Nutrient Accessibility Influences Stalk Formation in the Benthic Diatom Achnanthidium minutissimum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Cultivation Conditions
2.3. Extraction of the Stalk-Inducing Signal Molecule
2.4. Cultivation of Axenic A. minutissimum Under Nutrient Limitation
2.5. Stalk Induction Bioassay for A. minutissimum
2.6. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopic Analysis (EDX)
2.7. Statistical Analysis
3. Results
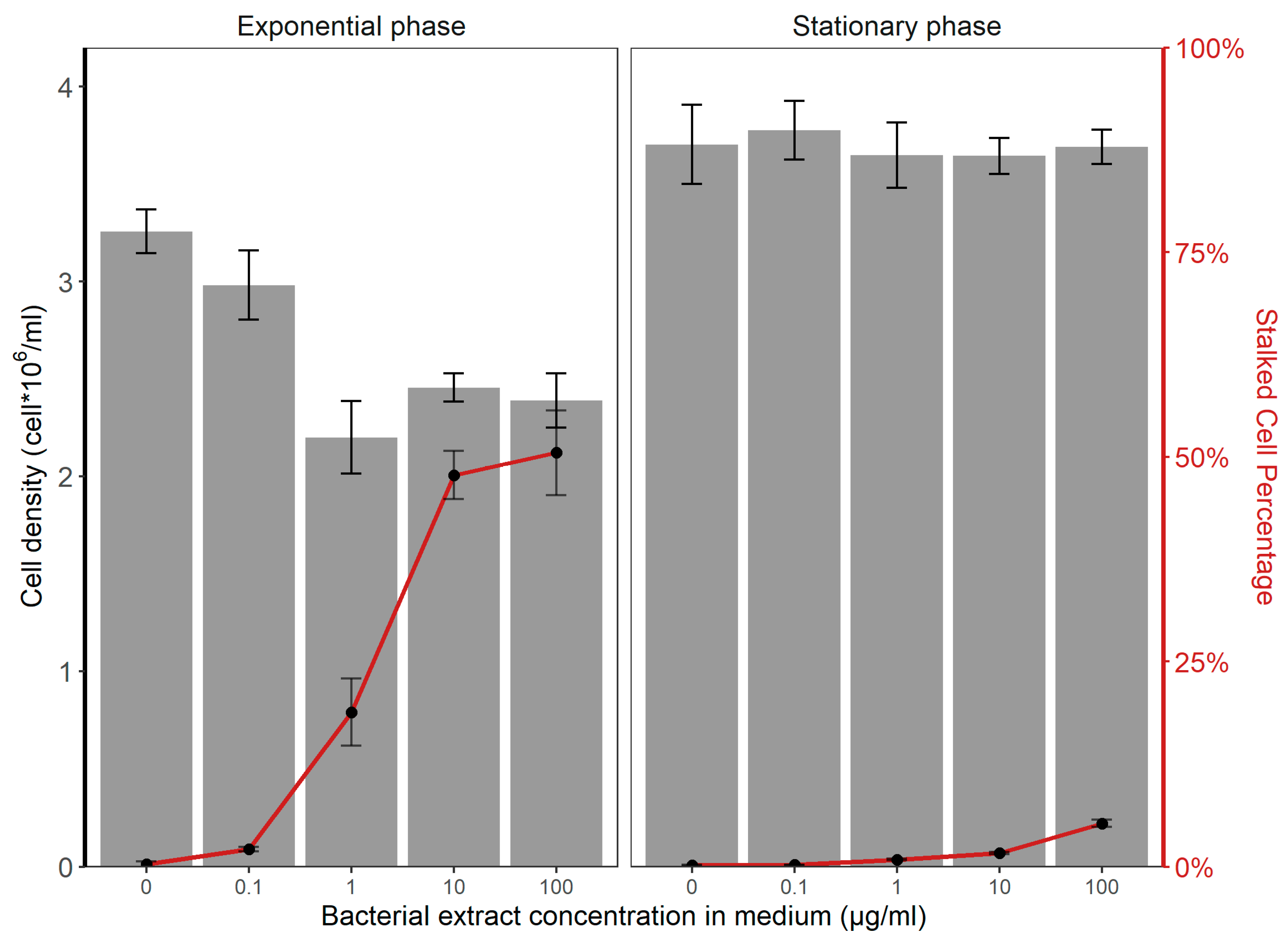
3.1. Induction of Stalk Formation in A. minutissimum by Dyadobacter sp. S32 Extracts
3.2. Differential Stalk Formation in Exponential and Stationary Cells
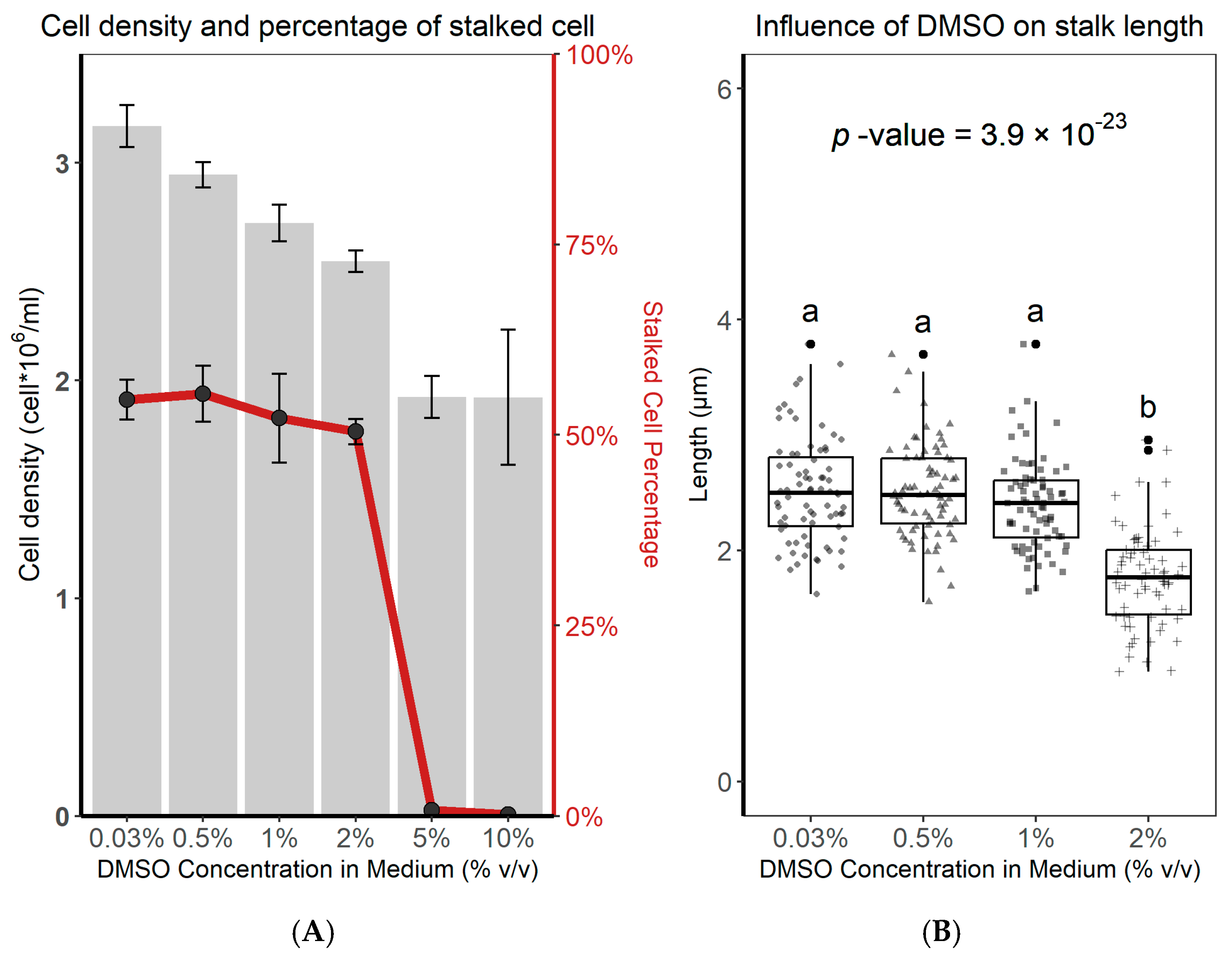
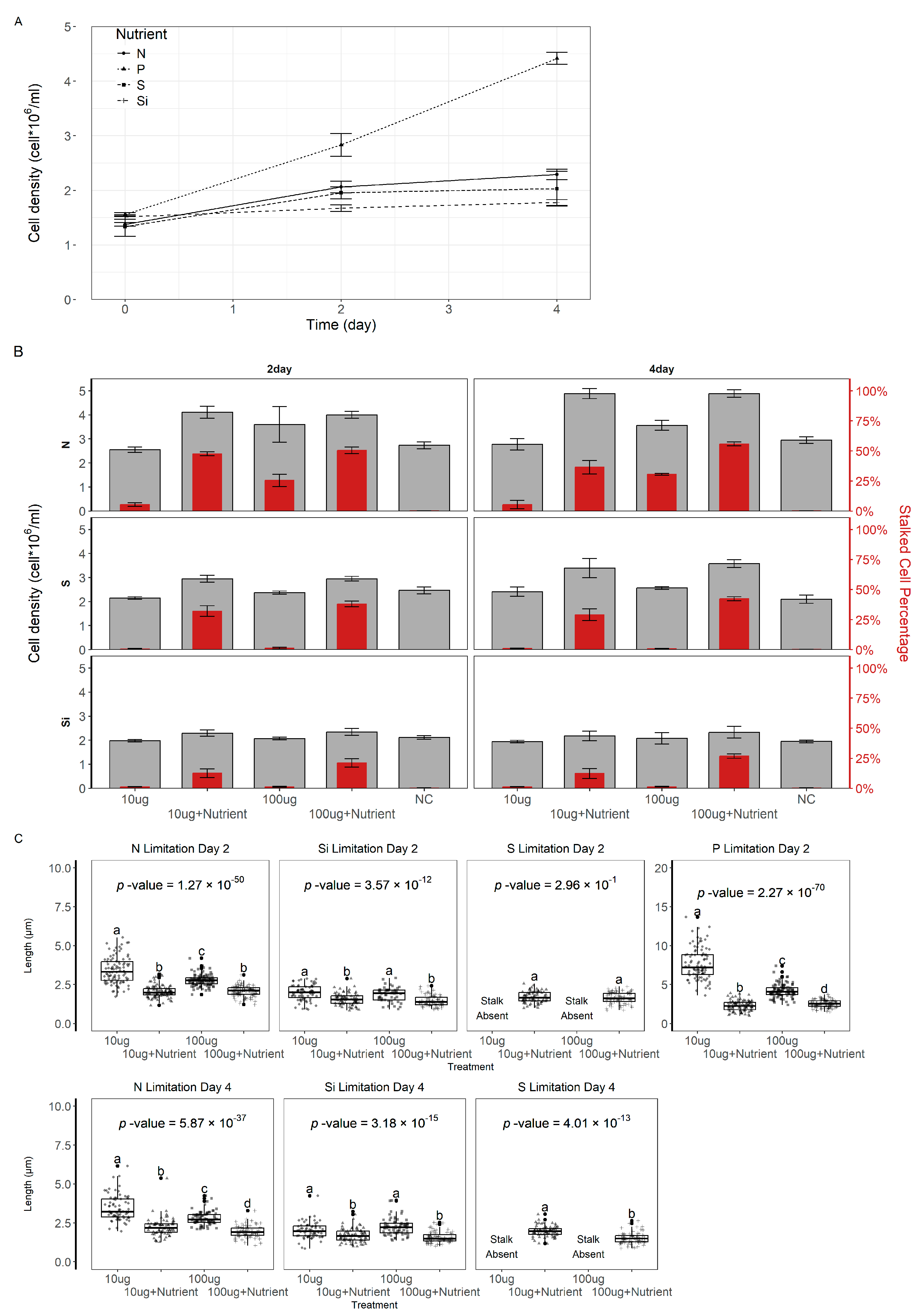
3.3. The Effect of Nutrient Limitation on Stalk Production
3.4. Nutrient Limitation Inhibits Stalk Production
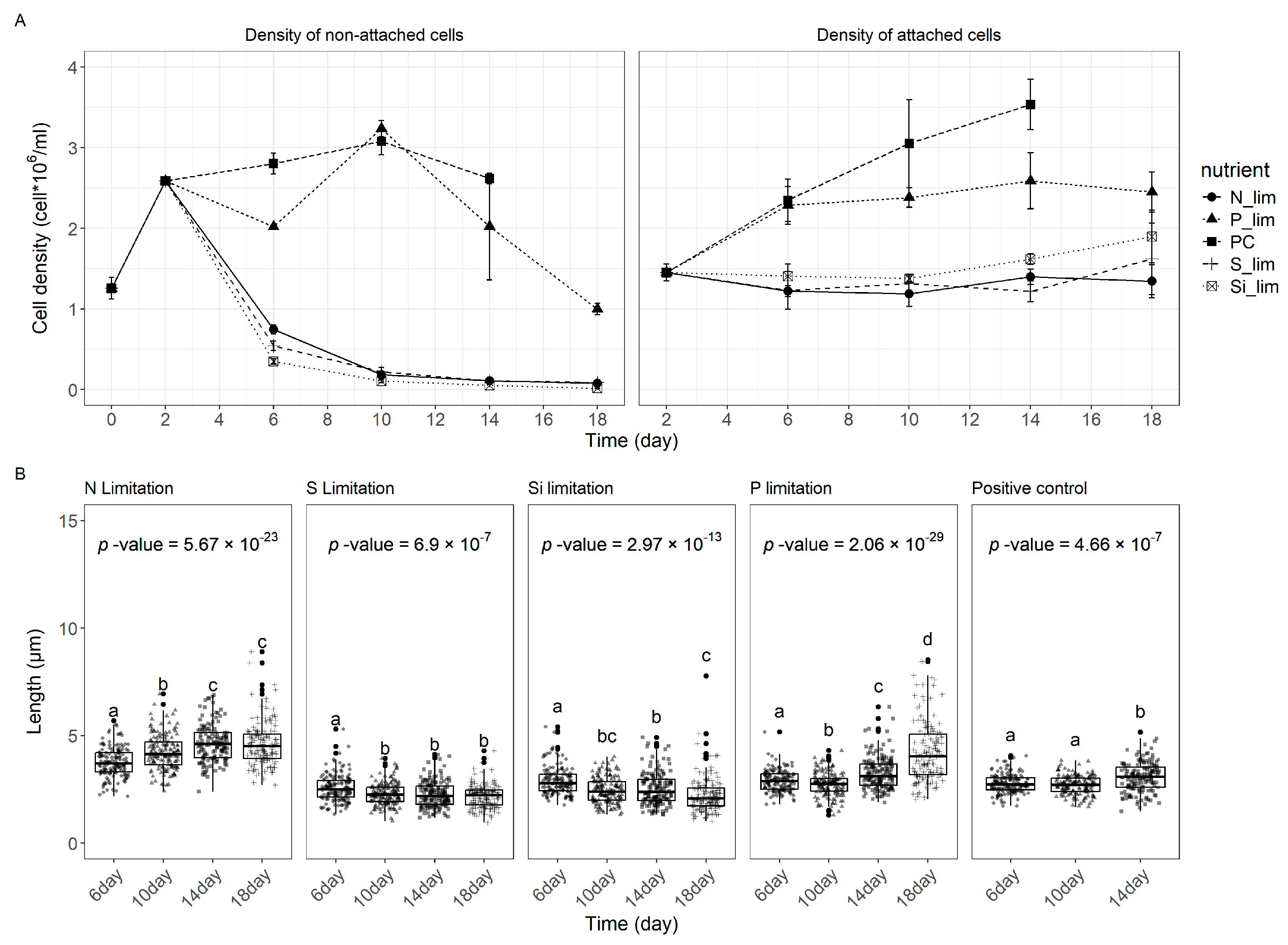
3.5. Nutrient Limitation on Stalked Cells
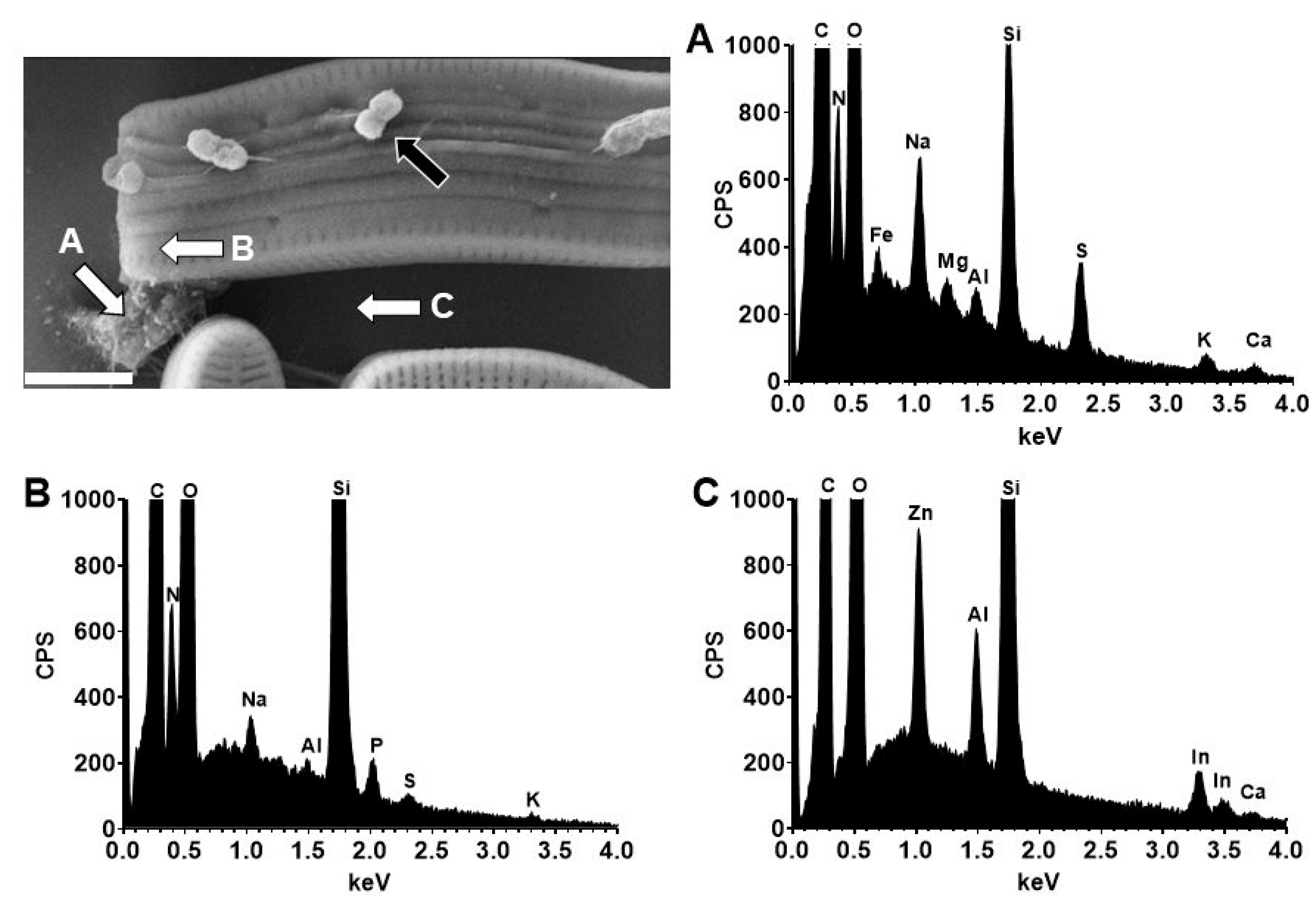
3.6. Identification of Sulfur in the Stalks of A. minutissimum
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Achnanthidium medium |
| FDC | Frequency of dividing cells |
| SEM | Scanning electron microscope |
| EDX | Energy dispersive X- ray analyses |
| DMSO | Dimethyl sulfoxide |
References
- Bruckner, C.G.; Kroth, P.G. Protocols for the Removal of Bacteria from Freshwater Benthic Diatom Cultures. J. Phycol. 2009, 45, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Bahulikar, R.A.; Kroth, P.G. Localization of EPS components secreted by freshwater diatoms using differential staining with fluorophore-conjugated lectins and other fluorochromes. Eur. J. Phycol. 2007, 42, 199–208. [Google Scholar] [CrossRef]
- Hoagland, K.D.; Rosowski, J.R.; Gretz, M.R.; Roemer, S.C. Diatom extracellular polymeric substances: Function, fine structure, chemistry, and physiology. J. Phycol. 1993, 29, 537–566. [Google Scholar]
- Wustman, B.A.; Gretz, M.R.; Hoagland, K.D. Extracellular matrix assembly in diatoms (Bacillariophyceae) (I. A model of adhesives based on chemical characterization and localization of polysaccharides from the marine diatom Achnanthes longipes and other diatoms). Plant Physiol. 1997, 113, 1059–1069. [Google Scholar] [PubMed]
- Bahulikar, R.A.; Kroth, P.G. The Complex Extracellular Polysaccharides of Mainly Chain-Forming Freshwater Diatom Species from Epilithic Biofilms. J. Phycol. 2008, 44, 1465–1475. [Google Scholar] [CrossRef]
- Dütsch, L.; Brendler, E.; Zuber, J.; Viehweger, C.; Ehrlich, H.; Jesionowski, T.; Vogt, C. Structural characterization of the extracellular stalk material of the diatom Didymosphenia geminata. Anal. Bioanal. Chem. 2024, 416, 4341–4352. [Google Scholar] [CrossRef] [PubMed]
- Blunn, G.; Evans, L. Microscopical observations on Achnanthes subsessilis, with particular reference to stalk formation. Bot. Mar. 1981, 24, 193–199. [Google Scholar]
- Molino, P.J.; Wetherbee, R. The biology of biofouling diatoms and their role in the development of microbial slimes. Biofouling 2008, 24, 365–379. [Google Scholar] [CrossRef]
- Kawamura, T.; Roberts, R.D.; Nicholson, C.M. Factors affecting the food value of diatom strains for post-larval abalone Haliotis iris. Aquaculture 1998, 160, 81–88. [Google Scholar]
- Karouna, N.K.; Fuller, R.L. Influence of four grazers on periphyton communities associated with clay tiles and leaves. Hydrobiologia 1992, 245, 53–64. [Google Scholar] [CrossRef]
- Ellwood, N.; Whitton, B. Importance of organic phosphate hydrolyzed in stalks of the lotic diatom Didymosphenia geminata and the possible impact of atmospheric and climatic changes. Hydrobiologia 2007, 592, 121–133. [Google Scholar] [CrossRef]
- Aboal, M.; Marco, S.; Chaves, E.; Mulero, I.; García-Ayala, A. Ultrastructure and function of stalks of the diatom Didymosphenia geminata. Hydrobiologia 2012, 695, 17–24. [Google Scholar] [CrossRef]
- Bray, J.; O’Brien, J.; Harding, J.S. Production of phosphatase and extracellular stalks as adaptations to phosphorus limitation in Didymosphenia geminata (Bacillariophyceae). Hydrobiologia 2017, 784, 51–63. [Google Scholar] [CrossRef]
- Brogan, M.S.; Peckarsky, B.L.; Resasco, J. Consequences of nuisance algal blooms of Didymosphenia geminata on invertebrate communities in Rocky Mountain streams. Freshw. Sci. 2024, 43, 52–64. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.; Holm, E.; Hertel, W. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.; Fletcher, R.; Jones, E. Diatom fouling of in-service shipping with particular reference to the influence of hydrodynamic forces. In Proceedings of the Ninth International Diatom Symposium, Bristol, UK, 24–30 August 1986; Biopress: Bristol, UK, 1986; pp. 49–59. [Google Scholar]
- Zargiel, K.A.; Swain, G.W. Static vs dynamic settlement and adhesion of diatoms to ship hull coatings. Biofouling 2014, 30, 115–129. [Google Scholar] [CrossRef]
- Perkins, K.; Sargison, J.; Hallegraeff, G. Diatom Fouling Problems in a Tasmanian Hydro Canal, Including the Description of Gomphomena tarraleahae sp. nov. Diatom Res. 2009, 24, 377–391. [Google Scholar] [CrossRef]
- Wysokowski, M.; Bartczak, P.; Żółtowska-Aksamitowska, S.; Chudzińska, A.; Piasecki, A.; Langer, E.; Bazhenov, V.V.; Petrenko, I.; Noga, T.; Stelling, A.L. Adhesive stalks of diatom Didymosphenia geminata as a novel biological adsorbent for hazardous metals removal. CLEAN–Soil Air Water 2017, 45, 1600678. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, Ł. The role of lignin and lignin-based materials in sustainable construction–a comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Smith, D.J.; Underwood, G.J. The production of extracellular carbohydrates by estuarine benthic diatoms: The effects of growth phase and light and dark treatment. J. Phycol. 2000, 36, 321–333. [Google Scholar]
- Kilroy, C.; Bothwell, M. Environmental Control of Stalk Length in the Bloom-Forming, Freshwater Benthic Diatom Didymosphenia geminata (Bacillariophyceae). J. Phycol. 2011, 47, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, C.; Bothwell, M.L. Attachment and short-term stalk development of Didymosphenia geminata: Effects of light, temperature and nutrients. Diatom Res. 2014, 29, 237–248. [Google Scholar] [CrossRef]
- Hagström; Larsson, U.; Hörstedt, P.; Normark, S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl. Environ. Microbiol. 1979, 37, 805–812. [Google Scholar]
- Cantonati, M.; Angeli, N.; Virtanen, L.; Wojtal, A.Z.; Gabrieli, J.; Falasco, E.; Lavoie, I.; Morin, S.; Marchetto, A.; Fortin, C. Achnanthidium minutissimum (Bacillariophyta) valve deformities as indicators of metal enrichment in diverse widely-distributed freshwater habitats. Sci. Total Environ. 2014, 475, 201–215. [Google Scholar] [CrossRef]
- Hlúbiková, D.; Ector, L.; Hoffmann, L. Examination of the type material of some diatom species related to Achnanthidium minutissimum (Kütz.) Czarn.(Bacillariophyceae). Algol. Stud. 2011, 136, 19. [Google Scholar] [CrossRef]
- Windler, M.; Gruber, A.; Kroth, P.G. Purification of benthic diatoms from associated bacteria using the antibiotic imipenem. J. Endocytobiosis Cell Res. 2012, 22, 62–65. [Google Scholar]
- Ponader, K.C.; Potapova, M.G. Diatoms from the genus Achnanthidium in flowing waters of the Appalachian Mountains (North America): Ecology, distribution and taxonomic notes. Limnologica 2007, 37, 227–241. [Google Scholar] [CrossRef]
- Windler, M. Bacterial Influence on Diatoms from Photoautotrophic Freshwater Biofilms. Dissertation. 2014. Available online: http://nbn-resolving.de/urn:nbn:de:bsz:352-0-287999 (accessed on 1 March 2025).
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 1 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 1 March 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Windler, M.; Leinweber, K.; Bartulos, C.R.; Philipp, B.; Kroth, P.G. Biofilm and capsule formation of the diatom Achnanthidium minutissimum are affected by a bacterium. J. Phycol. 2015, 51, 343–355. [Google Scholar] [CrossRef]
- Astafyeva, Y.; Gurschke, M.; Streit, W.R.; Krohn, I. Interplay between the microalgae Micrasterias radians and its symbiont Dyadobacter sp. HH091. Front. Microbiol. 2022, 13, 1006609. [Google Scholar] [CrossRef]
- Gao, J.-l.; Sun, P.; Wang, X.-M.; Qiu, T.-L.; Lv, F.-Y.; Yuan, M.; Yang, M.-M.; Sun, J.-G. Dyadobacter endophyticus sp. nov., an endophytic bacterium isolated from maize root. Int. J. Syst. Evol. Microbiol. 2016, 66, 4022–4026. [Google Scholar] [CrossRef]
- Leinweber, K.; Kroth, P.G. Capsules of the diatom Achnanthidium minutissimum arise from fibrillar precursors and foster attachment of bacteria. PeerJ 2015, 3, e858. [Google Scholar] [CrossRef]
- Koedooder, C.; Stock, W.; Willems, A.; Mangelinckx, S.; De Troch, M.; Vyverman, W.; Sabbe, K. Diatom-bacteria interactions modulate the composition and productivity of benthic diatom biofilms. Front. Microbiol. 2019, 10, 1255. [Google Scholar] [CrossRef]
- Figueroa, F.A.; Abdala-Díaz, R.; Hernández, V.; Pedreros, P.; Aranda, M.; Cabrera-Pardo, J.R.; Pérez, C.; Becerra, J.; Urrutia, R. Invasive diatom Didymosphenia geminata as a source of polysaccharides with antioxidant and immunomodulatory effects on macrophage cell lines. J. Appl. Phycol. 2020, 32, 93–102. [Google Scholar] [CrossRef]
- Bishop, I.W.; Spaulding, S.A. Life cycle size dynamics in Didymosphenia geminata (Bacillariophyceae). J. Phycol. 2017, 53, 652–663. [Google Scholar] [CrossRef]
- Lapointe, A.; Kocademir, M.; Bergman, P.; Ragupathy, I.C.; Laumann, M.; Underwood, G.J.; Zumbusch, A.; Spiteller, D.; Kroth, P.G. Characterization of polyphosphate dynamics in the widespread freshwater diatom Achnanthidium minutissimum under varying phosphorus supplies. J. Phycol. 2024, 60, 624–638. [Google Scholar] [CrossRef]
- Jüttner, I.; Hamilton, P.B.; Wetzel, C.E.; Van De Vijver, B.; King, L.; Kelly, M.G.; Williams, D.M.; Ector, L. A study of the morphology and distribution of four Achnanthidium Kütz. species (Bacillariophyta), implications for ecological status assessment, and description of two new European species. Cryptogam. Algol. 2022, 43, 147–176. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Mollet, J.-C.; Gretz, M.R.; Hoagland, K.D. Extracellular matrix assembly in diatoms (Bacillariophyceae) (II. 2, 6-Dichlorobenzonitrile inhibition of motility and stalk production in the marine diatom Achnanthes longipes). Plant Physiol. 1997, 113, 1071–1080. [Google Scholar]
- Abdullahi, A.S.; Underwood, G.J.; Gretz, M.R. Extracellular matrix assembly in diatoms (bacillariophyceae). v. environmental effects on polysaccharide synthesis in the model diatom, Phaeodactylum tricornutum. J. Phycol. 2006, 42, 363–378. [Google Scholar]
- Brzezinski, M.A.; Olson, R.J.; Chisholm, S.W. Silicon availability and cell-cycle progression in marine diatoms. Mar. Ecol. Prog. Ser. 1990, 67, 83–96. [Google Scholar]
- Lapointe, A. Strategies of the Ubiquitous Freshwater Diatom Achnanthidium minutissimum in Response to Phosphate Limitation. Dissertation University of Konstanz. 2022. Available online: http://nbn-resolving.de/urn:nbn:de:bsz:352-2-15l1qqyc8r6w98 (accessed on 1 March 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, P.; Lapointe, A.; Hartenberger, K.; Spiteller, D.; Kroth, P.G. Nutrient Accessibility Influences Stalk Formation in the Benthic Diatom Achnanthidium minutissimum. Phycology 2025, 5, 13. https://doi.org/10.3390/phycology5020013
Ji P, Lapointe A, Hartenberger K, Spiteller D, Kroth PG. Nutrient Accessibility Influences Stalk Formation in the Benthic Diatom Achnanthidium minutissimum. Phycology. 2025; 5(2):13. https://doi.org/10.3390/phycology5020013
Chicago/Turabian StyleJi, Pengyu, Adrien Lapointe, Katja Hartenberger, Dieter Spiteller, and Peter G. Kroth. 2025. "Nutrient Accessibility Influences Stalk Formation in the Benthic Diatom Achnanthidium minutissimum" Phycology 5, no. 2: 13. https://doi.org/10.3390/phycology5020013
APA StyleJi, P., Lapointe, A., Hartenberger, K., Spiteller, D., & Kroth, P. G. (2025). Nutrient Accessibility Influences Stalk Formation in the Benthic Diatom Achnanthidium minutissimum. Phycology, 5(2), 13. https://doi.org/10.3390/phycology5020013





