Antimicrobial Efficacy of a Portable UV-C-Based Coating Activation Device against Candida albicans Biofilm and SARS-CoV-2 as an Additional Feature: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
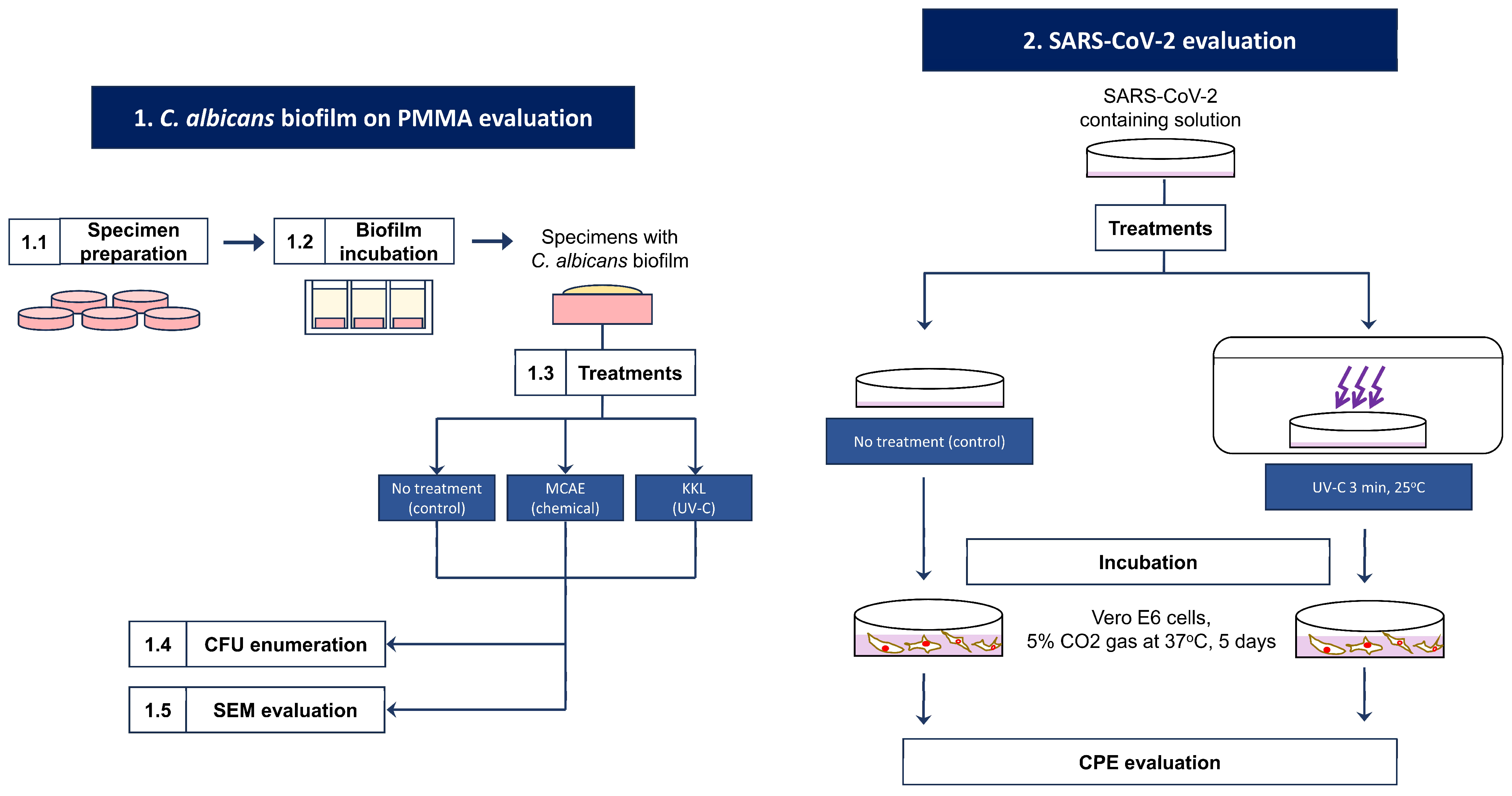
2.1. C. albicans Biofilm on PMMA Evaluation
2.1.1. Specimen Preparation
2.1.2. Biofilm Incubation
2.1.3. Treatments
2.1.4. Colony-Forming Unit (CFU) Enumeration
2.1.5. Scanning Electron Microscope (SEM) Image Evaluation
2.2. SARS-CoV-2 Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeed, F.; Muhammad, N.; Khan, A.S.; Sharif, F.; Rahim, A.; Ahmad, P.; Irfan, M. Prosthodontics dental materials: From conventional to unconventional. Mater. Sci. Eng. C. 2020, 106, 110167. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Reichl, F.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear simulation methods—A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Dental Biofilm and Laboratory Microbial Culture Models for Cariology Research. Dent. J. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, P.; Milward, P.; McAndrew, R. Denture cleanliness and hygiene: An overview. Br. Dent. J. 2022, 233, 20–26. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, L.E.; Robertson, D.; Nile, C.J.; Cross, L.J.; Riggio, M.; Sherriff, A.; Bradshaw, D.; Lambert, M.; Malcolm, J.; Buijs, M.J.; et al. The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS ONE 2015, 10, 0137717. [Google Scholar] [CrossRef]
- Yoshijima, Y.; Murakami, K.; Kayama, S.; Liu, D.; Hirota, K.; Ichikawa, T.; Miyake, Y. Effect of substrate surface hydrophobicity on the adherence of yeast and hyphal Candida. Mycoses 2010, 53, 221–226. [Google Scholar] [CrossRef]
- Le Bars, P.; Kouadio, A.A.; Bandiaky, O.N.; Le Guéhennec, L.; de La Cochetière, M.-F. Host’s Immunity and Candida Species Associated with Denture Stomatitis: A Narrative Review. Microorganisms 2022, 10, 1437. [Google Scholar] [CrossRef]
- Gomes, S.C.; Fachin, S.; da Fonseca, J.G.; Angst, P.D.M.; Lamers, M.L.; da Silva, I.S.B.; Nunes, L.N. Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J. Clin. Periodontol. 2021, 48, 880–885. [Google Scholar] [CrossRef]
- Riad, A.; Gad, A.; Boccuzzi, M.; Cosola, S. Are ‘family bubbles’ safe? Br. Dent. J. 2020, 229, 147. [Google Scholar] [CrossRef]
- Jerônimo, L.S.; Esteves Lima, R.P.; Suzuki, T.Y.U.; Discacciati, J.A.C.; Bhering, C.L.B. Oral Candidiasis and COVID-19 in Users of Removable Dentures: Is Special Oral Care Needed? Gerontology 2022, 68, 80–85. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Colnaghi, A.; Morittu, S.; Barbieri, S.; Ricci, M.; Guerrisi, G.; Piloni, D.; et al. Assessment of Oral Microbiome Changes in Healthy and COVID-19-Affected Pregnant Women: A Narrative Review. Microorganisms 2021, 9, 2385. [Google Scholar] [CrossRef]
- Dioguardi, M.; Cantore, S.; Scacco, S.; Quarta, C.; Sovereto, D.; Spirito, F.; Alovisi, M.; Troiano, G.; Aiuto, R.; Garcovich, D.; et al. From Bench to Bedside in Precision Medicine: Diabetes Mellitus and Peri-Implantitis Clinical Indices with a Short-Term Follow-Up: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 235. [Google Scholar] [CrossRef]
- Schmutzler, A.; Rauch, A.; Nitschke, I.; Lethaus, B.; Hahnel, S. Cleaning of removable dental prostheses—A systematic review. J. Evid. Based Dent. Pract. 2021, 21, 101644. [Google Scholar] [CrossRef]
- Bajunaid, S.O. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, Y.; Fukuyama, T.; Hamano, N.; Iwashita, H.; Watanabe, M.; Ino, S. The stain resistant effect of an ultraviolet curable coating material on denture base resin. Dent. Mater. J. 2023, 42, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, K.; Fukunishi, M.; Iwasa, F.; Inoue, Y.; Ishihara, K.; Baba, K. 2-methacryloyloxyethyl phosphorylcholine polymer treatment of complete dentures to inhibit denture plaque deposition. J. Vis. Exp. 2016, 118, e54965. [Google Scholar] [CrossRef]
- Putra Wigianto, A.Y.; Ishida, Y.; Iwawaki, Y.; Goto, T.; Watanabe, M.; Sekine, K.; Hamada, K.; Murakami, K.; Fujii, H.; Ichikawa, T. 2-methacryloyloxyethyl phosphorylcholine polymer treatment prevents Candida albicans biofilm formation on acrylic resin. J. Prosthodont. Res. 2023, 67, 384–391. [Google Scholar] [CrossRef] [PubMed]
- International Ultraviolet Association. What is UV? Available online: https://www.iuva.org/What-is-UV (accessed on 26 January 2024).
- Food and Drug Administration. Ultraviolet (UV) Radiation. Available online: https://www.fda.gov/radiation-emitting-products/tanning/ultraviolet-uv-radiation (accessed on 26 January 2024).
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV irradiation and TiO2-Photocatalysis on airborne bacteria and viruses: An overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Matsuura, R.; Iimura, K.; Wada, S.; Shinjo, A.; Benno, Y.; Nakagawa, M.; Takei, M.; Aida, Y. UVC disinfects SARS-CoV-2 by induction of viral genome damage without apparent effects on viral morphology and proteins. Sci. Rep. 2021, 11, 13804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hirota, K.; Yumoto, H.; Matsuo, T.; Miyake, Y.; Ichikawa, T. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J. Appl. Microbiol. 2010, 109, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Murakami, K.; Yoshida, K.; Sakurai, S.; Kudo, Y.; Ozaki, K.; Hirota, K.; Fujii, H.; Suzuki, M.; Miyake, Y.; et al. Suppressive effects of 2-methacryloyloxyethyl phosphorylcholine (MPC)-polymer on the adherence of Candida species and MRSA to acrylic denture resin. Heliyon 2020, 6, e04211. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-2:2013; Microbiology of the Food Chain, Horizontal Method for the Enumeration of Microorganisms Part 2: Colony Count at 30 °C by the Surface Plating Technique 4833-2. International Organization for Standardization: Geneva, Switzerland, 2013.
- Factors Affecting the Efficacy of Disinfection and Sterilization. Centers for Disease Control and Prevention, Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/efficacy.html (accessed on 3 July 2023).
- Harada, K.; Horinouchi, N.; Yamashita, Y.; Murakami, M.; Nishi, Y.; Nishimura, M. Sterilization effect of ultraviolet irradiation system against Candida albicans and its application to dentures. In Proceedings of the 15th Scientific Meeting of Japan Denture Care Society, Iwate University, Iwate (Online Conference), Online, 22 January 2023. [Google Scholar]
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartagenes, M.S.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, R.; Gao, L.; Gao, X.; Wang, D.; Cao, J. SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Front. Cell. Infect. Microbiol. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.; Abid, M. Mechanistic understanding of Candida albicans biofilm formation and approaches for its inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef]
- Théraud, M.; Bédouin, Y.; Guiguen, C.; Gangneux, J.P. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J. Med. Microbiol. 2004, 53, 1013–1018. [Google Scholar] [CrossRef]
- Duering, H.; Westerhoff, T.; Kipp, F.; Stein, C. Short-Wave Ultraviolet-Light-Based Disinfection of Surface Environment Using Light-Emitting Diodes: A New Approach to Prevent Health-Care-Associated Infections. Microorganisms 2023, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Asano, K.; Naito, K.; Ohashi, H.; Sasaki, M.; Morimoto, Y.; Igarashi, T.; Nakane, A. Ultraviolet C light with wavelength of 222 nm inactivates a wide spectrum of microbial pathogens. J. Hosp. Infect. 2020, 105, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Rane, H.S.; Bernardo, S.M.; Walraven, C.J.; Lee, S.A. In vitro analyses of ethanol activity against Candida albicans biofilms. Antimicrob. Agents. Chemother. 2012, 56, 4487–4489. [Google Scholar] [CrossRef] [PubMed]



| Artificial Dental Materials | Properties | Applications |
|---|---|---|
| Metals | High strength, hardness, and durability Low esthetics and biocompatibility |
|
| Ceramics | High esthetics, biocompatibility, and wear resistance Low toughness and fracture resistance |
|
| Polymers | High flexibility, elasticity, and biocompatibility Low strength, hardness, and wear resistance |
|
| Composites | Combination of the different properties of different materials |
|
| Type | Composition | Effects |
|---|---|---|
| Supragingival biofilm | Mainly aerobic and facultative anaerobic bacteria, such as Streptococcus spp., Actinomyces spp., and Veillonella spp. |
|
| Subgingival biofilm | Mainly anaerobic and microaerophilic bacteria, such as Porphyromonas spp., Prevotella spp., and Fusobacterium spp. |
|
| Cariogenic biofilm | Mainly acidogenic and aciduric bacteria, such as Streptococcus mutants, Lactobacillus spp., and Actinomyces spp. |
|
| Endodontic biofilm | Mainly anaerobic and polymicrobial bacteria, such as Enterococcus spp., Porphyromonas spp., and Prevotella spp. |
|
| Denture plaque | Bacteria:
|
|
Fungi:
|
|
| Method | Device or Material | Advantages |
|---|---|---|
| Mechanical | Manual (brush) Vibrational based (ultrasonic bath) | The most common method and usually combined with standard denture cleaning paste which is easy to obtain |
| Chemical | Solution (ex.: bleach, mineral acids, enzymes, oral rinsing, and denture cleaners) Effervescent (peroxide, bicarbonate, percarbonate, and persulphate) | Commonly used, high efficacy, plenty of commercial products are available on the market. Direct chemical contact and adjustable concentration and immersion time |
| Irradiation | Microwave oven, LED, and UV-C | Does not contact the denture surface directly, minimizing the risk of increased surface roughness. Can reach the areas that are difficult to clean mechanically |
| Material properties modification | Incorporated antimicrobial polymers (ex.: polymers, nanoparticles, silver oxides, protein-repellent biocides, and natural agents) embedded in the material or applied as a coating | Modifies the material properties that favor denture plaque adhesion either during the fabrication step or during routine control visits |
| UV Type | Wavelength (nm) | Absorption | Hygiene Application |
|---|---|---|---|
| UV-A | 315–400 | Absorbed by the dermis, causing sun tanning | Can be used for phototherapy |
| UV-B | 280–315 | Absorbed by the epidermis, causing sunburn | Can be used for phototherapy and disinfection |
| UV-C | 200–280 | Absorbed by DNA: mutation, cancers, and disinfection | Effective disinfectant against a wide range of microorganisms |
| Vacuum UV | 100–200 | Absorbed only in vacuum conditions (water and air) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra Wigianto, A.Y.; Watanabe, M.; Iwawaki, Y.; Goto, T.; Otsuki, T.; Ichikawa, T. Antimicrobial Efficacy of a Portable UV-C-Based Coating Activation Device against Candida albicans Biofilm and SARS-CoV-2 as an Additional Feature: An In Vitro Study. Hygiene 2024, 4, 93-102. https://doi.org/10.3390/hygiene4010006
Putra Wigianto AY, Watanabe M, Iwawaki Y, Goto T, Otsuki T, Ichikawa T. Antimicrobial Efficacy of a Portable UV-C-Based Coating Activation Device against Candida albicans Biofilm and SARS-CoV-2 as an Additional Feature: An In Vitro Study. Hygiene. 2024; 4(1):93-102. https://doi.org/10.3390/hygiene4010006
Chicago/Turabian StylePutra Wigianto, Adityakrisna Yoshi, Megumi Watanabe, Yuki Iwawaki, Takaharu Goto, Tamaki Otsuki, and Tetsuo Ichikawa. 2024. "Antimicrobial Efficacy of a Portable UV-C-Based Coating Activation Device against Candida albicans Biofilm and SARS-CoV-2 as an Additional Feature: An In Vitro Study" Hygiene 4, no. 1: 93-102. https://doi.org/10.3390/hygiene4010006
APA StylePutra Wigianto, A. Y., Watanabe, M., Iwawaki, Y., Goto, T., Otsuki, T., & Ichikawa, T. (2024). Antimicrobial Efficacy of a Portable UV-C-Based Coating Activation Device against Candida albicans Biofilm and SARS-CoV-2 as an Additional Feature: An In Vitro Study. Hygiene, 4(1), 93-102. https://doi.org/10.3390/hygiene4010006







