Macroalgal Diseases: Exploring Biology, Pathogenesis, and Management Strategies
Abstract
1. Introduction
2. Pyropia/Porphyra Species
2.1. Red Rot Disease (RRD)
2.2. Olpidiopsis Disease (OD), Chytrid Disease (CD), Olpidiopsis-Blight (O-B)
2.3. Green Spot Disease
2.4. Cyanobacteria Felt
2.5. Diatom Felt
2.6. Genetic Toolkits against Common Diseases in Porphyra/Pyropia Species
3. Kappaphycus sp. and Eucheuma sp.
3.1. “Ice-Ice” Malaise
3.2. Goose Bumps Disease
4. Gracilaria sp.
5. Laminaria sp., Saccharina sp., and Undaria sp.
6. Ulva sp.
7. A Case Study on Kappaphycus: What We Can Learn from Past Mistakes?
- The main one is the neglect of cultivation rules (which are much more careful for the cultivation of terrestrial plants, agronomy, etc.), which occurs very often because you do not have the knowledge of certain pathologies or do not know the symptoms [53].
- Another reason is the low genetic variation and loss of strain vigour, which has further ramifications in that the biomass becomes susceptible to pathogens, diseases, and epi- or endophyte infestations [6].
- A third reason is lack of development in commercial utilization of local seaweed biodiversity leading to seemingly unnecessary introductions of non-indigenous Eucheumatoids and their unfettered expansion into new farming areas. Some of these introductions have caused serious environmental issues, such as an increased prevalence of invasive organisms. Also, a lot of the time, it is difficult to establish correlations with pathogens [54].
- The final reason is the failure to innovate new techniques for Eucheumatoid farming, and the indigenous utilization of raw materials merely fuels the expansion of commercial operations through the unregulated transfer of seedlings to new farming areas to meet increasing global demands [14].
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Oort, P.A.J.; Verhagen, A.; Van Der Werf, A.K. Can Seaweeds Feed the World? Modelling World Offshore Seaweed Production Potential. Ecol. Model. 2023, 484, 110486. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Cheung, W.W.L.; Sumaila, U.R. Global Estimates of Suitable Areas for Marine Algae Farming. Environ. Res. Lett. 2023, 18, 064028. [Google Scholar] [CrossRef]
- Kim, G.H.; Moon, K.-H.; Kim, J.-Y.; Shim, J.; Klochkova, T.A. A Revaluation of Algal Diseases in Korean Pyropia (Porphyra) Sea Farms and Their Economic Impact. Algae 2014, 29, 249–265. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Hurtado, A.Q.; Critchley, A.T. Impacts of AMPEP on Epiphytes and Diseases in Kappaphycus and Eucheuma Cultivation. In Tropical Seaweed Farming Trends, Problems and Opportunities; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 111–119. ISBN 978-3-319-63497-5. [Google Scholar]
- Che, S.; Du, G.; Wang, N.; He, K.; Mo, Z.; Sun, B.; Chen, Y.; Cao, Y.; Wang, J.; Mao, Y. Biomass Estimation of Cultivated Red Algae Pyropia Using Unmanned Aerial Platform Based Multispectral Imaging. Plant Methods 2021, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.Q.; Critchley, A.T.; Neish, I. (Eds.) Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce. In Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-63498-2. [Google Scholar]
- Largo, D.B. Recent Developments in Seaweed Diseases. In Proceedings of the National Seaweed Planning Workshop, Iloilo, Philippines, 2–3 August 2001. [Google Scholar]
- Gachon, C.M.M.; Sime-Ngando, T.; Strittmatter, M.; Chambouvet, A.; Kim, G.H. Algal Diseases: Spot-light on a Black Box. Trends Plant Sci. 2010, 15, 633–640. [Google Scholar] [CrossRef]
- Kirchman, D.L. (Ed.) Microbial Ecology of the Oceans, 2nd ed.; Thoroughly rev.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; ISBN 978-0-470-04344-8. [Google Scholar]
- Quéré, G.; Nugues, M.M. Coralline Algae Disease Reduces Survival and Settlement Success of Coral Planulae in Laboratory Experiments. Coral Reefs 2015, 34, 863–870. [Google Scholar] [CrossRef]
- Araújo, R. Algae Biomass Status in Europe. In Proceedings of the EABA Webinar Seaweed Valorization, Webinar, Online, 7 April 2020. [Google Scholar]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The Seaweed Holobiont: Understanding Seaweed–Bacteria Interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef]
- Egan, S.; Fernandes, N.D.; Kumar, V.; Gardiner, M.; Thomas, T. Bacterial Pathogens, Virulence Mechanism and Host Defence in Marine Macroalgae: Bacterial Pathogens of Macroalgae. Environ. Microbiol. 2014, 16, 925–938. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Neish, I.C.; Critchley, A.T. Phyconomy: The Extensive Cultivation of Seaweeds, Their Sustainability and Economic Value, with Particular Reference to Important Lessons to Be Learned and Transferred from the Practice of Eucheumatoid Farming. Phycologia 2019, 58, 472–483. [Google Scholar] [CrossRef]
- Ward, G.M.; Faisan, J.P.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A Review of Reported Seaweed Diseases and Pests in Aquaculture in Asia. J. World Aquac. Soc. 2019, 51, 815–828. [Google Scholar] [CrossRef]
- Badis, Y.; Han, J.W.; Klochkova, T.A.; Gachon, C.M.M.; Kim, G.H. The Gene Repertoire of Pythium porphyrae (Oomycota) Suggests an Adapted Plant Pathogen Tackling Red Algae. Algae 2020, 35, 133–144. [Google Scholar] [CrossRef]
- Yang, L.-E.; Deng, Y.-Y.; Xu, G.-P.; Russell, S.; Lu, Q.-Q.; Brodie, J. Redefining Pyropia (Bangiales, Rhodophyta): Four New Genera, Resurrection of Porphyrella and Description of Calidia pseudolobata sp. nov. from China. J. Phycol. 2020, 56, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Abd-Elsalam, K.; Ingle, A.P. (Eds.) Pythium: Diagnosis, Diseases and Management; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-367-25941-9. [Google Scholar]
- Arasaki, S. Studies on the Rot of Porphyra tenera by Pythium. Nippon Suisan Gakkaishi 1947, 13, 74–90. [Google Scholar] [CrossRef]
- Takahashi, M. Pythium porphyrae Takahashi & Sasaki, sp. nov. Causing Red Rot of Marine Red Algae Porphyra spp. Trans. Mycol. Soc. Jpn. 1977, 18, 279–285. [Google Scholar]
- Qiu, L.; Mao, Y.; Tang, L.; Tang, X.; Mo, Z. Characterization of Pythium chondricola Associated with Red Rot Disease of Pyropia yezoensis (Ueda) (Bangiales, Rhodophyta) from Lianyungang, China. J. Oceanol. Limnol. 2019, 37, 1102–1112. [Google Scholar] [CrossRef]
- Diehl, N.; Kim, G.H.; Zuccarello, G.C. A Pathogen of New Zealand Pyropia plicata (Bangiales, Rhodophyta), Pythium porphyrae (Oomycota). Algae 2017, 32, 29–39. [Google Scholar] [CrossRef]
- Mo, Z.; Li, S.; Kong, F.; Tang, X.; Mao, Y. Characterization of a Novel Fungal Disease That Infects the Gametophyte of Pyropia yezoensis (Bangiales, Rhodophyta). J. Appl. Phycol. 2016, 28, 395–404. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yokoo, K.; Tojo, M.; Hishiike, M. Distribution of Pythium porphyrae, the Causal Agent of Red Rot Disease of Porphyra spp., in the Ariake Sea, Japan. Plant Dis. 2005, 89, 1041–1047. [Google Scholar] [CrossRef]
- Ding, H.; Ma, J. Simultaneous Infection by Red Rot and Chytrid Diseases in Porphyra yezoensis Ueda. J. Appl. Phycol. 2005, 17, 51–56. [Google Scholar] [CrossRef]
- Weng, P.; Yang, H.; Mo, Z.; Zhang, W.; Yan, Y.; Rong, X.; Li, J. Application and Evaluation of Probiotics against Red Rot Disease in Pyropia. Aquaculture 2024, 578, 740050. [Google Scholar] [CrossRef]
- Lee, S.J.; Jee, B.Y.; Son, M.-H.; Lee, S.-R. Infection and Cox2 Sequence of Pythium chondricola (Oomycetes) Causing Red Rot Disease in Pyropia yezoensis (Rhodophyta) in Korea. Algae 2017, 32, 155–160. [Google Scholar] [CrossRef]
- Badis, Y.; Klochkova, T.A.; Brakel, J.; Arce, P.; Ostrowski, M.; Tringe, S.G.; Kim, G.H.; Gachon, C.M.M. Hidden Diversity in the Oomycete Genus Olpidiopsis is a Potential Hazard to Red Algal Cultivation and Conservation Worldwide. Eur. J. Phycol. 2019, 55, 162–171. [Google Scholar] [CrossRef]
- Sekimoto, S.; Yokoo, K.; Kawamura, Y.; Honda, D. Taxonomy, Molecular Phylogeny, and Ultrastructural Morphology of Olpidiopsis porphyrae sp. nov. (Oomycetes, Straminipiles), a Unicellular Obligate Endoparasite of Bangia and Porphyra spp. (Bangiales, Rhodophyta). Mycol. Res. 2008, 112, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Klochkova, T.A.; Shin, Y.J.; Moon, K.-H.; Motomura, T.; Kim, G.H. New Species of Unicellular Obligate Parasite, Olpidiopsis pyropiae sp. nov., That Plagues Pyropia Sea Farms in Korea. J. Appl. Phycol. 2016, 28, 73–83. [Google Scholar] [CrossRef]
- Wen, X.; Zuccarello, G.C.; Klochkova, T.A.; Kim, G.H. Oomycete Pathogens, Red Algal Defense Mechanisms and Control Measures. Algae 2023, 38, 203–215. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, R.; Shim, E.; Park, H.; Klochkova, T.A.; Kim, G.H. Control of Oomycete Pathogens during Pyropia Farming and Processing Using Calcium Propionate. Algae 2023, 38, 71–80. [Google Scholar] [CrossRef]
- Im, S.H.; Klochkova, T.A.; Lee, D.J.; Gachon, C.M.M.; Kim, G.H. Genetic Toolkits of the Red Alga Pyropia tenera against the Three Most Common Diseases in Pyropia Farms. J. Phycol. 2019, 55, 801–815. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, L.; Liu, C.; Du, G.; Mo, Z.; Tang, X.; Mao, Y. Transcriptomic Insights into Innate Immunity Responding to Red Rot Disease in Red Alga Pyropia yezoensis. Int. J. Mol. Sci. 2019, 20, 5970. [Google Scholar] [CrossRef]
- Mine, T.; Tanaka, S.; Kawamura, Y.; Kobayashi, G.; Kanda, K. Isolation and Application of Bacteriophages to Suminori Disease Control. Aquac. Sci. 2010, 58, 211–217. [Google Scholar]
- Guan, X.; Li, J.; Zhang, Z.; Li, F.; Yang, R.; Jiang, P.; Qin, S. Characterizing the Microbial Culprit of White Spot Disease of the Conchocelis Stage of Porphyra yezoensis (Bangiales, Rhodophyta). J. Appl. Phycol. 2013, 25, 1341–1348. [Google Scholar] [CrossRef]
- Liu, Q.; Zhi, Y.; He, Y.; Ren, Z.; Chen, H.; Yang, R. Changes in Phycospheric and Environmental Microbes Associated with an Outbreak of Yellow Spot Disease on Pyropia yezoensis. Aquaculture 2020, 529, 735651. [Google Scholar] [CrossRef]
- Azanza, R.V.; Escalona, K.S.; Largo, D.B. A sustainable and inclusive blue economy for the Philippine archipelago. Trans. NAST PHL 2022, 44, 1–11. [Google Scholar] [CrossRef]
- Yamamoto, K.; Endo, H.; Yoshikawa, S.; Ohki, K.; Kamiya, M. Various Defense Ability of Four Sargassacean Algae against the Red Algal Epiphyte Neosiphonia harveyi in Wakasa Bay, Japan. Aquat. Bot. 2013, 105, 11–17. [Google Scholar] [CrossRef]
- Borlongan, I.A.G.; Luhan, M.R.J.; Padilla, P.I.P.; Hurtado, A.Q. Photosynthetic Responses of ‘Neosiphonia sp. Epiphyte-Infected’ and Healthy Kappaphycus alvarezii (Rhodophyta) to Irradiance, Salinity and pH Variations. J. Appl. Phycol. 2016, 28, 2891–2902. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Yasir, S.M.; Critchley, A.T.; Hurtado, A.Q. Impacts of Ascophyllum Marine Plant Extract Powder (AMPEP) on the Growth, Incidence of the Endophyte Neosiphonia apiculata and Associated Carrageenan Quality of Three Commercial Cultivars of Kappaphycus. J. Appl. Phycol. 2018, 30, 1185–1195. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Chung, C.S.; Hurtado, A.Q.; Soya, F.E.; Lhonneur, G.B.; Critchley, A. Distribution and Symptoms of Epiphyte Infection in Major Carrageenophyte-Producing Farms. J. Appl. Phycol. 2008, 20, 477–483. [Google Scholar] [CrossRef]
- Muñoz, J.; Fotedar, R. Epiphytism of Gracilaria cliftonii (Withell, Millar & Kraft) from Western Australia. J. Appl. Phycol. 2010, 22, 371–379. [Google Scholar] [CrossRef]
- Lavilla-Pitogo, C.R. Agar-Digesting Bacteria Associated with ‘Rotten Thallus Syndrome’ of Gracilaria sp. Aquaculture 1992, 102, 1–7. [Google Scholar] [CrossRef]
- Sun, X.; He, Y.; Xu, N.; Xia, Y.; Liu, Z. Isolation and Identification of Two Strains of Pathogenic Bacteria and Their Effects on the Volatile Metabolites of Gracilariopsis lemaneiformis (Rhodophyta). J. Appl. Phycol. 2012, 24, 277–284. [Google Scholar] [CrossRef]
- Schroeder, D.C.; Jaffer, M.A.; Coyne, V.E. Investigation of the Role of a β(1–4) Agarase Produced by Pseudoalteromonas gracilis B9 in Eliciting Disease Symptoms in the Red Alga Gracilaria gracilis. Microbiology 2003, 149, 2919–2929. [Google Scholar] [CrossRef][Green Version]
- Weinberger, F.; Friedlander, M.; Gunkel, W. A Bacterial Facultative Parasite of Gracilaria conferta. Dis. Aquat. Org. 1994, 18, 135–141. [Google Scholar] [CrossRef]
- Vettori, D.; Nikora, V.; Biggs, H. Implications of Hyposaline Stress for Seaweed Morphology and Biomechanics. Aquat. Bot. 2020, 162, 103188. [Google Scholar] [CrossRef]
- Bernard, M.S.; Strittmatter, M.; Murúa, P.; Heesch, S.; Cho, G.Y.; Leblanc, C.; Peters, A.F. Diversity, Biogeography and Host Specificity of Kelp Endophytes with a Focus on the Genera Laminarionema and Laminariocolax (Ectocarpales, Phaeophyceae). Eur. J. Phycol. 2019, 54, 39–51. [Google Scholar] [CrossRef]
- Gauna, M.C.; Escobar, J.F.; Odorisio, M.; Cáceres, E.J.; Parodi, E.R. Spatial and Temporal Variation in Algal Epiphyte Distribution on Ulva sp. (Ulvales, Chlorophyta) from Northern Patagonia in Argentina. Phycologia 2017, 56, 125–135. [Google Scholar] [CrossRef]
- Siniscalchi, A.G.; Gauna, M.C.; Cáceres, E.J.; Parodi, E.R. Myrionema strangulans (Chordariales, Phaeophyceae) Epiphyte on Ulva spp. (Ulvophyceae) from Patagonian Atlantic Coasts. J. Appl. Phycol. 2012, 24, 475–486. [Google Scholar] [CrossRef]
- Herrero, M.-L.; Brurberg, M.B.; Ojeda, D.I.; Roleda, M.Y. Occurrence and Pathogenicity of Pythium (Oomycota) on Ulva Species (Chlorophyta) at Different Salinities. Algae 2020, 35, 79–89. [Google Scholar] [CrossRef]
- Mateo, J.P.; Campbell, I.; Cottier-Cook, E.J.; Luhan, M.R.J.; Ferriols, V.M.E.N.; Hurtado, A.Q. Analysis of Biosecurity-Related Policies Governing the Seaweed Industry of the Philippines. J. Appl. Phycol. 2020, 32, 2009–2022. [Google Scholar] [CrossRef]
- Hayashi, L.; Hurtado, A.Q.; Msuya, F.E.; Bleicher-Lhonneur, G.; Critchley, A.T. A Review of Kappaphycus Farming: Prospects and Constraints. In Seaweeds and their Role in Globally Changing Environments; Seckbach, J., Einav, R., Israel, A., Eds.; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2010; Volume 15, pp. 251–283. ISBN 978-90-481-8568-9. [Google Scholar]
- Hurtado, A.Q.; Critchley, A.T. Time for Applications of Biostimulants in Phyconomy: Seaweed Extracts for Enhanced Cultivation of Seaweeds (SEECS). In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–127. ISBN 978-0-12-817943-7. [Google Scholar]
- Neish, I.C. Adaptive Phyconomy for Sustainable Management of Coastal Ecoscapes in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 763, 012009. [Google Scholar] [CrossRef]
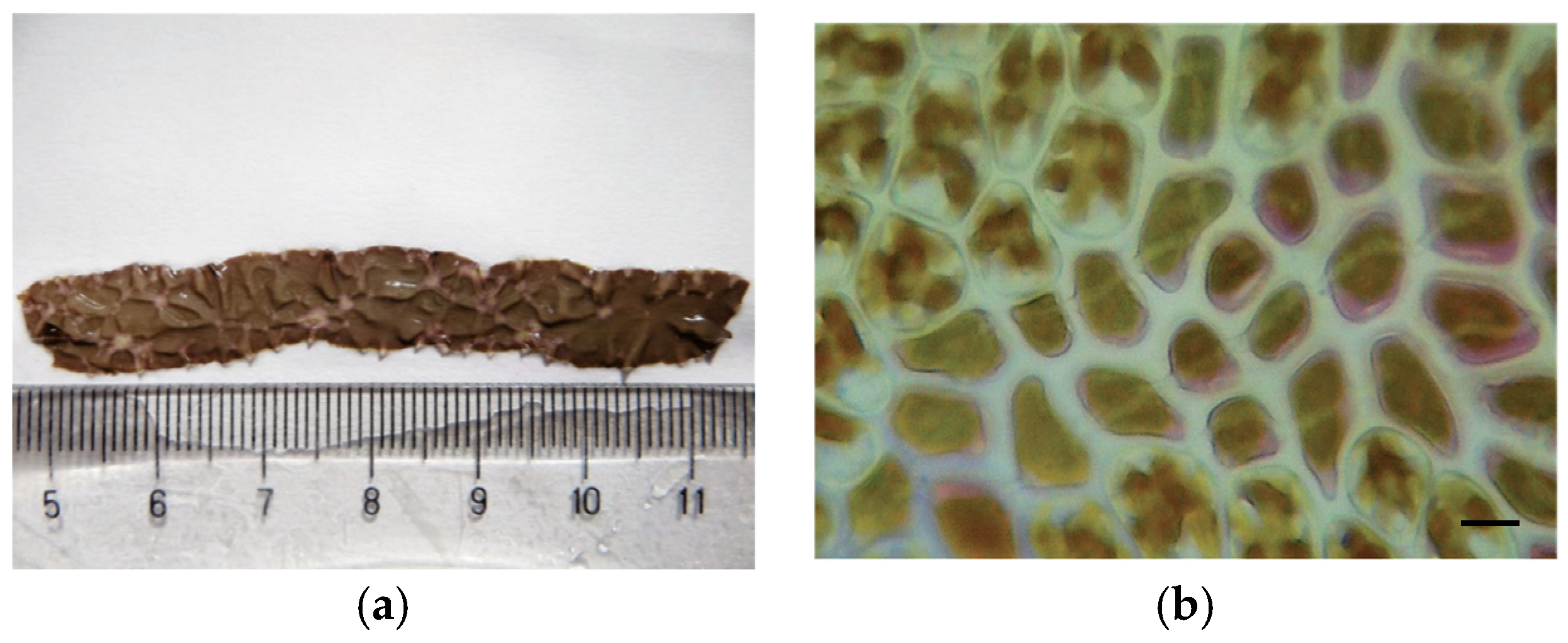
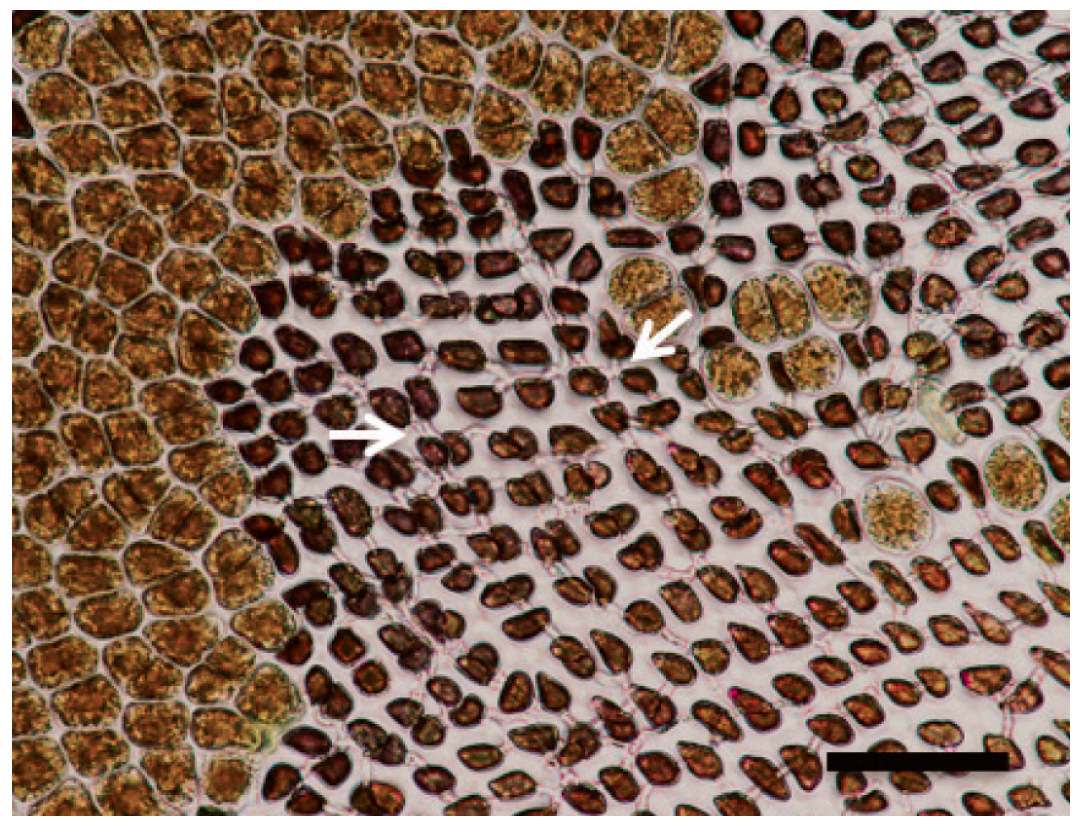
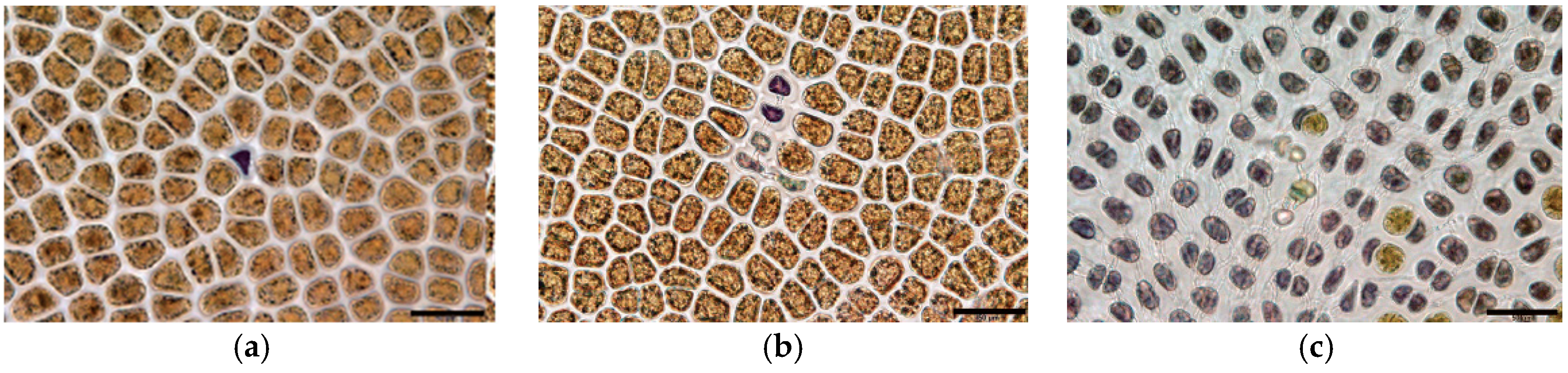
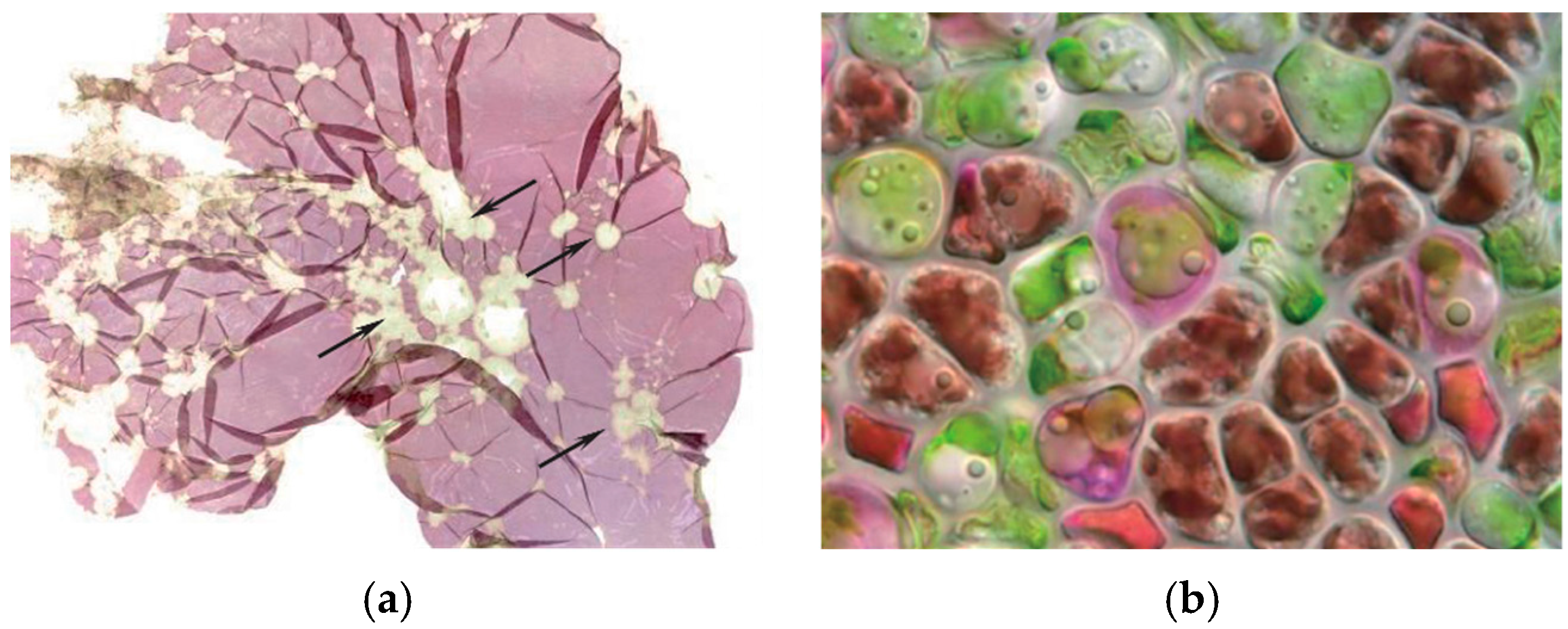




| Disease Name | Causative Organism/Taxonomy | Symptoms | Current Treatment | Effectiveness of Treatment | Severity ● ● ● | References |
|---|---|---|---|---|---|---|
| Red rot disease | Pythium porphyrae, Pythium chondricola/Oomycete Alternaria sp./Ascomycota | Red patches on the blade; blade’s colour changes from natural brownish-red to violet-red; formation of numerous holes, followed by disintegration of the blade | Exposure of culture nets to air; acid wash | Partially effective | High | [3,8,16,17,18,19,20,21,22,23,24,25,26,27] |
| Olpidiopsis disease | Olpidiopsis porphyrae, Olpidiopsis pyropiae, Olpidiopsis sp./Oomycete | Bleached portion on the blades; appearance of greenish lesions; formation of numerous holes, followed by disintegration of the entire blade | Exposure of culture nets to air; decrease in density of culture nets; acid wash; calcium propionate | No | High | [3,28,29,30,31,32] |
| Green-spot disease | Primary: PyroV1/Virus Secondary: Flavobacterium sp., Pseudoalteromonas sp., Vibrio sp./Gram-negative bacteria | Lesions with wide green borders; slimy rots and holes in the blade | Exposure of culture nets to air; acid wash | No | High | [3,33] |
| “Cyanobacteria felt” | Filamentous and coccoid blue-green algae/Cyanobacteria | Dirty blade surface; lesions and holes in the blade | Drying of culture nets; acid wash | Partially effective | Medium | [15] |
| “Diatom felt” | Fregellaria sp., Licmopohora flabellata, Melosira sp., Navicula sp./Bacillariophyceae | Dirty blade surface; blade bleaching; rust-coloured powder | Drying of culture nets; acid wash | Partially effective | Medium | [3] |
| White blight disease | ? | Random bleached areas on the blade; cell lysis | No treatment | No | Low | [15] |
| White rot disease | Vibrio sp./Gram-negative bacteria | Random circular bleached areas of thallus | No treatment | No | Low | [15] |
| “Suminori” disease | Gaetbulibacter saemankumensis, Arthrobacter tumbae, Flavobacterium spp., Vibrio spp./Gram-negative bacteria | Black lustreless colour of blade; plasmoptysis of blade cells | Exposure of culture nets to air; acid wash | Partially effective | Medium | [35] |
| “Anaaki” disease (often associated with green spot) | Flavobacterium sp., Pseudoalteromonas sp., Vibrio sp./Gram-negative bacteria | Random holes on the blade; fast degradation of the blade | Exposure of culture nets to air; acid wash | Partially effective | Medium | [3] |
| Unnamed disease | “Pseudomonas-like” bacteria/Gram-negative bacteria | Similarity to white rot disease | No treatment | No | Low | [15] |
| White spot disease | Phoma sp./Coelomycete | Bleaching of oyster shell with shell-boring conchocelis | Discarding infected oyster shells | Yes | Low | [36] |
| Yellow spot disease | Vibrio mediterranei 117-T6/Gram-negative bacteria | Yellow spots gradually spread around and form lesions of different sizes | / | / | n/a | [37] |
| Disease Name | Causative Organism /Taxonomy | Symptoms | Current Treatment | Effectiveness of Treatment | Severity ● ● ● | References |
|---|---|---|---|---|---|---|
| Epiphytes | Ceramium minuta, Polysiphonia forfex, Hypnea spp., and more species/Rhodophyta | Generally, epiphytes are attached superficially to the surface of the host; however, genera such as Polysiphonia spp. and Ceramium spp. can penetrate the host tissue, affecting its growth and, hence, its productivity | Control of nutrients; move and shift growing structures | Partially effective | Medium | [44] |
| Rotten thallus syndrome or “Thalluswhitening” | Vibrioparahaemolyticus, Vibrio spp., Thalassospira spp./Gram-negative bacteria (agarolytic) | Slow growth, whitening of axesand branches, increased thallusfragility | Transfer to areas with slightly greater water current | Partially effective | Medium | [45,46] |
| Bleaching Stripe Disease or “Cell-wall degradation” | Pseudoalteromonas spp./Gram-negative bacteria (agarolytic) | Cell wall degradation | / | / | n/a | [46] |
| White-tip disease | Bacterial strain OR-I1? | Fast development of white necrotic tissues, followed by thallus fragmentation | / | / | n/a | [44] |
| Brown points disease | Bacterial strain OR-I1? | “Tumour-like” growth, leading to proliferations of nearly1 mm diameter | / | / | n/a | [47] |
| Gracilaria Gall syndrome | Bacterial? | Small bump-like structures | / | / | Medium | [44] |
| Grazing | Fishes and invertebrates | Loss of biomass | Floating culture; control grazing | Yes | Medium | - |
| Country/Region/State | Start Massive Cultivation | First Report of Disease | Collapse | Recovered |
|---|---|---|---|---|
| Philippines | 1969 | 1975 (“ice-ice”) | 2002 | 2005/2008 |
| Indonesia | 1975 | 2000 | - | - |
| Malaysia | 1978 | - | 2012 | 2019/2022 |
| Tanzania | 1990 | 1995 | 2006 | Arguably never |
| South America | 2000 | 2010 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnuolo, D.; Genovese, G. Macroalgal Diseases: Exploring Biology, Pathogenesis, and Management Strategies. Phycology 2024, 4, 450-464. https://doi.org/10.3390/phycology4030026
Spagnuolo D, Genovese G. Macroalgal Diseases: Exploring Biology, Pathogenesis, and Management Strategies. Phycology. 2024; 4(3):450-464. https://doi.org/10.3390/phycology4030026
Chicago/Turabian StyleSpagnuolo, Damiano, and Giuseppa Genovese. 2024. "Macroalgal Diseases: Exploring Biology, Pathogenesis, and Management Strategies" Phycology 4, no. 3: 450-464. https://doi.org/10.3390/phycology4030026
APA StyleSpagnuolo, D., & Genovese, G. (2024). Macroalgal Diseases: Exploring Biology, Pathogenesis, and Management Strategies. Phycology, 4(3), 450-464. https://doi.org/10.3390/phycology4030026







