Optimizing Early Growth of Laminaria hyperborea in Controlled Settings: A Pathway to Improved Restoration Efforts
Abstract
1. Introduction
2. Materials and Methods
2.1. Fieldwork and Laboratory Procedure
2.2. Data Analysis
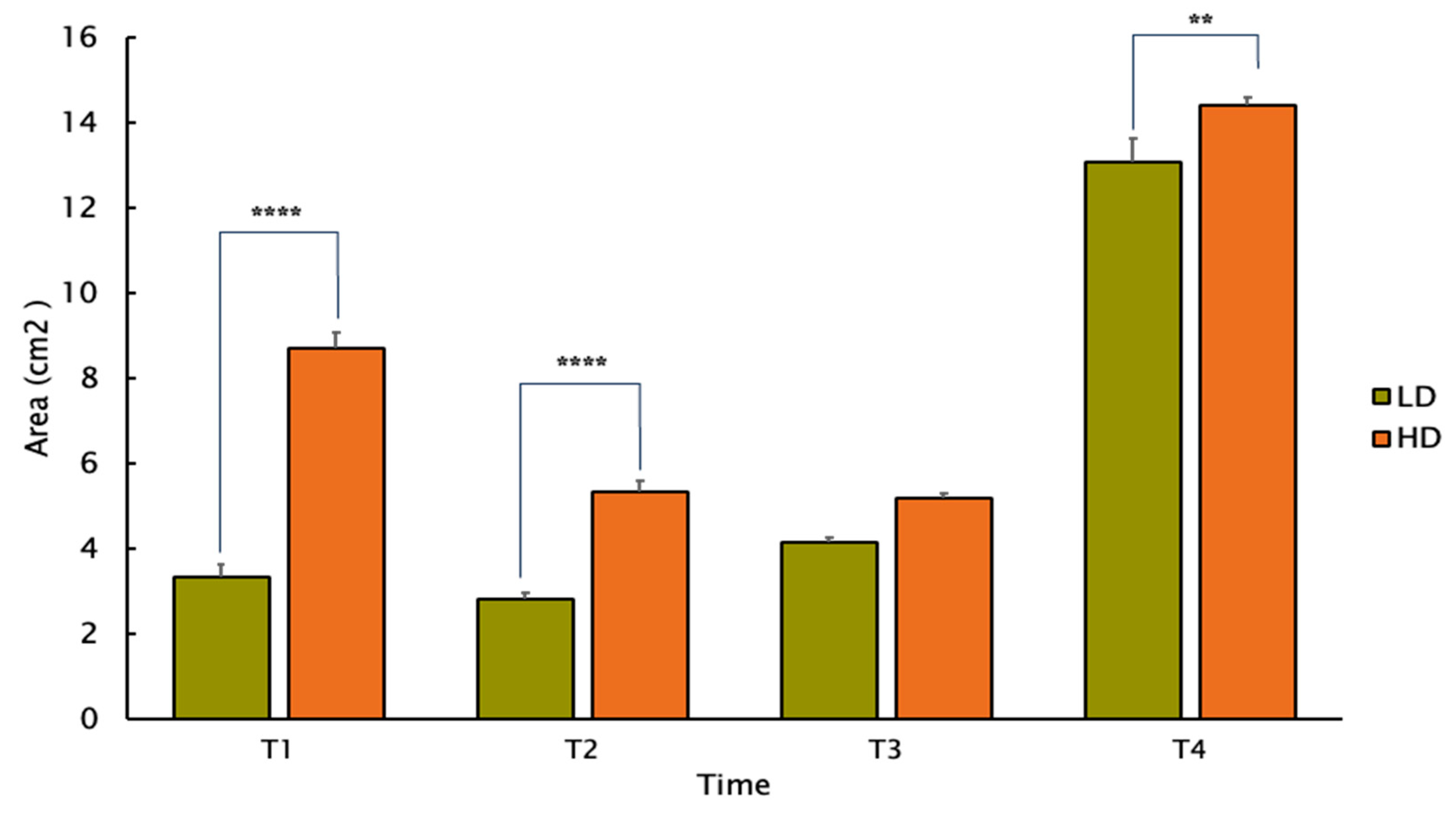
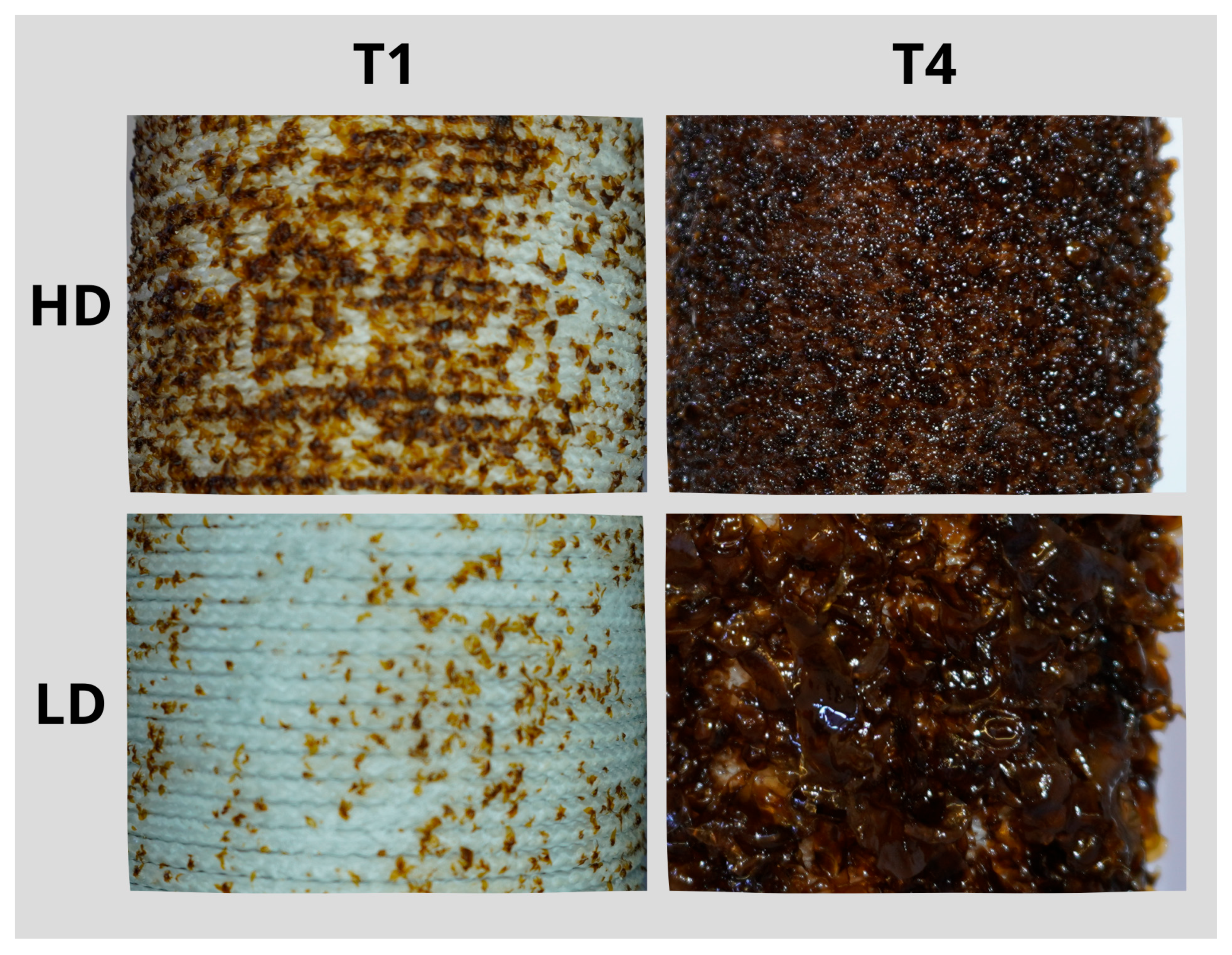
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Graham, M.H.; Steneck, R.S.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Wernberg, T.; Thomsen, M.S.; Tuya, F.; Kendrick, G.A.; Staehr, P.A.; Toohey, B.D. Decreasing resilience of kelp beds along a latitudinal temperature gradient: Potential implications for a warmer future. Ecol. Lett. 2010, 13, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.M.; Assis, J.; Aguillar, R.; Airoldi, L.; Bárbara, I.; Bartsch, I.; Bekkby, T.; Christie, H.; Davoult, D.; Derrien-Courtel, S.; et al. Status, trends and drivers of kelp forests in Europe: An expert assessment. Biodivers. Conserv. 2016, 25, 1319–1348. [Google Scholar] [CrossRef]
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefalì, M.E.; Cebrian, E. Restoration of a Canopy-Forming Alga Based on Recruitment Enhancement: Methods and Long-Term Success Assessment. Front. Plant Sci. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Araujo, R.M.; Bartsch, I.; Bekkby, T.; Erzini, K.; Sousa-Pinto, I. What is the impact of kelp forest density and/or area on fisheries? Environ. Evid. 2013, 2, 15. [Google Scholar] [CrossRef]
- Turner, R.K.; Burgess, D.; Hadley, D.; Coombes, E.; Jackson, N. A cost–benefit appraisal of coastal managed realignment policy. Glob. Environ. Chang. 2007, 17, 397–407. [Google Scholar] [CrossRef]
- Temmerman, S.; Meire, P.; Bouma, T.J.; Herman, P.M.J.; Ysebaert, T.; De Vriend, H.J. Ecosystem-based coastal defence in the face of global change. Nature 2013, 504, 79–83. [Google Scholar] [CrossRef]
- Belkin, I.M. Rapid warming of large marine ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Casado-Amezúa, P.; Araújo, R.; Bárbara, I.; Bermejo, R.; Borja, Á.; Díez, I.; Fernández, C.; Gorostiaga, J.M.; Guinda, X.; Hernández, I.; et al. Distributional shifts of canopy-forming seaweeds from the Atlantic coast of Southern Europe. Biodivers. Conserv. 2019, 28, 1151–1172. [Google Scholar] [CrossRef]
- Pinho, D.; Bertocci, I.; Arenas, F.; Franco, J.N.; Jacinto, D.; Castro, J.J.; Vieira, R.; Sousa-Pinto, I.; Wernberg, T.; Tuya, F. Spatial and temporal variation of kelp forests and associated macroalgal assemblages along the Portuguese coast. Mar. Freshw. Res. 2015, 67, 113–122. [Google Scholar] [CrossRef]
- Fernández, C. The retreat of large brown seaweeds on the north coast of Spain: The case of Saccorhiza polyschides. Eur. J. Phycol. 2011, 46, 352–360. [Google Scholar] [CrossRef]
- Tanaka, K.; Taino, S.; Haraguchi, H.; Prendergast, G.; Hiraoka, M. Warming off southwestern Japan linked to distributional shifts of subtidal canopy-forming seaweeds. Ecol. Evol. 2012, 2, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Wernberg, T.; de Bettignies, T.; Joy, B.A.; Finnegan, P.M. Physiological responses of habitat-forming seaweeds to increasing temperatures. Limnol. Oceanogr. 2016, 61, 2180–2190. [Google Scholar] [CrossRef]
- Smale, D.A.; Burrows, M.T.; Moore, P.; O’Connor, N.; Hawkins, S.J. Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast Atlantic perspective. Ecol. Evol. 2013, 3, 4016–4038. [Google Scholar] [CrossRef]
- Assis, J.; Lucas, A.V.; Bárbara, I.; Serrão, E.Á. Future climate change is predicted to shift long-term persistence zones in the cold-temperate kelp Laminaria hyperborea. Mar. Environ. Res. 2016, 113, 174–182. [Google Scholar] [CrossRef]
- García, L.M.; Rancel-Rodríguez, N.M.; Sangil, C.; Reyes, J.; Benito, B.; Orellana, S.; Sansón, M. Environmental and human factors drive the subtropical marine forests of Gongolaria abies-marina to extinction. Mar. Environ. Res. 2022, 181, 105759. [Google Scholar] [CrossRef]
- Vea, J.; Ask, E. Creating a sustainable commercial harvest of Laminaria hyperborea in Norway. J. Appl. Phycol. 2011, 23, 489–494. [Google Scholar] [CrossRef]
- Voerman, S.E.; Llera, E.; Rico, J.M. Climate driven changes in subtidal kelp forest communities in NW Spain. Mar. Environ. Res. 2013, 90, 119–127. [Google Scholar] [CrossRef]
- Eger, A.; Aguirre, J.D.; Altamirano, M.; Arafeh-Dalmau, N.; Arroyo, N.L.; Bauer-Civiello, A.M.; Beas-Luna, R.; Bekkby, T.; Bellgrove, A.; Bennett, S.; et al. The Kelp Forest Challenge: A collaborative global movement to protect and restore 4 million hectares of kelp forests. J. Appl. Phycol. 2024, 36, 951–964. [Google Scholar] [CrossRef]
- Harden, M.; Kovalev, M.; Molano, G.; Yorke, C.; Miller, R.; Reed, D.; Alberto, F.; Koos, D.S.; Lansford, R.; Nuzhdin, S. Heat stress analysis suggests a genetic basis for tolerance in Macrocystis pyrifera across developmental stages. Commun. Biol. 2024, 7, 1147. [Google Scholar] [CrossRef]
- Perrow, M.R.; Davy, A.J. Handbook of Ecological Restoration; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Bayraktarov, E.; Saunders, M.I.; Abdullah, S.; Mills, M.; Beher, J.; Possingham, H.P.; Mumby, P.J.; Lovelock, C.E. The cost and feasibility of marine coastal restoration. Ecol. Appl. 2016, 26, 1055–1074. [Google Scholar] [CrossRef]
- Ebbing, A.; Pierik, R.; Bouma, T.; Kromkamp, J.C.; Timmermans, K. How light and biomass density influence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae). J. Phycol. 2020, 56, 709–718. [Google Scholar] [CrossRef]
- Peteiro, C.; Freire, Ó. Biomass yield and morphological features ofthe seaweed Saccharina latissima cultivated at two different sites in acoastal bay in the Atlantic coast of Spain. J. Appl. Phycol. 2013, 25, 205–213. [Google Scholar] [CrossRef]
- Forbord, S.; Steinhovden, K.B.; Solvang, T.; Handå, A.; Skjermo, J. Effect of Seeding Methods and Hatchery Periods on Sea Cultivation of Saccharina latissima (Phaeophyceae): A Norwegian Case Study. J. Appl. Phycol. 2020, 32, 2201–2212. [Google Scholar] [CrossRef]
- Martins, N.; Tanttu, H.; Pearson, G.A.; Bartsch, I. Interactions of Daylength, Temperature and Nutrients Affect Thresholds for Life Stage Transitions in the Kelp Laminaria digitata (Phaeophyceae). Bot. Mar. 2017, 60, 109–121. [Google Scholar] [CrossRef]
- Kerrison, P.D.; Stanley, M.S.; Kelly, M.; MacLeod, A.; Black, K.D.; Hughes, A.D. Optimising the Settlement and Hatchery Culture of Saccharina latissima (Phaeophyta) by Manipulation of Growth Medium and Substrate Surface Condition. J. Appl. Phycol. 2016, 28, 1181–1191. [Google Scholar] [CrossRef]
- Nardelli, A.E.; Visch, W.; Farrington, G.; Sanderson, J.C.; Bellgrove, A.; Wright, J.T.; Macleod, C.; Hurd, C.L. A New Nursery Approach Enhances at—Sea Performance in the Kelp Lessonia corrugata. J. Appl. Phycol. 2023, 36, 591–603. [Google Scholar] [CrossRef]
- Dieck, T.I. North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): Hybridization experiments and temperature responses. Phycologia 1992, 31, 147–163. [Google Scholar]
- Harrison, P.J.; Berges, J.A. Marine Culture Media. In Algal Culturing Techniques; Andersen, R., Ed.; Academic Press: New York, NY, USA, 2005; pp. 21–33. [Google Scholar]
- Su, L.; Pang, S.J.; Shan, T.F.; Li, X. Large-scale hatchery of the kelp Saccharina japonica: A case study experience at Lvshun in northern China. J. Appl. Phycol. 2017, 29, 3003–3013. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Brawley, S.H.; Johnson, L.E. Survival Of Fucoid Embryos In The Intertidal Zone Depends Upon Developmental Stage And Microhabitat1. J. Phycol. 1991, 27, 179–186. [Google Scholar] [CrossRef]
- Steen, H.; Scrosati, R. Intraspecific competition in Fucus serratus and F. evanescens (Phaeophyceae: Fucales) germlings: Effects of settlement density, nutrient concentration, and temperature. Mar. Biol. 2004, 144, 61–70. [Google Scholar] [CrossRef]
- Arenas, F.; Viejo, R.M.; Fernández, C. Density-dependent regulation in an invasive seaweed: Responses at plant and modular levels. J. Ecol. 2002, 90, 820–829. [Google Scholar] [CrossRef]
- Alsuwaiyan, N.A.; Vranken, S.; Burkholz, C.; Cambridge, M.; Coleman, M.A.; Wernberg, T. Green gravel as a vector of dispersal for kelp restoration. Front. Mar. Sci. 2022, 9, 910417. [Google Scholar] [CrossRef]
- Lawton, R.J.; Magnusson, M. Effects of seeding twine type and seeding density on hatchery performance and initial at-sea cultivation performance of the kelp Ecklonia radiata. Alg. Res. 2024, 84, 103777. [Google Scholar] [CrossRef]
- Kerrison, P.D.; Twigg, G.; Stanley, M.; De Smet, D.; Buyle, G.; Martínez Pina, A.; Hughes, A.D. Twine selection is essential for successful hatchery cultivation of Saccharina latissima, seeded with either meiospores or juvenile sporophytes. J. Appl. Phycol. 2019, 31, 3051–3060. [Google Scholar] [CrossRef]
- Arantzamendi, L.; Andrés, M.; Basurko, O.C.; Suárez, M.J. Circular and lower impact mussel and seaweed aquaculture by a shift towards bio-based ropes. Rev. Aquac. 2023, 15, 1010–1019. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemello, S.; Coutinho, A.; Sá, M.F.; Borges, D.; Arenas, F.; Sousa-Pinto, I.; Costa, I. Optimizing Early Growth of Laminaria hyperborea in Controlled Settings: A Pathway to Improved Restoration Efforts. Phycology 2025, 5, 5. https://doi.org/10.3390/phycology5010005
Chemello S, Coutinho A, Sá MF, Borges D, Arenas F, Sousa-Pinto I, Costa I. Optimizing Early Growth of Laminaria hyperborea in Controlled Settings: A Pathway to Improved Restoration Efforts. Phycology. 2025; 5(1):5. https://doi.org/10.3390/phycology5010005
Chicago/Turabian StyleChemello, Sílvia, Ana Coutinho, M. Francisca Sá, Débora Borges, Francisco Arenas, Isabel Sousa-Pinto, and Isabel Costa. 2025. "Optimizing Early Growth of Laminaria hyperborea in Controlled Settings: A Pathway to Improved Restoration Efforts" Phycology 5, no. 1: 5. https://doi.org/10.3390/phycology5010005
APA StyleChemello, S., Coutinho, A., Sá, M. F., Borges, D., Arenas, F., Sousa-Pinto, I., & Costa, I. (2025). Optimizing Early Growth of Laminaria hyperborea in Controlled Settings: A Pathway to Improved Restoration Efforts. Phycology, 5(1), 5. https://doi.org/10.3390/phycology5010005






