Abstract
To account for the bell- or acorn-shaped glans penis, in 1995 Baker and Bellis put forward their ‘semen-displacement hypothesis’. They argued that the existence of the glans penis is indicative of a promiscuous phase in our evolutionary past, in which females would commonly mate with several males in rapid succession. They argued that within this promiscuous scenario the distinctive shape of the glans penis evolved so as to enable the displacement of rival males’ semen. The idea that there was an influential promiscuous phase in our evolutionary past has faced several powerful criticisms that are here briefly reviewed. However, the critics of the semen-displacement hypothesis have not put forward an alternative evolutionary explanation of the glans penis. I try to redress that here, albeit speculatively. I suggest an alternative hypothesis that may more convincingly account for the shape and texture of the human glans penis.
1. Introduction
In 1995 Baker and Bellis proposed an explanation for the bell- or acorn-shaped human glans penis: the semen-displacement hypothesis. As I explain below, this hypothesis has been discredited by primatologists such as R.D. Martin and A.F. Dixson. However, al-though the sperm-displacement hypothesis has, in my view, been discredited, it does raise an interesting question, namely: how are we to explain the shape and texture of the human glans penis?
In this short opinion piece, I provide a brief exposition of the semen-displacement hypothesis and of the criticisms that have been made of it before finally putting forward an alternative hypothesis that attempts to explain the shape and texture of the human glans penis.
2. The Semen-Displacement Hypothesis
Primate mating systems vary considerably, from monogamous to polygynous to promiscuous. At the most promiscuous end of the scale, it is a common occurrence for female chimpanzees to mate with several male chimpanzees in quick succession. When that happens, the sperm of the male chimpanzees competes, within the female, to fertilize the female ova. According to the semen-displacement thesis proposed by Baker and Bellis—developed from a suggestion by R.L. Smith [1]—there is evidence to suggest that the same was once also true of Homo sapiens.
In brief, they claim that there are two categories of human spermatozoa, ‘egg-getters’ and ‘kamikaze’, and that, as seen under the microscope, the latter sacrifice themselves in warfare with other spermatozoa. Furthermore, they argue that the shape of the glans is consistent with this envisaged scenario of sperm competition, in that the thrusting of a later male draws the semen of his earlier rival away from the cervix. Specifically, they argue that because the base of the glans, the coronal ridge, is wider than the shaft of the penis this makes it effective as a semen displacement device [2].
3. Criticisms
The claim that there are two categories of spermatozoa, ‘egg-getters’ and ‘kamikaze,’ has been disputed. After attempting in vain to corroborate this claim, Moore, Martin and Birkhead concluded unequivocally that “On the basis of several different in vitro assays our results provide no support for Baker and Bellis’s (1995) hypothesis that human sperm selectively make contact with sperm from a different male and incapacitate them” [3] (p. 2346).
Martin notes that our biological characteristics place us at the monogamous or polygynous end of the scale. “Primates with a unimale mating system (monogamous or polygynous) typically have fairly small testes, whereas testis size is relatively large in species with a multimale system that potentially involves sperm competition [. . .] human relative testis size seems to suggest adaptation to a mating system in which sperm competition was limited or completely lacking” [4] (p. 61). By contrast, promiscuous chimpanzees and bonobos have the largest testes of all primates [5].
The size of the sperm midpiece also place Homo sapiens at the monogamous or polygynous end of the scale. Anderson and Dixson found an association between the size of the midpiece and the mating system [6]. The mitochondria within the midpiece provide the energy to power the tail. In species where there is sperm competition (such as baboons, macaques and chimpanzees), the midpiece is larger than among monogamous species (such as gibbons) or polygynous species (such as gorillas)—“Confirming the inference based on testis size, the volume of the midpiece in human sperm falls into the range characteristic of primate species with a unimale mating system. In fact the midpiece volume of human sperm is among the smallest recorded for primates and well below the values typical of species with multimale mating systems” [4] (p. 62).
Furthermore, the glans penis is not uncommon among primates, and is certainly not confined to promiscuous species. According to Dixson, arguing against Baker and Bellis: “A helmet or acorn-shaped glans is common amongst Old World monkeys, such as various colobines, macaques, baboons, mangabeys, and guenons, regardless of whether they have polygynous or multi-male/multi-female mating systems” [7] (p. 342). See Figure 1.
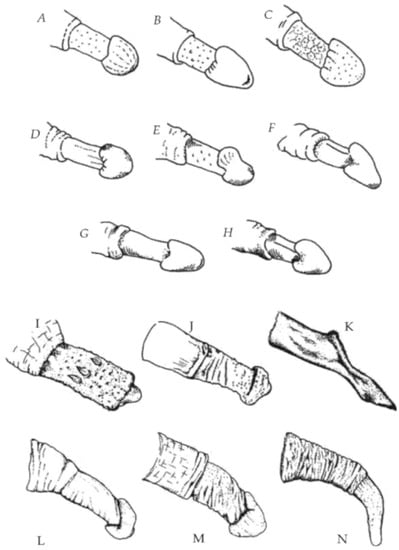
Figure 1.
Examples of penile morphology in primates which have primarily polygynous mating systems (A–H), or multi-male/multi-female mating systems (I–N). (A) Trachypithecus vetulus; (B) Presbytis thomasi; (C) Semnopithecus entellus; (D) Cercopithecus mona; (E) C. petaurista; (F) C. albogularis; (G) C. neglectus; (H) Erythrocebus patas; (I) Eulemur fulvis; (J) Saimiri boliviensis; (K) Macaca arctoides; (L) M. fascicularis; (M) Papio cynocephalus; (N) Pan troglodytes. Source: Dixson Primate Sexuality (1998).
Dixson suggests that specialisations for sperm competition, including the loss of the glans, “arose after the lineage which gave rise to Pan had separated from that which gave rise to the genus Homo. Indeed, although many authors have equated the common ancestor of chimpanzees and humans as being similar to the modern chimpanzee, this is misleading, especially where various features of its reproductive and sexual behaviour are concerned” [8] (p. 266). Moreover, although the common ancestor of humans, chimpanzees and gorillas is projected to have lived between five and eight million years ago [9] (p. 268), comparative analysis of semenogelin genes (the major proteins of semen) of these species suggests that, in relation to this common ancestor, these genes have changed relatively little in humans and gorillas. However, they have changed considerably in chimpanzees [9]. This change is likely to be related to chimpanzees’ evolved ability to form copulatory plugs.
4. An Alternative Hypothesis
The above criticisms of the semen-displacement hypothesis are, to my mind, convincing. They suggest that promiscuity played no role in the evolution of the glans penis. Yet, this hypothesis does raise an interesting question: how are we to explain the shape of the glans? It is certainly not obvious that it is an evolutionary spandrel, or by-product, of some other bodily development. Rightly or wrongly, it is perhaps the lack of an alternative evolutionary explanation of the glans that has contributed to the surviving popularity of the semen-displacement hypothesis.
A feature of the glans that is passed over by the semen-displacement hypothesis is that it has not only a distinctive shape but that it also is more labile than the shaft of the penis. Partly on this basis, the suggestion has been made that in humans (and, presumably, in other species) the glans may play a protective role in intercourse–protective of both male and female—analogous to a boxing glove which functions as a cushion and a shock absorber [10].
An alternative hypothesis—which, to the best of my knowledge, has not previously been suggested—is that, after ejaculation, as the penis withdraws from the vagina, the narrower entrance to the vagina (the introitus), involuntarily squeezes the labile glans, which in turn squeezes the urethra. In this way, together, through a parting squeeze, contact between the introitus and the glans ensures that the female extracts a final small fraction of semen. (As Alan Dixson notes: “[A] close fit between complementary genital structures is essential if copulation is to succeed” [8] (p. 275)).
According to this hypothesis, the shape and texture of the glans enables semen-displacement, but in an entirely different manner to that proposed by the original semen-displacement hypothesis. The semen-displacement of the sort here proposed is enabled by the shape and texture of glans penis in conjunction with the introitus, but it does not imply that the evolution of the glans was influenced by internal sperm competition (between the sperm of different males). On the contrary, it suggests that in our evolutionary history, the sperm of a particular male was, in effect, already selected via courtship before the act of sexual intercourse (however, that is not to suggest that females are necessarily selecting for potentially good fathers. The species that possess a glans penis do not, so far as I am aware, correlate with species known for a presence or absence of paternal behaviour).
The hypothesis of a parting squeeze complements the arguments, outlined above, that there are no biological adaptations in Homo sapiens that indicate sperm competition at any time in our formative past. It suggests that the glans probably evolved in a relatively non-promiscuous society.
Nonetheless, in the course of making a case for the semen-displacement hypothesis, Baker and Bellis note certain traits that can be usefully transferred to the new hypothesis. They note that within “less than a minute of insemination into the upper vagina, the human ejaculate coagulates to form a soft, spongy structure... After about 10–20 min, the structure decoagulates and, at least in part, is eventually ejected in flowback” [2] (p. 188). They calculate the median time from ejaculation to flowback is 30 minutes, at which event, approximately 35% of sperm are ejected [2] (p. 45). They note that the concentration of sperm decreases from the first part of the ejaculate to the last. “The first part of the human ejaculate is dominated by fluids from the prostate and Cowper’s gland and the later parts by fluids from the seminal vesicle” [2] (p. 91); [11]. The fluids from the prostate encourage decoagulation, whereas fluids from the seminal vesicle, which are more dominant in the final ejaculate, encourage coagulation. It is here suggested that it is this more readily coagulating semen that is extracted by the compression of the glans. This semen plays no direct role in fertilisation but, coagulating in the lower part of the vagina, it plays an indirect role, in that it delays flowback.
It is also the case that in chimpanzees the final ejaculate coagulates more readily than the first, but in chimpanzees, it coagulates to such an extent that, as noted above, it forms a copulatory plug, intended to deter future intercourse with other sexual partners. It has been argued that chimpanzee’s pointed penis evolved to dislodge these plugs. However, as suggested above, the evolved ability to form copulatory plugs is most likely an evolutionary development that takes chimpanzees further away from the common ancestor that they share with humans and gorillas.
In summary, this short opinion piece has focused on putting forward a hypothesis that explains the evolution of the human glans penis in a more satisfactory way than the semen-displacement hypothesis. As outlined above, the latter has faced several serious criticisms but there has been comparatively little work done with regard to suggesting an alternative explanation for the human glans. It may be for this reason that the semen-displacement hypothesis continues to enjoy some popularity. The hypothesis proposed here is not necessarily intended to apply to all species with a glans penis, nor does it claim to have discovered the one and only function of the human glans penis. It may be that the glans also plays a protective role in intercourse, as suggested by Hatzichristou et al., 2003 [10] (nonetheless, since promiscuous chimpanzees and bonobos manage without a protective glans, the protective role of the glans is unlikely to have been the driver behind its evolution.)
Although it is offered here only as a speculative hypothesis, it is, I believe, worth considering that the glans penis, in conjunction with the introitus, may enable a parting squeeze that extracts a final small fraction of semen that coagulates at the base of the vagina.
Funding
This research received no external funding.
Acknowledgments
My thanks to the two anonymous referees for their helpful comments and thanks also to Alan Dixson for his encouragement.
Conflicts of Interest
The author declares no conflict of interest.
References
- Smith, R.L. (Ed.) Human Sperm Competition. In Sperm Competition and the Evolution of Animal Mating Systems; Academic Press: London, UK, 1984; pp. 601–660. [Google Scholar]
- Baker, R.R.; Bellis, M.A. Human Sperm Competition: Copulation, Masturbation and Identity; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Moore, H.D.M.; Martin, M.; Birkhead, T.R. No Evidence for Killer Sperm or other selective interactions between human spermatozoa in ejaculates of different males in vitro. R. Soc. Proc. Biol. Sci. 1999, 266, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.D. The Evolution of Human Reproduction: A Primatological Perspective. Am. J. Phys. Anthropol. 2007, 134, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.H.; Harcourt, A.H. Sperm competition, testes size and breeding systems in primates. In Sperm Competition and the Evolution of Animal Mating Systems; Smith, R.L., Ed.; Academic Press: London, UK, 1984; pp. 589–600. [Google Scholar]
- Anderson, M.J.; Dixson, A.F. Motility and the midpiece in primates. Nature 2002, 416, 496. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.F. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes and Human Beings, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Dixson, A.F. Copulatory and Postcopulatory Sexual Selection in Primates. Folia Primatol. 2018, 89, 258–286. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Seaman, M.I.; Li, W.H. Evolution of the Hominoid Semenogelin Genes, the major proteins of ejaculated semen. J. Mol. Evol. 2003, 57, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hatzichristou, D.; Tzortzis, V.; Hatzimouratidis, K.; Apostolidis, A.; Panteliou, S. The Protective Role of the Glans Penis during Coitus. J. Impot. Res. 2003, 15, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Valsa, J.; Skandhan, K.P.; Khan, P.S.; Sumangala, B.; Gondalia, M. Split ejaculation study: Semen parameters and calcium and magnesium in seminal plasma. Cent. Eur. J. Urol. 2012, 65, 216–218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).