3. Criticisms
The claim that there are two categories of spermatozoa, ‘egg-getters’ and ‘kamikaze,’ has been disputed. After attempting in vain to corroborate this claim, Moore, Martin and Birkhead concluded unequivocally that “On the basis of several different in vitro assays our results provide no support for Baker and Bellis’s (1995) hypothesis that human sperm selectively make contact with sperm from a different male and incapacitate them” [
3] (p. 2346).
Martin notes that our biological characteristics place us at the monogamous or polygynous end of the scale. “Primates with a unimale mating system (monogamous or polygynous) typically have fairly small testes, whereas testis size is relatively large in species with a multimale system that potentially involves sperm competition [. . .] human relative testis size seems to suggest adaptation to a mating system in which sperm competition was limited or completely lacking” [
4] (p. 61). By contrast, promiscuous chimpanzees and bonobos have the largest testes of all primates [
5].
The size of the sperm midpiece also place
Homo sapiens at the monogamous or polygynous end of the scale. Anderson and Dixson found an association between the size of the midpiece and the mating system [
6]. The mitochondria within the midpiece provide the energy to power the tail. In species where there is sperm competition (such as baboons, macaques and chimpanzees), the midpiece is larger than among monogamous species (such as gibbons) or polygynous species (such as gorillas)—“Confirming the inference based on testis size, the volume of the midpiece in human sperm falls into the range characteristic of primate species with a unimale mating system. In fact the midpiece volume of human sperm is among the smallest recorded for primates and well below the values typical of species with multimale mating systems” [
4] (p. 62).
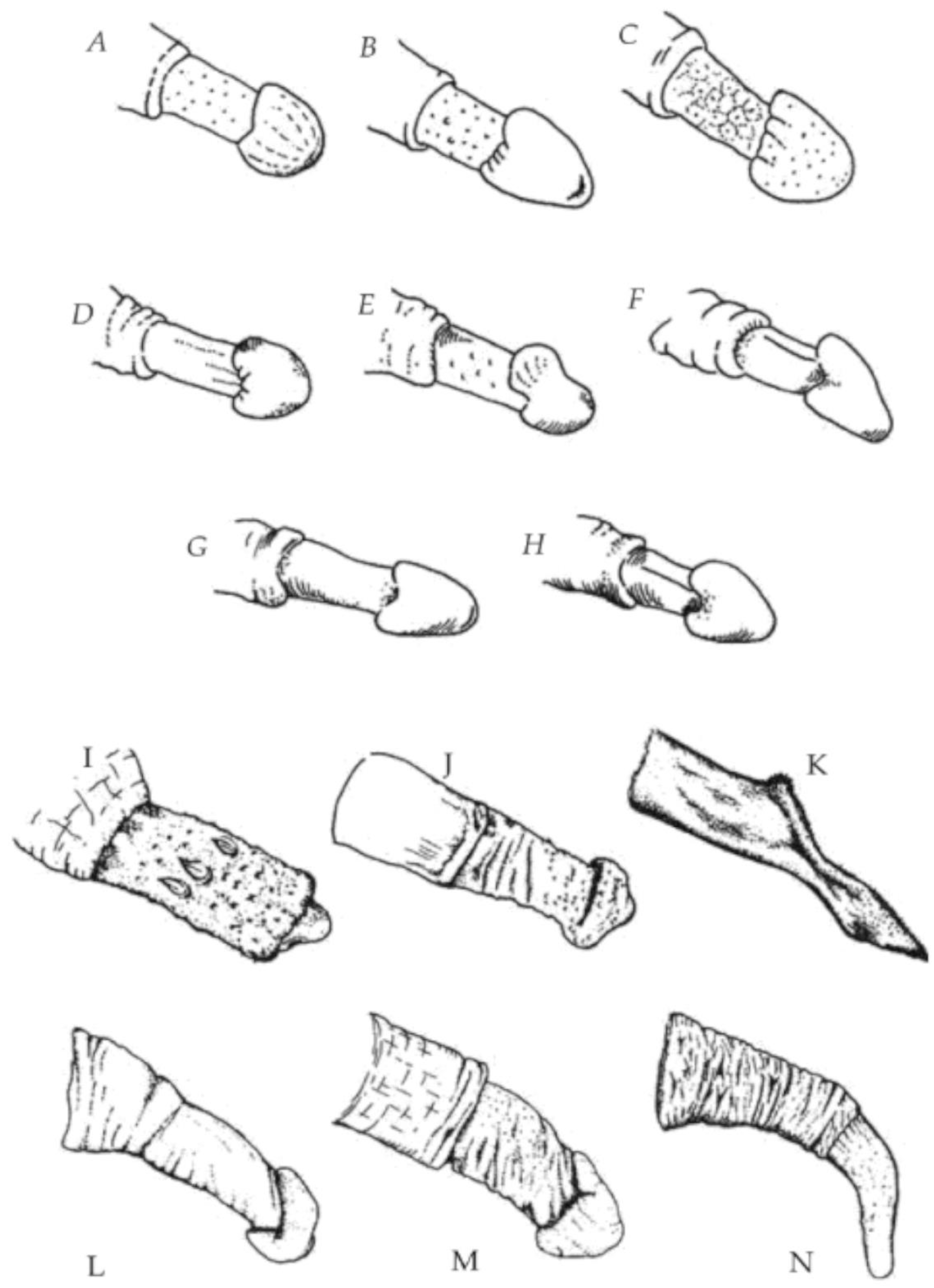
Furthermore, the glans penis is not uncommon among primates, and is certainly not confined to promiscuous species. According to Dixson, arguing against Baker and Bellis: “A helmet or acorn-shaped glans is common amongst Old World monkeys, such as various colobines, macaques, baboons, mangabeys, and guenons, regardless of whether they have polygynous or multi-male/multi-female mating systems” [
7] (p. 342). See
Figure 1.
Dixson suggests that specialisations for sperm competition, including the loss of the glans, “arose after the lineage which gave rise to
Pan had separated from that which gave rise to the genus
Homo. Indeed, although many authors have equated the common ancestor of chimpanzees and humans as being similar to the modern chimpanzee, this is misleading, especially where various features of its reproductive and sexual behaviour are concerned” [
8] (p. 266). Moreover, although the common ancestor of humans, chimpanzees and gorillas is projected to have lived between five and eight million years ago [
9] (p. 268), comparative analysis of semenogelin genes (the major proteins of semen) of these species suggests that, in relation to this common ancestor, these genes have changed relatively little in humans and gorillas. However, they have changed considerably in chimpanzees [
9]. This change is likely to be related to chimpanzees’ evolved ability to form copulatory plugs.
4. An Alternative Hypothesis
The above criticisms of the semen-displacement hypothesis are, to my mind, convincing. They suggest that promiscuity played no role in the evolution of the glans penis. Yet, this hypothesis does raise an interesting question: how are we to explain the shape of the glans? It is certainly not obvious that it is an evolutionary spandrel, or by-product, of some other bodily development. Rightly or wrongly, it is perhaps the lack of an alternative evolutionary explanation of the glans that has contributed to the surviving popularity of the semen-displacement hypothesis.
A feature of the glans that is passed over by the semen-displacement hypothesis is that it has not only a distinctive shape but that it also is more labile than the shaft of the penis. Partly on this basis, the suggestion has been made that in humans (and, presumably, in other species) the glans may play a protective role in intercourse–protective of both male and female—analogous to a boxing glove which functions as a cushion and a shock absorber [
10].
An alternative hypothesis—which, to the best of my knowledge, has not previously been suggested—is that, after ejaculation, as the penis withdraws from the vagina, the narrower entrance to the vagina (the introitus), involuntarily squeezes the labile glans, which in turn squeezes the urethra. In this way, together, through a parting squeeze, contact between the introitus and the glans ensures that the female extracts a final small fraction of semen. (As Alan Dixson notes: “[A] close fit between complementary genital structures is essential if copulation is to succeed” [
8] (p. 275)).
According to this hypothesis, the shape and texture of the glans enables semen-displacement, but in an entirely different manner to that proposed by the original semen-displacement hypothesis. The semen-displacement of the sort here proposed is enabled by the shape and texture of glans penis in conjunction with the introitus, but it does not imply that the evolution of the glans was influenced by internal sperm competition (between the sperm of different males). On the contrary, it suggests that in our evolutionary history, the sperm of a particular male was, in effect, already selected via courtship before the act of sexual intercourse (however, that is not to suggest that females are necessarily selecting for potentially good fathers. The species that possess a glans penis do not, so far as I am aware, correlate with species known for a presence or absence of paternal behaviour).
The hypothesis of a parting squeeze complements the arguments, outlined above, that there are no biological adaptations in Homo sapiens that indicate sperm competition at any time in our formative past. It suggests that the glans probably evolved in a relatively non-promiscuous society.
Nonetheless, in the course of making a case for the semen-displacement hypothesis, Baker and Bellis note certain traits that can be usefully transferred to the new hypothesis. They note that within “less than a minute of insemination into the upper vagina, the human ejaculate coagulates to form a soft, spongy structure... After about 10–20 min, the structure decoagulates and, at least in part, is eventually ejected in flowback” [
2] (p. 188). They calculate the median time from ejaculation to flowback is 30 minutes, at which event, approximately 35% of sperm are ejected [
2] (p. 45). They note that the concentration of sperm decreases from the first part of the ejaculate to the last. “The first part of the human ejaculate is dominated by fluids from the prostate and Cowper’s gland and the later parts by fluids from the seminal vesicle” [
2] (p. 91); [
11]. The fluids from the prostate encourage decoagulation, whereas fluids from the seminal vesicle, which are more dominant in the final ejaculate, encourage coagulation. It is here suggested that it is this more readily coagulating semen that is extracted by the compression of the glans. This semen plays no direct role in fertilisation but, coagulating in the lower part of the vagina, it plays an indirect role, in that it delays flowback.
It is also the case that in chimpanzees the final ejaculate coagulates more readily than the first, but in chimpanzees, it coagulates to such an extent that, as noted above, it forms a copulatory plug, intended to deter future intercourse with other sexual partners. It has been argued that chimpanzee’s pointed penis evolved to dislodge these plugs. However, as suggested above, the evolved ability to form copulatory plugs is most likely an evolutionary development that takes chimpanzees further away from the common ancestor that they share with humans and gorillas.
In summary, this short opinion piece has focused on putting forward a hypothesis that explains the evolution of the human glans penis in a more satisfactory way than the semen-displacement hypothesis. As outlined above, the latter has faced several serious criticisms but there has been comparatively little work done with regard to suggesting an alternative explanation for the human glans. It may be for this reason that the semen-displacement hypothesis continues to enjoy some popularity. The hypothesis proposed here is not necessarily intended to apply to all species with a glans penis, nor does it claim to have discovered the one and only function of the human glans penis. It may be that the glans also plays a protective role in intercourse, as suggested by Hatzichristou et al., 2003 [
10] (nonetheless, since promiscuous chimpanzees and bonobos manage without a protective glans, the protective role of the glans is unlikely to have been the driver behind its evolution.)
Although it is offered here only as a speculative hypothesis, it is, I believe, worth considering that the glans penis, in conjunction with the introitus, may enable a parting squeeze that extracts a final small fraction of semen that coagulates at the base of the vagina.