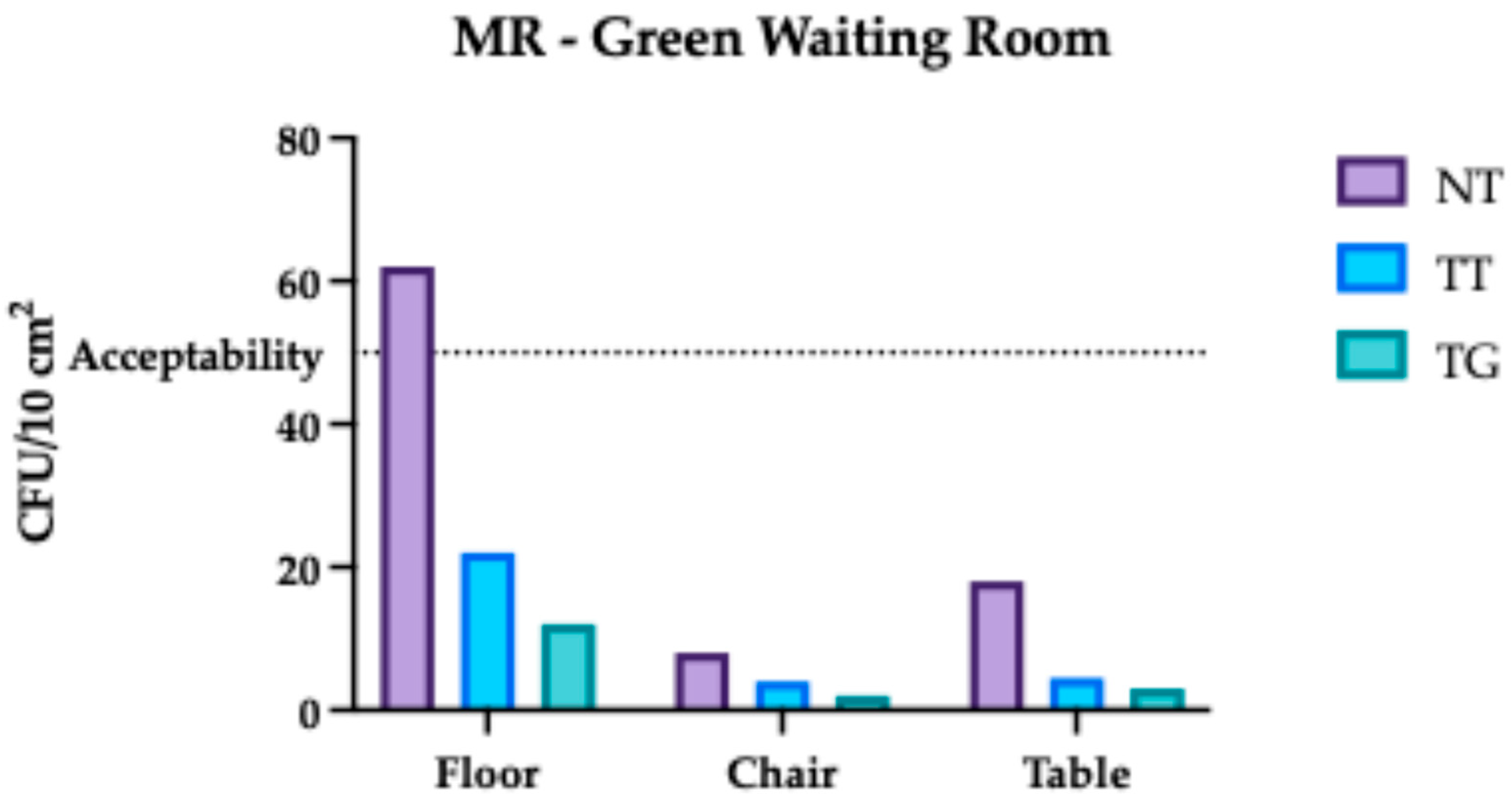
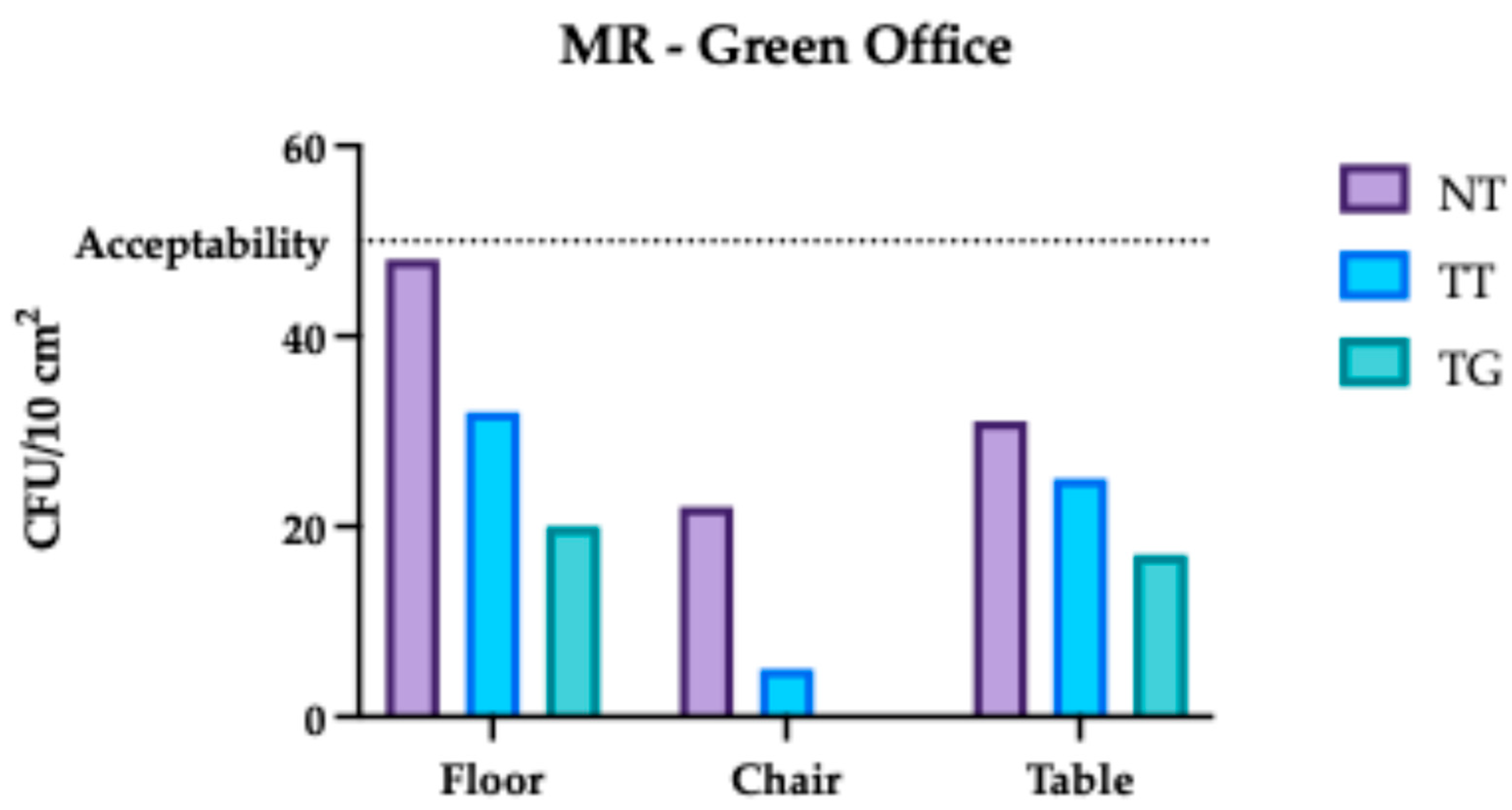
3.2. Monte Rocchetta Microbiological Assessment
3.3. Comparative Analysis Results of Monte Rocchetta
- For the green protocol, electricity consumption for laundry accounts for 51%, production of laundry chemicals accounts for 12%, trolley manufacturing accounts for 11%, production of cleaning chemicals accounts for 7%, and wastewater treatment from laundry accounts for 6%.
- In the traditional protocol, electricity consumption for laundry accounts for 48%, production of cleaning chemicals for 23%, production of laundry chemicals for 6%, trolley manufacturing for 5%, and wastewater treatment for 3%.
3.4. Comparative Analysis Results of Regina Margherita
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Everard, M. Assessment of the sustainable use of chemicals on a level playing field. Integr. Environ. Assess. Manag. 2023, 19, 1131–1146. [Google Scholar] [CrossRef] [PubMed]
- Sonnemann, G.; Gemechu, E.D.; Remmen, A.; Frydendal, J.; Jensen, A.A. Life Cycle Management: Implementing Sustainability in Business Practice; Springer: Berlin/Heidelberg, Germany, 2015; pp. 7–21. [Google Scholar] [CrossRef]
- Luthin, A.; Traverso, M.; Crawford, R.H. Circular life cycle sustainability assessment: An integrated framework. J. Ind. Ecol. 2024, 28, 41–58. [Google Scholar] [CrossRef]
- Zeug, W.; Bezama, A.; Thrän, D. A framework for implementing holistic and integrated life cycle sustainability assessment of regional bioeconomy. Int. J. Life Cycle Assess. 2021, 26, 1998–2023. [Google Scholar] [CrossRef]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, D.; Jovanović, S.; Skerlić, J.; Šušteršič, J.; Radulović, J. Methodology of Life Cycle Sustainability Assessment. Proc. Eng. Sci. 2019, 1, 793–800. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Cave, R.; Cole, J.; Mkrtchyan, H.V. Surveillance and prevalence of antimicrobial resistant bacteria from public settings within urban built environments: Challenges and opportunities for hygiene and infection control. Environ. Int. 2021, 157, 106836. [Google Scholar] [CrossRef]
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef]
- Klassert, T.E.; Zubiria-Barrera, C.; Neubert, R.; Stock, M.; Schneegans, A.; López, M.; Driesch, D.; Zakonsky, G.; Gastmeier, P.; Slevogt, H.; et al. Comparative analysis of surface sanitization protocols on the bacterial community structures in the hospital environment. Clin. Microbiol. Infect. 2022, 28, 1105–1112. [Google Scholar] [CrossRef]
- Kalita, I.; Kamilaris, A.; Havinga, P.; Reva, I. Assessing the Health Impact of Disinfection Byproducts in Drinking Water. ACS EST Water 2024, 4, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, R.F.; Alsadaan, N.; Alruwaili, A.N.; Alrumayh, A.G. Unveiling the Symbiosis of Environmental Sustainability and Infection Control in Health Care Settings: A Systematic Review. Sustainability 2023, 15, 15728. [Google Scholar] [CrossRef]
- Fontana, R.; Buratto, M.; Marzola, M.; Trioschi, G.; Bandera, B.; Buffone, C.; Vogli, L.; Marconi, P. An Evaluation of Hospital Cleaning Regimes—Microbiological Evaluation and LCA Analysis after Traditional and Sustainable/Green Procedures. Sustainability 2022, 14, 11465. [Google Scholar] [CrossRef]
- Dancer, S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004, 56, 10–15. [Google Scholar] [CrossRef]
- Griffith, C.J.; Cooper, R.A.; Gilmore, J.; Davies, C.; Lewis, M. An evaluation of hospital cleaning regimes and standards. J. Hosp. Infect. 2000, 45, 19–28. [Google Scholar] [CrossRef]
- La Contaminazione Microbiologica Delle Superfici Negli Ambienti Lavorativi. 2017. Available online: www.inail.it (accessed on 10 August 2022).
- Linee Guida Sugli Standard di Sicurezza e di Igiene del Lavoro nel Reparto Operatorio Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro Dipartimento Igiene del Lavoro. Available online: https://www.lisaservizi.it/wp-content/uploads/2024/05/20150820174253-ISPESL-LG-SaleOperatorie.pdf (accessed on 19 August 2025).
- O’Hara, C.M.; Rhoden, D.L.; Miller, J.M. Reevaluation of the API 20E identification system versus conventional biochemicals for identification of members of the family Enterobacteriaceae: A new look at an old product. J. Clin. Microbiol. 1992, 30, 123–125. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 10 August 2022).
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 10 August 2022).
- ISO 14067:2018; Greenhouse Gases—Carbon Footprint of Products—Requirements and Guidelines for Quantification. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71206.html (accessed on 10 August 2022).
- Product Category Rules–PCR Professional Cleaning Services for Buildings 2011:03, Version 3.1; EPD International: Stockholm, Sweden, 2011.
- ISO 14026:2017; Environmental Labels and Declarations—Principles, Requirements and Guidelines for Communication of Footprint Information. ISO: Geneva, Switzerland, 2017.
- Niyonzima, V.; Luwaga, R.; Beinempaka, F. Enablers and Barriers to Hand Hygiene among Health Workers at Mbarara Regional Referral Hospital. J. Health Med. Nurs. 2023, 9, 54–63. [Google Scholar] [CrossRef]
- Carling, P.C. Health Care Environmental Hygiene. Infect. Dis. Clin. N. Am. 2021, 35, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Otter, J.; Moldovan, A.; Parneix, P.; Voss, A.; Pittet, D. Keeping hospitals clean and safe without breaking the bank; summary of the Healthcare Cleaning Forum 2018. Antimicrob. Resist. Infect. Control 2018, 7, 132. [Google Scholar] [CrossRef]
- Doll, M.; Stevens, M.; Bearman, G. Environmental cleaning and disinfection of patient áreas. Int. J. Infect. Dis. 2018, 67, 52–57. [Google Scholar] [CrossRef]
- Kamaruddin, A.; Zainol, N.N.; Sulaiman, M.A.; Sukereman, A.S.; Hashim, H.A. Green Cleaning as a Tool in Combating COVID-19: A Content Validity Study for Malaysian Healthcare Facilities. IOP Conf. Ser. Earth Environ. Sci. 2022, 1067, 012081. [Google Scholar] [CrossRef]



| Area/Item to Clean | Tasks | Frequency |
|---|---|---|
| GREEN 1 Areas (administrative offices, educational areas and classrooms, meeting rooms and reception rooms, library, medical offices, duty doctor rooms, prayer rooms and accommodations for religious personnel, residential centers, day care centers, and family homes) | Dusting, wiping to remove fingerprints and stains, and disinfection of all horizontal and vertical surfaces (furniture, equipment, medical supplies, etc.) accessible without the use of ladders. | Daily |
| GREEN 2 Areas (waiting rooms, public and service access stairs, gyms, lounge, relaxation area, kitchenettes, reception, guard posts, internal pharmacy, entrances, ambulance access, elevators, etc.) | Same cleaning tasks as GREEN 1 area. | Daily |
| Steel Equipment | Cleaning of external parts of steel equipment using approved healthcare facility products. | Frequency determined with each healthcare facility |
| Floors | Dust removal, cleaning, and possible scraping of all protected and unprotected floors. | Daily |
| Sanitary Fixtures | Cleaning, descaling, and disinfection of sanitary fixtures, washable walls, showers, accessories, etc. | Daily |
| Area/Item to Clean | Tasks | Frequency |
|---|---|---|
| YELLOW Areas (laboratories, blood transfusion service, radiodiagnostics, radiotherapy, nuclear medicine, mortuary, pathological anatomy, employee and non-employee changing rooms, storage rooms) | Dusting, wiping to remove fingerprints and stains, and disinfection of all horizontal (furniture, equipment, medical supplies, including beds, tables, carts, etc.) and vertical surfaces (furniture, IV poles, both sides of doors, windows, switches, handles, etc.). Accessible areas cleaned without the use of ladders. | Once to twice daily (7/7–14/7), as determined by each healthcare facility |
| Steel Equipment | Cleaning of external parts of steel equipment (washing machines, autoclaves, dishwashers, etc.) using approved healthcare facility products. | Once every 60 days |
| Floors | Dust removal, cleaning (with disposable gauze if necessary), and disinfection upon request of all protected and unprotected floors. | Once to twice daily (7/7–14/7), as determined by each healthcare facility |
| Deep Cleaning | Thorough cleaning of all areas including moving furniture, cleaning walls, floors, fixtures, radiators, etc. Use of ladders if necessary. | Once every 90 days |
| Sanitary Fixtures | Cleaning, descaling, and disinfection of sanitary fixtures, washable walls, showers, shower stalls, accessories, etc. | Weekly |
| High-Level Surfaces | Vacuuming, wet dusting, and cleaning of all aerial parts including lighting fixtures, radiators, air conditioning units, internal blinds, ceilings, etc. | Frequency determined with each healthcare facility |
| Area/Item to Clean | Tasks | Frequency |
|---|---|---|
| ORANGE Areas (border areas and access to red zones such as filter zones and changing rooms, possible studies and administrative offices attached to operating rooms, emergency room, non-intensive surgical and medical care area, hospice, clinics, consulting rooms, day hospital, level 3 microbiology laboratories (P3), storage rooms. The internal corridors, stairs, paths and all the toilets related to the aforementioned areas) | Dusting, wiping to remove fingerprints and stains, and disinfection of all horizontal (furniture, equipment, medical supplies, including beds, tables, carts, etc.) and vertical surfaces (furniture, IV poles, both sides of doors, windows, switches, handles, etc.). Accessible areas cleaned without the use of ladders. | Once to twice daily (7/7–14/7), as determined by each healthcare facility |
| Steel Equipment | Cleaning of external parts of steel equipment (washing machines, autoclaves, dishwashers, etc.) using approved healthcare facility products. | Once every 60 days |
| Floors | Dust removal, cleaning (with disposable gauze if necessary), and disinfection upon request of all protected and unprotected floors. | Once to twice daily (7/7–14/7), as determined by each Healthcare Facility |
| Deep Cleaning | Thorough cleaning of all areas including moving furniture, cleaning walls, floors, fixtures, radiators, etc. Use of ladders if necessary. | Once every 90 days |
| Sanitary Fixtures | Cleaning, descaling, and disinfection of sanitary fixtures, washable walls, showers, shower stalls, accessories, etc. | Weekly |
| High-Level Surfaces | Vacuuming, wet dusting, and cleaning of all aerial parts including lighting fixtures, radiators, air conditioning units, internal blinds, ceilings, etc. | Frequency determined with each healthcare facility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, R.; Buratto, M.; Caproni, A.; Nordi, C.; Pappadà, M.; Facchini, M.; Buffone, C.; Bandera, B.; Vogli, L.; Marconi, P. Eco-Friendly vs. Traditional Cleaning in Healthcare Settings: Microbial Safety and Environmental Footprint. Hygiene 2025, 5, 37. https://doi.org/10.3390/hygiene5030037
Fontana R, Buratto M, Caproni A, Nordi C, Pappadà M, Facchini M, Buffone C, Bandera B, Vogli L, Marconi P. Eco-Friendly vs. Traditional Cleaning in Healthcare Settings: Microbial Safety and Environmental Footprint. Hygiene. 2025; 5(3):37. https://doi.org/10.3390/hygiene5030037
Chicago/Turabian StyleFontana, Riccardo, Mattia Buratto, Anna Caproni, Chiara Nordi, Mariangela Pappadà, Martina Facchini, Cesare Buffone, Beatrice Bandera, Luciano Vogli, and Peggy Marconi. 2025. "Eco-Friendly vs. Traditional Cleaning in Healthcare Settings: Microbial Safety and Environmental Footprint" Hygiene 5, no. 3: 37. https://doi.org/10.3390/hygiene5030037
APA StyleFontana, R., Buratto, M., Caproni, A., Nordi, C., Pappadà, M., Facchini, M., Buffone, C., Bandera, B., Vogli, L., & Marconi, P. (2025). Eco-Friendly vs. Traditional Cleaning in Healthcare Settings: Microbial Safety and Environmental Footprint. Hygiene, 5(3), 37. https://doi.org/10.3390/hygiene5030037










