Abstract
In recent decades, the world has observed the emergence and re-emergence of a multitude of previously non-existent or re-emerging infectious diseases, for which there is a paucity of timely and effective preventative measures. The WHO has published a catalogue of priority pathogens that are likely to trigger future epidemics, with the objective of designing effective prophylactic and therapeutic interventions. The rationale behind these interventions is rooted in a comprehensive understanding of the aetiology, epidemiology, and pathogenesis of the target diseases. While it is imperative to acknowledge the pivotal role that evolutionary changes in pathogens play, it is equally important to recognise the influence of a multifaceted interplay of factors on the emergence and re-emergence of infectious diseases. These include changes in human populations, the vectors and reservoirs of exposure, and environmental changes. This review summarises the aetiology, epidemiology, and pathogenesis of the ten WHO priority diseases, as well as those with high epidemic potential that are already the focus of specific control programme initiatives, such as HIV/AIDS, tuberculosis, and malaria diseases. Furthermore, this review concentrated on the means of addressing these infections through public health surveillance and response systems. Such systems must be designed to rapidly detect unusual and unexpected disease patterns, track and share information in real time, and rapidly mobilise global responses, which are the most important ways to effectively contain transmission.
1. Introduction
Infectious diseases have exerted a profound influence on the development of humanity [1]. The introduction of measures such as sanitation, improved nutrition, vaccines, and antimicrobials has led to the erroneous belief that humanity has definitively triumphed over pathogens, or that the risk of infection is confined to underdeveloped nations [2]. However, the assertion that the risk of infection is exclusive to developing nations is demonstrably erroneous, given the persistence of both emerging and re-emerging infectious epidemics [3]. ‘Emerging and re-emerging infectious diseases’ refers to two categories. Newly emerging infections are caused by novel pathogens. Re-emerging infectious diseases are caused by microorganisms resurging after a period of decline [4]. According to the CDC, the diseases of infectious origin are defined as those which have either increased in incidence among the human population within the past two decades or which are likely to do so in the future [3]. From an epidemiological perspective, infectious diseases exhibit different spread characteristics [5]. An epidemic is defined as the frequent and localised, albeit time-limited, manifestation of an infectious disease, which occurs when there is an increase, often sudden, in the frequency of cases beyond what is normally expected within a population [6]. In contrast, the term pandemic originates from the Greek pân, which means all, and dêmos, which means people, and signifies the widespread dissemination of a disease across multiple continents or, in more expansive terms, over vast geographical areas of the world [7]. In 2018, the World Health Organisation (WHO) published a plan which identified some diseases as the world’s most important, including the following: Ebola virus disease (EVD), malaria (MVD), Crimean–Congo haemorrhagic fever (CCHF), Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), Nipah virus (Nipah), henipaviruses, Rift Valley fever (RVF), Zika, and “Disease X”, a yet unknown disease [8]. The most recent pandemic was declared by the WHO on 11 March 2020 for the new strain of Coronavirus (SARS-CoV-2) [9]. In recent decades, the global community has experienced several outbreaks and re-emergences of infectious diseases, posing significant challenges to global health security and the global economy. Notable epidemics that have emerged include the following: SARS (severe acute respiratory syndrome), which emerged from China in 2002, H1N1 swine flu, a pandemic that originated from Mexico in 2009, and Middle East respiratory syndrome (MERS), which first emerged from Saudi Arabia in 2014 [10,11,12]. In addition to the aforementioned epidemics, the following diseases have also caused significant global health concerns: Ebola virus disease (2013–2016), Zika virus (2015), Lassa virus (2018), Marburg virus disease (2018), Crimean–Congo haemorrhagic fever (2022), and Rift Valley fever (2019) [13,14,15,16,17]. In addition to the diseases previously mentioned, it is also important to consider other deadly infectious diseases, such as monkeypox and hantavirus [18,19]. Mpox is a zoonotic viral disease caused by the Monkeypox virus (MPXV), which can be transmitted in two different ways: from animals to humans and from humans to humans. The WHO declared the mpox outbreak a public health emergency in July 2022, but reversed this status in May 2023 as the outbreak came under control. By August 2024, it had spread to multiple countries and the WHO declared it a public health emergency once more [18]. In view of the intricate and evolving epidemiological profile of the mpox virus, a subsequent review will focus on the pathogen. Hantaviruses are a class of emerging and lethal zoonotic viruses, primarily spread by rodents [19]. There has been a notable increase in the relevance of these viruses as human pathogens in recent years. In humans, the diseases caused by these viruses can result in two distinct clinical syndromes: haemorrhagic fever with renal syndrome (HFRS), whose most severe form is caused by the Hantaan virus, and cardiopulmonary syndrome (HCPS), mainly caused by the Sin Nombre virus. The overall mortality rate for these diseases is estimated to be approximately 35% [20]. People become infected through rodent bites or contact with rodent excreta, but human-to-human transmission is also possible. The displacement of both rodents and humans from their natural habitats due to climate change increases the likelihood of zoonotic infection with hantavirus. The absence of a vaccine or therapy and the identification of new hantaviruses in rodents linked to human diseases exacerbates this threat [21]. With regard to the diseases previously mentioned, it is of the utmost importance to consider other deadly infectious diseases that claim millions of victims worldwide each year, especially in low-income countries. These include HIV/AIDS, tuberculosis, and malaria [22] (Table 1).

Table 1.
The key emerging and re-emerging diseases.
Despite significant advancements in the field of medicine and public health, these diseases continue to pose significant challenges, particularly in low-income regions. Within the context of low-income nations, the predominant causes of mortality are infectious and parasitic diseases; in contrast, in high-income nations, non-communicable diseases such as heart disease and cancer prevail as the leading causes of death [52,53,54]. In this review, the focus is on three key factors that contribute to the emergence and re-emergence of infectious diseases. These factors are as follows: the agent, defined as the microbial risk; the host, defined as the human vulnerability; and the environment, defined as the human exposure [55,56]. The aforementioned factors include genetic, biological, social, and economic elements. The most significant of these determinants are as follows: international trade and commerce, human susceptibility to infection, poverty and social inequality, war and famine, changing ecosystems, and microbial change. Furthermore, the identification of the most efficacious courses of action to guide prevention and control strategies is imperative [56]. The enhancement of global surveillance, the utilisation of vaccines, the development of novel pharmaceuticals, the optimisation of vector and zoonotic control, and the dissemination of health education on a more extensive scale are some potential solutions to this issue.
2. Emerging and Re-Emerging Infectious Diseases: How and Why They Arise
Epidemics are a natural phenomenon in the context of human history. Despite the fact that infectious diseases have had a profound impact on the course of human history, the collective memory of these diseases remains limited. This is due to the rarity of pandemics [57]. Two notable examples are the Spanish flu pandemic of 1918–1920, which resulted in an estimated 40 million deaths worldwide, and the more recent pandemic, the global novel coronavirus crisis (SARS-CoV-2), which will result in approximately 6.7 million deaths between 2020 and 2022 [58]. Historical analysis indicates that the majority of infections have emerged over millennia, coinciding with the rise and evolution of interactions between humans, animals, and their environment [59]. The emergence and re-emergence of infectious diseases has been shown to be a multifaceted phenomenon [3,4,60] (Figure 1). It is estimated that between 60% and 80% of emerging infections are of animal origin, suggesting that zoonotic origin is a significant source of emerging diseases [61]. Through close contact with diseased animals carrying a potentially emerging pathogen, humans are susceptible to direct infection. Alternatively, indirect infection may occur through a vector or by the ingestion of contaminated water and food. In this respect, the ways in which water and food are procured, stored, or processed have a decisive influence on the transmission process [62,63]. Evolutionary changes in pathogens represent a pivotal element in the emergence of diseases, exerting influence on characteristics such as species specificity, geographical distribution, clinical manifestations (including virulence or immune evasion), transmission modalities, and resistance to therapeutic interventions [64,65] (Figure 1). The evolutionary process of a pathogen may involve a range of genetic alterations, including single nucleotide substitutions, gene acquisition from other organisms, and recombination and reassortment events [66]. These evolutionary changes can influence various epidemiological outcomes, such as spillover and outbreak occurrences. The selective pressure exerted by antimicrobials has led to the emergence of bacteria and parasites that are resistant to such agents [67]. Furthermore, pathogens with a high mutation rate can lead to the rapid evolution of new variants. This phenomenon is particularly evident in RNA viruses, including influenza, flavivirus, and coronavirus, which are distinguished by their high genetic variability and consequent impact on vector and/or host competence, resulting in significant consequences for their dissemination among human populations [68,69,70]. In addition to the evolution of pathogens, the emergence and re-emergence of infectious diseases are influenced by a multifaceted interplay of factors, which can be conceptualised as the human environment [55] (Figure 1). A multitude of factors exert a substantial influence on the emergence of such diseases, many of which are also subject to the impact of other conditions. These include, but are not limited to, the processes of globalisation, climate change, urbanisation, and population growth, which can lead to the concentration of populations in urban areas characterised by inadequate sanitation [71]. Additional host-related variables worthy of note include demographic shifts, such as an aged population and an increase in immune-compromised individuals [71,72].
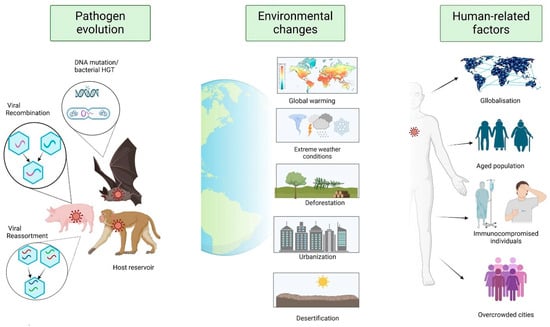
Figure 1.
The multifactorial nature of emergent and re-emergent infectious diseases. Genetic changes in pathogens affect their biology, including how easily they spread, how they evade the immune system, how harmful they are, and how resistant they become to antimicrobials. These changes allow the most adaptable pathogen to thrive in a specific environment. Environmental change can also impact the geographical distribution of reservoir hosts and vectors, increasing the likelihood of spread to new areas. Human activities, such as globalisation, help pathogens to spread quickly and extensively. Overcrowding and unsanitary conditions in cities increase the risk of new pathogens emerging and spreading.
3. Pathogens with Epidemic and Pandemic Potential
3.1. Zika Virus
The Zika virus (ZIKV) belongs to the genus Flavivirus. This enveloped, single-stranded RNA arbovirus was first isolated from a rhesus monkey in the Zika forest in Uganda in 1947 [73]. The virus was then isolated in the Aedes africanum mosquito in the same location the following year (1948) [74]. For the following several decades after its discovery, only sporadic and mild cases of infection were reported in Asia and Africa. Nevertheless, the first major outbreak occurred in 2007 outside of the African continent, taking place in the Yap Islands of Micronesia (Pacific Islands) [23]. This was followed by significant further outbreaks in French Polynesia (2013) and Brazil (2015), thus confirming the virus’s ability to emerge and spread among the human population [24]. The majority of ZIKV infections are asymptomatic or mild and non-specific. Manifestations may include fever, arthralgia, rash, and conjunctivitis [75]. These generally resolve within 2–7 days; however, in certain cases, more severe manifestations may emerge. ZIKV is transmitted through the bite of an infected mosquito. The Aedes genus of mosquitoes is responsible for the transmission of the Zika virus (ZIKV) on various continents. ZIKV is transmitted by mosquitoes in two cycles: the sylvatic cycle and the urban cycle. In the latter cycle, the main vectors of ZIKV are the Aedes aegypti and Aedes albopictus species. The transmission of infection is facilitated by the feeding of the vector on infected humans and the subsequent dissemination of the virus to other humans and mosquito populations [25]. However, in comparison with other arboviruses, ZIKV infection has been shown to involve additional transmission routes, including infected blood transfusion, sexual transmission, and maternal–foetal transmission. The Zika virus has been found in semen, indicating potential sexual transmission even where there are no Aedes mosquitoes [26]. Vertical transmission has also been confirmed, linking ZIKV infection to neonatal neurological complications. During the outbreak in Brazil, the Zika virus spread rapidly and led to an unexpected rise in the number of children born with microcephaly [76]. Furthermore, several countries, including Brazil, have reported a significant increase in Guillain–Barré syndrome and other neuronal disorders among adult patients infected with the Zika virus [76]. Consequently, in February 2016, the WHO formally declared this association of Zika infection with clusters of microcephaly to be a Public Health Emergency of International Concern [77]. WHO has classified the Zika virus as a priority because of its links to congenital defects and neurological impairments. However, there are also concerns about its potential for epidemics due to its widespread and abundant presence of Aedes spp. vectors [78]. The spread of ZIKV is being driven by several key factors, including poverty, inadequate/damaged infrastructure, deforestation, urbanisation, and climate change [77]. It is possible that the virus’s affinity with the urban vector, A. aegypti, and the efficiency of urban transmission between humans have been affected by an adaptive evolutionary process. This would explain the total absence of a ZIKV epidemic before 2007 [79]. The virus’s spread is challenging to predict, making planning difficult. The complexity of the virus’s epidemiology and transmission dynamics poses significant challenges in predicting future outbreaks and epidemics. The WHO has issued a call to the global research communities, urging them to prioritise the development of vaccines, improved diagnostics, and innovative vector control strategies.
3.2. SARS-CoV-2
Coronavirus disease (COVID-19) is an infectious disease from a new type of coronavirus (CoV) called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [27]. The initial cases of the as-yet unidentified respiratory illness were reported on 8 December 2019 in Wuhan, China. Local health authorities in China identified an outbreak of pneumonia of unknown origin in Wuhan. The disease then spread to several other countries, leading to the declaration of a Public Health Emergency of International Concern by the WHO on 31 January 2020 [27]. The disease then spread rapidly, resulting in significant mortality, and was characterised by the WHO as a pandemic. SARS-CoV-2 is an enveloped virus with a single-stranded positive-sense RNA genome. It belongs to the Betacoronavirus genus and the Coronaviridae family. Betacoronaviruses cause infections in a wide range of mammals [27]. Like other coronaviruses, they have a high mutation frequency and significant recombination events. This allows them to ‘jump’ from one species to another. Epidemiological studies suggest the Huanan Seafood Market in Wuhan was the early epicentre of SARS-CoV-2 infection due to its proximity to various animals. This is based on the similarity between SARS-CoV-2 and bat or pangolin CoVs [80]. It is thought that the pangolin may have acted as an intermediate host in the evolution of the virus, increasing its zoonotic potential and transmissibility [3]. As the virus replicates and spreads among humans, new variants such as Alpha, Beta, Delta, and Omicron have been detected, confirming the high mutation capacity of SARS-CoV-2 and posing new challenges in controlling the pandemic. SARS-CoV-2 is transmitted primarily through close contact with respiratory droplets from an infected person’s mouth or nose. Given the ability of the virus to remain on inanimate surfaces such as plastic, stainless steel, cardboard, and copper, the transmission can also occur by touching an infected surface and subsequently touching the eyes, nose, or mouth [28]. The virus can cause a range of symptoms, including fever, cough, and fatigue, which can progress to severe respiratory disease. However, some patients may experience no respiratory symptoms at onset and exhibit extra-pulmonary manifestations, including headache, diarrhoea, and vomiting [81]. SARS-CoV-2 infection can affect people of all ages, but the highest morbidity and mortality rates are observed in individuals over 65 years old or with other comorbidities (hypertension, diabetes, cardiovascular disorders, respiratory diseases), often requiring hospitalisation and intensive care [81]. “LongCoV” or “post-COVID-19 syndrome” is a recently identified chronic condition associated with a SARS-CoV-2 infection. It is characterised by prolonged symptoms in multiple systems that emerge after a SARS-CoV-2 infection. The syndrome can persist for months after the acute illness, affecting numerous organs, including the heart, lungs, immune system, digestive system, nervous system, blood vessels, kidneys, spleen, and liver. The overlap of symptoms can pose challenges in the management of the condition [82]. The WHO has identified COVID-19 as a priority disease due to its potentially devastating consequences. The potential pandemic of SARS-CoV-2 has resulted in a rapid global spread of the virus, leading to an overload of health systems ill prepared for an emergency of such magnitude [83]. The consequences resulting from the rapid transmission of the virus have been numerous. Healthcare facilities throughout countries have experienced critical shortages of personal protective equipment and ventilators, especially for patients exhibiting severe symptoms and requiring intensive treatment [84]. Furthermore, the virus’s capacity for mutation has engendered the development of new variants that have the potential to compromise vaccine efficacy or disease severity, necessitating ongoing global vigilance and representing a matter of urgent concern to the WHO [85]. The pandemic has had far-reaching consequences for public health, the economy, and society [84]. Lockdowns, travel restrictions, and quarantine have reduced SARS-CoV-2 transmission, but these measures have concomitantly precipitated profound economic adversity for millions of individuals worldwide [85].
3.3. Middle East Respiratory Disease (MERS) and Severe Acute Respiratory Syndrome (SARS)
Middle East respiratory syndrome (MERS), and severe acute respiratory syndrome (SARS) are two respiratory diseases caused by two viruses belonging to the Betacoronavirus genus [33]. MERS, first detected in Jordan in 2012, has had two major outbreaks: in 2013 in Saudi Arabia and in 2015 in South Korea [86]. Most patients become severely ill, with a fever, cough, and shortness of breath. There is currently no available vaccine or specific treatment. Treatment is therefore supportive [87]. According to the WHO, 2613 cases of Middle East respiratory syndrome (MERS) have been reported since April 2012. Most cases have been reported from the Arabian Peninsula, with 943 deaths (case fatality ratio of 36%—the highest among all coronaviruses) [29]. In recent times, there have been sporadic imported cases in Europe, North America, Africa, and Asia. MERS-CoV is transmitted between humans less often than other coronaviruses, but sporadic outbreaks still occur [30]. The virus is traced back to bats, with dromedary camels serving as intermediate hosts. These camels are a primary source of MERS-CoV transmission to humans [31]. Other livestock species, including wildlife, can also spread MERS-CoV. Humans can contract the virus from camels, through airborne transmission, or by eating contaminated milk and meat [32].
The first cases of SARS were identified among animal handlers in Guangzhou [88]. These individuals had been in close contact with wild game meat and subsequently infected healthcare workers. The first cases were reported in November 2002 in Guangdong, China, representing the first instance of severe human illness caused by a coronavirus [88]. The virus was traced back to bats and civets, with the spread to humans via both animal-to-human and human-to-human transmission, leading to its global spread [34].
Stringent public health measures, including quarantine and travel restrictions, led to the containment of the disease by 5 July 2003 [89]. By its conclusion, 8422 cases had been documented by the CDC and WHO, resulting in 916 deaths in 29 countries [11]. The exact means of transmission is unclear, but it is hypothesised that airborne droplets from infected patients may be the primary vector. SARS can cause pneumonia and respiratory failure, often leading to mortality [90]. Both SARS-CoV and MERS-CoV are a global health threat because they can spread quickly and lots of people die [91]. MERS-CoV is more deadly, but its spread has been more restricted. Both viruses have animal reservoirs, which can lead to new variants and outbreaks [91]. We need to understand how these viruses jump from animals to humans and have better countermeasures—including safe and effective vaccines and therapies—in place to stop a potential health crisis. The emergence of two highly pathogenic coronaviruses within ten years highlights the need for constant pandemic preparedness. This means that governments, health officials, and international agencies must remain vigilant to detect and respond to cases.
3.4. Ebola Virus Disease and Marburg Virus Disease
Ebola is a rare and serious illness caused by a filovirus. First reported in 1976 in South Sudan and the Democratic Republic of Congo, it takes its name from a village built near the Ebola River [35]. Since then, sporadic outbreaks have occurred, especially in Africa, where it is a serious public health problem. Guinea, Sierra Leone, and Liberia accounted for the highest number of deaths (28,000 cases and 11,000 deaths) between 2014 and 2016 [36]. The virus is transmitted through direct contact with the mucous membranes or wounds of infected individuals and through contact with the bodies of deceased individuals (Ebola virus also persists post-mortem) [37]. The Ebola virus can also be spread through contact with contaminated objects or surfaces or through contact with infected wild animals. The natural reservoir of the virus is believed to be fruit bats [92]. Ebola can persist in the blood of survivors and can last for months or years [37]. The precise mechanisms of resistance remain to be fully elucidated; however, emerging evidence suggests a potential role for macrophages. Notably, pregnant women who have recovered from Ebola appear to transmit the virus to their infants through breast milk, underscoring the complexity of viral transmission dynamics. These characteristics influence the occurrence of epidemics, which are likely generated by survivors infecting susceptible individuals [3]. Initial non-specific symptoms (2–21 days post-infection) overlap with those of numerous ailments. These are then followed by vomiting, diarrhoea, skin rashes, and sudden bleeding. Severe cases may lead to multi-organ failure, immunological disorders, shock, and death. The wide spectrum of symptoms makes the differentiation of the Ebola virus disease challenging [3]. The main diagnostic methods are ELISA and RT-PCR assays. The treatment of the disease involves the administration of monoclonal antibodies in combination with other therapeutic interventions. Currently, two authorised vaccines are in use. Despite the significant reduction in the mortality rate from EVD that has been observed with the administration of monoclonal antibodies (mAbs), the overall case fatality rate remains high at 30% [93].
Marburg virus disease (MVD) is a haemorrhagic fever caused by a filovirus. First identified in 1967 between Germany and present-day Serbia, the most recent outbreak occurred in Guinea in 2023 [76]. It is linked to caves and the habitat of the Rousettus aegyptiacus bat and has symptoms and fatality rates comparable to those of the Ebola virus [38]. These viruses can spread quickly and easily because they are very deadly, can lie hidden in the body for a long time, spread after healing, and do not have specific medicines. The WHO therefore considers them a priority [39]. It is very important for people to take part in stopping the spread of disease by being clean, staying away from infected people, and practicing safe funeral practices. The WHO’s strategy for monitoring and searching for these viral agents is based on anticipation, reinforcement, and cure [39]. There is no approved MVD vaccine; several are in clinical trials [39].
3.5. Crimean–Congo Haemorrhagic Fever
Crimean–Congo haemorrhagic fever (CCHF) is caused by a virus from the Nairovirus genus. It was discovered in Crimea in 1944 and in a Congolese child in 1956 [94]. The virus spreads to humans via tick bites or through contact with infected tissues/blood/body fluids, especially during breeding and slaughter. It infects many different types of wild and domestic animals. Since it can spread without symptoms, it is more infectious among animals than humans, and those involved in breeding, slaughter, and veterinary work are the most affected [43].
The lethality rate of this disease is between 10 and 40%. The virus is endemic in Africa, Asia, the Balkans, and the Middle East. The last outbreak was described in Iraq in 2022 [44]. The disease has a sudden onset with non-specific symptoms. The incubation period after a tick bite is between one and three days (nine max) and between five and six days after contact with infected tissue. Initially, there is fever, headache, dizziness, nausea, muscle pain and stiffness, and sensitivity to light [95]. Next comes the haemorrhagic phase, marked by the swelling of the liver, skin, and mucus membranes, bleeding from eyes, noses, and gums, and blood in the faeces and urine. Intravascular coagulopathy and cardiogenic shock can lead to an unfavourable outcome [95]. The disease is diagnosed microbiologically using ELISA and RT-PCR assays. However, in the early stages, patients do not develop an antibody response, so the diagnosis is made by detecting the virus RNA in the blood [95]. There is currently no specific therapy or effective vaccine for CCFH. The only possible means of reducing the infection in people is through basic preventive measures [95]. The intricate nature of infection control in animals and ticks, the often-challenging detection of the tick–animal–tick cycle, and the prevalence of asymptomatic animals underscore the complexity of the disease dynamics.
3.6. Rift Valley Fever (RVF)
Rift Valley fever (RVF) is a viral zoonosis transmitted by mosquitoes. It was discovered in 1931 and has since caused sporadic epidemics in Africa, Madagascar, Saudi Arabia, and Yemen [45]. A severe epidemic was reported in Kenya, Somalia, and Tanzania in 1997–98. The disease is endemic to domestic ruminants and is caused by an arbovirus belonging to the genus Phlebovirus (family Bunyaviridae). The disease has caused significant economic losses due to the death and/or abortion of infected animals. The virus is spread among animals by mosquitoes of the Aedes genus, which can also transmit it vertically [45]. Humans can become infected through the bite of infected mosquitoes or by direct contact with infected animals. There have been a few documented cases of infection resulting from the bite of hematophagous flies [46]. The most at-risk groups are breeders, slaughterers, and veterinarians. There is no documented evidence of human-to-human transmission of RVF. The disease can present with mild or severe symptoms, with serious consequences for survivors. The incubation period is 2–6 days. The symptoms of the mild form are comparable to those of influenza or meningitis and subside after about a week. At this point, an immune response becomes evident. Severe syndromes, on the other hand, may manifest as isolated episodes or concurrently. The severe ocular form affects the macula, causing blindness in half the patients. The meningoencephalitis form causes hallucinations, disorientation, confusion, and coma, leading to severe neurological deficits in survivors [96]. The haemorrhagic form causes severe liver failure, frequent bleeding, and a 50% mortality rate. These syndromes manifest one to three weeks after symptoms first appear.
RVF has non-specific clinical symptoms, so RVF is investigated using RT-PCR, antigen detection tests, or viral isolation. Antibodies are only detectable five to six days after onset [46]. Viral isolation, a common concern in all priority diseases, is difficult because it is highly biohazardous. This necessitates the use of laboratories with cutting-edge biocontainment capabilities, as this viral agent poses a serious threat as a biological weapon. The disease causes mild symptoms in humans and is short-lived, with no specific treatment available. In severe cases, intensive care and management are required; the most effective method for preventing the spread of the disease is the vaccination of cattle and strict adherence to safe husbandry and slaughter practices. A vaccine for humans is available but not authorised or available to buy [45]. Rainy periods are causing more cases of RVF, so we need better forecasting models to stop outbreaks in animals and humans.
3.7. Lassa Fever
Lassa fever is a viral haemorrhagic fever caused by a virus belonging to the Arenaviridae family. It is endemic to Benin, Guinea, Liberia, Nigeria, Mali, Sierra Leone, and Togo [96]. The precise incidence of infection is uncertain; however, it is estimated that cases range from 30,000 to 50,000 per year. The estimated mortality rate falls within the range of 5000 and 10,000, resulting in an estimated annual mortality rate of approximately 1 per cent (although it has been observed to reach as high as 15 per cent in hospitalised patients suffering from serious illnesses) [40]. The natural reservoir for the infection is the multimammate mouse. The prevalence of the disease in Africa is such that its eradication is impracticable. Humans contract the disease through contact with these rodents. Although rare, human-to-human transmission can occur through contact with blood or body fluids [40]. Unlike other haemorrhagic fevers, the onset of Lassa fever is gradual. The incubation period is the duration between infection and the onset of symptoms and can vary between 2 and 21 days. Initial symptoms are comparable to those of numerous other syndromes and include fever, general weakness, and malaise. These are followed by additional symptoms such as sore throat, abdominal and chest pain, vomiting, and diarrhoea. In severe cases, the disease can cause facial swelling, lung swelling, and internal bleeding. Recovery from Lassa fever is slow. Patients must be closely monitored for Lassa fever sequelae, which can cause sudden deafness and neurological disorders [41]. Pregnancy increases the risk of complications, with high maternal and foetal mortality in the third trimester. The disease must be microbiologically diagnosed using RT-PCR, enzyme immunoassays, or antigen detection. No infection tests are available for this or other haemorrhagic fevers; testing must be performed in specialised centres. There are no treatments or vaccines for Lassa fever. As mentioned, controlling the infection reservoir is impossible due to the huge spread of these rodents [97]. The only forms of prevention are hygiene and airtight storage.
3.8. Nipah and Henipaviral Diseases
It is a zoonotic disease caused by RNA viruses belonging to the genus Henipavirus and the Paramyxoviridae family. The most virulent and deadly viruses for humans include Nipah virus (NiV) and Hendra virus (HeV) [98]. The onset of henipaviral infection is characterised by flu-like symptoms. However, the major pathological consequence of infection is severe acute systemic vasculitis that affects many major organs, such as the brain and the lungs. This often leads to severe neurological and respiratory manifestations, including encephalitis and respiratory distress syndrome. The clinical course of the disease can vary in relation to the two different strains that have been identified, namely the Malaysian (Niv-M) and the Bangladesh strain (Niv-B), with the latter causing more severe respiratory troubles and having a higher case fatality. This infection is hard to detect because the symptoms are non-specific. The main known reservoir for henipaviruses is the Pteropus genus of flying fox species found in Southeast Asia, the Indian Ocean, Oceania, and Sub-Saharan Africa. Human infection can be transmitted via an intermediate host, such as pigs or horses, by direct contact with bats, or via human-to-human transmission. Body fluids and excreta play a crucial role in the various routes of infection. Cattle, horses, dogs, cats, and goats can also be exposed to NiV, as demonstrated by serological studies, though human transmission has yet to be shown [99]. The Hendra virus outbreak (1994) in Australia’s Hendra suburb killed twenty-one racehorses and two people. Since then, sporadic human cases have persisted. HeV’s pathogenesis (57% fatality rate) and frequent occurrence remain a concern [100]. The Nipah virus was first detected during the 1998–1999 outbreak in Kampung Sungai Nipah, Malaysia. Thereafter, it spread to Singapore in 1999 and has since caused outbreaks in Bangladesh, India, and the Philippines. Confirmed cases of the virus have been reported in these five countries, with the highest case fatality rates being reported from India (73%) and Bangladesh (71%). However, the case fatality rate for NiV infection can vary from less than 10% to over 100%, depending on the outbreak and the healthcare infrastructure available for managing cases. Its status as a priority for the WHO is due to the lack of effective treatment and vaccines and the possibility of the virus spreading further afield due to its global distribution and the fact that it can infect a wide range of animals [101].
3.9. “DISEASE X”
The term ‘Pathogen X’ is used by the World Health Organisation (WHO) to denote a hypothetical virus or bacterium that has the potential to cause a major new epidemic or pandemic [102]. The primary purpose of utilising this term is to encourage both scientists and governments to recognise the possibility of previously unknown microorganisms causing human diseases [102]. This realisation underscores the need for concerted efforts to be made in order to ensure preparedness for such eventualities. The adoption of this approach has the potential to facilitate a more expeditious and efficacious response to health threats through the implementation of prompt preventative measures and the acceleration of vaccine development and dissemination within a substantially reduced timeframe.
4. Overcoming Challenges: The Neglected Pandemics—HIV/AIDS, Tuberculosis, and Malaria
The ten diseases classified as ‘priority’ by the World Health Organisation (WHO) are deemed to be of such concern on account of their pandemic potential and the paucity of existing surveillance systems and specific action plans. In contrast to preventable diseases such as measles, pertussis, and so on, which can be prevented with vaccines and are not influenced by multiple factors, the emergence or re-emergence of ‘priority diseases’ as defined by the WHO is characterised by the lack of effective vaccines. Furthermore, the resurgence of diseases such as diphtheria and pertussis is linked to low vaccination rates [103]. The re-emergence of traditional infectious diseases can also be hypothesised to occur where pathogens exhibit augmented resistance to conventional antimicrobial therapy. Moreover, the re-emergence of diseases previously considered eliminated has been linked to bacteria that are resistant to common antibiotics. A notable example of this phenomenon is the emergence of the highly infectious, antibiotic-resistant strain of Clostridium difficile, which has resulted in a substantial increase in the number of cases and severity of infections over the past two decades [104]. Conversely, although surveillance systems and action plans for diseases such as HIV/AIDS, tuberculosis, and malaria have been implemented, death and illness rates remain high [105] (Figure 2).
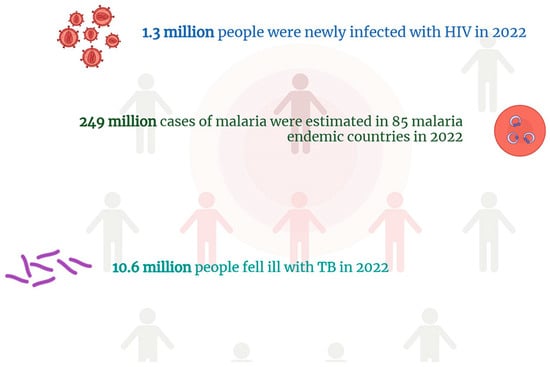
Figure 2.
The most recent WHO data on the worldwide incidence of HIV/AIDS, tuberculosis, and malaria. Numbers of this magnitude highlight how these diseases represent a serious public health problem despite the fact that they are well known and have been the subject of action plans for several years.
The WHO is working to address these diseases in impoverished communities, but they continue to be a leading cause of mortality in low-income countries. Nevertheless, as a consequence of the Global Fund initiative, which was launched in 2002, significant advances have been made in the reduction in the overall burden of HIV, malaria, and tuberculosis. Indeed, the rate of DALYS for these diseases was reduced by 56% between 2000 and 2021 [106]. However, it is evident that we are still far from achieving the objective of stopping these epidemics by 2030, which is part of the specific health goals of the Sustainable Development Goals [107].
4.1. HIV Infection and AIDS
Recent decades have seen notable shifts in the global HIV/AIDS pandemic. Effective antiretroviral therapy, prevention methodologies, and enhanced public consciousness regarding HIV transmission and treatment have contributed to a decrease in new infections and mortality. Globally, 2023 saw an estimated 39.9 million people living with HIV, including 1.4 million children (under 14) and 38.6 million adults (over 15) [108]. Key risk factors include behaviours such as sex with men, being transgender, injecting drugs, sex work, and imprisonment. The most impacted region by HIV is Sub-Saharan Africa, including South Africa, Nigeria, Mozambique, and Uganda [48]. Within this region, there are cultures where subjects relating to HIV and AIDS are often avoided, and misconceptions concerning transmission and prevention methods are prevalent. These factors also impede access to healthcare due to stigma and discrimination relating to gender identity, sexuality, prior incarceration, and HIV-infected status [48]. The lack of a vaccine and effective treatment means the disease can continue to spread. The development of new strategies to combat the spread of HIV has focused on enhancing data collection by national health services. This is a strategy that can be applied universally to any infection with a similar epidemiology. This approach is particularly relevant for ‘key’ populations, where the burden of HIV is high and access to health services is often constrained by social stigmatisation [109]. A comprehensive understanding of the habits of these individuals, along with access to their health data and the assurance of their safety, is paramount for the effective prevention and treatment of infections of this nature.
4.2. Malaria’s Impact Worldwide
The WHO’s World Malaria Report, updated to 2023, states that 263 million cases and 567 thousand deaths were caused by malaria that year [110]. Despite various malaria control programmes, including vector control, chemoprophylaxis, rapid diagnostic tests, and timely treatment, malaria remains a significant health problem. The African continent is the most affected, accounting for 95% of cases and 96% of deaths [49]. Following a decline in cases until 2015, there has been a resurgence, partly due to the emergence of artemisinin and insecticide resistance, and the SARS-coronavirus 2 (COVID-19) pandemic. This poses a threat to particularly vulnerable populations such as children under five and pregnant women. However, a vaccine offering moderate protection against malaria was approved in Oct 2021 [111]. Genetic studies on malaria vectors, in conjunction with geographical and environmental analyses of the endemic regions, are of pivotal importance in the development of surveillance systems. These systems employ the aforementioned characteristics, in conjunction with other socio-demographic factors, to create useful models for the development of elimination strategies.
4.3. Tuberculosis: Old Disease, New Threat
Tuberculosis is a preventable and treatable disease. In 2023, it re-emerged as the world’s leading infectious disease, following a three-year period in which it was overtaken by coronavirus disease (COVID-19) [112]. It is estimated that tuberculosis caused almost twice as many deaths as HIV/AIDS. In 2023, the global burden of tuberculosis was estimated to be the highest in 30 high-burden countries, with India (26%), Indonesia (10%), China (6.8%), the Philippines (6.8%), and Pakistan (6.3%) accounting for 56% of cases. The highest incident rate was observed in Africa [50]. The incidence and mortality of tuberculosis are linked to health coverage and access to services. Individuals identified as being most at risk include those who are imprisoned, displaced, economically disadvantaged, malnourished, HIV-positive, and those experiencing compromised immune systems. Eradicating tuberculosis remains far off, linked to inadequate progress towards universal health coverage [78]. Achieving the full potential of all strategies to halt TB requires universal access to treatment. These diseases are both the consequence and cause of poverty and inequality. Inadequate resources pose a significant threat to populations, leaving them vulnerable to the threat of disease. To eliminate outbreaks, services must be available to all, particularly the poor, and essential medicines and vaccines must be distributed fairly, especially in the most deprived countries [36]. Interventions in the health sector and those aimed at the social and cultural factors behind the diseases can tackle their causes and use health services more effectively.
5. Conflict and Infectious Diseases
A recent analysis of the European Centre for Disease Prevention and Control’s (ECDC) Surveillance Atlas of Infectious Diseases reveals a worrying trend in the continent’s recent outbreak of various infectious pathogens [113]. The atlas documents a resurgence of pathogens that had previously been in circulation, with some showing alarming trends in their prevalence. While certain diseases, including tuberculosis, a leading cause of mortality worldwide, show no signs of abatement, other diseases, such as sexually transmitted infections, are re-emerging with a resurgence in cases that have doubled or tripled in the post-COVID-19 era. In 2023, for instance, the European Union/European Economic Area (EU/EEA) reported 41,051 cases of syphilis, 230,199 cases of chlamydia, 96,969 cases of gonorrhoea, and 28,751 cases of hepatitis C virus infection [114]. In the context of the global spread of infectious diseases, it is imperative to acknowledge the significant yet often overlooked aspect of the numerous conflicts that have occurred and continue to occur in various parts of the world. There is a well-documented and well-evidenced history of the catastrophic consequences of the interconnection of infectious diseases and warfare [115] (Figure 3). It is therefore vital to recognise the relationship between these two phenomena. The advent of war gives rise to a plethora of conditions that engender the propagation of infectious diseases, chiefly by modifying the living conditions of the affected population. The consequences of war for public health are profound, and even infections that are typically preventable through vaccination can become problematic in the context of protracted conflict. This phenomenon is exemplified by the recent measles and hepatitis B epidemics in Syria and Yemen, respectively [116]. The destruction or overcrowding of sanitation facilities, shortages of food and clean water, and poor hygienic conditions are all factors that predispose us to the expansion of new or re-emerging pathogens (Figure 3). Furthermore, large-scale movements of people, whether military personnel or refugees, can lead to the dissemination of diseases across a wide geographical area. The impact of epidemics on conflict outcomes is significant, increasing the vulnerability of affected populations [115]. Historical examples of pandemics that have impacted world conflicts include the bubonic plague, cholera, the Spanish flu, and tuberculosis. The plague remains classified as an endemic disease in certain regions of the world, with documented outbreaks reported to the WHO from over 33 countries, including Madagascar, the Democratic Republic of the Congo, India, China, Peru, and sporadically, the USA [117]. If left undiagnosed, the bubonic plague can progress to a septicaemic infection or secondary pneumonic plague, in which Y. pestis, the causative agent, can be efficiently transmitted by respiratory droplets, potentially resulting in disease outbreaks, epidemics, and even pandemics. In regions where the plague remains endemic due to a combination of factors, including poverty and migration, the lack of an approved vaccine, and the risk of antimicrobial resistance, Y. pestis represents a significant and persistent biological threat [118]. The recent malaria outbreak in the Democratic Republic of Congo highlights the pressing need for interdisciplinary programmes that ensure prompt access to screening tests, isolation, and containment. Following a significant increase in mortality rates during the First World War, tuberculosis was recognised as a war disease. Given that the majority of contemporary conflicts are occurring in tuberculosis-endemic countries, tuberculosis continues to pose a significant threat not only to individuals residing in conflict-affected areas but also to the populations of neighbouring countries [119]. The internal displacement of significant numbers of people to safer locations within the country or internationally still poses a major threat today. Following the conflict that broke out in Ukraine in February 2022, the country is the fourth with the highest incidence of tuberculosis in Europe and the fifth globally in terms of drug-resistant tuberculosis cases [3]. The spread of tuberculosis is facilitated by two key factors: the lack of access to adequate medical care and the presence of large populations in confined spaces lacking proper ventilation [119]. The ongoing armed conflict has led to a resurgence in new cases, reversing the downward trend that had been observed until 2020. This situation underscores the critical importance of addressing not only the immediate health concerns posed by the pandemic but also the underlying factors that contribute to the high prevalence of MDR-TB. The armed conflict has the potential to severely impede efforts to combat the disease, primarily by limiting access to diagnostic services and anti-TB medications. Moreover, the substantial influx of refugees into neighbouring countries has placed considerable pressure on national health systems, further exacerbating the challenges posed by the pandemic [119]. It is therefore vital to emphasise the need for effective screening programmes to contain the disease, isolate those with active TB, and prevent its uncontrolled spread. The role of armed conflict as a driver for the spread of HIV and sexually transmitted diseases is well documented. A seminal study of the post-First World War period revealed that sexually transmitted infections (STIs) became the second most common cause of disability among American soldiers. A 15% increase in the incidence of HIV has been observed in certain regions of Ukraine since the onset of the Russian–Ukrainian conflict [120] (Figure 3). The factors contributing to this increase include uncontrolled sexual intercourse, reduced access to healthcare and contraception, abuse, and the spread of new viral strains, resulting from displacement. Moreover, the economic disadvantage engendered by war has been demonstrated to result in a significant number of women engaging in sex work as a means of income [120]. Moreover, the cessation of treatment in individuals with a positive diagnosis has been demonstrated to result in an escalation of viral load, which in turn engenders an elevated level of infectivity. Consequently, the implementation of programmes for the early detection of sexually transmitted diseases in migrants from war-affected countries is of paramount importance in order to counteract the spread of such diseases [115].
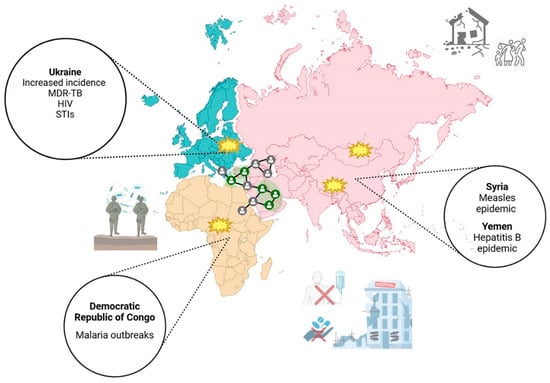
Figure 3.
The catastrophic consequences of the profound interconnection between infectious diseases and warfare. Infectious diseases have a significant impact during conflicts, spreading rapidly among military personnel and the population. History shows that warfare creates the perfect conditions for disease to spread due to its disruptive effect on living conditions. The destruction or overcrowding of health facilities, food shortages, drinking water shortages, and suboptimal hygiene all contribute to the spread of pathogens. Large-scale movements of people, whether military personnel or refugees, spread diseases, even in regions far from the conflict. The absence of access to healthcare services resurges vaccine-preventable diseases. Conflict increases unprotected sex, which transmits infections like STIs and HIV.
6. Conclusions
The emergence of new infectious diseases has been recognised as an unpredictable phenomenon for millennia, even prior to the discovery of causative infectious agents. Despite the significant advancements in developing countermeasures (diagnostic, therapeutic, and preventive), the ease of global travel and increasing global interdependence have greatly complicated the establishment of the necessary measures to contain such diseases. These diseases have the capacity to not only affect human health but also economic stability. Emerging and re-emerging infections pose a significant threat to global public health, as demonstrated by the recent 2019 novel coronavirus (nCoV) pandemic and the emergence and subsequent spread of other infectious pathogens. The ability of these pathogens to spread rapidly and cause large-scale outbreaks is due to a number of interconnected factors, including globalisation, climate change, deforestation, and drug resistance. In the context of the ongoing emergence, resurgence, and spread of infectious diseases, human interaction with domestic and wild animals and environmental changes resulting from human activities have become pivotal elements in understanding the epidemiology of these diseases. Furthermore, it is crucial to recognise the impact of these changes in terms of increasing opportunities for microbes to thrive. In light of the above, what efficacious strategies are currently available to combat these infections? In order to address these infections, it is imperative to employ a multifaceted approach that encompasses the following: (1) Rapidly detect unusual and unexplained disease outbreaks. (2) Track and exchange information in real time. (3) The mobilisation of a swift and decisive response effort that can rapidly extend on a global scale. (4) The containment of transmission with the objective of achieving rapid outcomes (Table 2).

Table 2.
Measures for the prevention and control of emerging and re-emerging infectious diseases.
The realisation of these points is contingent upon the successful resolution of the following national surveillance system deficiencies: (1) the lack of a comprehensive surveillance strategy for significant diseases; (2) the inadequate initial epidemiological investigations, laboratory diagnostics, and rapid field investigations; (3) the utilisation of erroneous case definitions; (4) delays in reporting and the inadequacy of data and information analysis at all levels; (5) the absence of feedback to peripheral levels; (6) the insufficiency of preparedness to control epidemics. The following measures are proposed to address the gaps: firstly, the integration of laboratory science and epidemiology with a view to enhance the effectiveness of public health practice. Secondly, the strengthening of public health infrastructures in order to provide support for surveillance, research, and the implementation of prevention and control programmes. Thirdly, the provision of the public health workforce with the knowledge and tools required. Fourthly, the prompt implementation of prevention strategies and the enhancement of the communication of public health information regarding emerging diseases (Figure 4).
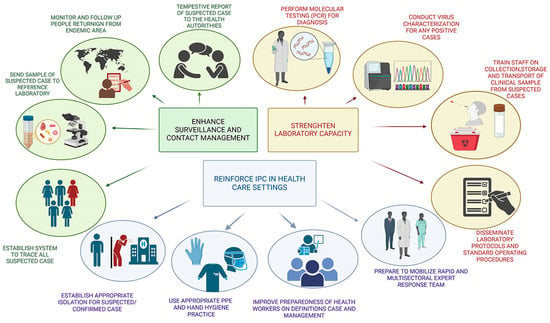
Figure 4.
Schematic representation of the measures deemed necessary for the enhancement of public health, with a focus on the timely diagnosis and prevention of infectious diseases.
The implementation of the aforementioned measures has been the result of a gradual and methodical process that requires careful planning. However, there are a number of measures that have the potential to be activated in a relatively short period of time. These include the following:
- -
- The enhancement of coverage and the acceptability of vaccines through the utilisation of nasal sprays, with the associated reduction in costs and reliance on “cold chain” logistics;
- -
- The reduction in antimicrobial usage in agriculture and food production;
- -
- The control of vector and zoonotic diseases by means of the use of safe insecticides.
Whatever measures are implemented to prevent epidemics from recurring, retraining medical personnel and enhancing diagnostics must be a priority. This is key to a fast and effective response to often non-specific clinical demands.
Author Contributions
Conceptualization, C.B., S.D.G. and E.P.; writing—original draft preparation, D.F., G.I., G.M., A.M. and S.Z.; review and editing, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karlsson, E.K.; Kwiatkowski, D.P.; Sabeti, P.C. Natural selection and infectious disease in human populations. Nat. Rev. Genet. 2014, 15, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Dharmarajan, G.; Khan, I. Evolution of pathogen tolerance and emerging infections: A missing experimental paradigm. eLife 2021, 10, e68874. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Wang, Z.; Yang, W.; Li, E.; Xia, X.; Yan, F.; Chiu, S. Emerging and reemerging infectious diseases: Global trends and new strategies for their prevention and control. Signal Transduct. Target. Ther. 2024, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Ristori, M.V.; Guarrasi, V.; Soda, P.; Petrosillo, N.; Gurrieri, F.; Longo, U.G.; Ciccozzi, M.; Riva, E.; Angeletti, S. Emerging Microorganisms and Infectious Diseases: One Health Approach for Health Shared Vision. Genes 2024, 15, 908. [Google Scholar] [CrossRef]
- Hauri, A.M.; Uphoff, H. Tasks, principles and methods of applied infectious disease epidemiology/field epidemiology. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz 2005, 48, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Talisuna, A.O.; Okiro, E.A.; Yahaya, A.A.; Stephen, M.; Bonkoungou, B.; Musa, E.O.; Minkoulou, E.M.; Okeibunor, J.; Impouma, B.; Djingarey, H.M.; et al. Spatial and temporal distribution of infectious disease epidemics, disasters and other potential public health emergencies in the World Health Organisation Africa region, 2016–2018. Glob. Health 2020, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Gollakner, R.; Capua, I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet. Ital. 2020, 56, 7–8. [Google Scholar] [CrossRef]
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef]
- Biondo, C.; Ponzo, E.; Midiri, A.; Ostone, G.B.; Mancuso, G. The Dark Side of Nosocomial Infections in Critically Ill COVID-19 Patients. Life 2023, 13, 1408. [Google Scholar] [CrossRef]
- Cunha, C.B.; Opal, S.M. Middle East respiratory syndrome (MERS): A new zoonotic viral pneumonia. Virulence 2014, 5, 650–654. [Google Scholar] [CrossRef]
- Cherry, J.D.; Krogstad, P. SARS: The first pandemic of the 21st century. Pediatr. Res. 2004, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mena, I.; Nelson, M.I.; Quezada-Monroy, F.; Dutta, J.; Cortes-Fernandez, R.; Lara-Puente, J.H.; Castro-Peralta, F.; Cunha, L.F.; Trovao, N.S.; Lozano-Dubernard, B.; et al. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. eLife 2016, 5, e16777. [Google Scholar] [CrossRef]
- Sayres, L.; Hughes, B.L. Contemporary Understanding of Ebola and Zika Virus in Pregnancy. Clin. Perinatol. 2020, 47, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.L.; Ly, H. Pathogenicity and virulence mechanisms of Lassa virus and its animal modeling, diagnostic, prophylactic, and therapeutic developments. Virulence 2021, 12, 2989–3014. [Google Scholar] [CrossRef] [PubMed]
- Cuomo-Dannenburg, G.; McCain, K.; McCabe, R.; Unwin, H.J.T.; Doohan, P.; Nash, R.K.; Hicks, J.T.; Charniga, K.; Geismar, C.; Lambert, B.; et al. Marburg virus disease outbreaks, mathematical models, and disease parameters: A systematic review. Lancet Infect. Dis. 2024, 24, e307–e317. [Google Scholar] [CrossRef]
- Alhilfi, R.A.; Khaleel, H.A.; Raheem, B.M.; Mahdi, S.G.; Tabche, C.; Rawaf, S. Large outbreak of Crimean-Congo haemorrhagic fever in Iraq, 2022. IJID Reg. 2023, 6, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Youssouf, H.; Subiros, M.; Dennetiere, G.; Collet, L.; Dommergues, L.; Pauvert, A.; Rabarison, P.; Vauloup-Fellous, C.; Le Godais, G.; Jaffar-Bandjee, M.C.; et al. Rift Valley Fever Outbreak, Mayotte, France, 2018–2019. Emerg. Infect. Dis. 2020, 26, 769–772. [Google Scholar] [CrossRef]
- Kozlov, M. Growing mpox outbreak prompts WHO to declare global health emergency. Nature 2024, 632, 718–719. [Google Scholar] [CrossRef]
- Vial, P.A.; Ferres, M.; Vial, C.; Klingstrom, J.; Ahlm, C.; Lopez, R.; Le Corre, N.; Mertz, G.J. Hantavirus in humans: A review of clinical aspects and management. Lancet Infect. Dis. 2023, 23, e371–e382. [Google Scholar] [CrossRef]
- Jacob, A.T.; Ziegler, B.M.; Farha, S.M.; Vivian, L.R.; Zilinski, C.A.; Armstrong, A.R.; Burdette, A.J.; Beachboard, D.C.; Stobart, C.C. Sin Nombre Virus and the Emergence of Other Hantaviruses: A Review of the Biology, Ecology, and Disease of a Zoonotic Pathogen. Biology 2023, 12, 1413. [Google Scholar] [CrossRef]
- Gutierrez-Jara, J.P.; Munoz-Quezada, M.T.; Cordova-Lepe, F.; Silva-Guzman, A. Mathematical Model of the Spread of Hantavirus Infection. Pathogens 2023, 12, 1147. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.T. Global, regional, and national age-specific progress towards the 2020 milestones of the WHO End TB Strategy: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 698–725. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Calvez, E.; Chouin-Carneiro, T.; Diallo, D.; Failloux, A.B. An overview of mosquito vectors of Zika virus. Microbes Infect. 2018, 20, 646–660. [Google Scholar] [CrossRef]
- Teixeira, F.M.E.; Pietrobon, A.J.; Oliveira, L.M.; Oliveira, L.; Sato, M.N. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front. Immunol. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Midiri, A.; Gerace, E.; Zummo, S.; Mancuso, G. SARS-CoV-2 Infection in Patients with Cystic Fibrosis: What We Know So Far. Life 2022, 12, 2087. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Oraby, T.; Tyshenko, M.G.; Balkhy, H.H.; Tasnif, Y.; Quiroz-Gaspar, A.; Mohamed, Z.; Araya, A.; Elsaadany, S.; Al-Mazroa, E.; Alhelail, M.A.; et al. Analysis of the Healthcare MERS-CoV Outbreak in King Abdulaziz Medical Center, Riyadh, Saudi Arabia, June-August 2015 Using a SEIR Ward Transmission Model. Int. J. Environ. Res. Public health 2020, 17, 2936. [Google Scholar] [CrossRef]
- Pavli, A.; Tsiodras, S.; Maltezou, H.C. Middle East respiratory syndrome coronavirus (MERS-CoV): Prevention in travelers. Travel Med. Infect. Dis. 2014, 12, 602–608. [Google Scholar] [CrossRef]
- Omrani, A.S.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): Animal to human interaction. Pathog. Glob. Health 2015, 109, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I. Zoonotic diseases transmitted from the camels. Front. Vet. Sci. 2023, 10, 1244833. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.X.; Liew, W.P.P.; Ong, H.K.; Yong, C.Y.; Shit, C.S.; Ho, W.Y.; Ng, S.Y.L.; Yeap, S.K. Current diagnostic approaches to detect two important betacoronaviruses: Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Pathol. Res. Pract. 2021, 225, 153565. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 2020, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G.; Heymann, D.L.; Lloyd, G.; McCormick, J.B.; Miatudila, M.; Murphy, F.A.; Muyembe-Tamfun, J.J.; Piot, P.; Ruppol, J.F.; Sureau, P.; et al. Discovery and Description of Ebola Zaire Virus in 1976 and Relevance to the West African Epidemic During 2013–2016. J. Infect. Dis. 2016, 214, S93–S101. [Google Scholar] [CrossRef]
- Den Boon, S.; Marston, B.J.; Nyenswah, T.G.; Jambai, A.; Barry, M.; Keita, S.; Durski, K.; Senesie, S.S.; Perkins, D.; Shah, A.; et al. Ebola Virus Infection Associated with Transmission from Survivors. Emerg. Infect. Dis. 2019, 25, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, F.; Bakhordarian, A.; Thames, A.D.; Du, A.M.; Jan, A.L.; Nahcivan, M.; Nguyen, M.T.; Sama, N.; Manfrini, E.; Piva, F.; et al. Ebola: Translational science considerations. J. Transl. Med. 2015, 13, 11. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, D.; Kumar, S.; Sharma, A.; Rijal, R.; Asija, A.; Adhikari, S.; Rustagi, S.; Sah, S.; Al-Qaim, Z.H.; et al. Emergence of Marburg virus: A global perspective on fatal outbreaks and clinical challenges. Front. Microbiol. 2023, 14, 1239079. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Souris, M.; Valdivia-Granda, W. Global Spread of Hemorrhagic Fever Viruses: Predicting Pandemics. Methods Mol. Biol. 2018, 1604, 3–31. [Google Scholar] [CrossRef]
- Reyna, R.A.; Littlefield, K.E.; Shehu, N.; Makishima, T.; Maruyama, J.; Paessler, S. The Importance of Lassa Fever and Its Disease Management in West Africa. Viruses 2024, 16, 266. [Google Scholar] [CrossRef]
- Shieh, W.J.; Demby, A.; Jones, T.; Goldsmith, C.S.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J.; Zaki, S.R. Pathology and Pathogenesis of Lassa Fever: Novel Immunohistochemical Findings in Fatal Cases and Clinico-pathologic Correlation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.E.; Walker, D.H. Pathogenesis of Lassa fever. Viruses 2012, 4, 2031–2048. [Google Scholar] [CrossRef]
- Papa, A.; Tsergouli, K.; Tsioka, K.; Mirazimi, A. Crimean-Congo Hemorrhagic Fever: Tick-Host-Virus Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Kodama, C.; Alhilfi, R.A.; Aakef, I.; Khamasi, A.; Mahdi, S.; Hasan, H.M.; Khaleel, R.I.; Naji, M.M.; Esmaeel, N.K.; Haji-Jama, S.; et al. Epidemiological analysis and potential factors affecting the 2022-23 Crimean-Congo hemorrhagic fever outbreak in Iraq. Eur. J. Public health 2025, 35, i6–i13. [Google Scholar] [CrossRef]
- Alkan, C.; Jurado-Cobena, E.; Ikegami, T. Advancements in Rift Valley fever vaccines: A historical overview and prospects for next generation candidates. NPJ Vaccines 2023, 8, 171. [Google Scholar] [CrossRef]
- Hartman, A. Rift Valley Fever. Clin. Lab. Med. 2017, 37, 285–301. [Google Scholar] [CrossRef]
- Li, H.; Kim, J.V.; Pickering, B.S. Henipavirus zoonosis: Outbreaks, animal hosts and potential new emergence. Front. Microbiol. 2023, 14, 1167085. [Google Scholar] [CrossRef] [PubMed]
- Kharsany, A.B.; Karim, Q.A. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016, 10, 34–48. [Google Scholar] [CrossRef]
- Oladipo, H.J.; Tajudeen, Y.A.; Oladunjoye, I.O.; Yusuff, S.I.; Yusuf, R.O.; Oluwaseyi, E.M.; AbdulBasit, M.O.; Adebisi, Y.A.; El-Sherbini, M.S. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Ann. Med. Surg. 2022, 81, 104366. [Google Scholar] [CrossRef]
- A spotlight on the tuberculosis epidemic in South Africa. Nat. Commun. 2024, 15, 1290. [CrossRef]
- Mancuso, G.; Midiri, A.; De Gaetano, S.; Ponzo, E.; Biondo, C. Tackling Drug-Resistant Tuberculosis: New Challenges from the Old Pathogen Mycobacterium tuberculosis. Microorganisms 2023, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Cao, B.; Varghese, C.; Mikkelsen, B.; Weiderpass, E.; Soerjomataram, I. Comparing cancer and cardiovascular disease trends in 20 middle- or high-income countries 2000-19: A pointer to national trajectories towards achieving Sustainable Development goal target 3.4. Cancer Treat. Rev. 2021, 100, 102290. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A. The double burden of communicable and non-communicable diseases in developing countries. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A.; Boutayeb, S. The burden of non communicable diseases in developing countries. Int. J. Equity health 2005, 4, 2. [Google Scholar] [CrossRef]
- Church, D.L. Major factors affecting the emergence and re-emergence of infectious diseases. Clin. Lab. Med. 2004, 24, 559–586. [Google Scholar] [CrossRef]
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef]
- Martin, P.M.; Martin-Granel, E. 2,500-year evolution of the term epidemic. Emerg. Infect. Dis. 2006, 12, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.T.; Liang, L.T.; Rosen, J.M. COVID-19: A comparison to the 1918 influenza and how we can defeat it. Postgrad. Med. J. 2021, 97, 273–274. [Google Scholar] [CrossRef]
- Weiss, R.A.; Sankaran, N. Emergence of epidemic diseases: Zoonoses and other origins. Fac. Rev. 2022, 11, 2. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Veiga, A.B.G.; Kaminski, V.L.; Valverde-Villegas, J.M.; Freitas, A.W.Q.; Chies, J.A.B. Control and prevention of infectious diseases from a One Health perspective. Genet. Mol. Biol. 2021, 44, e20200256. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44, e20200355. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lazaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A.; Nascimento, M.S.; D’Agostino, M.; Santos, R.; Saiz, J.C.; Rzezutka, A.; et al. Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 2012, 36, 786–814. [Google Scholar] [CrossRef] [PubMed]
- Endale, H.; Mathewos, M.; Abdeta, D. Potential Causes of Spread of Antimicrobial Resistance and Preventive Measures in One Health Perspective—A Review. Infect. Drug Resist. 2023, 16, 7515–7545. [Google Scholar] [CrossRef]
- Bonneaud, C.; Longdon, B. Emerging pathogen evolution: Using evolutionary theory to understand the fate of novel infectious pathogens. EMBO Rep. 2020, 21, e51374. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.; Hauser, H.; Sanders, M.; Ngo, T.H.; Cherevach, I.; Cronin, A.; Goodhead, I.; Mungall, K.; Quail, M.A.; Price, C.; et al. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS ONE 2009, 4, e6072. [Google Scholar] [CrossRef] [PubMed]
- LaTourrette, K.; Garcia-Ruiz, H. Determinants of Virus Variation, Evolution, and Host Adaptation. Pathogens 2022, 11, 1039. [Google Scholar] [CrossRef]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, A.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef]
- Howard, C.R.; Fletcher, N.F. Emerging virus diseases: Can we ever expect the unexpected? Emerg. Microbes Infect. 2012, 1, e46. [Google Scholar] [CrossRef]
- Enserink, M. Emerging infectious diseases. Nipah virus (or a cousin) strikes again. Science 2004, 303, 1121. [Google Scholar] [CrossRef]
- LeDuc, J.W. WHO program on emerging virus diseases. Arch. Virol. Suppl. 1996, 11, 13–20. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, C. Climate change and multiple emerging infectious diseases. Vet. J. 2018, 234, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Wikan, N.; Smith, D.R. Zika virus: History of a newly emerging arbovirus. Lancet. Infect. Dis. 2016, 16, e119–e126. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, E.B.; Kramer, L.D. Zika Virus Mosquito Vectors: Competence, Biology, and Vector Control. J. Infect. Dis. 2017, 216, S976–S990. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Choi, G.K.; Yip, C.C.; Cheng, V.C.; Yuen, K.Y. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J. Infect. 2016, 72, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Possas, C.; Brasil, P.; Marzochi, M.C.; Tanuri, A.; Martins, R.M.; Marques, E.T.; Bonaldo, M.C.; Ferreira, A.G.; Lourenco-de-Oliveira, R.; Nogueira, R.M.R.; et al. Zika puzzle in Brazil: Peculiar conditions of viral introduction and dissemination—A Review. Mem. Inst. Oswaldo Cruz 2017, 112, 319–327. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, B.; Endericks, T. The rise of Zika infection and microcephaly: What can we learn from a public health emergency? Public Health 2017, 150, 87–92. [Google Scholar] [CrossRef]
- Gardner, L.; Chen, N.; Sarkar, S. Vector status of Aedes species determines geographical risk of autochthonous Zika virus establishment. PLoS Neglected Trop. Dis. 2017, 11, e0005487. [Google Scholar] [CrossRef]
- Talavera-Aguilar, L.G.; Murrieta, R.A.; Kiem, S.; Cetina-Trejo, R.C.; Baak-Baak, C.M.; Ebel, G.D.; Blitvich, B.J.; Machain-Williams, C. Infection, dissemination, and transmission efficiencies of Zika virus in Aedes aegypti after serial passage in mosquito or mammalian cell lines or alternating passage in both cell types. Parasites Vectors 2021, 14, 261. [Google Scholar] [CrossRef]
- Worobey, M.; Levy, J.I.; Malpica Serrano, L.; Crits-Christoph, A.; Pekar, J.E.; Goldstein, S.A.; Rasmussen, A.L.; Kraemer, M.U.G.; Newman, C.; Koopmans, M.P.G.; et al. The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic. Science 2022, 377, 951–959. [Google Scholar] [CrossRef]
- Baj, J.; Karakula-Juchnowicz, H.; Teresinski, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakula, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; McNab, C.; Olson, R.M.; Bristol, N.; Nolan, C.; Bergstrom, E.; Bartos, M.; Mabuchi, S.; Panjabi, R.; Karan, A.; et al. How an outbreak became a pandemic: A chronological analysis of crucial junctures and international obligations in the early months of the COVID-19 pandemic. Lancet 2021, 398, 2109–2124. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y. WHO International Health Regulations Emergency Committee for the COVID-19 outbreak. Epidemiol. Health 2020, 42, e2020013. [Google Scholar] [CrossRef]
- Omrani, A.S.; Shalhoub, S. Middle East respiratory syndrome coronavirus (MERS-CoV): What lessons can we learn? J. Hosp. Infect. 2015, 91, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Gerber, S.I.; Swerdlow, D.L. Middle East respiratory syndrome coronavirus: Update for clinicians. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1686–1689. [Google Scholar] [CrossRef]
- Xu, R.H.; He, J.F.; Evans, M.R.; Peng, G.W.; Field, H.E.; Yu, D.W.; Lee, C.K.; Luo, H.M.; Lin, W.S.; Lin, P.; et al. Epidemiologic clues to SARS origin in China. Emerg. Infect. Dis. 2004, 10, 1030–1037. [Google Scholar] [CrossRef]
- Wilder-Smith, A. The severe acute respiratory syndrome: Impact on travel and tourism. Travel Med. Infect. Dis. 2006, 4, 53–60. [Google Scholar] [CrossRef]
- Luu, B.; McCoy-Hass, V.; Kadiu, T.; Ngo, V.; Kadiu, S.; Lien, J. Severe Acute Respiratory Syndrome Associated Infections. Physician Assist. Clin. 2023, 8, 495–530. [Google Scholar] [CrossRef]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Delicat, A.; Paweska, J.T.; Gonzalez, J.P.; Swanepoel, R. Fruit bats as reservoirs of Ebola virus. Nature 2005, 438, 575–576. [Google Scholar] [CrossRef] [PubMed]
- El Ayoubi, L.W.; Mahmoud, O.; Zakhour, J.; Kanj, S.S. Recent advances in the treatment of Ebola disease: A brief overview. PLoS Pathog. 2024, 20, e1012038. [Google Scholar] [CrossRef] [PubMed]
- Okesanya, O.J.; Olatunji, G.D.; Kokori, E.; Olaleke, N.O.; Adigun, O.A.; Manirambona, E.; Lucero-Prisno, D.E., 3rd. Looking Beyond the Lens of Crimean-Congo Hemorrhagic Fever in Africa. Emerg. Infect. Dis. 2024, 30, 1319–1325. [Google Scholar] [CrossRef]
- Frank, M.G.; Weaver, G.; Raabe, V.; State of the Clinical Science Working Group of the National Emerging Pathogens Training; Education Center’s Special Pathogens Research Network. State of the Clinical Science Working Group of the National Emerging Pathogens Training Education Center’s Special Pathogens Research, N. Crimean-Congo Hemorrhagic Fever Virus for Clinicians-Epidemiology, Clinical Manifestations, and Prevention. Emerg. Infect. Dis. 2024, 30, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, M.A.; Abdel-Hamid, Y.M.; Beier, J.C. Rift Valley Fever in Egypt and other African countries: Historical review, recent outbreaks and possibility of disease occurrence in Egypt. Acta Trop. 2018, 181, 40–49. [Google Scholar] [CrossRef]
- Marien, J.; Borremans, B.; Kourouma, F.; Baforday, J.; Rieger, T.; Gunther, S.; Magassouba, N.; Leirs, H.; Fichet-Calvet, E. Evaluation of rodent control to fight Lassa fever based on field data and mathematical modelling. Emerg. Microbes Infect. 2019, 8, 640–649. [Google Scholar] [CrossRef]
- Kaza, B.; Aguilar, H.C. Pathogenicity and virulence of henipaviruses. Virulence 2023, 14, 2273684. [Google Scholar] [CrossRef]
- Branda, F.; Ceccarelli, G.; Giovanetti, M.; Albanese, M.; Binetti, E.; Ciccozzi, M.; Scarpa, F. Nipah Virus: A Zoonotic Threat Re-Emerging in the Wake of Global Public Health Challenges. Microorganisms 2025, 13, 124. [Google Scholar] [CrossRef]
- Peel, A.J.; Yinda, C.K.; Annand, E.J.; Dale, A.S.; Eby, P.; Eden, J.S.; Jones, D.N.; Kessler, M.K.; Lunn, T.J.; Pearson, T.; et al. Novel Hendra Virus Variant Circulating in Black Flying Foxes and Grey-Headed Flying Foxes, Australia. Emerg. Infect. Dis. 2022, 28, 1043–1047. [Google Scholar] [CrossRef]
- Singh, R.K.; Dhama, K.; Chakraborty, S.; Tiwari, R.; Natesan, S.; Khandia, R.; Munjal, A.; Vora, K.S.; Latheef, S.K.; Karthik, K.; et al. Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies—A comprehensive review. Vet. Q. 2019, 39, 26–55. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.; Heung, M.; Nave, J.; Henkel, C.; Escudero-Perez, B. The natural virome and pandemic potential: Disease X. Curr. Opin. Virol. 2023, 63, 101377. [Google Scholar] [CrossRef] [PubMed]
- Lubanga, A.F.; Bwanali, A.N.; Kangoma, M.; Matola, Y.; Moyo, C.; Kaonga, B.; Ssebibubbu, S.; Makole, T.J.; Kambili, F.; Chumbi, G.D.; et al. Addressing the re-emergence and resurgence of vaccine-preventable diseases in Africa: A health equity perspective. Hum. Vaccines Immunother. 2024, 20, 2375081. [Google Scholar] [CrossRef] [PubMed]
- Aptekorz, M.; Sacha, K.; Gofron, Z.; Kabala, M.; Harmanus, C.; Kuijper, E.; Martirosian, G. Antibiotic Resistance Profile of RT 027/176 Versus Other Clostridioides difficile Isolates in Silesia, Southern Poland. Pathogens 2022, 11, 949. [Google Scholar] [CrossRef]
- Bell, D.; Schultz Hansen, K. Relative Burdens of the COVID-19, Malaria, Tuberculosis, and HIV/AIDS Epidemics in Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2021, 105, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.L. Human rights and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. Health Hum. Rights 2014, 16, 134–147. [Google Scholar] [PubMed]
- Strong, K.; Noor, A.; Aponte, J.; Banerjee, A.; Cibulskis, R.; Diaz, T.; Ghys, P.; Glaziou, P.; Hereward, M.; Hug, L.; et al. Monitoring the status of selected health related sustainable development goals: Methods and projections to 2030. Glob. Health Action 2020, 13, 1846903. [Google Scholar] [CrossRef] [PubMed]
- Payagala, S.; Pozniak, A. The global burden of HIV. Clin. Dermatol. 2024, 42, 119–127. [Google Scholar] [CrossRef]
- Schwartz, S.R.; Rao, A.; Rucinski, K.B.; Lyons, C.; Viswasam, N.; Comins, C.A.; Olawore, O.; Baral, S. HIV-Related Implementation Research for Key Populations: Designing for Individuals, Evaluating Across Populations, and Integrating Context. J. Acquir. Immune Defic. Syndr. 2019, 82 (Suppl. 3), S206–S216. [Google Scholar] [CrossRef]
- Ba Konko Cire, E.H.; Roh, M.E.; Diallo, A.; Gadiaga, T.; Seck, A.; Thiam, S.; Gaye, S.; Diallo, I.; Lo, A.C.; Diouf, E.; et al. Mass drug administration to reduce malaria incidence in a low-to-moderate endemic setting: Short-term impact results from a cluster randomised controlled trial in Senegal. medRxiv 2024. [Google Scholar] [CrossRef]
- Segala, F.V.; Di Gennaro, F.; Ictho, J.; L’Episcopia, M.; Onapa, E.; Marotta, C.; De Vita, E.; Amone, J.; Iacobelli, V.; Ogwang, J.; et al. Impact of antimalarial resistance and COVID-19 pandemic on malaria care among pregnant women in Northern Uganda (ERASE): Protocol of a prospective observational study. BMC Infect. Dis. 2022, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Falzon, D.; Zignol, M.; Bastard, M.; Floyd, K.; Kasaeva, T. The impact of the COVID-19 pandemic on the global tuberculosis epidemic. Front. Immunol. 2023, 14, 1234785. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.J.; Kumar, P.; Griffith, M.P.; Waggle, K.D.; Srinivasa, V.R.; Raabe, N.; Mills, E.G.; Coyle, H.; Ereifej, D.; Creager, H.M.; et al. Genomic Surveillance for Enhanced Healthcare Outbreak Detection and Control. medRxiv 2024. [Google Scholar] [CrossRef]
- Nerlander, L.; Champezou, L.; Gomes Dias, J.; Aspelund, G.; Berlot, L.; Constantinou, E.; Diaz, A.; Epstein, J.; Fogarassy, E.; Hernando, V.; et al. Sharp increase in gonorrhoea notifications among young people, EU/EEA, July 2022 to June 2023. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. = Eur. Commun. Dis. Bull. 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Marou, V.; Vardavas, C.I.; Aslanoglou, K.; Nikitara, K.; Plyta, Z.; Leonardi-Bee, J.; Atkins, K.; Condell, O.; Lamb, F.; Suk, J.E. The impact of conflict on infectious disease: A systematic literature review. Confl. Health 2024, 18, 27. [Google Scholar] [CrossRef]
- Mehtar, S.; AlMhawish, N.; Shobak, K.; Reingold, A.; Guha-Sapir, D.; Haar, R.J. Measles in conflict-affected northern Syria: Results from an ongoing outbreak surveillance program. Confl. Health 2021, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Butler, T. Plague Gives Surprises in the Second Decade of the Twenty-First Century. Am. J. Trop. Med. Hyg. 2023, 109, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.D.; Kilgore, P.B.; Hendrix, E.K.; Neil, B.H.; Sha, J.; Chopra, A.K. Progress on the research and development of plague vaccines with a call to action. NPJ Vaccines 2024, 9, 162. [Google Scholar] [CrossRef]
- Gebreyohannes, E.A.; Wolde, H.F.; Akalu, T.Y.; Clements, A.C.A.; Alene, K.A. Impacts of armed conflicts on tuberculosis burden and treatment outcomes: A systematic review. BMJ Open 2024, 14, e080978. [Google Scholar] [CrossRef]
- Kvasnevska, Y.; Faustova, M.; Voronova, K.; Basarab, Y.; Lopatina, Y. Impact of war-associated factors on spread of sexually transmitted infections: A systemic review. Front. Public Health 2024, 12, 1366600. [Google Scholar] [CrossRef]
- Norris, S.L.; Louis, H.; Sawin, V.I.; Porgo, T.V.; Lau, Y.H.A.; Wang, Q.; Ferri, M. An evaluation of WHO emergency guidelines for Zika virus disease. J. Evid.-Based Med. 2019, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Marcassoli, A.; Leonardi, M.; Passavanti, M.; De Angelis, V.; Bentivegna, E.; Martelletti, P.; Raggi, A. Lessons Learned from the Lessons Learned in Public Health during the First Years of COVID-19 Pandemic. Int. J. Environ. Res. Public health 2023, 20, 1785. [Google Scholar] [CrossRef] [PubMed]
- Group, F.-O.-W.M.T.W. MERS: Progress on the global response, remaining challenges and the way forward. Antivir. Res. 2018, 159, 35–44. [Google Scholar] [CrossRef]
- Wang, T.H.; Wei, K.C.; Hsiung, C.A.; Maloney, S.A.; Eidex, R.B.; Posey, D.L.; Chou, W.H.; Shih, W.Y.; Kuo, H.S. Optimizing severe acute respiratory syndrome response strategies: Lessons learned from quarantine. Am. J. Public Health 2007, 97 (Suppl. 1), S98–S100. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. Exploring the Importance of Infection Prevention and Control Measures in the 2014 Outbreak of Ebola. Int. J. Prev. Med. 2015, 6, 96. [Google Scholar] [CrossRef]
- Wirsiy, F.S.; Nkfusai, C.N.; Bain, L.E. The SPIN framework to control and prevent the Marburg virus disease outbreak in Equatorial Guinea. Pan Afr. Med. J. 2023, 44, 110. [Google Scholar]
- Isere, E.E.; Fatiregun, A.A.; Omorogbe, N.E.; Oluwole, M.T. Preventive practices by households against Lassa fever transmission in Ondo State, Southwest, Nigeria. Pan Afr. Med. J. 2022, 43, 176. [Google Scholar] [CrossRef]
- de la Fuente, J.; Ghosh, S.; Lempereur, L.; Garrison, A.; Sprong, H.; Lopez-Camacho, C.; Maritz-Olivier, C.; Contreras, M.; Moraga-Fernandez, A.; Bente, D.A. Interventions for the control of Crimean-Congo hemorrhagic fever and tick vectors. NPJ Vaccines 2024, 9, 181. [Google Scholar] [CrossRef]
- Fawzy, M.; Helmy, Y.A. The One Health Approach is Necessary for the Control of Rift Valley Fever Infections in Egypt: A Comprehensive Review. Viruses 2019, 11, 139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).