Abstract
The human pelvis is adapted to accommodate bipedal locomotion while retaining a wide enough pelvic canal to birth large babies. Many forces act on the pubic bone, with the pelvis being in charge of supporting the organs of the abdominopelvic cavity. In this research, we investigate whether increases in gut volume (GV) and number of births (parity) impact the skeletal morphology of the pubic bone at two regions: the pubic symphysis and the pubic arch. Our results indicate that in our female sample, the pubic symphysis width increased with increased GV and parity, while the pubic arch width decreased with increased GV and parity, although not significantly. In the male sample, there was almost no effect of increased GV on the pubic symphysis, while the pubic arch width increased in response to increased GV. We hypothesize that while significance is not present for this entire data set, these pelvic structures are impacted by GV and parity, and these changes should be investigated further. These changes in the structure can impact the function of the pelvic girdle and result in pain and changes to mobility. Pelvic girdle pain may be one result of these structural changes due to increased forces, and thus it is vital to investigate what factors may or may not contribute to these bone morphology changes.
1. Introduction
Amongst the primates, the human pelvis is uniquely adapted to bipedal locomotion (Lewis et al., 2017; Lovejoy, 2005; Lovejoy et al., 2009; Tague & Lovejoy, 1986). This adaptation has shaped the human pelvic girdle in a way to accommodate birthing a large infant, as well as functioning to support viscera, and providing attachments for muscles, ligaments, and tendons for movement and locomotion (Lewis et al., 2017; Lovejoy, 2005; Lovejoy et al., 2009; Tague & Lovejoy, 1986; Wobser et al., 2023). The ‘pelvic floor hypothesis’ suggests that the structure and stability of the pelvic floor are an adaptation for bipedal locomotion, but they also constrain the birth canal (Abitbol, 1988; Haeusler et al., 2021). The pelvic girdle is comprised of three bones: the ilium, ischium, and pubis. The anterior connection of the two pubic bones forms the pubic symphysis joint (Figure 1A). The pubic symphysis absorbs forces during locomotion and expands during childbirth (Becker et al., 2010). Inferior to the pubic symphysis is the pubic arch, also formed by the anterior articulation of the pubic bones (Figure 1B). The width of the pubic arch provides structural support in response to changes to the body’s mass by both widening and narrowing, for example, widening in females to accommodate childbirth or narrowing in response to hormonal changes (Oladipo, 2010). The pubic bone, therefore, represents an important adaptive anatomical structure in humans.
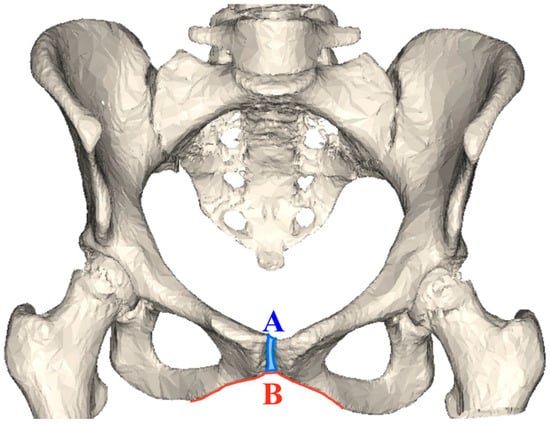
Figure 1.
Human female pelvis displaying the two structures examined in this research: (A) Pubic symphysis (blue shaded) and (B) pubic arch (red line).
Prior research on the pubic bones has focused on pain and mobility issues, while neglecting the impact gut volume and parity may have on the shape and size of the pubis. “Gut volume” is defined in this study as the volume taken up in the abdominal cavity by the large and small intestines. “Parity” refers to the number of live births a woman has experienced. This study uses parity values ranging from 0 to 5+ live births to examine the impact reproduction may have on these pelvic structures. Additionally, anatomical markers for measurement of the pubic structures vary between studies, leading to conflicting results (Alicioglu et al., 2008; Becker et al., 2010; Roberts, 1934). In this study, we employ the same methodology at both the pubic symphysis and pubic arch, with three data points per structure. Widths were measured at a superior, middle, and inferior point for both the pubic symphysis and pubic arch. This methodology allows for an investigation into how gut volume and parity may alter the width of these two structures. Changing widths of these structures may inform us of issues regarding how pelvic morphology may contribute to pelvic girdle pain and mobility.
1.1. Sex Differences of the Pubic Symphysis and Pubic Arch
The pubic symphysis (PS) and pubic arch (PA) differ, on average, between male and female pelves. In one study, the average pubic symphysis width (PSW) was 5.9 mm in men versus 4.9 mm in women, but in another study, the average width was significantly greater at 11.8 mm in men versus 12.6 mm in women (Alicioglu et al., 2008; Vix & Ryu, 1971). Among these studies, there is no consensus on how to measure pubic structures, especially at the pubic symphysis width (PSW). This results in a lack of a clear definition of what a “normal” PSW is in the human body, even when examining sex differences at the pubis. The pubic arch also exhibits differences in males and females, allowing its use in medicine, anthropology, and forensics (Buikstra & Ubelaker, 1994; Kurihara et al., 1996; Memarian et al., 2017; Oladipo, 2010; Washburn, 1948). Indeed, measures of the pelvis to identify males and females in forensics, such as the pubic angle, subpubic angle, and pubic ramus angle, have a 98% accuracy (Memarian et al., 2017). Men have an average pubic arch angle of 75–130 degrees, and females have an average pubic arch angle of 90–150 degrees (Oladipo, 2010).
Differences in the anatomy of male and female pelves may be due to the demands of reproduction. In the final weeks of pregnancy, the pubic symphysis (PS) widens, which may be a response to the hormone relaxin, although there is disagreement on this mechanism’s effect on the PS (Kristiansson et al., 1999; Leadbetter et al., 2004; MacLennan, 1991). Kristiansson et al. (1999) also found that serum concentrations of relaxin were significantly correlated with pelvic pain, as a result of more laxity at the joints. Connective tissue is also remodeled during pregnancy to allow for the delivery of a baby, promoting more joint mobility and possibly, pelvic pain (Kristiansson et al., 1999). Other possible mechanisms for the widening of the PS during pregnancy include mechanical strain caused by weight gain (Leadbetter et al., 2004). Dysfunction due to pubic symphysis relaxation usually goes away after delivery, but for some individuals, symptoms persist, resulting in long-term negative effects such as localized shooting and/or stabbing pain in the pubic symphysis region, radiating discomfort, and difficulty during movement, and even effects beyond impaired mobility, such as depression (Becker et al., 2010; Jain et al., 2006).
As a result of the PS widening during pregnancy, the pubic arch (PA) also increases its width progressively throughout pregnancy (Martelli et al., 2020). Research has found that before pregnancy occurs, the female PA is naturally wider than the male PA on average, and from the first to second trimesters, the average PA angle increased from 121.8 to 123.5 degrees. Similarly, from the second to the third trimester, the average angle increased from 123.5 to 125.3 degrees (Martelli et al., 2020). This slight widening is thought to be due to intrinsic mechanisms in preparation for childbirth and may contribute to the negative symptoms women experience. The exact mechanism of widening is not fully known, but two main contributors are the hormone relaxin and the shift of the pelvic organs, altering the center of gravity (Kristiansson et al., 1999; Leadbetter et al., 2004; MacLennan, 1991; Martelli et al., 2020; Sakamoto et al., 2021).
While research has documented the anatomical sex differences and the consequences of female reproduction and pregnancy on the pubic arch, most studies direct their focus on other components of the pelvic girdle. Due to the direct relationship between these two pelvic structures, the pubic symphysis and the pubic arch, this study investigates how these structures adapt in response to changes in gut volume and parity. Both gut volume and parity may act similarly on the pelvis; gut volume will display the effects mass may have on this region of the pelvis, and parity allows an investigation of potential pregnancy hormone effects, in addition to the increased mass effects.
1.2. Gut Volume and Parity Variables
Investigating parity may show how the weight and the hormones from pregnancy may impact pelvic structures. However, pregnancy is not the only mechanism of increasing mass, and thus it is important to investigate gut volume as well. Increased mass affects one’s whole body, including the PS and PA (Alicioglu et al., 2008; Becker et al., 2010; Rustamova et al., 2009). Because the pelvis works to anchor the pelvic floor muscles, which, in turn, support the abdominopelvic organs (Abitbol, 1988; Haeusler et al., 2021; Uy & Laudicina, 2021), this research investigates how increased mass of these organs, or gut volume, may affect the structures of the pelvis. Individuals with a greater abdominal volume, such as from pregnancy, may have a greater risk of their pubic symphysis and arch widening, leading to symptoms that range from uncomfortable to debilitating pain (Becker et al., 2010; Kristiansson et al., 1999; Leadbetter et al., 2004; MacLennan, 1991; Martelli et al., 2020; Sakamoto et al., 2021). There are currently few studies investigating the relationship between the pubis and changes in mass (Alicioglu et al., 2008; Becker et al., 2010; Rustamova et al., 2009). Exploring the relationship of gut size and parity with the widths of these pubic structures will help elucidate if abdominal mass increases relate to PS and PA width changes. In this study, gut volume (the volume of the small and large intestines) was used (instead of BMI) in an attempt to limit racial disparities in health care related to adiposity (Dougherty et al., 2020).
Although it is difficult to make direct links between bony morphology and pain causation, this research attempts to understand how changes in force (gut volume) and volume (space due to parity) at the pubic region may inform us about issues of and causes of pelvic girdle pain.
2. Materials and Methods
2.1. Materials
2.1.1. Gut Volume Sample
The gut volume sample contained 44 males and 48 females for a total of 92 adults (Table 1). These samples were retrieved from scans originally obtained for another study (Uy & Laudicina, 2021) collected from the University of Wisconsin-Madison School of Medicine and Public Health (archival database). The Institutional Review Board (IRB) did not require a new review for this present study, as the individuals were previously de-identified, and we used secondary data from the previous study (Uy & Laudicina, 2021). To account for changes that may occur during growth and development, the ages of the individuals for this analysis ranged from 18 to 25 years. Gut volume was divided into small (<4670cc) and large (>4670cc) categories for analysis following protocols established by previous research (Uy & Laudicina, 2021). The “small” group was comprised of 47 pelves (13 male, 34 female). The “large” group consisted of 47 pelves (32 male, 15 female). It is important to note that in the “small” pelvic shape group, the sample is predominantly female, while the “large” group is predominantly male. Sex differences may play a role in the data, which is why this study examines overall trends in addition to trends based on sex alone.

Table 1.
Descriptive statistics for gut volume study sample (from Uy & Laudicina, 2021, Table 1).
2.1.2. Parity Sample
The parity sample (N = 64, all females) was obtained from the New Mexico Decedent Image Database (NMDID), a free Computed Tomography (CT) scan database (Edgar et al., 2020). All individuals in the NMDID were obtained from the New Mexico medical examiner’s office from 2010 to 2017 (Edgar et al., 2020), and permission to access the CT scans was granted to the authors by the NMDID. IRB was not required for this sample, as all individuals were de-identified prior to our access to the material. Parity status was divided into four groups based upon the reported number of live births: nulliparous (no births), 1–2 births, 3–4 births, and 5 or greater births reported (Table 2). Individuals were selected by searching the metadata on the NMDID using the following inclusion criteria: individuals aged 18–50, assigned female at birth, and no chromosomal abnormalities. All individuals selected were then controlled for differences in ancestry to avoid confounding effects, and Indigenous populations were excluded from the study to avoid using material from a vulnerable population. Due to this criterion selection, the sample size for the largest parity group (>5 births) was small. Individuals in the final parity study sample ranged from 18 to 50 years old to account for age-related changes to pelvic morphology due to puberty and menopause, and the average age of individuals was 37.67 years old (Table 2).

Table 2.
Descriptive statistics for the parity study sample. CT images obtained from the New Mexico Decedent Image Database (Edgar et al., 2020).
2.2. Methods
2.2.1. Pelvic 3D Models
The 3D images of the pelves associated with the gut volume measurements were obtained previously by Uy and Laudicina (2021) and used for this study. CT images for the parity data were obtained from the NMDID (Edgar et al., 2020). The pelvis from each individual was then isolated and exported as an .STL 3D model using Slicer (3D Slicer Image Computing Platform, n.d.; Fedorov et al., 2012). Using CloudCompare software 2.13.2 (Girardeau-Montaut, 2016), these 3D models were measured at the pubic symphysis and pubic arch. Two authors (E.L. and E.P.) conducting the pelvic measurements were not made aware of the gut volume, gender, or other metadata of each specimen until after the measurements were conducted to avoid bias.
2.2.2. Measurement Methods
Pubic Symphysis Width (PSW)
To measure the pubic symphysis width, three measurements (superior, middle, and inferior) were taken on the anterior side of the pubis (Figure 2A). These three measures were taken between the 1. most anterosuperior edge of the pubis (noted as superior PSW), 2. the most anteroinferior edge of the pubis (inferior PSW), and 3. the middle PSW was taken at the midpoint between the superior and inferior PSW (Figure 2A).
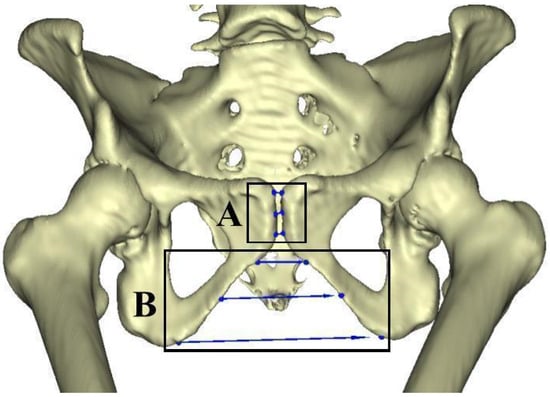
Figure 2.
(A) Pelvis model with the superior, middle, and inferior measurements for the pubic symphysis noted (box A). (B) Pelvis model with the superior, middle, and inferior measurements of the pubic arch noted (box B).
Pubic Arch Width (PAW)
To measure the pubic arch width (PAW), three measurements (superior, middle, and inferior) were taken from the anterior side of the pelvis (Figure 2B). The superior measurement was taken at the uppermost portion of the arch, where the curvature begins inferior to the pubic symphysis. The middle measurement was taken at the midpoint of the curvature, and the inferior measurement was taken at the base of the arch at the point of maximum curvature before the curve changes direction posterior to the ischium. Different measurement techniques have been developed to investigate the pubic arch, sometimes taking into account the height of the arch, other times the width, or a combination of the two (Buikstra & Ubelaker, 1994; Mohd Ali et al., 2019). In this study, we focus on the pubic arch width, rather than the arch height, to investigate the bones’ response to stressors, rather than focusing on the sex differences.
Body Size (Stature)
Multiple linear regression analyses were conducted to assess the relationship of the two pubic measurements (PSW, PAW) and gut size and parity, using stature as a covariate. Previous research has shown that while female body size does not impact gut volume size, in males, there is a relationship between body size and pelvic morphology (Uy et al., 2020).
3. Results
3.1. Gut Volume Results
3.1.1. Pubic Symphysis Width (PSW)
Logarithmic values of the PSW and gut volume (GV) were used to normalize the sample. t-tests were conducted to compare the relationship of PSW to gut volume in a combined male and female sample and then compared to males and females separately to look at sex differences (Table 3). Using the standard significance value of p < 0.05, there was found to be no significant difference in the superior, middle, and inferior PSW depending on small or large gut volume in the combined gender group (Table 3).

Table 3.
Results of PSW and gut volume statistical analyses. Bolded values represent a significant p-value (<0.05).
A linear regression was then conducted (Figure 3, Table 3). In the overall group, with combined male and female samples, gut volume size and all three measurements, superior, middle, and inferior, have a slightly positive relationship based on the positive slope from the graphs (Figure 3A). As gut volume increased, so did the pubic symphysis width at all three measures (superior, middle, inferior). For the combined gender group using linear regression (Figure 3A), for superior PSW, 0.1% (R2) of the variability in PSW can be explained by gut volume. For middle PSW, 0.5% (R2) of the variability in PSW can be explained by gut volume. Finally, for inferior PSW, 1.9% (R2) of the variability in PSW can be explained by gut volume. In this combined sample, none of the linear regression models were statistically significant (Table 3).
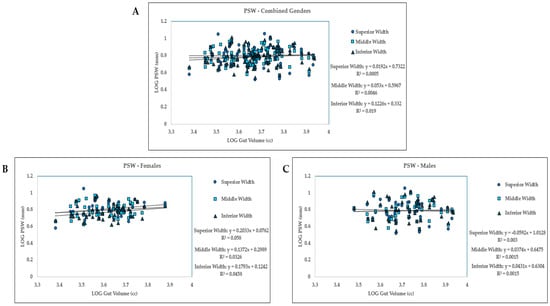
Figure 3.
Gut volume measures compared to pubic symphysis widths for (A) combined gender group (females and males); (B) females only; (C) males only, at three PSW measures: superior, middle, and inferior width measurements.
Next, we analyzed the data to see if there were differences in gender groups. t-tests and linear regression models were run for this purpose (Table 3, Figure 3B,C). Similar to results in the overall combined gender group, there was no significant difference in superior, middle, and inferior PSW and small or large gut volume in the female groups. Trends indicate that in the female sample, as gut volume increased, so did the width of the pubic symphysis (Figure 3B). In the male group, there was almost a flat trendline indicating no change at the pubic symphysis width with increasing gut volume (Figure 3C). The superior and middle PSW regression models were not statistically significant in the male sample, but the inferior PSW linear regression model was significant (p = 0.003, Table 3).
3.1.2. Pubic Arch Width (PAW)
Comparing the pubic arch width (PAW) and gut volume (GV) samples, logarithmic values were taken of each of the three measurements to normalize the sample. t-tests were performed to determine if there is a relationship between gut volume and pubic arch width (Table 4). In the combined gender group, the superior width PAW was significantly different (p = 0.041) between gut volume groups (small/large), while the middle and inferior widths were not statistically significant (Table 4).

Table 4.
Results of PAW and gut volume statistical analyses. Bolded values represent a significant p-value (<0.05).
In the combined gender group, PAW and gut volume have a slight positive relationship (Figure 4A). At the pubic arch, 1.1% (R2) of superior width variability is explained by gut volume (Figure 4A). In the middle width, 0.72% (R2) of variability can be explained by gut volume. In the inferior width, 0.16% (R2) of variability is explained by gut volume. However, none of these linear regression models were statistically significant (Table 4).
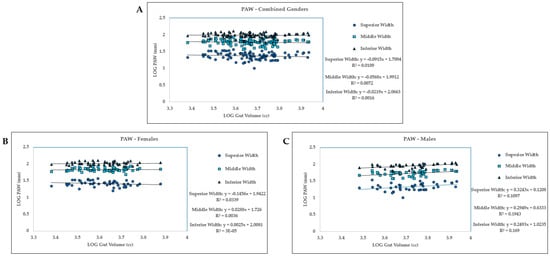
Figure 4.
For pubic arch width, LOG Gut Volume vs LOG width for superior width, middle width, and inferior width measurements. (A) Combined gender group. (B) Females. (C) Males.
Separating the individuals by gender yielded one significant relationship in males (inferior PAW: p = 0.047; Table 4) but no significance within females, although the superior PAW was nearing significance (p = 0.056; Table 4). The male PAW and gut volume relationship appears to be similar to the overall gender group, slightly positive, where with an increase in the gut volume, the pubic arch width increases (Figure 4A,C). All three linear regression models for the male sample were statistically significant (Table 4). Interestingly, the female sample exhibits a slight negative relationship where with increasing gut volume, the PAW decreases, although these linear regression models were not statistically significant (Figure 4B; Table 4).
3.1.3. Body Size
Finally, to account for the potential influence of the PSW and PAW due to body size differences, a multiple regression analysis was conducted (Table 5). Stature (living height) was used for both the PSW and PAW analyses. Because both the pubis metrics and height are linear measurements, while gut size is a volumetric (cubic centimeters) measurement, the cubed root of GV was used to keep all the variables linear. These results illustrate that at the pubic symphysis measures, no models were significant (Table 5). At the pubic arch width, the male PAW regression models were significant at all three measures (superior, middle, inferior widths), while the female models were not statistically significant (Table 5).

Table 5.
Summary of results from multiple regression analyses comparing pubic symphysis width (PSW) and pubic arch width (PAW) with gut volume (GV). Each regression model uses stature as a covariate (e.g., SPSW~GV + Stature). The beta coefficients (slopes) of each predictor variable and their p-values, the r2 values of the regression models, and the p-values of the models are reported; bolded p-values with an asterisk * indicate a p < 0.05.
3.2. Parity Results
An ANOVA test was conducted to see if there were any differences in PSW when comparing all of the parity groups together. ANOVA tests are similar to t-tests in that they analyze the difference between the means of groups to determine the impact of one variable on another. P-values were not significant between parity groups and PSW (Table 6). ANOVA tests were also performed to compare PAW measurements and parity (Table 6). None of the resulting p-values were statistically significant between the parity groups and the three PAW measures (Table 6).

Table 6.
Results of PSW/PAW and parity statistical analysis. Three measures (superior, middle, inferior) were conducted at both the PSW and PAW.
There is a positive relationship between the number of births and pubic symphysis width (PSW) at all three width measures (superior, middle, inferior) (Figure 5A). For both superior and middle PSW, 3.9% (R2) of the variability in PSW can be explained by the number of births (parity). When looking at inferior PSW, 1.2% (R2) of the variability in PSW can be explained by the number of births (parity). None of these linear regression models were significant based on an alpha of 0.05 (Figure 5, Table 6).
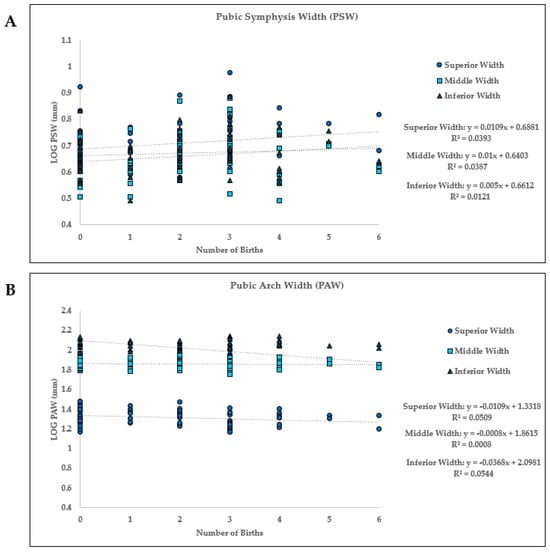
Figure 5.
Linear regression between the number of births (parity) and the pubic symphysis width (PSW) and pubic arch width (PAW) at each of the three measurements (superior width (SW), middle width (MW), and inferior width (IW)). (A) PSW: Parity vs. LOG width three measurements, (B) PAW: Parity vs. LOG width for three measurements.
At all three pubic arch width (PAW) measures (superior, middle, and inferior), there is a negative relationship between the number of births and the width of the pubic arch. As parity increased, the pubic arch width decreased, as seen by the negative slope values (Figure 5B). The middle PAW’s negative correlation is minor, and almost near 0. The 5.1% (R2) of variability in the superior width can be attributed to parity. Furthermore, the middle PAW width shows that 0.1% (R2) of the variability in width is explained by parity, while at the inferior PAW, 5.4% of the variability is explained by parity. As with the PSW, these PAW regression analyses were not statistically significant (Figure 5, Table 6).
Body Size
Using stature as a covariate in the parity multiple linear regression analysis shows that parity has no relationship with either the pubic symphysis width or pubic arch width (Table 7).

Table 7.
Summary of results from multiple regression analyses comparing pubic symphysis width (PSW) and pubic arch width (PAW) with parity. Each regression model uses stature as a covariate (e.g., SPSW~Parity + Stature). The beta coefficients (slopes) of each predictor variable and their p-values, the r2 values of the regression models, and the p-values of the models are reported.
4. Discussion
While many of the pubic symphysis width (PSW) and pubic arch width (PAW) measures were not significantly associated with differences in gut volume or parity, based on an alpha level of 0.05, a few measures were (Table 4 and Table 5). The regression models’ R2 values are low, ranging from 0 to 0.058 (Figure 3, Figure 4 and Figure 5). However, there are trends exhibited at the PSW and PAW that are important to highlight. At the PSW, in both the female gut volume and parity analyses, there appeared to be a positive relationship, indicating that as gut volume and parity increased, so did the PSW. This indicates that increased forces due to gut volume load and pelvic expansion due to childbirth may be contributing to an increased width at the pubic symphysis. However, the small sample size of the highest parity group (n > 5 births) warrants further study to confirm these results. For the male sample, as gut volume increased, there was very little effect at the PSW.
For female PAW, there appeared to be a negative relationship, indicating that as gut volume and parity increased, PAW decreased. This indicates that the pelvic girdle is accommodating the larger forces put upon the symphysis by reducing the space at the inferior aspect of the pelvic canal, potentially increasing stability of the region. With the male sample, we see an opposite result with a slight positive relationship between gut volume and pubic arch width. We see that gut volume predicts the male PAW relatively well, even without stature (Table 5). In fact, 10–24% of the PAW variation in the male sample is due to increasing gut size (Table 5). We hypothesize that the male pelvis has more flexibility in the amount of pelvic expansion available compared to the already expanded female pelvis. However, further study should examine this relationship between increased mass and PAW in both males and females.
The trends in the data show increased gut volume and parity leading to a wider female pubic symphysis and a narrower female pubic arch. Changes in the pelvic girdle may result in impaired mobility or pain. Larger width in the pubic symphysis with a narrowed arch could result in decreased stability, ultimately leading to mobility issues and potentially pelvic girdle pain (PGP). In one study, pregnancy was “well-established” as a leading factor of PGP, as it alters structures of the pelvis, specifically at the pubic symphysis (Ali et al., 2020). Joint pain, lower back pain, and instability are three symptoms of PGP (Ali et al., 2020). PGP increases the risk for joint and other bone injuries, as well as mental health deficits due to the pain and decreased mobility (Ali et al., 2020). Ali et al. (2020) also show how the soft tissue surrounding the pelvis plays an important role in stabilization. In pregnancy, the hormone relaxin is released and is believed to remodel collagen, leading to deficits in the connective tissue around the pelvis, which could ultimately lead to dysfunction in the pelvic structures, and PGP (Ali et al., 2020). This relaxin hormone theory is supported by some research (Ali et al., 2020; Kristiansson et al., 1999) but contraindicated by others (Leadbetter et al., 2004; MacLennan, 1991), highlighting that there are still many unknowns regarding the exact mechanisms causing PGP. This research on the PSW and PAW highlights the importance of the pelvic girdle’s bony structures and investigates how two variables (gut volume and parity) may alter these pelvic structures. Analyzing the pubic symphysis and pubic arch structures, and how their widths may change with respect to increased gut volume and parity, can allow for predictions about PGP and how an individual’s stability and mobility may be affected.
There are likely other extrinsic factors, or a combination of factors, that contribute to changes at the PSW and PAW beyond the factors (gut volume and parity) examined in this study. In this study, body size (stature) was used as a covariate for both the gut volume and parity samples and showed sex differences, as has been noted in previous studies (Uy et al., 2020). Future studies could examine other factors, including body weight or overall pelvic size, to investigate how differences in gut volume or parity impact pelvic measures.
5. Conclusions
This research contributes to the prediction that an increase in mass, due to either gut volume or pregnancy mass, will change the morphology of the pubis, even if in small ways. Further studies may look to explore what other regions of the pelvis, or even other parts of the body, may be affected by an increase in gut mass, or body mass in general, as well as the effects of pregnancy. This can contribute to discussions on the management and prevention of issues such as pelvic girdle pain and mobility issues.
Author Contributions
Conceptualization, E.L., E.P., J.U., and N.L.; methodology, E.L., E.P., J.U., and N.L.; software, E.L., E.P., J.U., and N.L.; validation, E.L., E.P., J.U., and N.L.; formal analysis, E.L., E.P., J.U., and N.L.; investigation, E.L., E.P., J.U., and N.L.; resources, E.L., E.P., J.U., and N.L.; data curation, E.L., E.P., J.U., and N.L.; writing—original draft preparation, E.L., E.P., and N.L.; writing—review and editing, E.L., E.P., J.U., and N.L.; visualization, E.L., E.P., J.U., and N.L.; supervision, J.U. and N.L.; project administration, E.L., E.P., J.U., and N.L.; funding acquisition, J.U. and N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
IRB did not require a new review for this present study, as the individuals were previously de-identified, and we used secondary data from the previous study (Uy & Laudicina, 2021). Metadata, including subject and case information, from the New Mexico Decedent Image Database (NMDID) were de-identified prior to us receiving the information. The NMDID approval board granted us permission to use the CT scans. Each subject’s accession number anonymized their identification.
Informed Consent Statement
Patient consent was waived, as all individuals were previously de-identified prior to the study.
Data Availability Statement
Data are available upon request. Scans obtained through the New Mexico Decedent Database are limited in access and cannot be shared without prior consent from the NMDID.
Acknowledgments
We thank the University of Wisconsin School of Medicine and the New Mexico Decedent Database for access to data used in this study. We thank the three anonymous reviewers who contributed feedback and comments that improved this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PS | Pubic symphysis |
| PA | Pubic arch |
| PSW | Pubic symphysis width |
| PAW | Pubic arch width |
| GV | Gut volume |
| PGP | Pelvic girdle pain |
| NMDID | New Mexico Decedent Database |
References
- 3D Slicer Image Computing Platform. (n.d.). Computer software. Available online: https://slicer.org/ (accessed on 14 November 2024).
- Abitbol, M. M. (1988). Evolution of the ischial spine and of the pelvic floor in the Hominoidea. American Journal of Physical Anthropology, 75(1), 53–67. [Google Scholar] [CrossRef]
- Ali, A., Andrzejowski, P., Kanakaris, N. K., & Giannoudis, P. V. (2020). Pelvic girdle pain, hypermobility spectrum disorder and hypermobility-type ehlers-danlos syndrome: A narrative literature review. Journal of Clinical Medicine, 9(12), 3992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alicioglu, B., Kartal, O., Gurbuz, H., & Sut, N. (2008). Symphysis pubis distance in adults: A retrospective computed tomography study. Surgical and Radiologic Anatomy, 30(2), 153–157. [Google Scholar] [CrossRef]
- Becker, I., Woodley, S. J., & Stringer, M. D. (2010). The adult human pubic symphysis: A systematic review: The pubic symphysis. Journal of Anatomy, 217(5), 475–487. [Google Scholar] [CrossRef]
- Buikstra, J. E., & Ubelaker, D. H. (1994). Standards for data collection from human skeletal remains. Arkansas Archaeological Survey Research Series, 44, 44. [Google Scholar]
- Dougherty, G. B., Golden, S. H., Gross, A. L., Colantuoni, E., & Dean, L. T. (2020). Measuring structural racism and its association with BMI. American Journal of Preventive Medicine, 59(4), 530–537. [Google Scholar] [CrossRef]
- Edgar, H. J. H., Daneshvari Berry, S., Moes, E., Adolphi, N. L., Bridges, P., & Nolte, K. B. (2020). New Mexico decedent image database. Office of the Medical Investigator, University of New Mexico. [Google Scholar] [CrossRef]
- Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J. C., Pujol, S., Bauer, C., Jennings, D., Fennessy, F. M., Sonka, M., Buatti, J., Aylward, S. R., Miller, J. V., Pieper, S., & Kikinis, R. (2012). 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging, 30(9), 1323–1341. [Google Scholar] [CrossRef]
- Girardeau-Montaut, D. (2016). CloudCompare. EDF R&D Telecom ParisTech. [Google Scholar]
- Haeusler, M., Grunstra, N. D. S., Martin, R. D., Krenn, V. A., Fornai, C., & Webb, N. M. (2021). The obstetrical dilemma hypothesis: There’s life in the old dog yet. Biological Reviews, 96(5), 2031–2057. [Google Scholar] [CrossRef]
- Jain, S., Eedarapalli, P., Jamjute, P., & Sawdy, R. (2006). Symphysis pubis dysfunction: A practical approach to management. The Obstetrician & Gynaecologist, 8(3), 153–158. [Google Scholar] [CrossRef]
- Kristiansson, P., Svärdsudd, K., & Von Schoultz, B. (1999). Reproductive hormones and aminoterminal propeptide of type III procollagen in serum as early markers of pelvic pain during late pregnancy. American Journal of Obstetrics and Gynecology, 180(1), 128–134. [Google Scholar] [CrossRef]
- Kurihara, Y., Kurihara, Y., Ohashi, K., Kitagawa, A., Miyasaka, M., Okamoto, E., & Ishikawa, T. (1996). Radiologic evidence of sex differences: Is the patient a woman or a man? American Journal of Roentgenology, 167(4), 1037–1040. [Google Scholar] [CrossRef]
- Leadbetter, R., Mawer, D., & Lindow, S. (2004). Symphysis pubis dysfunction: A review of the literature. The Journal of Maternal-Fetal & Neonatal Medicine, 16(6), 349–354. [Google Scholar] [CrossRef]
- Lewis, C. L., Laudicina, N. M., Khuu, A., & Loverro, K. L. (2017). The human pelvis: Variation in structure and function during gait. The Anatomical Record, 300(4), 633–642. [Google Scholar] [CrossRef]
- Lovejoy, C. O. (2005). The natural history of human gait and posture: Part 2. Hip and thigh. Gait & Posture, 21(1), 113–124. [Google Scholar]
- Lovejoy, C. O., Suwa, G., Spurlock, L., Asfaw, B., & White, T. D. (2009). The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science, 326(5949), 71–71.e6. [Google Scholar] [CrossRef]
- MacLennan, A. H. (1991). The role of the hormone relaxin in human reproduction and pelvic girdle relaxation. Scandinavian Journal of Rheumatology Supplement, 88, 7–15. [Google Scholar]
- Martelli, F., Youssef, A., Capogna, M. V., Bruno, A., Bruno, V., Dodaro, M. G., Ticconi, C., Ghi, T., Piccione, E., & Pietropolli, A. (2020). Longitudinal changes of subpubic arch angle throughout pregnancy. Gynecologic and Obstetric Investigation, 85(1), 100–106. [Google Scholar] [CrossRef]
- Memarian, A., Aghakhani, K., Mehrpisheh, S., & Fares, F. (2017). Gender determination from diagnostic factors on anteroposterior pelvic radiographs. Journal of the Chinese Medical Association, 80(3), 161–168. [Google Scholar] [CrossRef]
- Mohd Ali, S. H., Omar, N., Shafie, M. S., Nik Ismail, N. A., Hadi, H., & Mohd Nor, F. (2019). Comparison of two methods for subpubic angle measurement from reconstructed three-dimensional pelvic model. Egyptian Journal of Forensic Sciences, 9(1). [Google Scholar] [CrossRef]
- Oladipo, G. (2010). Comparative study of the sub-pubic angles of adult Ikwerres and Kalabaris. Asian Journal of Medical Sciences, 2(107), 10. [Google Scholar]
- Roberts, R. E. (1934). The physiology and pathology of the pelvic joints in relation to child-bearing: Section of obstetrics and gynaecology. Proceedings of the Royal Society of Medicine, 27(9), 1211–1230. [Google Scholar]
- Rustamova, S., Predanic, M., Sumersille, M., & Cohen, W. R. (2009). Changes in symphysis pubis width during labor. Journal of Perinatal Medicine, 37(4), 51. [Google Scholar] [CrossRef]
- Sakamoto, A., Watanabe, G., Morito, T., Katayama, K., Kumagai, H., & Gamada, K. (2021). Changes in pelvic alignment in a woman before and after childbirth, using three-dimensional pelvic models based on magnetic resonance imaging: A longitudinal observation case report. Radiology Case Reports, 16(12), 3955–3960. [Google Scholar] [CrossRef]
- Tague, R. G., & Lovejoy, C. O. (1986). The obstetric pelvis of AL 288-1 (Lucy). Journal of Human Evolution, 15(4), 237–255. [Google Scholar] [CrossRef]
- Uy, J., Hawks, J., & VanSickle, C. (2020). Sexual dimorphism of the relationship between the gut and pelvis in humans. American Journal of Physical Anthropology, 173(1), 130–140. [Google Scholar] [CrossRef]
- Uy, J., & Laudicina, N. M. (2021). Assessing the role of the pelvic canal in supporting the gut in humans. PLoS ONE, 16(10), e0258341. [Google Scholar] [CrossRef]
- Vix, V. A., & Ryu, C. Y. (1971). The adult symphysis pubis: Normal and abnormal. American Journal of Roentgenology, 112(3), 517–525. [Google Scholar] [CrossRef]
- Washburn, S. L. (1948). Sex differences in the pubic bone. American Journal of Physical Anthropology, 6(2), 199–208. [Google Scholar] [CrossRef]
- Wobser, A., Adkins, Z., & Wobser, R. (2023). Anatomy, abdomen and pelvis: Bones (ilium, ischium, and pubis). Available online: https://www.ncbi.nlm.nih.gov/books/NBK519524/ (accessed on 12 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).