1. Introduction
The water of aquatic systems has its characteristics, which are unique and make it differentiable. The water characteristics are sustainable and can only be changed once intentional–unintentional, direct–indirect external substances are added to it [
1]. Therefore, it is assumed that the reflectance from the water surface will be even and symmetrical. Increased algae growth, oil spills, and sediment runoff can alter the natural colour of water bodies and spectral reflectance [
2]. Remote sensing instruments identify these changes and also map their extent. Industrial discharges and similar phenomena lead to changes in water temperature due to exothermic and endothermic chemical reactions. Thermal sensors can detect these anomalies that indicate pollution [
3].
Remote sensing has become popular for studying algal growth and monitoring water quality across lakes, rivers, and coastal areas. It is now possible to track key indicators such as chlorophyll-a, turbidity, and suspended sediments over large regions with sensors like Sentinel-2 and Landsat. Specialised spectral indices designed for aquatic environments have made it easier to identify algal blooms under different water conditions [
4,
5]. At the same time, new approaches such as machine learning and bio-optical models are helping to improve the accuracy of these measurements [
6,
7]. There are still specific challenges, including the complex nature of water optics and atmospheric effects, but remote sensing still provides a cost-effective and scalable way to monitor water systems. Expanding the introduction to highlight these methods would give the study a stronger scientific base and clearly show why the research is relevant.
Moreover, remote sensing techniques help pinpoint the origin of pollution and monitor the spread of the pollution. This article mainly focuses on the algal bloom or eutrophication from the three significant aquatic pollutions: algal bloom, oil spills, and plastic pollution. A significant category of marine pollution is the anthropogenic loading of nutrients in the rivers and lakes [
8]. The process is known as eutrophication [
9,
10]. Eutrophication is a naturally occurring phenomenon, but due to anthropogenic activities, it has undergone drastic change in terms of increase in the occurrence of toxic algae, its intensity, and the species composition like blue-green algae (or cyanobacteria) in coastal and inland water bodies [
11,
12]. Eutrophication is a process that naturally occurs in lakes, rivers, and coastal waters, leading to periodic nutrient build-up and algal blooms [
13]. The scale and intensity of these events have changed in recent decades. Human activities such as fertiliser runoff from agriculture, untreated sewage, and industrial discharges add extra nutrients to the water, which can make blooms larger, more frequent, and sometimes toxic [
14,
15]. In other words, algal blooms are a natural phenomenon, but their increasing severity in many parts of the world is closely tied to human-driven nutrient loading and climate change [
12]. Algal blooms are lasting longer and occur more often. They are dominated by toxic algae. Toxins pose a direct health risk to both humans and animals. Also, they result in an unpleasant sight and obstruct many economic activities like boating, fishing, etc. Therefore, the monitoring of algae concentration in lakes has become a topic of interest. A report by the World Bank estimated that agricultural sources contribute roughly 60% of global nitrogen pollution entering waterways [
16]. A 2017 UN report highlights the substantial impact of industrial activities, stating that an estimated 80% of industrial wastewater is discharged untreated globally [
17].
1.1. Background on Algal Blooms
Algal blooms refer to the rapid proliferation of algae and are often triggered by favourable conditions such as increased nutrients (notably nitrogen and phosphorus), sunlight, warm temperatures, and stagnant or slow-moving waters. While not all algal blooms are harmful, a significant subset harmful algal blooms (HABs) produce toxins that can adversely affect aquatic ecosystems, human health, and local economies.
Eutrophication, mainly driven by anthropogenic nutrient enrichment from agricultural runoff, sewage discharge, and industrial effluents, is a major contributor to algal bloom formation [
18,
19]. Climate change is another driving factor for HABs; rising temperatures enhance stratification of water bodies, reduce vertical mixing, and increase the metabolic rates of algae, thereby favoring bloom conditions [
20].
The ecological impacts of HABs include the depletion of dissolved oxygen (leading to hypoxia and fish kills), disruption of food webs, and loss of biodiversity. On the socio-economic side, algal blooms affect fisheries, recreational water use, and tourism while also increasing the cost of water treatment for human consumption [
12]. Moreover, toxins produced by certain HABs (e.g.,
Microcystis spp.) can cause severe health issues ranging from skin irritation to liver damage in humans and animals [
14].
Given the frequency, scale, and impact of HABs, monitoring and early detection are critical. Traditional field-based studies are accurate, but remote sensing techniques offer cost-effective, timely, and broad-scale solutions for algal bloom detection and monitoring, particularly those using Multispectral Imaging (MSI) and Synthetic Aperture Radar (SAR) data.
1.2. Role of Remote Sensing
Monitoring algal blooms traditionally relies on in situ data and laboratory analysis, which offer high accuracy with constraints of limited spatial and temporal coverage that are overcome by remote sensing technologies which enables frequent, efficient, and large-scale monitoring [
7,
21]. Satellite data from MSIs and SAR sensors offer several capabilities in terms of dealing with algal blooms:
Broad spatial coverage: MODIS, Sentinel, and Landsat provide spatial resolutions of 250 m to 1 km, 10 m to 60 m, and 15 m to 30 m, respectively, facilitating observation of remote, inaccessible, and larger geographical regions.
High temporal resolution from sensors like MODIS, Sentinel-2, Sentinel-1, and Landsat-8 and Landsat-9 have temporal resolutions of 1-day, 5-day and 16-day, offers regular monitoring.
Long-term archives for retrospective analysis of bloom patterns and seasonality.
1.2.1. Multispectral Imaging (MSI)
Multispectral sensors such as MODIS, Sentinel-2 MSI, Landsat-8 OLI, and VIIRS capture data across visible, near-infrared (NIR), and shortwave infrared (SWIR) spectrums, which are quite effective in detecting water quality parameters such as chlorophyll-a, turbidity, and surface scum [
22,
23]. These spectral bands are also useful in formulating spectral indices such as Floating Algae Index (FAI), Normalized Difference Chlorophyll Index (NDCI), and Chlorophyll Index (CI) that are effective tools of remote sensing for detection and monitoring of algal bloom.
1.2.2. Synthetic Aperture Radar (SAR)
SAR sensors such as Sentinel-1, RADARSAT-2, and TerraSAR-X provide microwave data that can penetrate cloud cover and are independent of solar illumination. While SAR does not directly measure biological properties, it can detect surface anomalies such as dampening caused by dense algal biomass or slick formation [
24].
Recent research exhibits the increased efficiency of integrating MSI and SAR data in bloom detection, especially in optically complex or coastal waters where optical signals alone may be insufficient [
25].
1.3. Objectives and Scope
The primary objective of this review is to provide a comprehensive synthesis of how MSI and SAR remote sensing techniques have been utilised to detect, monitor, and assess algal blooms across various aquatic environments. This article aims to review MSI and SAR platforms for bloom detection, including spectral indices and surface roughness analysis; examining optical–radar data fusion to enhance HAB monitoring in complex or cloud-covered waters; and summarising satellite mission features, algorithmic advances, and application contexts and to identify key research gaps and challenges such as atmospheric effects and SAR interpretation limits.
By addressing these objectives, this review contributes to the growing body of research focused on operationalizing remote sensing-based early warning systems for HABs and improving our understanding of how remote sensing platforms can support sustainable water quality monitoring practices [
12,
21].
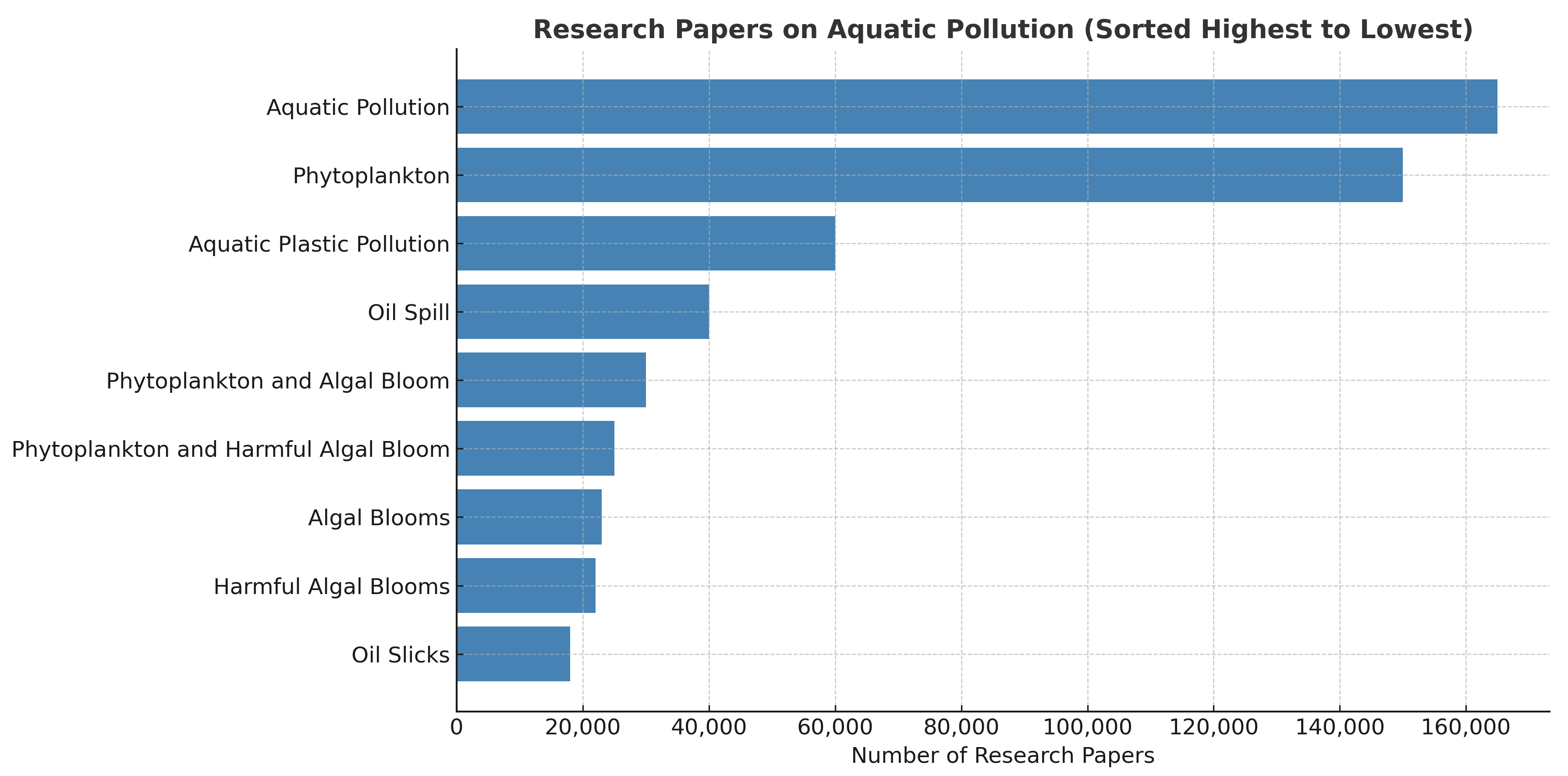
In this review paper, research articles on the most significant portal, Google Scholar, were searched with the keywords “Aquatic Pollution”, “Algal Blooms”, “Harmful Algal Blooms”, “Phytoplankton”, “Phytoplankton and Algal Bloom”, “Phytoplankton and Harmful Algal Bloom|”, “Oil Spill”, “Oil Slicks”, and “Aquatic Plastic Pollution”. It has been observed that this is an active research field, and numerous articles have been published, as shown in
Figure 1. The entire data since 2015 from Google Scholar are tabulated in
Table 1. In
Table 1, the term ‘No Data’ refers to studies that discussed HABs without incorporating remote sensing datasets such as MSI or SAR.
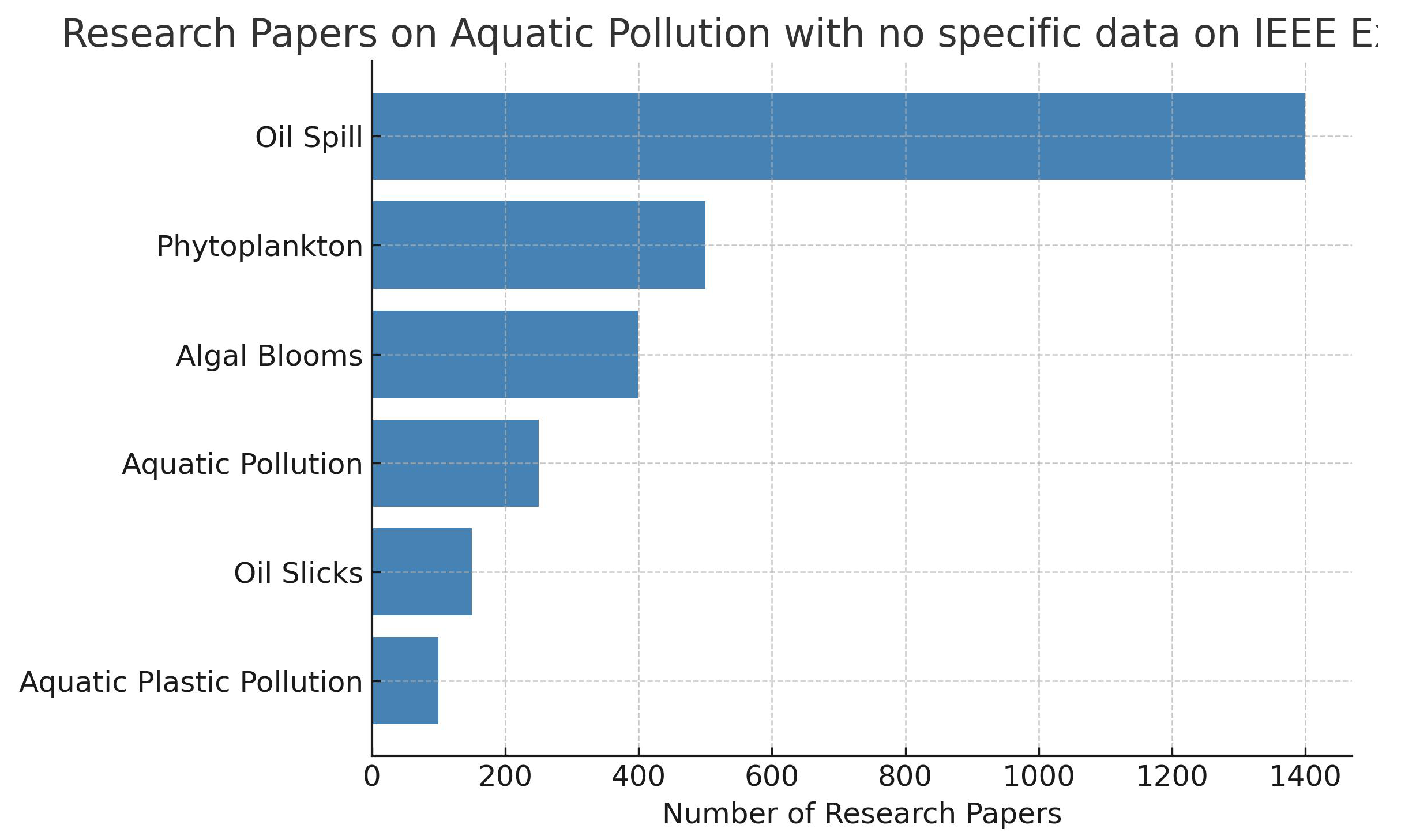
In similar context, the Institute of Electrical and Electronics Engineering (IEEE) is also a significant database for research articles. The above keywords were optimised, as the IEEE explore uses an advanced search engine and is able to catch the keywords perfectly. For example, harmful algal blooms includes all article related to algal blooms, which was used to search the number of articles and is depicted in
Figure 2. The entire matrix of articles with respect to type of pollutants against different data utilised is shown in
Table 2, NA refers to the Not Available in that category of data shown in the columns. The algal blooms are also referred to with different names such as phytoplankton, cyanobacteria, red tides, and harmful algal blooms. The articles were searched with these keywords and also with their combinations. Similarly, an oil spill has been sometimes referred as an oil slick.
In the recent past, marine pollution detection has received a great attention as it is a matter of public concern. Marine pollution has a strong influence on human health, and it environmentally and economically impacts the marine environment, fisheries, wildlife, tourism, benthic communities, forests, and human settlement on the beaches.
3. Techniques and Indices in Remote Sensing Used for the Detection and Monitoring of Algal Blooms
A great aid in the effort of monitoring sea surface pollution comes from remote sensing techniques. Nowadays, SAR and PolSAR data are also extensively used for monitoring eutrophication and harmful algal blooms. The algal bloom usually scatters the incoming electromagnetic wave, therefore mainly resulting in volume scattering phenomenon. However, the river or lake is usually dominant in surface scattering phenomena. Therefore, based on the scattering behaviour of these two, one can identify the algal bloom in a river or lake. The increase in algae concentration in a lake can be detected by observing the increase in the volume scattering phenomenon. The remote sensing-based algal blooms detection leverages various spectral indices, image processing techniques, and bio-optical models. Multiple complementary techniques have evolved given the variability in water composition, bloom types, and environmental conditions.
3.1. Techniques Used in Algal Bloom Detection Using SAR Data
Synthetic Aperture RADAR (SAR) has become a well-established remote sensing technology due to its day–night and all-weather sensing capabilities, as well as high-resolution image data [
40,
41,
42,
43]. Recent advances in SAR, namely, wide spectral band; multi-angle view; short revisit time; improved spatial resolution, swath width, and polarimetric capabilities; and imaging at different bands and acquisition modes have made it even more popular and convenient [
44,
45]. Depending on the capabilities and functionality, such as for the study of topographic information, topographic change information, geometric characterisation, and characterisation of volumetric structures, SAR interferometry (InSAR) [
46,
47], differential SAR interferometry (DInSAR) [
48], polarimetric SAR (PolSAR) [
49], and polarimetric interferometric SAR (PolInSAR) [
50] are used, respectively.
SAR transmits either vertical or horizontal polarized radio waves and measures the reflected energy, which describes the intensity and backscattering of the target. SAR generates 2D imagery of the backscattered energy, further revealing the target’s shape, size, and location on the Earth. SAR data are helpful in LULC mapping, change detection, and object detection. PolSAR transmits and receives multiple polarized radio waves (horizontal, vertical, and a combination of both); the received signals are used to measure the target’s intensity, phase, and polarisation information. PolSAR is richer in information generation; it helps understand the scattering mechanism of different objects based on their polarization response. This is further useful in analysing the properties of the materials, discriminating against lookalike objects, and understanding surface roughness. Due to enhanced capabilities, PolSAR is used in specialised applications such as vegetation classification, determining the level of soil moisture in agriculture, distinguishing man-made objects, monitoring forest health, and biomass estimation. PolSAR is more complex than SAR in data acquisition and preprocessing techniques. The additional information in PolSAR allows for a more nuanced understanding of the scene.
PolSAR data can be used for algal bloom detection, but they suffer from certain limitations that reduce their reliability compared to optical sensors. Studies show that blooms often appear as dark features in SAR imagery due to the dampening of capillary waves; however, similar signatures can also be caused by low wind zones or oil films, leading to ambiguity in detection [
51]. The calm surface of water reflects very little radar energy, which makes PolSAR analysis more difficult. Since algal blooms create only slight variations in surface texture, the scattering information is limited, and standard decomposition methods struggle to capture them effectively. In addition, SAR is unable to detect blooms beneath the water surface, and its performance is strongly influenced by wind conditions, revisit frequency, and calibration accuracy, which restrict its use mainly to visible surface scums [
52]. Although new techniques such as deep learning and multi-sensor data fusion are being explored to improve detection, they are computationally intensive and rely heavily on large, high-quality training datasets [
53]. As a result, PolSAR is best seen as a supporting tool—particularly valuable when clouds or rain obscure optical sensors—rather than a standalone solution for reliable algal bloom monitoring.
While MSI reigns supreme in the field of algal bloom detection due to its ability to capture variations in water color, SAR imagery offers a complementary perspective [
54]. Unlike MSI, SAR functions independently of weather conditions [
54,
55]. This allows for consistent monitoring regardless of weather patterns. Algal blooms can cause the water surface to become smoother, dampening capillary waves [
51]. SAR imagery excels at detecting changes in surface roughness; smoother surfaces indicate the presence of algal blooms. SAR data, when combined with MSI, can provide a more robust picture [
54,
55]. Researchers can enhance detection accuracy by correlating smooth surfaces in SAR images with spectral signatures of blooms in multispectral data.
Critical methods that deal with SAR data for the study of algal bloom are as follows:
Surface Smoothness Detection: The extraction of smoother surface that could be a probable algal bloom region [
51,
52].
Thresholding and Segmentation: Algorithms can be used to group pixels with similar characteristics, helping to delineate potential bloom regions [
54,
55].
Change Detection: Monitoring changes in surface smoothness over time using sequential SAR images can strengthen bloom detection [
52].
Integration with Other Data Sources: SAR data are often most effective when combined with other sources of information [
51].
3.1.1. Surface Smoothness Detection
Research on algal bloom detection using remote sensing has extensively explored surface smoothness as a critical indicator. SAR imagery plays a prominent role, with studies like Zhang et al. demonstrating how smoother areas in SAR images correspond to higher bloom concentrations in Chinese lakes [
51]. Wang et al. investigated high-resolution SAR for early bloom detection using similar principles [
56]. The effectiveness of this method in the Baltic Sea was evaluated by Elyouncha et al. (2019) using Envisat ASAR data [
57].
While PolSAR offers promise for bloom detection due to its richer information content, research in this area is still evolving. Studies like Meng et al. on compact PolSAR for wind and current measurements hint at the potential for integrating smoothness analysis with these factors for future bloom detection using PolSAR [
58]. Similarly, Tian et al. explored multi-temporal PolSAR for sea ice classification, highlighting the analysis of surface scattering properties, which could be adapted for bloom detection [
59]. Sun et al. also acknowledged the potential of PolSAR to distinguish smooth surfaces caused by blooms but emphasized the need for further investigation [
60]. It is essential to consider the complementary approach of Debnath et al., who showcased how SAR can be combined with optical imagery for improved bloom detection, going beyond just surface smoothness [
54]. Hughes et al. provides a valuable perspective on limitations and various techniques for algal bloom detection using remote sensing, including SAR-based surface smoothness detection. The main limitations of SAR for algal bloom studies are its inability to detect subsurface blooms, low sensitivity to pigment composition, strong influence of wind and surface conditions on backscatter, and the risk of confusing algal signals with other smooth-surface phenomena such as low wind or oil films [
61]. This collection of research highlights the potential and ongoing development of using surface smoothness from SAR and PolSAR imagery for algal bloom detection.
3.1.2. Thresholding and Segmentation
Li et al. proposed a self-learning thresholding algorithm that learns optimal thresholds for improved accuracy [
62]. Several studies explored segmentation techniques, with Li et al. combining k-means clustering with support vector machine classification and Liu et al. introducing a region-growing segmentation approach for automatic bloom detection [
63,
64]. Li et al. also investigated thresholding methods for oil spill detection in SAR images, with potential applications for bloom detection [
65]. Furthermore, Li et al. introduced a fuzzy C-means clustering approach for unsupervised change detection in SAR images, which is potentially helpful for bloom monitoring [
66]. The possibility of utilizing deep learning for segmentation in SAR imagery was explored by Gao et al., paving the way for Li et al. to propose a deep learning framework designed explicitly for algal bloom detection in SAR data [
65,
67]. Li et al. further explored bloom detection in SAR images using a novel local binary pattern method and a deep ensemble learning framework [
67,
68]. Li et al. introduced a multi-scale saliency detection model for bloom detection in SAR data, showcasing the continuous development in this area [
69].
While research on thresholding and segmentation [
70] specifically for algal bloom detection using PolSAR imagery remains limited, there is growing interest in exploring PolSAR’s potential for this application. Studies like Chen et al. demonstrate the concept of object-based change detection in PolSAR data for wetland monitoring, which could be adapted for future bloom detection using segmentation [
71]. Texture analysis, as explored by Li et al. for land-cover classification using PolSAR, could also be valuable for characterising blooms in segmentation approaches [
63]. Understanding PolSAR scattering mechanisms of marine phenomena, as investigated by Zhang et al. for oil spills, is crucial for developing a similar analysis of algal blooms [
72]. These studies, along with advancements in deep learning for remote sensing segmentation by Ma et al. and SAR image classification by Zhao et al., pave the way for future research on PolSAR-based bloom detection with thresholding and segmentation techniques [
73,
74].
3.1.3. Change Detection
Significant research has focused on change detection techniques using SAR imagery for algal bloom monitoring. Li et al. explored various approaches, including self-learning thresholding algorithms, unsupervised change detection with fuzzy C-Means clustering, and deep learning frameworks [
66,
75,
76]. Other studies investigated region-growing segmentation by Liu et al. [
64], ensemble learning by Li et al. [
76], and local binary pattern methods by Li et al. [
68] for bloom detection in SAR data. Combining SAR with MSI, the work of Cao et al. also shows promise for improved change analysis of algal blooms [
77]. These advancements demonstrate the effectiveness of SAR-based change detection techniques for algal bloom monitoring.
Research on PolSAR-based change detection [
78] for algal bloom detection is limited compared to SAR. However, some studies lay the groundwork for future exploration. Chen et al. demonstrated object-based change detection in PolSAR data for wetland monitoring, a concept potentially adaptable for algal blooms using SAR [
71]. Tian et al. explored multi-temporal PolSAR data analysis for sea ice classification, highlighting the importance of analysing temporal changes for change detection, which could be applied to future studies on PolSAR-based bloom monitoring [
59].
3.2. Techniques for Algal Bloom Detection Using MSI Data
In MSI, the width of the electromagnetic spectrum is divided into narrower recording bands between 4 to 15, and each recording band is recorded separately to generate gray-scale images. The combination of any three bands at a time displays a colour composite image. In the case of MSI, the spectral information, such as colour and hue are utilised in the interpretation.
Multispectral data capture information beyond what our eyes can see [
79]. They record reflected energy across multiple, specific wavelengths of light, often exceeding the visible spectrum. Here is the breakdown: (i). Sensors: Specialised sensors on satellites, aeroplanes, or drones collect this data. These sensors have multiple channels, each tuned to capture a particular wavelength range. (ii). Wavelengths: Visible light is just a tiny portion of the electromagnetic spectrum. Multispectral sensors can capture data in the infrared, ultraviolet, and other invisible regions. (iii). Spectral Signature: Every object has a unique spectral signature, essentially a fingerprint based on how it reflects or absorbs light across different wavelengths.
Multispectral satellite imagery has magical capabilities to enhance a particular terrestrial feature and suppress others. There are different ways of utilizing the multispectral data, which can be categorized as, viz., (a) digitizing through visual interpretation [
80], (b) density slicing of a single band/spectral band analysis [
81], (c) calculating spectral indices, which combines more than two bands using some mathematical ratios [
82,
83], (d) unsupervised [
84,
85] and supervised [
86,
87] classification of multispectral data, and (e) machine learning methods. Spectral band analysis takes a closer look at individual bands of satellite imagery. By understanding how water and pollutants interact with light at different wavelengths, scientists can identify potential trouble spots. For example, chlorophyll, a sign of algal blooms, absorbs specific wavelengths, while sediment scatters light differently. Analysing the reflectance patterns across these bands (blue, green, red, near-infrared) helps identify areas where the spectral signature deviates from the norm, potentially indicating pollution. MSI is able to peer into the health of our oceans, lakes, and rivers from space.
3.2.1. Spectral Indices
Spectral indices are like mathematical recipes, combining information from multiple bands to highlight specific terrestrial feature. The Normalised Difference Vegetation Index (NDVI) [
88] is a popular example, acting like a neon sign for areas with high chlorophyll content, which is a telltale sign of algal blooms. Other indices, like the Forel–Ule Index (FUI), Normalised Difference MODIS Algae Index (NDMAI) [
89] and the Coloured Dissolved Organic Matter (CDOM) Index, target specific pollutants or water quality issues.
Researchers have used various spectral indices for discriminating algae from the water in the aquatic ecosystems, viz., the Normalised Difference Vegetation Index (NDVI) [
88], Leaf Area Index (LAI) [
90], Normalised Difference Chlorophyll Index (NDCI) [
91], Floating Algae Index (FAI) [
92], Multi Sensor Water Index (MSWI) [
93], Coloured Dissolved Organic Matter (CDOM) [
94], Adjusted Floating Algae Index (AFAI) [
83], Fluorescence Detection Index (FDI) [
95], Maximum Chlorophyll Index (MCI) [
96], and Algal Bloom Detection Index (ABDI) [
97] between the years 1973 and 2021. The entire list of such indices, successfully utilised in delineating the algal bloom and similar features over the water surface, is tabulated in
Table 5.
The most suitable spectral index for algal bloom detection depends on factors like the type of algae, satellite sensor characteristics, and environmental conditions. These indices, their combinations, and some additional data (e.g., water temperature and wind patterns) are often used for more accurate bloom identification.
3.2.2. Machine Learning
Recent advances in machine learning have provided powerful approaches for algal bloom detection and monitoring using multispectral satellite imagery. Among the most widely used methods are Support Vector Machines (SVMs), Random Forests (RFs), and deep learning architectures such as Long Short-Term Memory (LSTM) networks and Convolutional Neural Networks (CNNs) [
34,
98,
99,
100,
101].
Traditional classifiers like SVMs and RFs remain popular due to their efficiency, interpretability, and relatively low computational cost. They can identify the most relevant spectral bands, making them practical for large-scale satellite data analysis. However, their performance may decline under highly variable environmental conditions, and they often require careful parameter tuning.
In contrast, deep learning methods offer superior scalability and the ability to capture complex spatiotemporal patterns. LSTMs are particularly effective for time-series data, enabling both detection and forecasting of bloom dynamics. CNNs and other architectures (e.g., GANs, ResNets, Capsule Networks) excel at learning features automatically but demand larger datasets, higher computational resources, and often lack interpretability.
To provide a synthesis,
Table 6 summarizes key algorithms and their applications, advantages, and limitations.
3.3. Bio-Optical Algorithms
Bio-optical algorithms are fundamental tools for estimating biophysical parameters of water bodies using remote sensing reflectance data. These algorithms exploit the interaction of light with water constituents such as phytoplankton, coloured dissolved organic matter (CDOM), and suspended sediments to infer concentrations of chlorophyll-a and other indicators of algal biomass [
117].
There are two main categories of bio-optical algorithms: empirical and semi-analytical. Empirical algorithms, such as the Ocean Chlorophyll 4 (OC4) algorithm developed for SeaWiFS, establish statistical relationships between reflectance at specific wavelengths and chlorophyll-a concentrations [
118]. These are often simple to implement and provide acceptable accuracy in open ocean waters. However, they may not perform well in optically complex coastal and inland waters where multiple constituents influence the reflectance signal.
On the other hand, semi-analytical algorithms are based on radiative transfer theory and attempt to model the inherent optical properties (IOPs) of water components [
119]. These algorithms decompose the remote sensing reflectance into contributions from water itself, phytoplankton, non-algal particles, and CDOM, enabling a more accurate estimation of chlorophyll-a and other biogeochemical variables in Case 2 waters [
120].
The Quasi-Analytical Algorithm (QAA) remains a foundational semi-analytical method for assessing water quality parameters. Over the years, it has been tailored to work effectively across multiple satellite sensors and aquatic environments [
121]. Rather than relying on empirical relationships alone, QAA interprets the ratio of water-leaving reflectances to estimate key optical properties—namely, the total absorption and backscattering coefficients. These parameters form the basis for determining chlorophyll-a concentrations, a key indicator of algal presence.
In recent years, the emergence of machine learning-assisted bio-optical models with leverage ground-truth (in situ) measurements alongside satellite data have enabled more flexible and precise estimations [
122,
123] of algal bloom detection. Especially in regions with dense observational networks, such hybrid approaches adapt well to spatial and temporal variations in water constituents, offering notable advantages over traditional analytical techniques.
Bio-optical algorithms have been operationalised in various satellite missions, including MODIS, MERIS, Sentinel-3 OLCI, and VIIRS. For example, the OC3M algorithm is widely applied to MODIS data to estimate surface chlorophyll concentrations in oceanic and freshwater bodies [
124]. Sentinel-2 and Sentinel-3 platforms have enabled finer spatial resolution and extended applicability to small water bodies and reservoirs [
125].
3.4. Spatial Resolution, Georeferencing Accuracy, and Error Mitigation
To ensure reliable monitoring of harmful algal blooms (HABs), careful attention was given to the spatial resolution and georeferencing accuracy of the satellite data. Sentinel-2 MSI imagery with a spatial resolution of 10–20 m (depending on the band) and Sentinel-1 SAR data at 10 m resolution were co-registered prior to analysis to enable pixel-level comparability. Geometric corrections were applied using pre-processed Level-2A products (for MSI) and terrain-corrected GRD products (for SAR). When available, ground control points (GCPs) and shoreline reference data were used to validate the co-registration accuracy. The resulting root mean square error (RMSE) of image alignment was kept below one pixel to minimize spatial mismatches between datasets. This approach is consistent with geospatial best practices in hydrological monitoring studies [
126].
3.4.1. Correction for Wind-Driven Surface Waves and Glint Effects
Wind-driven surface waves can introduce distortions in both optical and radar observations. Capillary waves often increase surface reflectance, creating sunglint effects in multispectral data, while gravity waves can modify the backscatter response in SAR imagery. To mitigate these effects, Level-2A MSI products processed with Sen2Cor were employed, which perform atmospheric and surface reflectance correction. Additionally, median compositing was used across multiple acquisitions within a short time window to minimize outliers caused by transient surface roughness. For SAR data, multi-look filtering and speckle reduction techniques were applied to smooth high-frequency noise. Where available, wind speed data from ECMWF ERA5 reanalysis were used to interpret anomalous radar backscatter patterns and exclude wind-contaminated scenes from analysis.
3.4.2. Incorporation of NDWI for Water Body Enhancement
To improve water body delineation, the Normalised Difference Water Index (NDWI) was incorporated into the processing chain. The NDWI is calculated as
where
and
represent reflectance in the green and near-infrared bands, respectively. The NDWI enhances water features by maximizing the contrast between water and vegetation/soil, making it particularly effective in coastal or turbid regions. The NDWI was combined with indices such as NDCI and FAI to improve classification performance and reduce the misclassification of land–water boundaries. The combined approach results in more robust detection of bloom-affected water pixels under optically complex conditions.
3.4.3. Continental Distribution of Studies
To provide a comprehensive geographical context, a summary table (
Table 7) has been added to organise the reviewed studies by continent, study area, sensor type, methods used, and key findings. This highlights regional research strengths and gaps, supporting future research prioritisation.
5. Challenges and Limitations
While remote sensing technologie like MSI and SAR have revolutionised algal bloom monitoring, they also have certain constraints. This section outlines the key challenges that impact data reliability, accuracy, and large-scale operational deployment.
5.1. Atmospheric and Water Column Effects
Atmospheric interference is a primary concern when working with MSI data. Scattering and absorption by atmospheric constituents such as aerosols, thin cloud layers, and water vapour can distort the top-of-atmosphere radiance, leading to errors in retrieving water-leaving reflectance [
21,
141]. Cloud cover limits the number of usable images and introduces gaps in time-series data, hampering early detection and forecasting systems in regions with persistent cloudiness, such as tropical and coastal monsoon areas [
20].
In optically complex waters, the reflectance from algal pigments is often masked by signals from suspended sediments, coloured dissolved organic matter (CDOM), and variable bottom reflectance in shallow waters [
142,
143]. Such interferences reduce the reliability of chlorophyll-a or phycocyanin retrievals and increase the risk of false positives or underestimations.
Moreover, bathymetry and turbidity influence the light penetration depth, which alters reflectance values detected by satellite sensors. For example, shallow regions with sandy or rocky bottoms may be misinterpreted as bloom-affected areas due to elevated reflectance in green and red bands.
5.2. SAR Limitations
SAR sensors provide significant advantages by offering all-weather, day-and-night imaging capabilities and detect variations in surface roughness rather than direct spectral signatures of algal pigments. The presence of surface slicks caused by blooms suppressing capillary waves can manifest itself as dark patches in SAR imagery [
29]. Unfortunately, these features are non-specific and may also arise from other sources such as oil spills, floating debris, calm wind zones, or even aquaculture activity [
144].
SAR observations are influenced by prevailing wind conditions; calm winds diminishes the surface roughness and can mimic the radar signature typically associated with algal slicks, potentially leading to false positives, whereas strong winds may disperse or obscure the surface features related to blooms, making them undetectable in SAR imagery. Due to this dependence on wind, SAR data are therefore best used in conjunction with optical imagery or meteorological datasets to ensure accurate interpretation [
145].
5.3. Validation Issues
The validation of remote sensing-derived bloom products relies heavily on in situ observations of chlorophyll a, phycocyanin, turbidity, temperature, and nutrient concentrations. However, these ground truth data are often limited in temporal and spatial extent due to financial, logistical, and operational constraints [
20]. Consistent water quality monitoring programs are either absent or poorly maintained in many developing regions and inland systems. This limitation is significant for ML and bio-optical algorithms that require robust training datasets. Model calibration becomes sensor- or region-specific without sufficient field data, leading to poor generalisability [
146]. The time mismatch between satellite overpasses and field sampling can also introduce temporal discrepancies in dynamic aquatic environments where bloom conditions change within hours or days.
Efforts such as the Global HAB Observation Network (GlobalHAB) and regional initiatives like NOAA’s NCCOS in the U.S. or the Indian National Centre for Ocean Information Services (INCOIS) are improving the availability of ground-based observations. Still, their integration with satellite data remains a work in progress, and there is a strong need for harmonised protocols for validation across spatial and temporal scales.
6. Future Perspectives
6.1. Advancements in Sensor Technologies
The future of algal bloom monitoring is closely tied to the advancement of satellite missions and innovations in sensor technology, especially in hyperspectral imaging [
147]. Forthcoming spaceborne missions such as EnMAP (Environmental Mapping and Analysis Program, Germany) and PRISMA (PRecursore IperSpettrale della Missione Applicativa, Italy) are expected to deliver high spectral resolution data across the visible to shortwave infrared spectrum. These data will facilitate more accurate identification of algal pigments, suspended particles, and dissolved organic matter [
148,
149].
Unlike traditional multispectral sensors, these hyperspectral platforms are capable of detecting subtle spectral variations in phytoplankton groups [
25], enabling earlier and more precise detection of harmful algal blooms (HABs), particularly in optically complex water bodies such as estuaries and eutrophic lakes [
150,
151].
Further improvements in spatial, spectral, and temporal resolutions—exemplified by sensor constellations like PlanetScope and the upcoming Landsat Next—will support high-frequency, localised bloom monitoring [
152,
153]. These advancements will aid in the development of early warning systems and dynamic response mechanisms at regional and watershed scales.
6.2. AI and Deep Learning Applications
Artificial intelligence (AI) and deep learning techniques are revolutionizing the way algal blooms are detected and predicted from satellite data. Algorithms such as Convolutional Neural Networks (CNNs), Random Forests, and Long Short-Term Memory (LSTM) networks have demonstrated considerable promise in classifying satellite imagery and identifying bloom events by recognizing complex patterns within large, multidimensional datasets.
Machine learning models have been successfully applied to identify proxy indicators of blooms and outperform conventional threshold-based approaches. In particular, hybrid approaches that merge remote sensing inputs with meteorological and hydrological parameters—such as rainfall, temperature, and nutrient runoff—have shown marked improvements in spatiotemporal bloom prediction capabilities.
Cloud-based geospatial platforms like Google Earth Engine have further democratized access to satellite imagery and advanced analytics, enabling research institutions and agencies of varying capacities to develop and deploy machine learning models for local and regional water quality monitoring initiatives.
6.3. Policy Integration and Decision Support
Translating technological advancements into societal benefits requires the integration of satellite-derived insights into environmental policy and decision-making frameworks. Several countries, including the United States and members of the European Union, have initiated efforts to incorporate satellite observations into water quality monitoring, early warning systems, and emergency response strategies.
Operational systems such as the Environmental Sample Processor (ESP) in Lake Erie and the GOCI-based HAB monitoring program along the Korean Peninsula exemplify how remote sensing can be aligned with public health advisories and ecosystem management policies.
Moreover, decision-support tools are being developed to link satellite-based bloom forecasts with agriculture, fisheries, tourism, and water resource planning. Integrating predictive models with near-real-time data feeds from autonomous sensing platforms such as buoys and autonomous underwater vehicles (AUVs) will significantly enhance alert systems for government authorities, public health agencies, and the general population.
Continued collaboration between scientific communities, space agencies (e.g., NOAA, ESA, NASA, ISRO), local governments, and citizen scientists remains essential for transforming satellite observations into actionable knowledge that supports sustainable environmental policy.
7. Conclusions
This review shows that combining MSI and SAR sensors creates a stronger approach to monitoring harmful algal blooms (HABs). MSI can pick up key pigments such as chlorophyll-a and phycocyanin, making it highly effective for identifying bloom composition. At the same time, SAR fills in the gaps by providing round-the-clock coverage, even under cloud cover. It offers a more precise and more dependable view of bloom dynamics.
Progress in bio-optical algorithms and indices like NDVI, NDCI, FAI, AFAI, and NDMAI—together with machine learning techniques—has led to better bloom mapping and biomass estimation, including in challenging, optically complex waters. The combined use of MSI and SAR data, paired with AI models, allows researchers to observe blooms at finer spatial and temporal scales than before.
Case studies from Lake Erie, Vembanad Lake, and the Korean coast highlight how these technologies are being applied in practice, helping to track seasonal trends, issue early warnings, and guide water management strategies. Platforms such as GOCI and ESP are proving that satellite observations can directly support decision making and public health actions. Despite these advances, key hurdles remain: atmospheric effects can obscure observations, SAR data can be tricky to interpret, and there is still a shortage of in situ validation data. Tackling these challenges will require improved algorithms, better standardisation, and more field measurements.
Future hyperspectral missions such as EnMAP and PRISMA, AI-based analytics, and cloud processing promise faster, near-real-time bloom detection. Turning these advances into actionable solutions will depend on close collaboration among scientists, space agencies, policymakers, and local communities to protect ecosystems, fisheries, and public health.