Exposure to Lead Compounds in an Industrial Setting and the Effects on the Thyroid Gland: A Pilot Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Under Study
- (1)
- Male or female workers;
- (2)
- Aged between 18 and 67 years;
- (3)
- Occupational exposure to lead, with at least one blood lead analysis conducted in the past year;
- (4)
- No history of thyroidectomy;
- (5)
- No use of drugs that could replace thyroid hormones;
- (6)
- Ability to participate in the study (e.g., adequate understanding of the Italian language and normal cognitive function).
2.2. Analytical Methods
2.3. Statistical Analysis
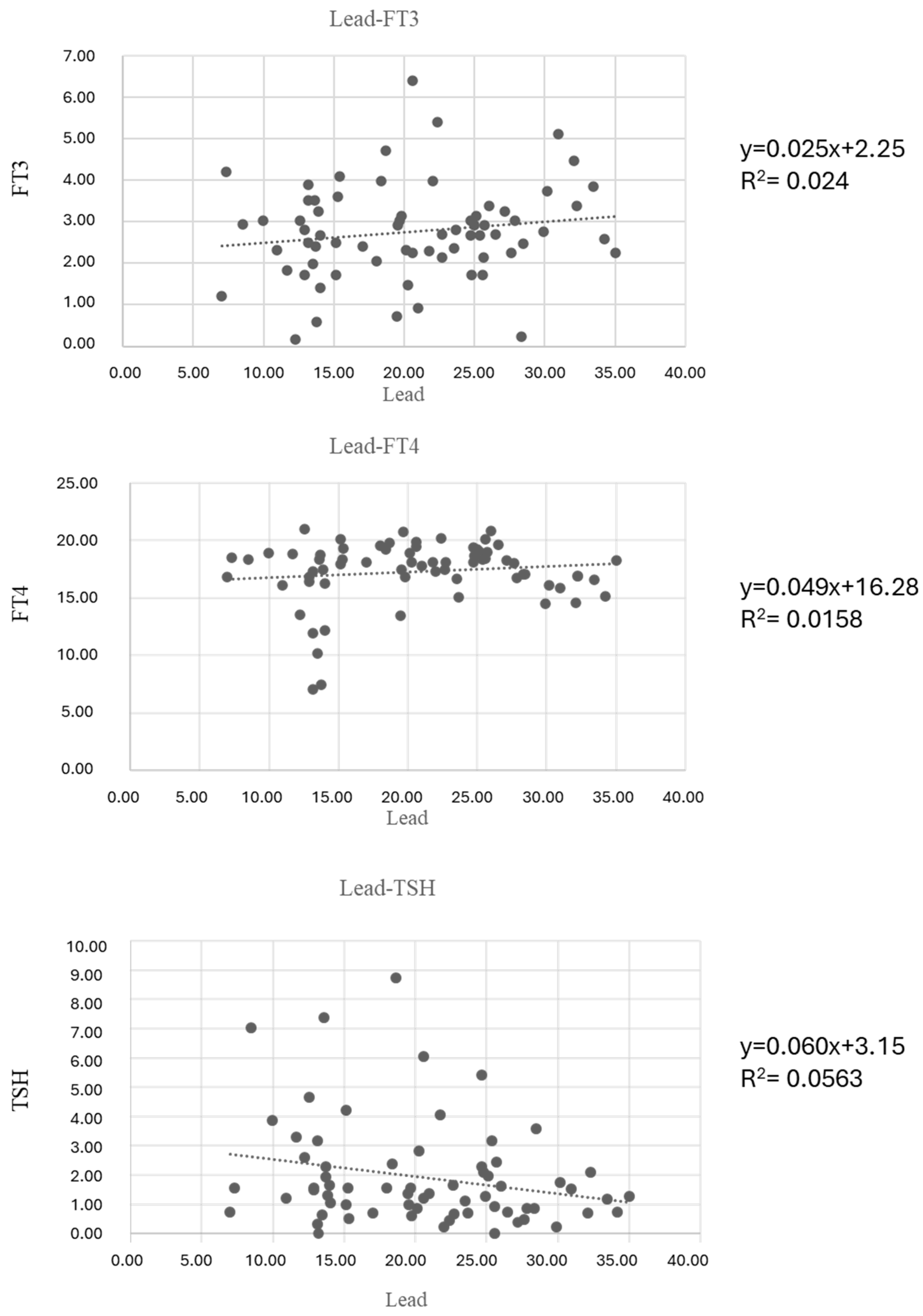
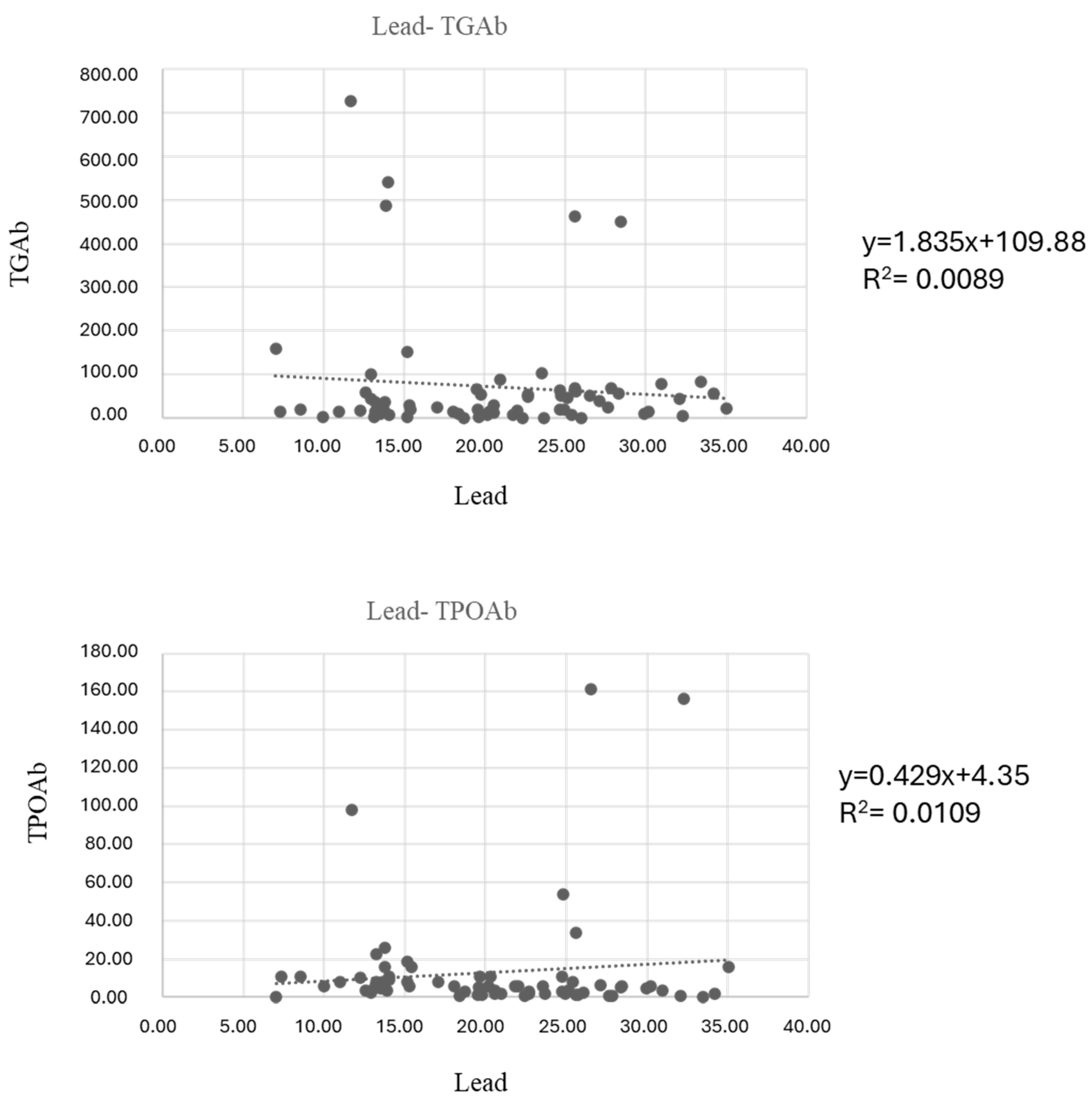
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| TSH | thyroid-stimulating hormone |
| TGAb | thyroglobulin antibody |
| TPOAb | thyroid peroxidase antibody |
| GH | growth hormone |
| FSH | follicle-stimulating hormone |
| TRH | thyrotropin-releasing hormone |
| LH | luteinizing hormone |
| GHRH | growth hormone-releasing hormone |
| GnRH | gonadotropin-releasing hormone |
| ELISA | enzyme-linked-immunosorbent-assay |
| EDTA | ethylenediaminetetraacetic acid. |
| LOD | limit of detection |
| GFAAS | graphite furnace atomic absorption spectrometer |
| BMI | body mass index |
References
- Pekcici, R.; Kavlakoglu, B.; Yilmaz, S.; Sahin, M.; Delibasi, T. Effects of lead on thyroid function of occupationally exposed workers. Cent. Eur. J. Med. 2010, 5, 215–218. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Lv, Q.; Chen, G.; Chen, J.; Li, S.; Mo, Y.; Ou, S.; Yuan, Z.; Lu, G.; et al. Relationship of lead and essential elements in whole blood from school-age children in Nanning. China. J. Trace Elem. Med. Biol. 2015, 32, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Olympio, K.P.K.; Silva, J.; Silva, A.S.D.; Souza, V.C.O.; Buzalaf, M.A.R.; Barbosa, F., Jr.; Cardoso, M.R.A. Blood lead and cadmium levels in preschool children and associated risk factors in Sao Paulo, Brazil. Environ. Pollut. 2018, 240, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yabe, J.; Nakayama, S.M.M.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-Sam, N.; Kabalo, A.N.; Ntapisha, J.; Mizukawa, H.; Umemura, T.; Ishizuka, M. Lead and cadmium excretion in feces and urine of children from polluted townships near a lead-zinc mine in Kabwe, Zambia. Chemosphere 2018, 202, 48–55. [Google Scholar] [CrossRef]
- Treu, G.; Drost, W.; Stock, F. An evaluation of the proposal to regulate lead in hunting ammunition through the European Union’s REACH regulation. Environ. Sci. Eur. 2020, 32, 68. [Google Scholar] [CrossRef]
- EFSA. Panel on contaminations in the food chain (CONTAM). Scientific opinion on lead in food. EFSA J. 2010, 4, 1570. [Google Scholar] [CrossRef]
- Staessen, J.A.; Nawrot, T.; Hond, E.D.; Thijs, L.; Fagard, R.; Hoppenbrouwers, K.; Koppen, G.; Nelen, V.; Schoeters, G.; Vanderschueren, D.; et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: A feasibility study of biomarkers. Lancet 2001, 357, 1660–1669. [Google Scholar] [CrossRef]
- Needleman, H. Lead poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef]
- Rousseau, M.C.; Straif, K.; Siemiatycki, J. IARC carcinogen update. Environ. Health Perspect. 2005, 113, A580–A581. [Google Scholar] [CrossRef]
- National Toxicology Program. Lead and Lead compounds. In 15th Report on Carcinogens; National Institute of Environmental Health Sciences: Research Triangle Parck, NC, USA, 2021. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, A.J. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Wu, H.M.; Lin Tan, D.T.; Wang, M.L.; Huang, H.Y.; Lee, C.L.; Wang, H.S.; Soong, Y.K.; Lin, J.L. Lead level in seminal plasma may affect semen quality for men without occupational exposure to lead. Reprod. Biol. Endocrinol. 2012, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Undaryati, Y.M.; Sudjarwo, S.A.; l’thisom, R. Literature review: Effect of lead toxicity on reproductive system. J. Glob. Res. Public Health 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.; Kshetrimayum, C. Environmental & occupational exposure & female reproductive dysfunction. Indian J. Med. Res. 2019, 150, 532–545. [Google Scholar] [CrossRef]
- Silver, M.K.; Li, X.; Liu, Y.; Li, M.; Mai, X.; Kaciroti, N.; Kileny, P.; Tardif, T.; Meeker, J.D.; Lozoff, B. Low-level prenatal lead exposure and infant sensory function. Environ. Health 2016, 15, 65. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, B.; Huo, W.; Cao, Z.; Liu, W.; Liao, J.; Xia, W.; Xu, S.; Li, Y. Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries. Int. J. Hyg. Environ. Health 2017, 220, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.B.; Reddy, G.A.; Krishna, V.A.; Quadri, S.S.Y.; Kumar, S.P. Developmental neurotoxicity of monocrotophos and lead is linked to thyroid disruption. Vet. World 2016, 9, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, F.; Abdollahi, O.; Rafeemanesh, E.; Shabnam, N. Evaluation the Effect of Serum Lead Levels on Thyroid Function in Battery Industry Workers. Indian J. Occup. Environ. Med. 2023, 27, 120–125. [Google Scholar] [CrossRef]
- Doumouchtsis, K.K.; Doumouchtsis, S.K.; Doumouchtsis, E.K.; Perrea, D.N. The Effect of lead intoxication on endocrine functions. J. Endocrinol. Investig. 2009, 32, 175–183. [Google Scholar] [CrossRef]
- Slingerland, D.W. The influence of various factors on the uptake of iodine by the thyroid. J. Clin. Endocrinol. Metab. 1955, 15, 131–141. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Stant, E.G.; Brill, A.B.; Arias, L.I.; Terry, R.T. Lead intoxication and the thyroid. Arch. Intern. Med. 1969, 123, 632–635. [Google Scholar] [CrossRef]
- Dundar, B.; Oktem, F.; Arslan, M.K.; Delibas, N.; Baykal, B.; Arslan, C.; Gultepe, M.; Ilhan, I.E. The effect of long-term low dose lead exposure on thyroid function in adolescents. Environ. Res. 2006, 101, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Yousif, A.S.; Ahmed, A.A. Effects of cadmium (Cd) and lead (Pb) on the structure and function of thyroid gland. Adv. J. Environ. Sci. Technol. 2020, 11, 1–8. [Google Scholar]
- Gharaibeh, M.Y.; Alzoubi, K.H.; Khabour, O.F.; Khader, S.Y.; Gharaibeh, A.M.; Matarneh, S.K. Lead exposure among five distinct occupational groups: A comparative study. Pak. J. Pharm. Sci. 2014, 27, 39–43. [Google Scholar] [PubMed]
- Rezaei, M.; Javadmoosavi, S.Y.; Mansouri, B.; Azadi, A.N.; Mehrpour, O.; Nakhaee, S. Thyroid dysfunction: How concentration of toxic and essential elements contributes to risk of hypothyroidsm, hyperthyroidism and thyroid cancer. Environ. Sci. Pollut. Res. Int. 2019, 26, 35787–35796. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, J.; Jin, L.; Yang, F.; Wang, J.; Wang, O.; Gao, Y. Correlation between serum lead and thyroid diseases: Papillary thyroid carcinoma, nodular goiter, and thyroid adenoma. Int. J. Environ. Health Res. 2017, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Barbalace, M.C.; Croce, L.; Malaguti, M.; Campennì, A.; Rotondi, M.; Cannavò, S.; Hrelia, S. Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients 2023, 15, 3953. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommended Actions Based on Blood Lead Level. 2024. Available online: https://www.cdc.gov/lead-prevention/hcp/clinical-guidance/index.html?utm_medium=email&utm_source=transaction (accessed on 3 March 2024).
- Siegel, M.; Forsyth, B.; Siegel, L.; Cullen, M.R. The effect of lead on thyroid function in children. Environ. Res. 1989, 49, 190–196. [Google Scholar]
- Chaurasia, S.S.; Kar, A. Influence of Lead on type-I iodothyronine 5′-monodeiodinase activity in male mouse. Horm. Metab. Res. 1997, 29, 532–533. [Google Scholar]
- Chaurasia, S.S.; Kar, A. Lead induced oxidative damage to the membrane associated type I iodothyronine-monodeiodinase activity in chicken liver homogenate. Fresenius Environ. Bull. 1998, 7, 209–215. [Google Scholar]
- Swarup, D.; Naresh, R.; Varshney, V.P.; Balagangatharathilagar, M.; Kumar, P.; Nandi, D.; Patra, R.P. Changes in plasma hormones profile and liver function in cows naturally exposed to lead and cadmium around different industrial areas. Res. Vet. Sci. 2007, 82, 16–21. [Google Scholar] [CrossRef]
- Husernan, C.A.; Moriarty, C.M.; Angle, C.R. Childhood lead toxicity and impaired release of thyrotropin-stimulating hormone. Environ. Res. 1987, 42, 524–533. [Google Scholar] [CrossRef]
- Robins, J.M.; Cullen, M.R.; Connors, B.B.; Kayne, R.D. Depressed thyroid indexes associated with occupational exposure to inorganic lead. Arch. Intern. Med. 1983, 143, 220–224. [Google Scholar] [CrossRef]
- Tuppurainen, M.; Wägar, G.; Kurppa, K.; Sakari, W.; Wambugu, A.; Fröseth, B.; Alho, J.; Nykyri, E. Thyroid function as assessed by routine laboratory tests of workers with long-term lead exposure. Scand. J. Work Environ. Health 1988, 14, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Shumacher, C.; Brodkin, C.A.; Alexander, B.; Cullen, M.; Rainey, P.M.; van Netten, C.; Faustman, E.; Checkoway, H. Thyroid function in lead smelter workers: Absence of subacute or cumulative effects with moderate lead burdens. Int. Arch. Occup. Environ. Health 1998, 71, 453–458. [Google Scholar] [CrossRef]
- Erfurth, E.M.; Gerhardsson, L.; Nilsson, A.; Rylander, L.; Schütz, A.; Skerfving, S.; Börjessonet, J. Effects of lead on the endocrine system in lead smelter workers. Arch. Environ. Health 2001, 56, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, A.; Hedner, P.; Schütz, A.; Skerfving, S. Occupational lead exposure and pituitary function. Int. Arch. Occup. Environ. Health 1989, 61, 277–281. [Google Scholar] [CrossRef]
- Krieg, E.F.J. The relationships between blood lead levels and serum thyroid stimulating hormone and total thyroxine in the third National Health and Nutrition Examination Survey. J. Trace Elem. Med. Biol. 2019, 51, 130–137. [Google Scholar] [CrossRef]
- Rivera-Buse, J.E.; Patajalo Villalta, S.J.; Donadi, E.A.; Barbosa, F.J.; Magalhaes Ribeiro, K.P.; Zanini, M.L.A. Impact of lead exposure on the thyroid glands of individuals living in high-or low-lead exposure areas. Medicine 2023, 102, e33292. [Google Scholar] [CrossRef]
- Memon, N.S.; Kazi, T.G.; Afridi, H.I.; Baig, J.A.; Arain, S.S.; Sahito, O.M.; Baloch, S.; Waris, M. Evaluation of calcium and lead interaction, in addition to their impact on thyroid functions in hyper and hypothyroid patients. Environ. Sci. Pollut. Res. 2016, 23, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, Y.; Chen, Y.; Chen, C.; Han, B.; Li, Q.; Zhu, C.; Xia, F.; Zhai, H.; Wang, N.; et al. Lead and cadmium exposure higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ Pollut 2017, 230, 320–328. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Mergler, D.; Takser Labde, V.C.; St-Jean, M.; Baldwin, M.; Spear, P.A.; Chan, H.M. Gender differences in the effects of organochlorines, mercury and lead on thyroid hormone levels in lakeside communities of Quebec (Canada). Environ. Res. 2008, 107, 390–392. [Google Scholar] [CrossRef]
- Singh, B.; Chandran, V.; Bandhu, H.K.; Mittal, B.R.; Bhattacharya, A.; Jindal, S.K.; Varma, S. Impact of lead exposure on pituitary-thyroid axis in humans. Biometals 2000, 13, 187–192. [Google Scholar] [CrossRef]
- Sherif, M.; Mohammed, Y.; Zedan, H.; Kheder, M.; Mohammed, A. Toxic effect of some heavy metals (cadmium and lead) on thyroid function. Egypt. J. Hosp. Med. 2017, 69, 2512–2515. [Google Scholar] [CrossRef]
- Mendy, A.; Gasana, J.; Vieira, E.R. Low blood lead concentrations and thyroid function of American adults. Int. J. Environ. Health Res. 2013, 23, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M. Relationship between blood cadmium, lead and serum thyroid measures in US adults—The National Health and Nutrition examination survey (NHANES) 2007–2010. Int. J. Environ. Health Res. 2014, 24, 125–136. [Google Scholar] [CrossRef]
- Krieg, E.F.J. A meta-analysis of studies investigating the effects of occupational lead exposure on thyroid hormones. Am. J. Ind. Med. 2016, 59, 583–590. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Blood lead levels in young children and selected sites, 1996–1999. MMWR Morb. Mortal Wkly. Rep. 2000, 49, 244–245. [Google Scholar]
- Shen, X.M.; Wu, S.H.; Yan, C.H. Impacts of low-level lead exposure on development of children: Recent studies in China. Clin. Chim. Acta 2001, 313, 217–220. [Google Scholar] [CrossRef]
- Fels, L.M.; Wünsch, M.; Baranowski, J.; Norska-Borowka, I.; Price, R.G.; Taylor, S.A.; Patel, S.; De Broe, M.; Elsevier, M.M.; Lauwerys, R.; et al. Adverse effects of chronic low level lead exposure on kidney function—A risk group study in children. Nephrol. Dial. Transplant. 1998, 13, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.M.; Vyskocil, A.; Roles, H.; Kriz, J.; Kodl, M.; Lauwerys, R. Renal effects in children living in the vicinity of a lead smelter. Environ. Res. 1995, 68, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.S. Lead toxicity: Problems of definition and laboratory evaluation. Ann. Clin. Biochem. 1984, 21, 2248–2256. [Google Scholar] [CrossRef]
- Payton, M.; Hu, H.; Sparrow, D.; Weiss, S.T. Low-level lead exposure and renal function in the normative aging study. Am. J. Epidemiol. 1994, 140, 821–829. [Google Scholar] [CrossRef]
| FT3 (pg/mL) | FT4 (pg/mL) | TSH (µlU/mL) | TGAb (IU/mL) | TPOAb (IU/mL) | |
|---|---|---|---|---|---|
| Normal range | 2.2–5.3 | 7.0–22.0 | 0.3–5.0 | <100 | <50 |
| Borderline | 100–150 | 50–75 | |||
| Positive | >150 | >75 | |||
| Precision intraday 1 | 9.7 | 4.9 | 7.7 | 2.6 | 1.6 |
| Precision interday 1 | 8.6 | 11.5 | 12.3 | 5.7 | 3.1 |
| LOD 2 | 0.3 | 1.0 | 0.1 | 10 | 5 |
| LOQ 3 | 0.6 | 2.0 | 0.2 | 30 | 15 |
| Characteristics | Males (68) |
|---|---|
| Age (range) | 55.82 (25–66) |
| BMI(%) | |
| Normal | 39.7 |
| Overweight | 38.2 |
| First degree obesity | 14.7 |
| Second grade obesity | 2.9 |
| Unknown | 4.4 |
| Actual smokers (%) | 26.5 |
| Previously smokers (%) | 30.9 |
| Alcohol consumption (%) | |
| Daily | 11.8 |
| Weekly | 4.4 |
| Occasional | 52.9 |
| Never | 22.1 |
| Missing | 8.8 |
| Working seniority (years) and range | 22.58 (3–40) |
| Working seniority class (%) | |
| ≤20 years | 33.8 |
| >20 years | 50.0 |
| Missing | 16.2 |
| FT3 (pg/mL) | FT4 (pg/mL) | TSH (µLU/mL) | TGAb (IU/mL) | TPOAb (IU/mL) | Pb (µg/dL) | ||
|---|---|---|---|---|---|---|---|
| Average ± SD | 2.76 ± 1.14 | 17.28 ± 2.75 | 1.92 ± 1.78 | 72.33 ± 137.76 | 13.14 ± 29.04 | 20.47 ± 7.07 | |
| Out of range | 27.9% | 0.0% | 13.2% | 13.2% | 5.9% | >15 µg/dL | 69.1% |
| >60 µg/dL | 0.0% | ||||||
| Unit | Exposure Level | Mann–Whitney Test | ||
|---|---|---|---|---|
| ≤15 µg/dL | >15 µg/dL | p Value | ||
| FT3 | pg/mL | 2.56 ± 1.15 | 2.84 ± 1.133 | 0.550 |
| FT4 | pg/mL | 15.80 ± 3.93 | 17.94 ± 1.68 | 0.044 * |
| TSH | µLU/mL | 2.68 ± 2.42 | 1.59 ± 1.30 | 0.061 |
| TGAb | IU/mL | 110.95 ± 205.54 | 55.07 ± 91.03 | 0.770 |
| TPOAb | IU/mL | 12.94 ± 20.52 | 13.22 ± 32.33 | 0.42 * |
| Unit | Working Seniority Class | Mann–Whitney Test | ||
|---|---|---|---|---|
| ≤20 | >20 | p Value | ||
| FT3 | pg/mL | 2.60 ± 0.99 | 2.90 ± 1.23 | 0.897 |
| FT4 | pg/mL | 17.18 ± 2.75 | 17.50 ± 2.61 | 0.678 |
| TSH | µLU/mL | 1.92 ± 1.44 | 1.85 ± 1.89 | 0.765 |
| TGAb | IU/mL | 104.31 ± 187.25 | 52.80 ± 93.32 | 0.793 |
| TPOAb | IU/mL | 10.49 ± 19.88 | 12.12 ± 28.12 | 0.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporossi, L.; Di Renzi, S.; De Rosa, M.; Capanna, S.; Partenzi, E.; D’Alessandro, I.; Papaleo, B. Exposure to Lead Compounds in an Industrial Setting and the Effects on the Thyroid Gland: A Pilot Cohort Study. Hygiene 2025, 5, 13. https://doi.org/10.3390/hygiene5020013
Caporossi L, Di Renzi S, De Rosa M, Capanna S, Partenzi E, D’Alessandro I, Papaleo B. Exposure to Lead Compounds in an Industrial Setting and the Effects on the Thyroid Gland: A Pilot Cohort Study. Hygiene. 2025; 5(2):13. https://doi.org/10.3390/hygiene5020013
Chicago/Turabian StyleCaporossi, Lidia, Simona Di Renzi, Mariangela De Rosa, Silvia Capanna, Elisa Partenzi, Iacopo D’Alessandro, and Bruno Papaleo. 2025. "Exposure to Lead Compounds in an Industrial Setting and the Effects on the Thyroid Gland: A Pilot Cohort Study" Hygiene 5, no. 2: 13. https://doi.org/10.3390/hygiene5020013
APA StyleCaporossi, L., Di Renzi, S., De Rosa, M., Capanna, S., Partenzi, E., D’Alessandro, I., & Papaleo, B. (2025). Exposure to Lead Compounds in an Industrial Setting and the Effects on the Thyroid Gland: A Pilot Cohort Study. Hygiene, 5(2), 13. https://doi.org/10.3390/hygiene5020013