Reservoir and Riverine Sources of Cyanotoxins in Oregon’s Cascade Range Rivers Tapped for Drinking Water Supply
Abstract
1. Introduction
2. Study Background
- (1)
- identify the planktonic and benthic cyanobacteria present and measure concentrations of four cyanotoxins from concentrated “grab” samples of cyanobacteria and plankton net tows;
- (2)
- examine the occurrence of cyanotoxins at or near DWTPs in the dissolved phase using passive samplers; and
- (3)
- characterize the spatiotemporal occurrence and transport of cyanotoxins using multiple lines of evidence from the three methods to identify upstream sources within each of three intensively studied reservoir-river systems.
3. Field and Laboratory Methods
Sample Collection, Processing, and Analyses
4. Results
4.1. Cyanotoxin Occurrence
4.2. Sources of Cyanotoxins at Three Water Supply Intakes
5. Discussion
5.1. Cyanobacteria and Cyanotoxin Production in the Cascade Range
5.2. Potential Impacts of Cyanotoxins on Recreation and Drinking Water
6. Detailed Assessment of Three River Basins
6.1. Clackamas River Basin
6.2. North Santiam River Basin
6.3. McKenzie River Basin
7. Study Limitations
8. Major Findings and Next Steps
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holcomb, B.; Stanton, B.; Baysinger, C.; Carpenter, K.D. Strategies for Preventing and Managing Harmful Benthic Cyanobacterial Blooms (CHB-2); Interstate Technology & Regulatory Council: Washington, DC, USA, 2022; Available online: https://hcb-2.itrcweb.org/ (accessed on 27 December 2024).
- U.S. Environmental Protection Agency. Analyzing and Assessing Benthic Harmful Algal Blooms. 2023. Available online: https://www.epa.gov/system/files/documents/2022-12/Benthic%20HCB%20Fact%20Sheet_Final_12.21.22.pdf (accessed on 20 January 2023).
- Quiblier, C.; Wood, S.A.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.F. A review of current knowledge on toxic benthic freshwater cyanobacteria–ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Smith, R.B.; Bass, B.; Sawyer, D.; Depew, D.; Watson, S.B. Estimating the economic costs of algal blooms in the Canadian Lake Erie Basin. Harmful Algae 2019, 87, 101624. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; World Health Organization: London, UK, 1999. [Google Scholar]
- Wood, S.A.; Kelly, L.T.; Bouma-Gregson, K.; Humbert, J.; Laughinghouse, H.D., IV; Lazorchak, J.; McAllister, T.G.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic benthic freshwater cyanobacterial proliferations—Challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.; Wood, S.; Hawes, I. The rise of toxic benthic Phormidium proliferations—A review of their taxonomy, distribution, toxin content and factors regulating prevalence and increased severity. Harmful Algae 2016, 55, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Summary Report—One Health Harmful Algal Bloom System (OHHABS), United States, 2021. Available online: https://www.cdc.gov/ohhabs/data/summary-report-united-states-2021.html?CDC_AAref_Val=https://www.cdc.gov/habs/data/2021-ohhabs-data-summary.html (accessed on 27 December 2024).
- Carmichael, W.W.; Azevedo, S.M.F.O.; Ji, S.A.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria—Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Oregon Health Authority. Public Health Advisory Guidelines for Harmful Algae Blooms in Freshwater Bodies. 2018. Available online: https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/RECREATION/HARMFULALGAEBLOOMS/Documents/Advisory%20Guidelines%20for%20Harmful%20Cyanobacteria%20Blooms%20in%20Recreational%20Waters.2024_Final.pdf (accessed on 27 December 2024).
- U.S. Environmental Protection Agency. Drinking Water Health Advisories for Two Cyanobacterial Toxins, EPA Office of Water Fact Sheet 820F15003. 2015. Available online: https://www.epa.gov/sites/default/files/2017-06/documents/cyanotoxins-fact_sheet-2015.pdf (accessed on 27 December 2024).
- U.S. Environmental Protection Agency. Fact Sheet: Fifth Contaminant Candidate List (CCL 5), EPA 815-F-22-005. 2022. Available online: https://www.epa.gov/ccl/contaminant-candidate-list-5-ccl-5 (accessed on 27 December 2024).
- Stancheva, R.; Brown, S.; Boyer, G.L.; Wei, B.; Goel, R.; Henry, S.; Kristan, N.V.; Read, B. Effect of salinity stress and nitrogen depletion on growth, morphology and toxin production of freshwater cyanobacterium Microcoleus anatoxicus. Hydrobiologia 2025, 852, 561–574. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. (Eds.) Toxic Cyanobacteria in Water, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; 858p. [Google Scholar]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Oregon Health Authority. HABs Website. 2023. Available online: http://public.health.oregon.gov/HealthyEnvironments/Recreation/HarmfulAlgaeBlooms/Pages/index.aspx (accessed on 27 December 2024).
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.K.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Kudela, R.M.; Power, M.E. Widespread anatoxin-a detection in benthic cyanobacterial mats throughout a river network. PLoS ONE 2018, 13, e0197669. [Google Scholar] [CrossRef]
- Fadness, R.; Thomas, M.; Bouma-Gregson, K.; van Dyke, M. Benthic cyanobacteria and cyanotoxin monitoring in northern California rivers, 2016–2019. In Freshwater Harmful Algal Bloom Monitoring and Response Program Report SWAMP-MR-RB1-2022-0001; North Coast Regional Water Quality Control Board: Santa Rosa, CA, USA, 2022; 132p. [Google Scholar]
- Genzoli, L.; Hall, R.O.; Otten, T.G.; Johnson, G.; Blaszczak, J.R.; Kann, J. Benthic Cyanobacteria Proliferations Drive Anatoxin Production throughout the Klamath River Watershed, California. Freshw. Sci. 2024, 43, 307–324. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms—Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.; Fastner, J.; Bartha-Dima, B.; Breuer, W.; Falkenau, A.; Mayer, C.; Raeder, U. Mass occurrence of anatoxin-a- and dihydroanatoxin-a-producing Tychonema sp. in mesotrophic Reservoir Mandichosee (River Lech, Germany) as a cause of neurotoxicosis in dogs. Toxins 2020, 12, 726. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids—Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Gad, S.E. Saxitoxin. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 218–220. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Bothwell, M.L.; Lowe, R.L. Algal Ecology—Freshwater Benthic Ecosystems; Academic Press: San Diego, CA, USA, 1996; 753p. [Google Scholar]
- Wood, S.A.; Heath, M.W.; Holland, P.T.; Munday, R.; McGregor, G.B.; Ryan, K.G. Identification of a benthic microcystin-producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.O.C.; Landsberg, J.H.; Miller, M.; Keel, K.; Taylor, T.K. Canine cyanotoxin poisonings in the United States (1920s–2012)—Review of suspected and confirmed cases from three data sources. Toxins 2013, 5, 1597–1628. [Google Scholar] [CrossRef] [PubMed]
- Oregon Health Authority. Current Cyanobacteria Advisories. 2025. Available online: https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/RECREATION/HARMFULALGAEBLOOMS/Pages/Blue-GreenAlgaeAdvisories.aspx (accessed on 2 April 2025).
- National Park Service. Toxic Cyanobacteria Bloom in the Virgin River and the Streams of Zion National Park, NPS Web Page. 2022. Available online: https://www.nps.gov/zion/planyourvisit/toxic-cyanobacteria-bloom-in-the-virgin-river-and-the-streams-of-zion-national-park.htm (accessed on 27 December 2024).
- National Public Radio. More Dogs Dead from Exposure to Toxic Algae in the Columbia River. 2021. Available online: https://www.kuow.org/stories/more-dogs-dead-from-exposure-to-toxic-algae-in-the-columbia-river (accessed on 27 December 2024).
- Department of Ecology (DOE) for Washington State. Northwest Toxic Algae Database. 2023. Available online: https://www.nwtoxicalgae.org/ (accessed on 27 December 2024).
- Fetscher, A.E.; Howard, M.D.A.; Stancheva, R.; Kudela, R.M.; Stein, E.D.; Sutula, M.A.; Busse, L.B.; Sheath, R.G. Wadeable streams as widespread sources of benthic cyanotoxins in California, USA. Harmful Algae 2015, 49, 105–116. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Power, M.E.; Bormans, M. Rise and fall of toxic benthic freshwater cyanobacteria (Anabaena spp.) in the Eel River—Buoyancy and dispersal. Harmful Algae 2017, 66, 79–87. [Google Scholar] [CrossRef]
- Fadness, R.; Thomas, M.; Bouma-Gregson, K. Cyanotoxin Monitoring with SPATT Passive Samplers in Northern California Rivers, 2019: Freshwater Harmful Algal Bloom Monitoring and Response Program Report SWAMP-MR-RB1-2022-0002; California North Coast Regional Water Quality Control Board: Santa Rosa, CA, USA, 2022; 68p. [Google Scholar]
- Kelly, L.T.; Bouma-Gregson, K.; Puddick, J.; Fadness, R.; Ryan, K.G.; Davis, T.W.; Wood, S.A. Multiple cyanotoxin congeners produced by sub-dominant cyanobacterial taxa in riverine cyanobacterial and algal mats. PLoS ONE 2019, 14, e0220422. [Google Scholar] [CrossRef]
- Loftin, K.A.; Clark, J.M.; Journey, C.A.; Kolpin, D.W.; Van Metre, P.C.; Bradley, P.M. Spatial and temporal variation in microsystins occurrence in wadeable streams in the southeastern USA. Environ. Toxicol. Chem. 2016, 35, 2281–2287. [Google Scholar] [CrossRef]
- Rosen, B.H.; MacLeod, B.W.; Simpson, M.R. Accumulation and release of geosmin during the growth phases of Anabaena circinalis. Water Science Technol. 1992, 25, 185–190. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. Endangered and Threatened Wildlife; Final Rule to Revise the Code of Federal Regulations for Species Under the Jurisdiction of the National Marine Fisheries Service, 50 CFR Parts 223 and 224. 2024. Available online: https://www.federalregister.gov/documents/2014/04/14/2014-08347/endangered-and-threatened-wildlife-final-rule-to-revise-the-code-of-federal-regulations-for-species (accessed on 20 April 2025).
- Sweet, J.W. A Survey and Ecological Analysis of Oregon and Idaho Phytoplankton; Final Report (Variously Paged); U.S. Environmental Protection Agency: Seattle, WA, USA, 1986.
- Eilers, J.M.; Cramer, D.P. Cyanobacterial Investigations for Timothy Lake and North Fork Reservoir, Oregon 2013; Portland General Electric: Portland, OR, USA, 2014; 66p. [Google Scholar]
- Carpenter, K.D. Water-quality and algal conditions in the Clackamas River basin, Oregon, and their relations to land and water management. In Water-Resources Investigations Report 02-4189; U.S. Geological Survey: Reston, VA, USA, 2003; 114p. Available online: https://pubs.usgs.gov/wri/WRI02-4189/ (accessed on 27 December 2024).
- Eilers, J.M.; Loomis, D.; Amand, A.S.; Vogel, A.; Jackson, L.; Kann, J.; Eilers, B.; Truemper, H.; Cornett, J.; Sweets, R. Biological effects of repeated fish introductions in a formerly fishless lake: Diamond Lake, USA. Fundam. Appl. Limnol. 2007, 169, 265–277. [Google Scholar] [CrossRef]
- Youchul, J.; Struewing, I.; Clauson, K.; Reetz, N.; Fairchild, N.; Goeres-Priest, L.; Carpenter, K.D.; Labiosa, R.; Villegas, E.; Lu, J. Dominant Dolichospermum and microcystin production in Detroit Lake (Oregon, USA). Harmful Algae 2025, 142, 102802. [Google Scholar]
- Dreher, T.W.; Davis, E.W.; Mueller, R.S.; Otten, T.G. Comparative genomics of the ADA clade within the Nostocales. Harmful Algae 2021, 104, 102037. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W.; Davis, E.W.; Mueller, R.S. Complete genomes derived by directly sequencing freshwater bloom populations emphasize the significance of the genus level ADA clade within the Nostocales. Harmful Algae 2021, 103, 102005. [Google Scholar] [CrossRef]
- Dreher, T.W.; Foss, A.J.; Davis, E.W.; Mueller, R.S. 7-epi-cylindrospermopsin and microcystin producers among diverse Anabaena/Dolichospermum/Aphanizomenon CyanoHABs in Oregon, USA. Harmful Algae 2022, 116, 102241. [Google Scholar]
- Portland General Electric. Cyanotoxin Monitoring Data for Timothy Lake and North Fork Reservoir, Report Submitted to the Clackamas River Water Providers; Clackamas River Water Providers: Oregon City, OR, USA, 2015. [Google Scholar]
- Oregon Health Authority. OHA Drinking Water Advisory. 2018. Available online: https://yourwater.oregon.gov/advisorydetails.php?ISN=371 (accessed on 27 December 2024).
- Marion County. Drinking Water Advisory Issued to City of Salem, City of Turner, Suburban East Salem, Water District, and Orchard Heights Water Association 2018. Available online: https://www.co.marion.or.us/PW/EmergencyManagement/PublishingImages/Pages/default/CityofSalem_drinking_water_advisory_cyanotoxins_2018May418pm.pdf (accessed on 27 December 2024).
- Oregon Health Authority. Oregon Cyanobacteria Harmful Algae Bloom Surveillance Program—Advisory Guidelines for Cyanobacteria Blooms in Recreational Waters; Oregon Health Authority: Salem, OR, USA, 2021; 25p. Available online: https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/RECREATION/HARMFULALGAEBLOOMS/Documents/Advisory%20Guidelines%20for%20Harmful%20Cyanobacteria%20Blooms%20in%20Recreational%20Waters.pdf (accessed on 2 April 2025).
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of Cylindrospermopsin, the Toxin from the Cyanobacterium, Cylindrospermopsis raciborskii—Effect of pH, Temperature, and Sunlight on Decomposition. Environ. Toxicol. 1999, 14, 155–161. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Summary of Cyanotoxins Treatment in Drinking Water. 2024. Available online: https://www.epa.gov/ground-water-and-drinking-water/summary-cyanotoxins-treatment-drinking-water (accessed on 27 December 2024).
- Carpenter, K.D.; Kraus, T.E.C.; Goldman, J.H.; Saraceno, J.F.; Downing, B.D.; McGhee, G.; Triplett, T. Sources and characteristics of organic matter in the Clackamas River, Oregon, related to the formation of disinfection by-products in treated drinking water. In Scientific Investigations Report 2013–5001; U.S. Geological Survey: Reston, VA, USA, 2013; 78p. Available online: https://pubs.usgs.gov/sir/2013/5001/ (accessed on 27 December 2024).
- Carpenter, K.D.; Waite, I.R. Relations of algal assemblages to land use and water chemistry in the Willamette Basin, Oregon. Environ. Monit. Assess. 2000, 64, 247–257. [Google Scholar]
- Carpenter, K.D.; Wise, D.R. Cyanotoxin concentrations in extracts from cyanobacteria colonies, plankton net tows, and Solid-Phase Adsorption Toxin Tracking (SPATT) samplers in western rivers, lakes, and reservoirs, including drinking water sources in the Oregon Cascades: 2016–2020. In Science Base Data Release; U.S. Geological Survey: Reston, VA, USA, 2023. [Google Scholar] [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar]
- Lane, J.Q.; Roddam, M.; Langlos, G.W.; Kudela, R. Application of Solid Phase Adsorption Toxin Tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California. Limnol. Oceanogr. Methods 2010, 8, 645–660. [Google Scholar] [CrossRef]
- Kudela, R. Characterization and deployment of Solid Phase Adsorption Toxin Tracking (SPATT) resin for monitoring of microcystins in fresh and saltwater. Harmful Algae 2011, 11, 117–125. [Google Scholar] [CrossRef]
- Howard, M.D.A.; Hayashi, K.; Smith, J.; Kudela, R.; Caron, D. Standard Operating Procedure for Solid Phase Adsorption Toxin Testing (SPATT) Assemblage and Extraction of HAB Toxins; University of California: Oakland, CA, USA; University of Southern California: Los Angeles, CA, USA, 2019; 14p. [Google Scholar] [CrossRef]
- Roue, M.; Darius, H.T.; Chinain, M. Solid Phase Adsorption Toxin Tracking (SPATT), Technology for the monitoring of aquatic toxins—A review. J. Toxins 2018, 10, 167. [Google Scholar] [CrossRef]
- Gold Standard Diagnostics. ABRAXIS® Anatoxin-a ELISA Microtiter Plate User Guide. 2021. Available online: https://www.goldstandarddiagnostics.us/media/15640/ug-21-057-rev-03-abraxis-anatoxin-a-elisa_520060.pdf (accessed on 27 December 2024).
- Gold Standard Diagnostics. ABRAXIS® Cylindrospermopsin ELISA (Microtiter Plate) User Guide. 2021. Available online: https://www.goldstandarddiagnostics.us/media/16556/ug-21-059-rev-02-abraxis-cylindrospermopsin-elisa_522011.pdf (accessed on 27 December 2024).
- Gold Standard Diagnostics. ABRAXIS® Microcystin-ADDA ELISA Microtiter Plate User Guide. 2021. Available online: https://www.goldstandarddiagnostics.us/media/15635/ug-21-052-rev-01-abraxis-microcystins-adda-elisa_520011.pdf (accessed on 27 December 2024).
- Gold Standard Diagnostics. ABRAXIS® Saxitoxin (PSP) ELISA Microtiter Plate User Guide. 2021. Available online: https://www.goldstandarddiagnostics.us/media/15661/ug-21-081-rev-03-abraxis-saxitoxin-elisa_52255b.pdf (accessed on 27 December 2024).
- Bernard, C.; Ballot, A.; Thomazeau, S.; Maloufi, S.; Furey, A.; Mankiewicz-Boczek, J.; Pawlik-Skowrońska, B.; Capelli, C.; Salmaso, N. Appendix 2: Cyanobacteria Associated with the Production of Cyanotoxins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 501–525. [Google Scholar] [CrossRef]
- Chapman, A.; Foss, A. Greenwater Labs Potentially Toxigenic (PTOX) Cyanobacteria List; GreenWater Laboratories: Palatka, FL, USA, 2020. [Google Scholar]
- Belykh, O.; Tikhonova, I.; Kuzmin, A.V.; Sorokovikova, E.G.; Fedorova, G.A.; Khanaev, I.V.; Sherbakova, T.A.; Timoshkin, O.A. First detection of benthic cyanobacteria in Lake Baikal producing paralytic shellfish toxins. Toxicon 2016, 121, 36–40. [Google Scholar]
- Oregon Health Authority. Oregon Public Health—Drinking Water Data Online. 2024. Available online: https://yourwater.oregon.gov/ (accessed on 27 December 2024).
- Johnson, H. Natural Phosphorus Sources for the Pacific Northwest; U.S. Geological Survey Science: Reston, VA, USA, 2011. [CrossRef]
- Jansen, L.; Sobota, D.; Pan, Y.; Strecker, A.L. Watershed, lake, and food web factors influence diazotrophic cyanobacteria in mountain lakes. Limnol. Oceanogr. 2024, 69, 681–699. [Google Scholar] [CrossRef]
- Piper, A.M. Ground-water resources of the Willamette Valley, Oregon. In Water-Supply Paper 890; U.S. Geological Survey: Reston, VA, USA, 1942; 194p. [Google Scholar]
- U.S. Geological Survey. National Water Information System (NWIS). 2024. Available online: https://waterdata.usgs.gov/nwis/uv?site_no=14211010 (accessed on 2 April 2025).
- Triska, F.J.; Sedell, J.R.; Cromack, K., Jr.; Gregory, S.V.; McCorison, F.M. Nitrogen budget for a small coniferous forest stream. Ecol. Monogr. 1984, 54, 119–140. [Google Scholar] [CrossRef]
- Gregory, S.V. Willamette River Basin Study—Periphyton algal dynamics. In Final Report Submitted to the Oregon Department of Environmental Quality; Oregon Department of Environmental Quality: Portland, OR, USA, 1993; 112p. [Google Scholar]
- McHugh, R.A. An Interim Study of Some Physical, Chemical, and Biological Properties of Selected Oregon Lakes; Oregon Department of Environmental Quality: Portland, OR, USA, 1972; 109p.
- Oregon Health Authority. Oregon Public Health—HAB Advisory Archive. 2024. Available online: https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/RECREATION/HARMFULALGAEBLOOMS/Pages/archive.aspx (accessed on 27 December 2024).
- Jones, M.; Eilers, J.; Kann, J. Water quality effects of blue-green algal blooms in Diamond Lake, Oregon. In Advancing the Fundamental Sciences, Proceedings of the Forest Service National Earth Sciences Conference, San Diego, CA, USA, 18–22 October 2004; U.S. Forest Service, Pacific Northwest Research Station: Portland, OR, USA,, 2004; pp. 102–110. [Google Scholar]
- Palacio, H.M.; Palacio, J.A.; Echenique, R.O.; Sant’Anna, C.L.; Ramirez, J. Dolichospermum lemmermannii (Cyanobacteria)—A temperate species in a neotropical, eutrophic reservoir. Bol. Soc. Argent. Bot. 2015, 50, 309–321. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ogawa, K.; Kimura, Y.; Murata, H.; Suzuki, M.; Thorn, P.M.; William, R.; Evans, W.R.; Carmichael, W.W. Microcystins from Anabaena flos-aquae NRC 525-17. Chem. Res. Toxicol. 1991, 4, 535–540. [Google Scholar] [CrossRef]
- Fogg, G.E.; Stewart, W.D.P.; Fay, P.; Walsby, A.E. The Blue-Green Algae; Academic Press, Inc.: New York, NY, USA, 1973; 249p. [Google Scholar]
- Walsby, A. Gas vesicles. Microbiol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: New York, NY, USA, 2006; 535p. [Google Scholar]
- Dyer, S.W.; Needoba, J.A. Use of high-resolution pressure nephelometry to measure gas vesicle collapse as a means of determining growth and turgor changes in planktonic cyanobacteria. Appl. Environ. Microbiol. 2020, 86, e01790-19. [Google Scholar] [CrossRef]
- Wehr, J.D.; Descy, J.P. Use of phytoplankton in large river management. J. Phycol. 1998, 34, 741–749. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology, 2nd ed.; Saunders College Publishing: Philadelphia, PA, USA, 1983; 858p. [Google Scholar]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- U.S. Geological Survey. National Water Information System (NWIS). 2024. Available online: https://or.water.usgs.gov/projs_dir/habs/lakeprofiler.html?site=444306122144600&numberOfDays=879 (accessed on 2 April 2025).
- Graham, J.L.; Ziegler, A.C.; Loving, B.L.; Loftin, K.A. Fate and transport of cyanobacteria and associated toxins and taste-and-odor compounds from upstream reservoir releases in the Kansas River, Kansas, September and October 2011. In Scientific Investigations Report 2012–5129; U.S. Geological Survey: Reston, VA, USA, 2012; 65p. Available online: https://pubs.usgs.gov/sir/2012/5129/ (accessed on 27 December 2024).
- Rounds, S.A.; Carpenter, K.D.; Fesler, K.J.; and Dorsey, J.L. Upstream factors affecting Tualatin River algae—Tracking the 2008 Anabaena algae bloom to Wapato Lake, Oregon. In Scientific Investigations Report 2015–5178; U.S. Geological Survey: Reston, VA, USA, 2015; 41p. [Google Scholar] [CrossRef]
- Anderson, C.W.; Carpenter, K.D. Water Quality and Algal Conditions in the North Umpqua River Basin, Oregon, 1992–95, and Implications for Resource Management; Water-Resources Investigations Report 98–4125; U.S. Geological Survey: Reston, VA, USA, 1998; p. 78. Available online: https://pubs.usgs.gov/publication/wri984125 (accessed on 27 December 2024).
- Ward, A.K.; Dahm, C.N.; Cummins, K.W. Nostoc (Cyanophyta) productivity in Oregon stream ecosystems—Invertebrate influences and differences between morphological types. J. Phycol. 1985, 21, 223–227. [Google Scholar] [CrossRef]
- Robichon, C.; Robin, J.; Dolédec, S. Relative effect of hydraulics, physico-chemistry, and other biofilm algae on benthic cyanobacteria assemblages in a regulated river. Sci. Total Environ. 2023, 872, 162142. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.J.F. Patterns in Benthic Algae in Streams, in Stevenson. In Algal Ecology—Freshwater Benthic Ecosystems: San Diego, California; Bothwell, J.R., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1996; pp. 31–56. [Google Scholar]
- Oregon Veterinary Medical Association, Toxic Algae Advisories Web Site. Available online: https://www.oregonvma.org/toxic-algae-advisories (accessed on 27 December 2024).
- U.S. Environmental Protection Agency. Cyanobacterial Harmful Algal Blooms (CyanoHABs) in Water Bodies. 2024. Available online: https://www.epa.gov/cyanohabs (accessed on 27 December 2024).
- D’Anglada, L.V.; Donohue, J.W.; Strong, J.; Hawkins, B. Health Effects Support Document for the Cyanobacterial Toxin Microcystins; U.S. Environmental Protection Agency: Washington, DC, USA, 2015. Available online: https://www.epa.gov/sites/default/files/2017-06/documents/microcystins-support-report-2015.pdf (accessed on 27 December 2024).
- Oregon Department of Environmental Quality. Ambient Water Quality Monitoring System. 2024. Available online: https://www.oregon.gov/deq/wq/pages/wqdata.aspx (accessed on 18 February 2025).
- Carpenter, K.D.; Sobieszczyk, S.; Arnsberg, A.J.; Rinella, F.A. Pesticide occurrence and distribution in the lower Clackamas River basin, Oregon, 2000–2005. In Scientific Investigations Report 2008–5027; U.S. Geological Survey: Reston, VA, USA, 2008; 98p. Available online: https://pubs.usgs.gov/sir/2008/5027/pdf/sir20085027.pdf (accessed on 27 December 2024).
- Carpenter, K.D.; McGhee, G. Organic compounds in Clackamas River water used for public supply near Portland, Oregon, 2003–2005. In Fact Sheet 2009–3030; U.S. Geological Survey: Reston, VA, USA, 2009; 6p. Available online: https://pubs.usgs.gov/fs/2009/3030/ (accessed on 27 December 2024).
- Kelly, V.J.; Anderson, C.W.; Morgenstern, K. Reconnaissance of land-use sources of pesticides in drinking water, McKenzie River, Oregon. In Scientific Investigations Report 2012-5091; U.S. Geological Survey: Reston, VA, USA, 2012; 46p. Available online: https://pubs.usgs.gov/sir/2012/5091/pdf/sir20125091.pdf (accessed on 27 December 2024).
- Bradley, P.M.; Kolpin, D.W.; Romanok, K.M.; Smalling, K.L.; Focazio, M.J.; Brown, J.B.; Cardon, M.C.; Carpenter, K.D.; Corsi, S.R.; DeCicco, L.A.; et al. Reconnaissance of mixed organic and inorganic chemicals in private and public supply tapwaters at selected residential and workplace sites in the United States. Environ. Sci. Technol. 2018, 52, 13972–13985. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards—Impacts and implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at Low Doses Induce Apoptosis and Inflammatory Effects in Murine Brain Cells—Potential Implications for Neurodegenerative Diseases: Toxicology Reports, v. 3. 2016. Available online: https://www.scipedia.com/public/Takser_et_al_2016a (accessed on 27 December 2024).
- Anderson, B.; Voorhees, J.; Phillips, B.; Fadness, R.A.; Stancheva, R.; Nichols, J.; Orr, D.; Wood, S.A. Extracts from benthic anatoxin-producing Phormidium are toxic to 3 macroinvertebrate taxa at environmentally relevant concentrations. Environ. Toxicol. Chem. 2018, 37, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Catherine, A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Hoboken, NJ, USA, 2017; pp. 526–538. [Google Scholar]
- Jokela, J.; Heinilä, L.M.P.; Shishido, T.K.; Wahlsten, M.; Fewer, D.P.; Fiore, M.F.; Wang, H.; Haapaniemi, E.; Permi, P.; Sivonen, K. Production of high amounts of hepatotoxin nodularin and new protease inhibitors pseudospumigins by the Brazilian benthic Nostoc sp. CENA543. Front. Microbiol. 2017, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal. Bioanal. Chem. 2010, 397, 1705–1714. [Google Scholar] [CrossRef]
- Oregon Department of Environmental Quality. Harmful Algal Bloom (HAB) Strategy. 2011. Available online: https://www.oregon.gov/deq/wq/pages/harmful-algal-blooms.aspx (accessed on 27 December 2024).
- Carmichael, W.W.; Biggs, D.F.; Peterson, M.A. Pharmacology of anatoxin-a produced by the freshwater cyanophyte Anabaena flos-aquae NRC-44–1. Toxicon 1979, 17, 229–236. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health—A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 39515. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Health Effects Support Document for the Cyanobacterial Toxin Anatoxin-a: EPA-820R15104. 2015. Available online: https://www.epa.gov/sites/default/files/2017-06/documents/anatoxin-a-report-2015.pdf (accessed on 27 December 2024).
- Yang, X. Occurrence of the Cyanobacterial Neurotoxin, Anatoxin—A, in New York State Waters. Ph.D. Dissertation, State University of New York College of Environmental Science and Forestry, Syracuse, NY, USA, 2007; 230p. [Google Scholar]
- Strunecky, O.; Komarek, J.; Johansen, J.; Lukesova, A.; Elster, J. Molecular and morphological criteria for revision of the genus Microcoleus (Oscillatoriales, Cyanobacteria). J. Phycol. 2013, 49, 1167–1180. [Google Scholar] [CrossRef]
- Komarek, J.; Kastovsky, J.; Mares, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Wehr, J.D.; Sheath, R.G. Freshwater Algae of North America—Ecology and Classification; Academic Press: San Francisco, CA, USA, 2003; 918p. [Google Scholar]
- Conklin, K.Y.; Stancheva, R.; Otten, T.G.; Fadness, R.; Boyer, G.L.; Read, R.; Zhang, X.; Sheath, R.G. Molecular and morphological characterization of a novel dihydroanatoxin-a producing Microcoleus species (cyanobacteria) from the Russian River, California, USA. Harmful Algae 2020, 93, 101767. [Google Scholar] [CrossRef]
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P.; et al. Acute toxicity of dihydro-anatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a. Chemosphere 2021, 263, 127937. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.D.; Anderson, C.W.; Jones, M.E. Water quality and algal conditions in the North Umpqua River, Oregon, 1995–2007, and their response to Diamond Lake restoration. In Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2014; 87p. Available online: https://pubs.usgs.gov/of/2014/1098/pdf/ofr2014-1098.pdf (accessed on 2 April 2025).
- Asarian, J.E.; Pan, Y.; Gillett, N.D.; Kann, J. Spatial and Temporal Variation of Periphyton Assemblages in the Klamath River, 2004–2012: Report Prepared by Kier Associates, Portland State University, and Aquatic Ecosystem Sciences LLC for the Klamath Basin Tribal Water Quality Work Group; Kier Associates: San Rafael, CA, USA, 2014; 50p. [Google Scholar]
- Legleiter, C.J.; King, T.V.; Slonecker, T.; Hall, N.; Mumford, A.; Carpenter, K.D.; Simon, N.; and Rosen, B. Spectral mixture analysis for surveillance of harmful algal blooms (SMASH): A field-, laboratory-, and satellite-based approach to identifying algal genera from remotely sensed data. Remote Sens. Environ. 2022, 279, 113089. [Google Scholar] [CrossRef]
- Legleiter, C.J.; Stegman, T.K.; Overstreet, B.T. Spectrally based mapping of riverbed composition. Geomorphology 2016, 264, 61–79. [Google Scholar] [CrossRef]
- Hall, N.C.; Kishimoto, J.J.; Shtabnoy, A.; Legleiter, C.J.; King, T.V.; Mumford, A.C.; Spaulding, S.A.; Carpenter, K.D.; Slonecker, T. Hyperspectral Profiles of Harmful Algal Blooms (HABs) and Other Algae, 2022; U.S. Geological Survey: Reston, VA, USA, 2024. [CrossRef]
- Clark, G.D.; Murphy, S.F.; Skalak, K.; Clow, D.W.; Akie, G.; Carpenter, K.D.; Payne, S.E.; Ebel, B.E. Hysteretic response of suspended-sediment in wildfire affected watersheds of the Pacific Northwest and Southern Rocky Mountains. Earth Surf. Process. Landf. 2025, 50, e6067. [Google Scholar] [CrossRef]
- Swann, M.M.; Cortes, A.; Forrest, A.L.; Framsted, N.; Sadro, S.; Schladow, S.G.; De Palma-Dow, A. Internal phosphorus loading alters nutrient limitation and contributes to cyanobacterial blooms in a polymictic lake. Aquat. Sci. 2024, 86, 46. [Google Scholar] [CrossRef]
- U.S. Army Corps of Engineers. An Environmental Impact Statement on Operations and Maintenance of the Willamette Reservoir System; U.S. Army Engineer District, Portland, Corps of Engineers: Portland, OR, USA, 1980.
- U.S. Army Corps of Engineers. The Willamette Valley Project. 2019. Available online: https://usace.contentdm.oclc.org/digital/collection/p16021coll6/id/2151 (accessed on 27 December 2024).
- Paerl, H.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Carpenter, K.D. Indicators of Nutrient Limited Phytoplankton Growth in Lakes Near Mount Saint Helens, Washington. Master’s Thesis, Portland State University, Portland, OR, USA, 1995; 188p. [Google Scholar]
- Petersen, R.; Carpenter, K. Nutrient limitation in five lakes near Mount St. Helens, Washington. Proc. Int. Assoc. Theor. Appl. Limnol. 1997, 26, 377–380. [Google Scholar]
- U.S. National Weather Service. Average Annual Air Temperature. 2023. Available online: https://www.weather.gov/media/slc/ClimateBook/Annual%20Average%20Temperature%20By%20Year.pdf (accessed on 27 December 2024).
- Sobota, D. Oregon DEQ Freshwater Cyanobacteria Harmful Algal Blooms (CyanoHABs) Strategy. 2023. Available online: https://www.oregon.gov/deq/wq/Documents/habFwCyanobacHABstrat.pdf (accessed on 27 December 2024).
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-Based Cyanotoxin Guideline Values Allow for Cyanotoxin-Based Monitoring and Efficient Public Health Response to Cyanobacterial Blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef] [PubMed]
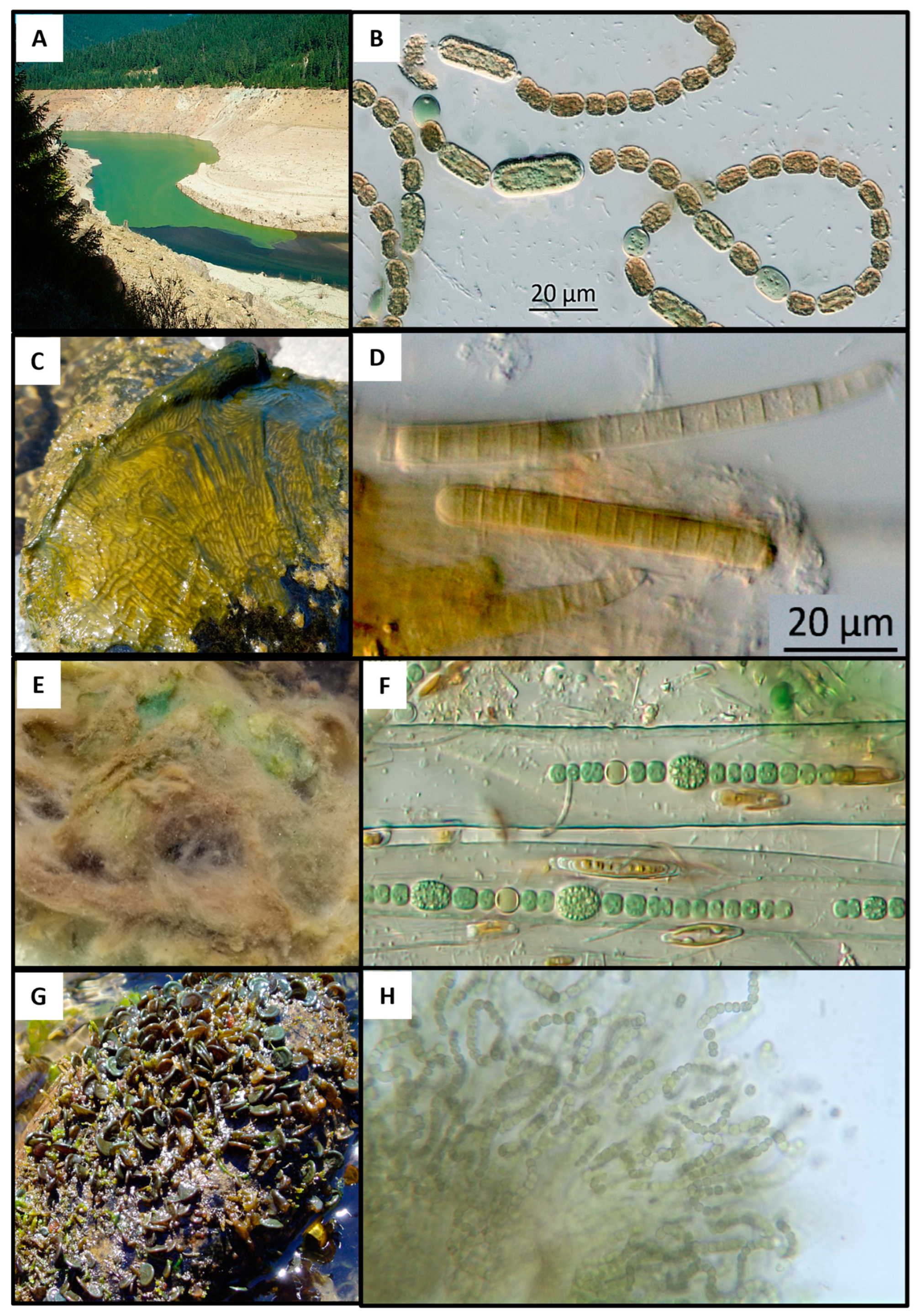

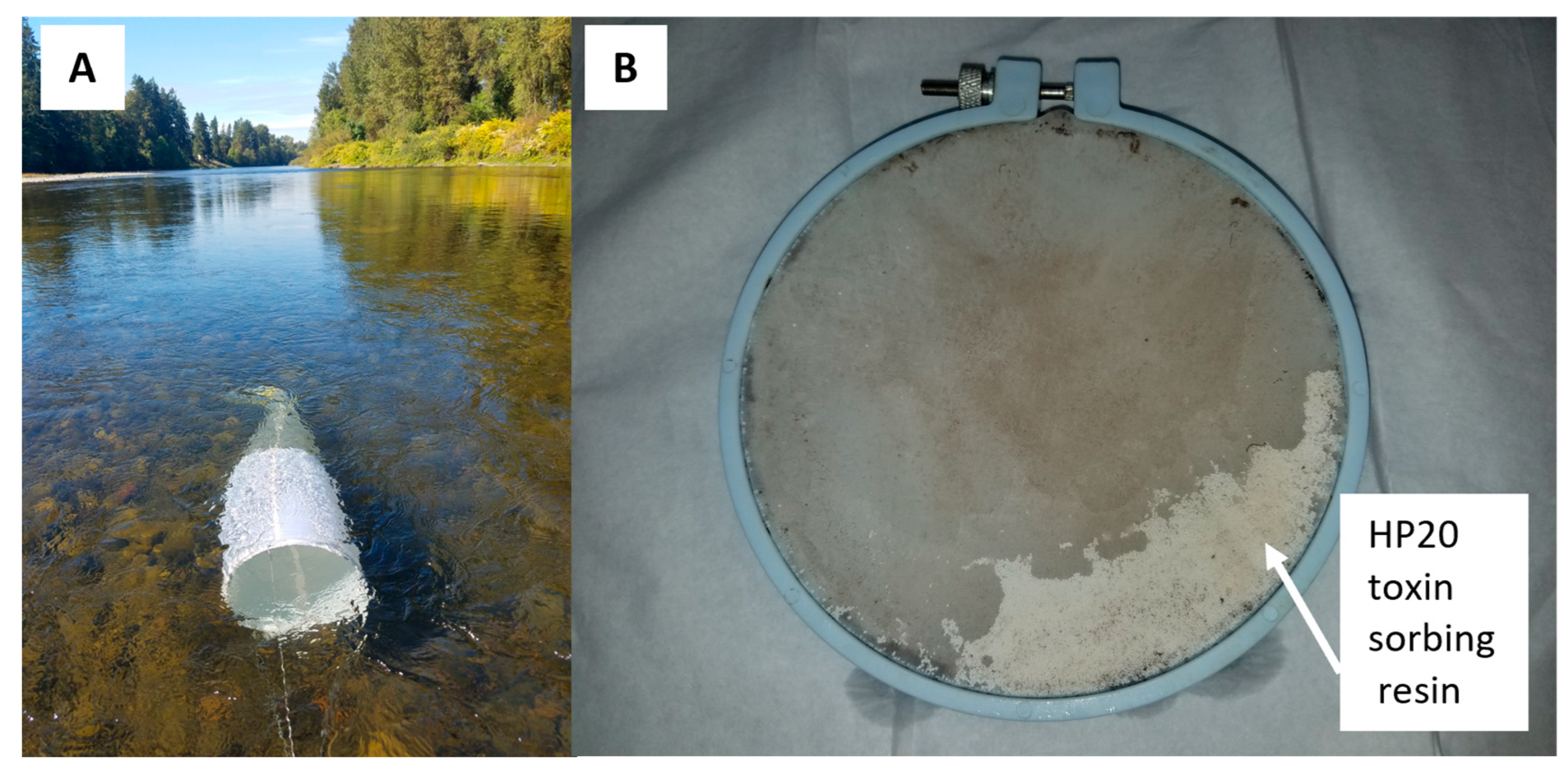

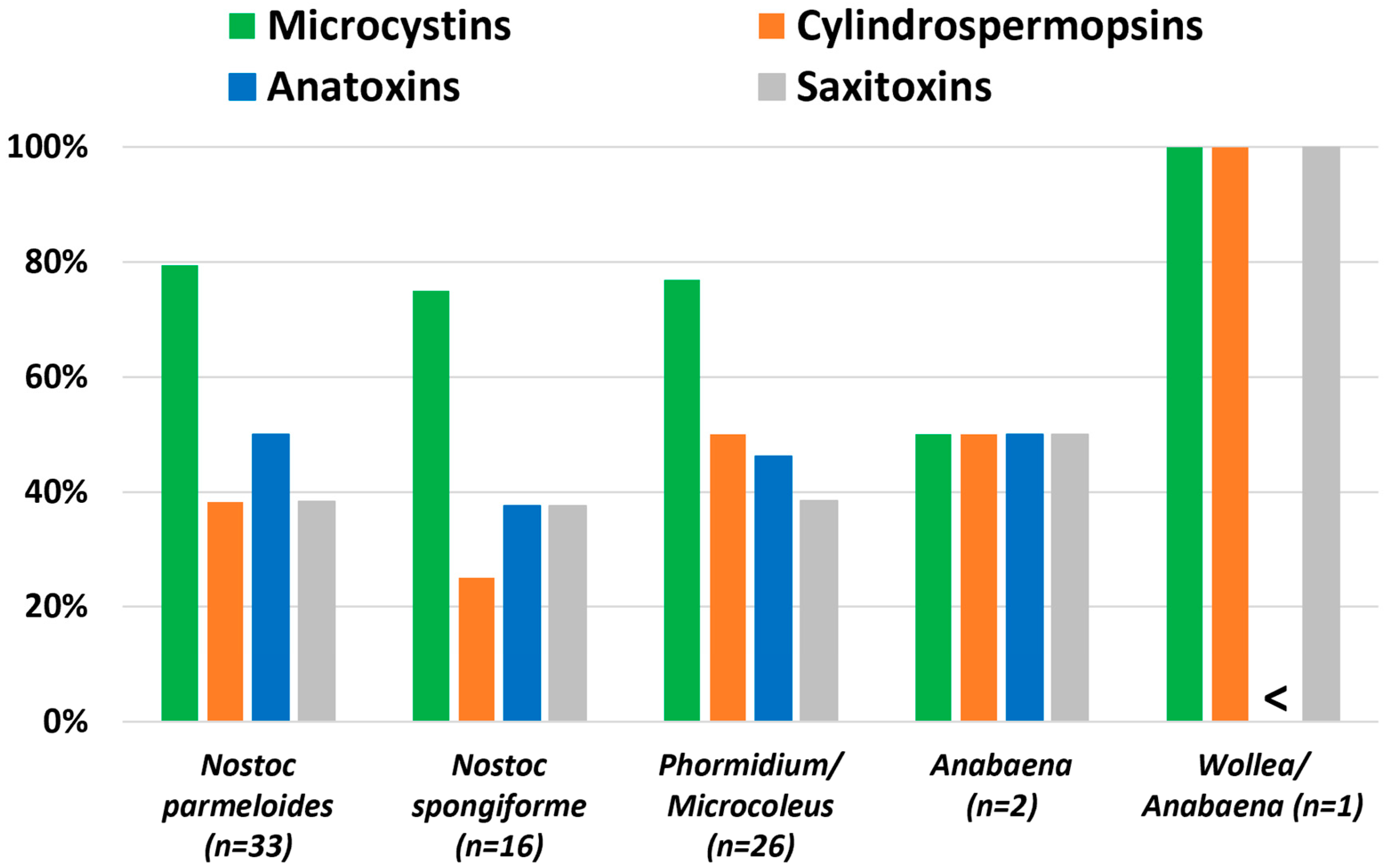
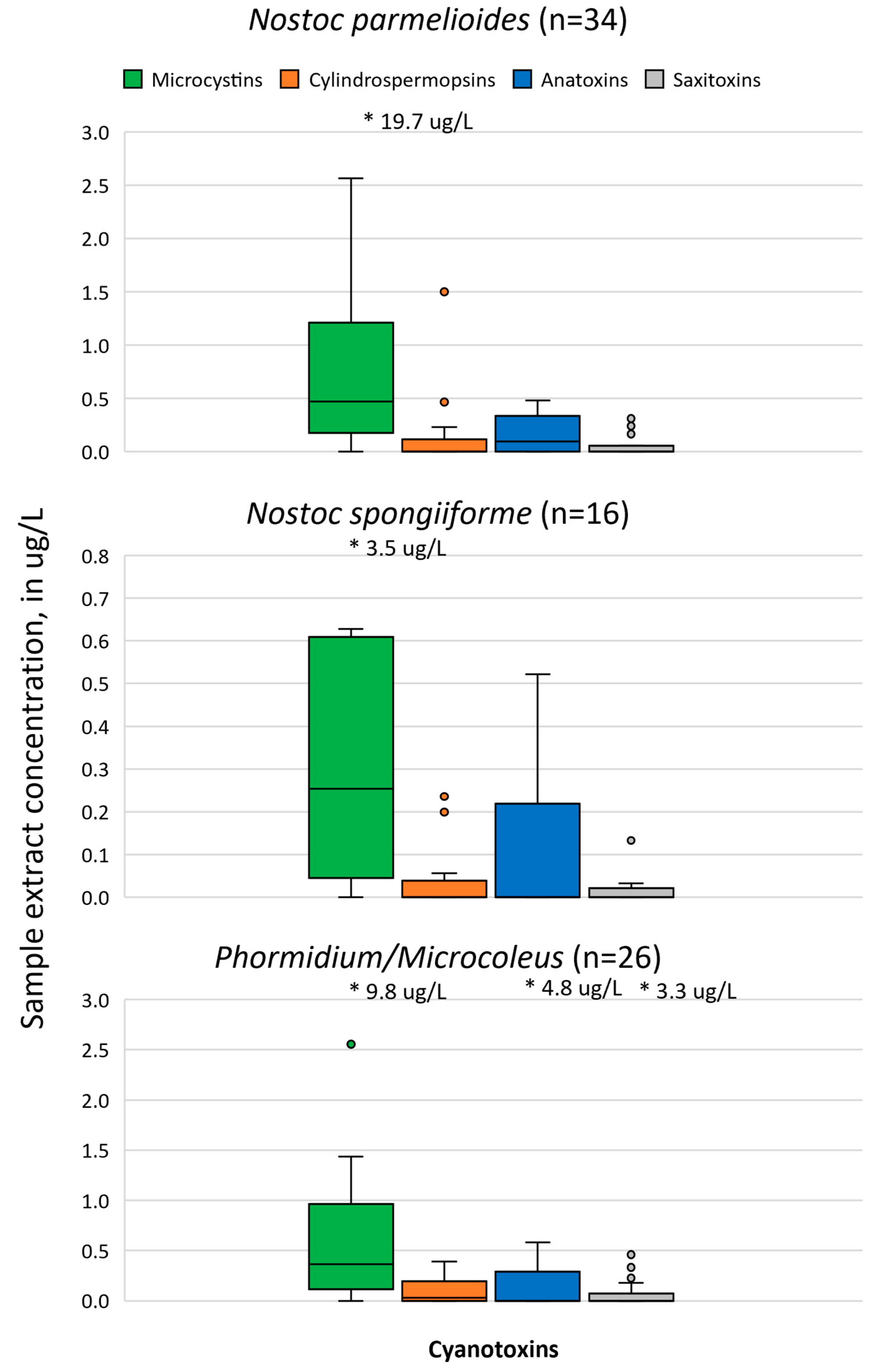
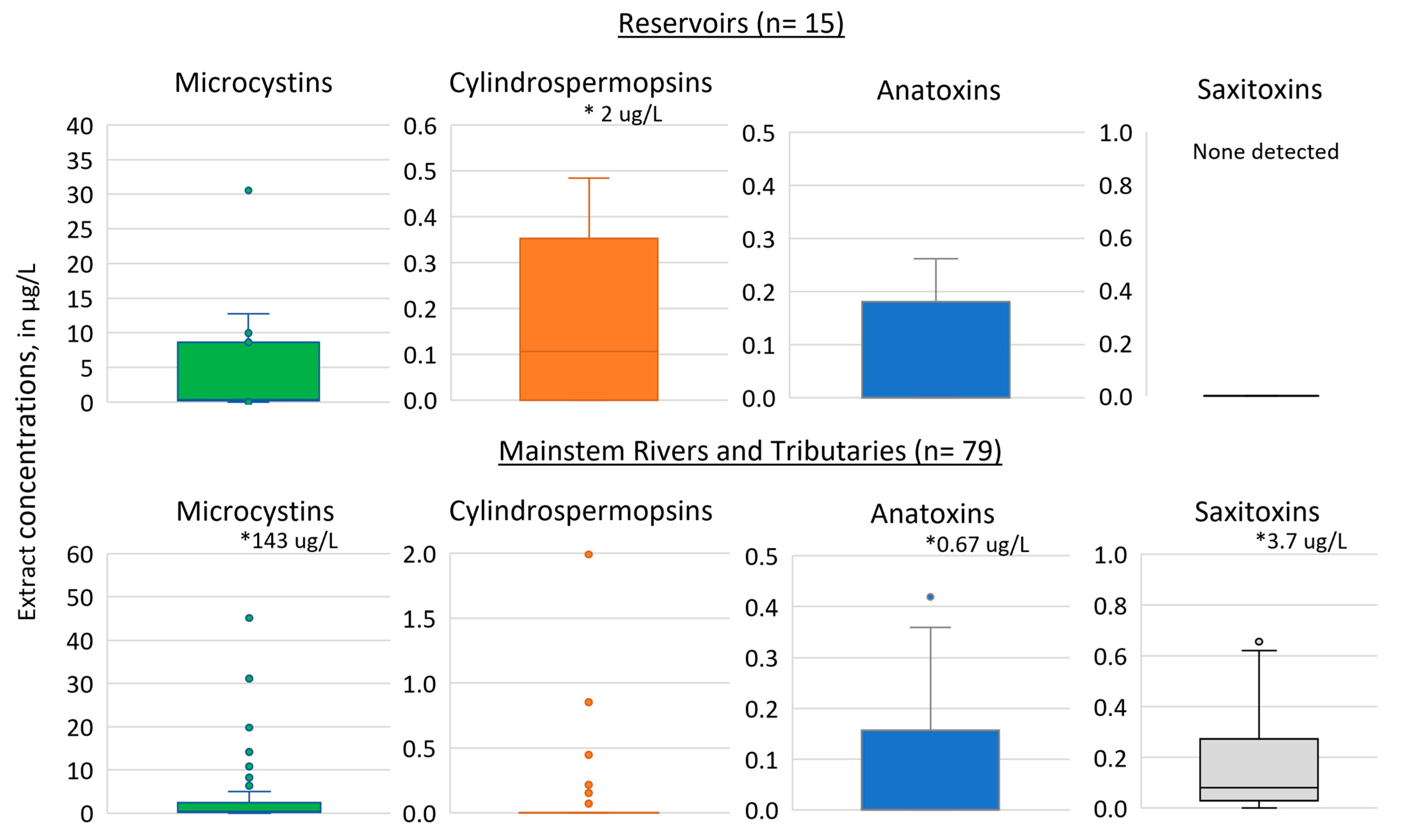

| Sample Type/Waterbody | Number of Samples (n) | Microcystins | Cylindrospermopsins | Anatoxins | Saxitoxins |
|---|---|---|---|---|---|
| All samples | 320 | 73% | 33% | 57% | 47% |
| Benthic cyanobacteria | 80 | 76% | 41% | 45% | 39% |
| Plankton net tows | 94 | 79% | 30% | 29% | 70% |
| SPATT samplers | 146 | 66% | 32% | 81% | 37% |
| Benthic cyanobacteria (n = 80) | |||||
| Clackamas River Basin | 56 | 73% | 45% | 50% | 45% |
| North Santiam River Basin | 4 | 100% | 100% | 50% | 17% |
| McKenzie River Basin | 19 | 80% | 25% | 30% | 25% |
| Coast Fork Willamette | 1 | 100% | 0% | 100% | 0% |
| Plankton net tows (n = 94) | |||||
| Reservoirs | 15 | 87% | 73% | 47% | 0% |
| Mainstems and Tributaries | 79 | 77% | 22% | 25% | 84% |
| Clackamas River Basin | 51 | 78% | 6% | 10% | 73% |
| North Santiam River Basin | 11 | 56% | 78% | 33% | 56% |
| McKenzie River Basin | 29 | 86% | 54% | 57% | 86% |
| Dexter Reservoir | 1 | 100% | 0% | 0% | 0% |
| Diamond Lake | 2 | 50% | 0% | 50% | 0% |
| Solid-Phase Adsorption Toxin Tracking (SPATT) samplers (n = 146) | |||||
| Clackamas River Basin | 70 | 59% | 9% | 77% | 16% |
| North Santiam River Basin | 17 | 88% | 88% | 94% | 82% |
| McKenzie River Basin | 39 | 67% | 41% | 79% | 46% |
| Upper Willamette River | 3 | 33% | 0% | 67% | 0% |
| Middle Fork Willamette | 5 | 80% | 0% | 80% | 80% |
| Coast Fork Willamette | 3 | 33% | 0% | 67% | 0% |
| North Umpqua River | 9 | 100% | 100% | 100% | 100% |
| Drinking Water Sources | Percent Detection | ||||
|---|---|---|---|---|---|
| Number of SPATT Samplers | Microcystins | Cylindrospermopsins | Anatoxins | Saxitoxins | |
| Clackamas River | 31 | 52% | 10% | 77% | 13% |
| North Santiam River | 2 | 100% | 100% | 50% | 100% |
| McKenzie River | 9 | 78% | 22% | 78% | 33% |
| Middle Fork Willamette River & Upper Willamette River | 8 | 63% | 0% | 75% | 50% |
| North Umpqua River | 5 | 100% | 100% | 100% | 100% |
| Site Types/Dominant Land Use | ||||
|---|---|---|---|---|
| Microcystins | Cylindrospermopsins | Anatoxins | Saxitoxins | |
| Concentration, in µg/L | ||||
| Benthic cyanobacteria | ||||
| Forested tributary (n = 13) | 1.8 | 0.05 | 0.24 | 0.29 |
| Spring (n = 1) | 0.0 | 0.19 | 0.00 | 0.00 |
| Upper mainstem (n = 22) | 1.7 | 0.14 | 0.13 | 0.37 |
| Reservoir outflow (n = 5) | 0.2 | 0.11 | 0.13 | < |
| Mixed ag/private/industrial (n = 2) | 3.3 | < | 1.1 | 0.01 |
| Lower mainstem/raw water (n = 37) | 1.2 | 0.07 | 0.24 | 0.04 |
| Plankton net tows | ||||
| Forested tributary (n = 8) | 1.4 | < | < | 0.11 |
| Upper mainstem (n = 24) | 5.7 | 0.12 | 0.02 | 0.72 |
| Reservoir (n = 14) | 4.6 | 0.51 | 0.17 | < |
| Reservoir outflow (n = 10) | 0.21 | 1.11 | 0.32 | 0.47 |
| Mixed ag/private/industrial (n = 5) | 0.07 | < | < | < |
| Lower mainstem/raw water (n = 32) | 6.3 | 0.23 | 0.05 | 0.06 |
| Solid-Phase Adsorption Toxin Tracking (SPATT) | ||||
| Concentration, in ng/g/day | ||||
| Forested tributary (n = 15) | 0.04 | 0.001 | 0.02 | 0.0005 |
| Upper mainstem (n = 32) | 0.02 | 0.002 | 0.12 | 0.0016 |
| Reservoir (n = 14) | 137 | 0.17 | 0.34 | 0.0012 |
| Reservoir outflow (n = 12) | 0.07 | 0.03 | 0.03 | 0.0014 |
| Mixed ag/private/industrial (n = 14) | 0.01 | < | 0.01 | 0.0002 |
| Lower mainstem/raw water (n = 59) | 0.36 | 0.01 | 0.07 | 0.005 |
| Cyanobacteria | Number of Samples | Microcystins | Cylindrospermopsins | Anatoxins | Saxitoxins |
|---|---|---|---|---|---|
| Reservoir plankton net tows | |||||
| Aphanizomenon | 2 | x 1 | x 1 | x 1 | x 1 |
| Dolichospermum | 33 | x 1,2 | x 1,2 | x 1,2 | x 1,2 |
| Gloeotrichia | 7 | x 2,3 | -- | -- | x 1,2 |
| Benthic cyanobacteria | |||||
| Cylindrospermum | 1 | x 2 | x 1,2 | x 1 | |
| Nostoc parmelioides | 34 | x 1,2 | -- | -- | -- |
| Nostoc spongiiforme | 16 | x 1,2 | -- | -- | -- |
| Oscillatoria | 1 | x 1,2 | x 1,2 | x 1,2 | -- |
| Phormidium/Microcoleus | 26 | x 1,2 | x 2 | x 1,2 | x 1,2 |
| Mixed assemblage in riverine plankton net tows | |||||
| Nostoc | 41 | x 1,2 | x 3 | -- 4,5 | |
| Tolypothrix | 48 | x 1,2 | - | x 5,6 | |
| Mixed assemblage in stalked diatom masses | |||||
| Anabaena | 4 | x 2 | x 2 | x 2 | x 2 |
| Wollea saccata | 1 | -- | -- | -- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpenter, K.D.; Rosen, B.H.; Donahue, D.; Duncan, K.; Hilbrandt, B.; Lewis, C.; Swan, K.; Triplett, T.; Welch, E. Reservoir and Riverine Sources of Cyanotoxins in Oregon’s Cascade Range Rivers Tapped for Drinking Water Supply. Phycology 2025, 5, 16. https://doi.org/10.3390/phycology5020016
Carpenter KD, Rosen BH, Donahue D, Duncan K, Hilbrandt B, Lewis C, Swan K, Triplett T, Welch E. Reservoir and Riverine Sources of Cyanotoxins in Oregon’s Cascade Range Rivers Tapped for Drinking Water Supply. Phycology. 2025; 5(2):16. https://doi.org/10.3390/phycology5020016
Chicago/Turabian StyleCarpenter, Kurt D., Barry H. Rosen, David Donahue, Kari Duncan, Brandin Hilbrandt, Chris Lewis, Kim Swan, Tracy Triplett, and Elijah Welch. 2025. "Reservoir and Riverine Sources of Cyanotoxins in Oregon’s Cascade Range Rivers Tapped for Drinking Water Supply" Phycology 5, no. 2: 16. https://doi.org/10.3390/phycology5020016
APA StyleCarpenter, K. D., Rosen, B. H., Donahue, D., Duncan, K., Hilbrandt, B., Lewis, C., Swan, K., Triplett, T., & Welch, E. (2025). Reservoir and Riverine Sources of Cyanotoxins in Oregon’s Cascade Range Rivers Tapped for Drinking Water Supply. Phycology, 5(2), 16. https://doi.org/10.3390/phycology5020016






