Abstract
As a producer of pigments with known bioactive potential, cyanobacteria are a great source of active ingredients for cosmetics (i.e., carotenoids and phycobiliproteins). Multiple phases in the cyanobacteria-based bioprocess led to the obtention of these compounds. The marine Cyanobium sp. LEGE 06113 has been proposed as a promising source for pigments for cosmetic uses, and it has been optimized in the past few years in terms of production, extraction, and application of pigment extracts. This report aims at providing an overview of the cyanobacteria-based bioprocess, regarding optimization strategies, consolidating into a proposed bioprocess for this cyanobacterium. The optimization of Cyanobium sp. included strategies regarding its production (culture medium, light, temperature, pH and salinity) and extraction (successive solvent extraction and ohmic heating). After the optimization, the two pigment-rich extracts (carotenoids and phycobiliproteins) were assessed in terms of their cosmetic potential and compatibility as an ingredient. Finally, aiming a scale-up proposal, life cycle assessment (LCA) was used as tool for a sustainable process. Ultimately, the proposed process gives the possibility to obtain two stable cosmetic ingredients from the same biomass and applied as anti-agent agents, especially due to their high anti-hyaluronidase capacity. Moreover, there remain challenges and information regarding novel cosmetic ingredient regulations were also discussed.
1. Introduction
Colour is believed to be the primary element seen by the senses, and it has played an important part in consumers acceptance for millennia in order to improve their actual appearance and quality [1]. Photosynthetic organisms such as cyanobacteria, microalgae, macroalgae, and plants can synthesise a wide range of light-absorbing compounds. Pigments are molecules that can absorb light in the visible spectrum, resulting in a colour reflection visible to the human eye [2]. Natural pigments have an important function not only for these organisms (which cannot survive or produce energy without them), but also for the environment (as oxygen and biomass primary producers) [3], and for society (since these species have been utilised as colorants for millennia) [4]. More recently, the push towards green-labelled products, sustainability and environmental awareness has resulted in a shift in industry expectations, as well as the rescue of hitherto known natural pigment sources that may be used to replace synthetic colourants [5].
Natural ingredients have been used for cosmetic reasons since before the notion of cosmetics, and they have long been prepared from mineral materials, herbal pastes, and oils [6]. With the concept of cosmeceuticals, the use of cyanobacteria as a source of components has even more potential due to their diverse range of produced compounds. Fatty acids, polyphenols, peptides, polysaccharides, and pigments are examples of chemicals with clearly delineated potential for health uses that can be found in these organisms [7].
The usage of Arthrospira spp. (commercial Spirulina) dates back to the Aztec Empire, while widespread recognition of its bioactive characteristics and increased utilisation did not occur until the 20th century [8]. Three types of pigments may be found in cyanobacteria: chlorophylls, carotenoids, and phycobiliproteins, each with various colours ranging from blue to red and a variety of physiochemical properties for use in food, feed, nutraceuticals, and cosmetics [5].
When it comes to cyanobacterial pigments, such as carotenoids and phycobiliproteins, it is possible to combine their bioactive capacity, varied colours, and cosmetic enhancement characteristics (e.g., moisturiser, stabilising agents) to pique the interest of the natural cosmetics industry, which typically apply these compounds in the form of extracts [5,9,10].
Because of their highly efficient antioxidant capability, carotenoids can be found in sunscreens, anti-aging, and antioxidant compositions. Inside cyanobacteria cells, these compounds are in charge of collecting exceeded energy from photosynthetic metabolism, preventing detrimental consequences and cell damage [11], and can be isolated and employed for a similar purpose in human skin, where extended exposure to sunlight (UV radiation and high light intensity) can cause cell damage [5,12,13].
Phycobiliproteins can be used in a similar way as functional additives because they have been linked to a number of bioactivities, including antioxidants, antivirals, antimicrobials, and anticancer, among others [14]. These compounds can also be utilised as natural colours to reduce skin toxicity and damage, as well as allergies to synthetic dyes [15]. Because of their water solubility, these blue hydrophilic pigments can be used in skincare products, particularly serum and lotions [5].
Despite the increased potential of cyanobacteria as a natural source of pigments for industrial use, there is still a gap in terms of technology scale-up, resulting in lower biomass output and higher production costs. As a result, there are still limitations to cyanobacteria-based processes in terms of biological, engineering, and economic factors [16,17]. The high installation and operation costs, the challenges in managing culture growth, and contamination by other microorganisms are just a few of the major issues [18]. One of the key restrictions in industrial production is a lack of knowledge of cyanobacteria basic biology, particularly in terms of biosynthesis and regulation of bioproducts [2]. An in-depth study of the growth and metabolic features of cyanobacteria is still required for industrial-scale culture [19].
Furthermore, the high cost of extraction and purification is the principal obstacle to the application of cyanobacteria pigments in industry. Additionally, since cosmetic formulations require a consistent and stable active ingredient, the large variety in pigment concentration and profile can be a major constraint for the cosmetic sector. Standardisation of growing and extraction processes, as well as a greater understanding of the biological characteristics of cyanobacteria pigments, are required to overcome these limitations [14].
Nevertheless, despite the wide variety of cyanobacteria species and biochemical profiles, Arthrospira platensis (Spirulina) remains the most popular source to cosmetic ingredients. Other cyanobacteria species, on the other hand, have been suggested in more fundamental studies. For the past few years, Pagels et al. have been studying and optimising the bioprocess of a marine cyanobacterium isolated in northern Portugal coast, Cyanobium sp. LEGE 06113. This report aims at providing an overview of the cyanobacteria-based bioprocess, regarding optimization strategies, consolidating into a proposed bioprocess for this cyanobacterium.
2. Cyanobacteria in Biotechnological Processes
Cyanobacteria are the only known prokaryote organisms capable of oxygenic photosynthesis, making them a unique class of organisms [20]. In nature, cyanobacteria can take many forms, including unicellular and filamentous, marine and freshwater, free-living and symbiotic, edible and hazardous species. They can be found in a wide range of habitats, including deserts, lakes, oceans, soil, glaciers, streams, and hot springs, due to their vast range of physiological properties and resilience to environmental stress [21,22].
Numerous studies from diverse fields, particularly those that specialise in healthcare ingredients, have been interested in marine cyanobacteria since they produce a variety of compounds. They have important biological qualities, such as antibacterial, antifungal, anticancer, antituberculosis, immunosuppressive, anti-inflammatory, and antioxidant attributes [23].
Arthrospira spp. are the most studied cyanobacteria and the only ones that are mass-produced on a significant scale around the world [24,25]. For commercial or industrial purposes, Arthrospira are normally produced in open raceway ponds, while greenhouses have been employed to enhance cost economics and annual Spirulina biomass productivity [24]. Inorganic compounds are used in these industrial-scale Arthrospira cultivations, which are expensive and require medium concentration adjustments to avoid contamination. Furthermore, temperature, light, pH, and aeration must all be regulated in the Arthrospira growth system to optimal biomass output [25].
Spirulina (as product) has achieved widespread appeal as a food supplement, being one of the most protein-dense foods known and recognised as a rich source of vitamins, amino acids, fatty acids and pigments [24].
Multiple stages lead to the synthesis of a given product in a cyanobacteria-based bioprocess. It begins with the identification and screening of strains with promising characteristics (quick growth, morphological advantages, and target compounds), and then moves on to biomass production, harvesting, cell disruption and extraction, and, if necessary, purification [26]. The bioprocess (Figure 1) varies depending on the end goal; for example, in pigments, the purification phase is sometimes overcome due to the high final cost, and most products are formulated with extracts [14].
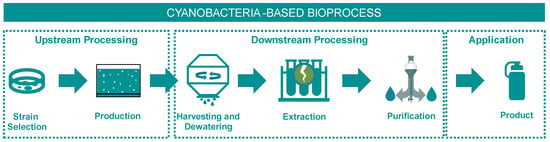
Figure 1.
Overview of a general cyanobacteria-based bioprocess.
More specifically for pigments production, the bioprocess requires special features due to the chemical characteristics of these molecules. Cyanobacteria, like all organisms, are influenced by a variety of abiotic and biotic stimuli, which can affect their growth and metabolite synthesis. Abiotic elements that might affect the organism’s metabolism include light, temperature, nutrient content, pH, salinity, and the chemical constitution of the culture medium. Biotic factors, on the other hand, can include pathogens (bacteria, fungi, and viruses) as well as various kinds of intra/interspecific competition, causing the organism’s response to the environment to fluctuate [27]. Cyanobacteria emerged as organisms that could adapt to a wide range of environments. These adaptations are frequently linked to the development of defensive apparatus, which results in the synthesis of several natural products and high-valued molecules. The stress that an organism is exposed to regulates the production of such metabolites and adjusting the conditions for the production of a target metabolite must take these various elements into account [28,29].
One of the most important aspects of the pigment manufacturing process is light optimization. Light, in a broad and general sense, is critical to the survival of many species. Cyanobacteria, like other photosynthetic organisms, grow in response to light. Furthermore, in a photoautotrophic system, the use and source of light is a major challenge in large-scale cyanobacteria production [2].
The main metabolic process, from biomass density change to compound accumulation, occurs during photosynthetic activity and other light-regulated pathways [30]. Thus, increasing pigment production can be primarily related to light quality (spectral composition), intensity, or photoperiod.
Other important factors to the production are the bioreactor size and design. For the production of cyanobacterial biomass, a variety of closed photobioreactors have been suggested, including flat-plate, tubular, vertical-column, and internally lit photobioreactors. These have all been reviewed in-depth elsewhere [31].
On the other hand, the extraction process is very important in obtaining natural pigments. The extraction of pigments can be influenced by a number of factors, including the target pigment, strain, market trends, available technology, and costs. Extraction usually demands the use of a cell disruption method and a compatible solvent. Furthermore, energy requirements are a critical component of the process’s long-term viability [32]. Extraction, according to Molina-Grima et al. [33], can account for up to 60% of the total expenditures. To reduce those costs, existing procedures must be optimized as well as new procedures developed.
Furthermore, using an optimized extraction method can reduce the need for purification. Purification may or may not be required depending on the final application (for example, in the case of food, feed, nutraceuticals, and cosmetics). The requirement for purification can increase the final price by a factor of a hundred [34].
Finally, the best scenario for cyanobacteria valorisation is the use of a biorefinery process, which increases economic feasibility by allowing the exploitation of various co-products that can be used individually [35].
3. Cyanobium sp. a Novel Biotechnological Cyanobacteria
The marine cyanobacterium Cyanobium sp. LEGE 06113, isolated in northern Portugal [36], is the subject of this case study (Figure 2). It is unicellular, has a small size (about 1 μm), does not form biofilms, and can be easily harvested by centrifugation.
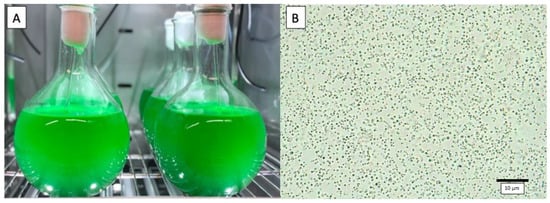
Figure 2.
Cyanobium sp. LEGE 06113 culture. (A) 2 L round flask during optimization trials (B) Optical microscope view (400×).
Its potential was first proposed as a producer of bioactive compounds with anticancer and antimalarial activity, such as hierridin B and C [37,38,39], including an already granted patent [40]. However, the content of such compounds is extremely low, reducing its interest in favour of an already described synthetic route [41]. Another way of valorising this strain could be to find alternative metabolites and new applications, as is the case of pigments. The production and extraction processes of pigments with antioxidant and anti-inflammatory properties have been investigated in the past years by Pagels et al. [42,43,44,45,46,47,48,49], also including the submission of a patent for cosmetic ingredients (Portuguese Provisional Patent Application no. 117951) and a scale-up proposal based on environmental evaluation. Furthermore, the Cyanobium-based bioprocess was optimized by focusing on two bioactive pigment extracts from the same biomass (carotenoids-rich and phycobiliproteins-rich extracts). Both pigment extracts can be especially used in the cosmetic industry.
The genus Cyanobium was proposed by Rippka and Cohen-Bazire [50] when a more precise classification of cyanobacteria strains was attempted. These cyanobacteria previously belonged to the genus Synechococcus, which includes organisms of varying sizes, chemical and physiological features, and a wide range of mean DNA-base composition [50]. The new genus Cyanobium is now utilised as a taxonomic rank that includes 14 species, with Cyanobium gracile Rippka & Cohen-Bazire serving as the type species [51]. The full genomes of a few Cyanobium strains, such as those obtained in Brazil and Japan, have previously been sequenced [52,53]. Cyanobium spp. can be found in a variety of habitats, including brackish water, freshwater, and marine environments, and when grown in culture, it has the unique ability to form a homogeneous suspension without producing mucilage [36,50,54]. Overall, the cyanobacteria of this genus are usually oval to ellipsoid single cells, with 1.2 ± 0.2 μm in diameter, but might show up in pairs, right after division, and rarely appear in small pseudofilaments. In the division process, it is symmetrical and occurs by binary fission in only one plane, parallel to the shorter cell axis. [36].
Other reports of Cyanobium cyanobacteria have been identified in a variety of biological applications. Pérez-Portilla et al. [55] described an isolated Cyanobium strain from the Salado River in Chile’s Atacama Desert that was more resistant to arsenic and cadmium compounds than other metal (loid)s-bioremediation species. In alkaline circumstances, up to 90 % of arsenic was removed during the first 3 h of exposure, suggesting that Cyanobium sp. isolated from the Atacama Desert should be researched further for biotechnological reasons and to better understand the evolutionary principles of dry adaptation. In another study, Armstrong et al. [56] observed the presence of volatile compound in Cyanobium sp. CENA178 (isolated in Brazil), including myristic acid, adipic acid, phytol, among others, which have been described with pharmaceutical relevance and other biotechnological applications.
Cyanobium sp. strains have been also proposed as source of carotenoids and with cosmetic potential, as in the case of Cyanobium gracile LEGE 12431 (isolated in Chile) evaluated by Lopes et al. [57], where this cyanobacterium showed the presence of several carotenoids (incl. lutein, β-carotene and echinenone) and antioxidant capacity against superoxide radical anion; and Cyanobium sp. LEGE 07175 (isolated in Portugal), which showed antioxidant and anti-hyaluronidase capacities in a cosmetic potential screening study [58].
Regarding process optimization for biomass production, another strain of Cyanobium have been also studied by a Brazilian group [59,60], which will be discussed further. Overall, Cyanobium sp. seem to have a great potential as a source of natural bioactive compounds due to the biological and biotechnological features.
4. Production Strategies
When it comes to industrial-scale bioprocesses, it is critical to optimize the processing parameters in order to enhance biomass/products output. The extraction is also influenced by the growing circumstances, which might alter the cell structure. As already mentioned, both abiotic (e.g., light, temperature, pH) and biotic elements should be considered in detail as much as possible, and in most cases, the optimisation is species- or strain specific, although some metabolic responses can be shared between species.
The optimization of the production of pigments by Cyanobium sp. was performed using with four different and complementary strategies: (i) complete multi-factor optimization of culture medium, light source, and intensity [42]; (ii) use of light supplements for metabolic activation [43]; (iii) factorial design optimization of temperature, pH and salinity [44]; and (iv) use of a two-phase light regime using white and red LEDs [45].
In the first strategy [42], Cyanobium sp. was grown in 16 conditions varying the light source (fluorescent lamp and low-pressure sodium lamp), light intensity (50 to 300 μmol photons m−2 s−1) and culture medium (marine BG11 and BG11+ doubled in nitrate (NaNO3, 3.00 g L−1) and phosphate (K2HPO4, 0.08 g L−1) concentration). The optimization considered growth, photosynthetic activity, antioxidant capacity, and biomass composition and productivity. The results showed a significant interaction among light quality, irradiance, and culture medium in all the analysed parameters, with the light irradiance being the one with most impact in the culture, as shown by a principal component analysis. For the production of carotenoids and phycobiliproteins, the optimal culture conditions include the use of a low-pressure sodium lamp, the BG11+ culture media, and a light irradiation of 200 μmol photons m−2 s−1. Overall, this study created a foundation for a potential application of this species, since it appears promising for the production of a variety of compounds also evaluated (soluble proteins, carbohydrates, phenolic compounds and lipids).
The second strategy [43] was to use light quality supplements in the optimal conditions selected. This strategy was created on the fact that four kinds of chromatic acclimation are known in cyanobacteria, with the type of response varying by the species and depending on the pigment composition of the organism [61]. Therefore, this strategy was based in the hypothesis that by supplementing the culture with residual amounts of red, green, blue light and UV radiation, it was possible to observe the main effects in terms of chromatic acclimation in Cyanobium sp. The results of this study show that red, green, and blue light supplementation had no effect on photosynthetic rate when compared to the control (only low-pressure sodium lamp), indicating that the photobiological response on biochemical profile induced by light qualities is due to the action of a specific photomorphogenic photoreceptor rather than a photosynthetic response. This finding suggests that the additional light was not used as active energy for photosynthesis. Supplemented UV radiation, on the other hand, reduced photosynthetic activity, most likely because of photo damage induced by this radiation. Overall, Cyanobium sp. demonstrated that red light is mostly responsible for positive photoactivation in the response of pigment synthesis. The production of lipids, phycocyanin, and carotenoids increased when Cyanobium sp. was exposed to red light irradiance. In addition, red light supplementation appears to stimulate the formation of bioactive molecules with a higher antioxidant capacity than the control.
Sequentially, the third strategy [44] was to use a response surface factorial design to optimize temperature, pH and salinity in Cyanobium sp. cultures. The application of factorial statistic optimization allows for the observation of both the interaction and individual effects of the factors being evaluated. Furthermore, with a smaller number of experimental trials, higher-order response surfaces can be generated. The Box–Behnken model looks to be a suitable tool for making cyanobacterial culture optimization more accessible and cost-effective. In this study, temperature (20 to 30 °C), pH (6 to 9) and NaCl concentration (10 to 30 g L−1) where optimized. The results showed that Cyanobium sp. growth was not inhibited in any of the tested conditions, with significative quadratic models for all the evaluated parameters. For phycobiliproteins and carotenoids co-production, the optimal condition was found at (20 °C, pH 9 and 10 g L−1 of NaCl). One of the main advantages of using these response surface models is that the optimal condition can be between tested conditions, what is not possible is extrapolating from multi-factor studies such as strategy one.
Finally, the last strategy [45] was to apply a two-phase light regime, with a growth phase in white LED and an accumulation phase in red LED. In this study, seven conditions were evaluated, varying the time of each phase, in a total of 21 days. In this study, the results revealed that Cyanobium sp. had two separate metabolisms, one under white LED and the other under red LED. Due of the wide spectral range of white LEDs, numerous photoreceptors are activated, perhaps leading to a larger biomass production [62]. In the case of red LEDs, the monochromatic spectrum activates only a portion of light responses, mostly phytochrome-related reactions [63]. Moreover, the optimal condition attained was 10 days of white plus 11 days of red led, with the highest productivity of biomass and pigments being achieved at day 14. Furthermore, the optimal culture process was set as 10 days of growth under white LED plus 4 days of pigments accumulation under red LED, with no further production of biomass nor pigment accumulation in the following days.
In terms of optimization strategies, processing parameters can be evaluated individually, in a sequential way, where one parameter is changed while all others are kept as optimal/sub-optimal [64], or simultaneously, in factorial optimizations, such as the response surface methodology, which considers the synergetic effect of two or more factors [65]. Overall, factorial optimization has received attention in recent years as a more reliable strategy, as it considers a large variety of processing parameters; however, the response surface models still have a biological limitation as it is set through mathematical models. Sequential optimization, on the other hand, is still used for many applications and is sometimes easier to be performed. Moreover, when optimizing pigments, it is recommended that light should be evaluated and optimized individually, as it is the factor with the greatest influence. Optimal parameters for the production are summarised in Table 1.

Table 1.
Optimized parameters for Cyanobium sp. pigments production and final biomass output [42,43,44,45].
In terms of phycobiliproteins accumulation, light must be adjusted within for different species as the response might vary dramatically. Keithellakpam et al. [66] found that larger amounts of phycocyanin are produced in Nostoc muscorum when exposed to red light, and Mishra et al. [67] reported the similar impact in Pseudanabaena sp. On the other hand, some Anabaena species have been shown to enhance overall phycobiliprotein productivity when exposed to blue light [64,68,69] as well as in Nostoc sphaeroides [70] and Synechococcus sp. [71]. This unusual change in phycobiliprotein levels could be linked to photoreceptors in the blue and violet zones — cryptochrome-like receptors – which could trigger the phycobiliprotein synthesis pathway. This can also be corroborated by greater phycobiliprotein production in Phormidium foveolarum, Nostoc muscorum, and Arthrospira platensis when UV-B radiation is supplemented [72].
Furthermore, red light has been shown to induce carotenogenesis in plants, algae, and cyanobacteria. Red light triggers a signalling response from red/far-red photoreceptors, as well as the expression of the CrtB gene (in cyanobacteria), allowing carotenoids to accumulate [73]. In the cyanobacterium Arthrospira platensis, the use of red light for the formation of carotenoids has also been described [74]. Olaizola and Duerr [75], who assessed the potential of Arthrospira platensis cultivated under white, blue, and red lighting, found a comparable favourable effect of red light, with an increase in β-carotene. On the other hand, red light was deleterious in Pseudanabaena sp., reducing the total carotenoids content, as reported by Mishra et al. [67].
5. Downstream Strategies
When a predefined molecule or combination of molecules is targeted as the ultimate product in a cyanobacteria-based bioprocess, downstream processing is necessary. The extraction procedure is composed of a cell disruption procedure and a suitable solvent, and it must be designed for each strain (based on morphological traits) and application (due to solvent compatibility and purity required).
Many extraction procedures have been developed and optimized in recent years as a result of technological advancements. Non-mechanical (chemical, thermal and enzymatic) and mechanical (such as pressured systems, ultrasonication, microwave, and electric fields) methods of extraction are extensively utilised [76].
Traditionally, cyanobacteria products have been extracted using a solvent extraction method that is typically combined with thermal processing. Solvent extraction entails combining the solid and liquid phases in order for the solute to interact with the solvent until equilibrium is reached. Transference from one phase to the other is induced by chemical affinities [77]. The cheaper cost of infrastructure and operating operations is a major advantage of this form of extraction. On the other hand, it frequently requires large amounts of (often organic) solvents and a long processing time. As a result, efficiency is frequently insufficient for industrial use. When selecting a solvent, it is necessary to take into account the compound’s solubility, toxicity, and environmental impact of residues.
The optimization of the extraction of pigments by Cyanobium sp. was performed with two different strategies: i) solvent selection and successive extraction proposal [46]; ii) factorial design optimization of a continuous pressurized system extraction (CPSE) and an electric fields-assisted extraction (ohmic heating), followed by a comparison between optimized methods [47].
In the first strategy [46], four different solvents were proposed for the extraction of pigments from Cyanobium sp. biomass, including water (targeting phycobiliproteins), and ethanol, acetone, and ethyl acetate (targeting carotenoids). These solvents were chosen because they are Generally Recognized as Safe (GRAS) solvents, and because they were previously described as compatible with the targeted pigments. The use of GRAS solvents allows a better acceptance of the industry and overcoming further processing challenges. Furthermore, aiming at a biorefinery concept, a second extraction was performed targeting the opposite group of pigments. Results showed that the successive acetonic extract after water (W-A) was the best in terms of carotenoids content and bioactive capacity among organic solvents, and the successive water extract after ethanol (E-W) was the best in terms of phycobiliproteins content and purity, while all aqueous extracts had similar antioxidant capacity. When considering the yield of extract and the toxicity of extracts, the route Ethanol -> Water (E-W) was the one allowing an extraction of about 50 % of the biomass, and no cytotoxic effect, being the chosen one for further optimizations. Furthermore, it appears that successive extractions are a viable method for maximising biomass utilisation, minimising waste and improving the value of the bioprocess connected with this cyanobacterium.
Then, the second strategy [47] aimed at optimizing two different cell disruption methodologies, one based on pressure (CPSE) and the other based on electric fields. These methods were chosen due to the scalability and advantages in terms of processing. The CPSE has the benefit of easy ethanolic extract recovery since the biomass is maintained in an extraction chamber after extraction, eliminating the need for centrifugation, whereas ohmic heating requires a second step of centrifugation or filtering. CPSE, on the other hand, operates in batches of precise amounts of biomass and cannot be applied continuously, whereas ohmic heating is easier to handle and can be applied continuously. In the study using Cyanobium sp. biomass, CPSE and ohmic heating were optimized in terms of temperature, time and flow or frequency, using a central composite design (factorial modelling). Results showed that optimal conditions for co-extraction of carotenoids and phycobiliproteins using CPSE were 70 °C, for 20 min at a pressure of ca. 110 bar (flow of 1.5 mL min−1), while the conditions for ohmic heating were 70 °C, for 5 min, with a frequency of 20 kHz. All evaluated parameters resulted in a quadratic model, validated afterwards by experimental trials in the optimal conditions. The comparison between methods—using homogenisation as reference—showed that ohmic heating induced a higher content of carotenoids and total antioxidant capacity compared to CPSE and homogenisation, and both CPSE and ohmic heating had superior phycobiliproteins content when compared to homogenisation. Overall, it is suggested to use ohmic heating for the extraction of carotenoids, phycobiliproteins, and co-extraction of the two groups of pigments, considering all of the results achieved in this study and the subsequent discussion. Optimal parameters for the extraction are summarized in Table 2.

Table 2.
Optimized parameters for Cyanobium sp. pigments extraction and final extract output [46,47].
Again, because extraction methods directly affect the concentration of cyanobacterial products, optimization and method selection are usually focused on a specific compound and/or strain. For example, Sarada et al. [78] assessed the extraction effectiveness of different technologies, in Arthrospira sp., the maximum extraction rate was achieved using freeze–thawing and homogenization, when combined with a hydrochloric acid pre-treatment; however, the pre-treatment led to a decrease in the purity of the compound. In the case of extraction of carotenoids, Dey and Rathod [79] optimised an ultrasound-assisted extraction of β-carotene from Arthrospira platensis, with optimal condition found using heptane at 30 °C and 167 W cm−2.
Regarding the technologies used in Cyanobium sp. similar systems were used in other cyanobacteria. Rodríguez-Meizoso et al. [80] used pressurized liquid extraction for the obtention of carotenoids from Phormidium spp., being the optimal condition found at 150 °C, for 20 min, at 100 bar. In another study, Martínez et al. [81] used electric-fields for the extraction of phycocyanin from Arthrospira platensis, with the optimal condition being 40 °C, for 50 pulses of 3 μs of 25 kV cm−1.
6. Applicability in Cosmetic Industry
After the optimization of production and extraction of Cyanobium sp., and obtention of two pigment-rich extracts (carotenoids and phycobiliproteins), the cosmetic potential of these pigments needed to be assessed [48]. For this, the two pigment-rich extracts were evaluated regarding its in vitro safety through cytotoxicity assays with skin-related cell lines—keratinocyte cell line (HaCat), fibroblast cell line (3T3L1) and endothelial cell line (hCMEC/D3)—and regarding the capacity of inhibition of cosmetic-related enzymes (hyaluronidase, tyrosinase, collagenase and elastase). The results showed that the extract showed no cytotoxic effects in skin-related cell lines and both of them had high anti-hyaluronidase capacity; moreover, the phycobiliproteins extract had also collagenase inhibition capacity [48].
Anti-hyaluronidase activity in cyanobacteria extracts and purified molecules has previously been documented [82]. In comparison to what was found for Cyanobium sp. extracts, isolated peptides from Arthrospira platensis showed an IC50 10 times higher [83]. In another study, Morone et al. [58] reported an anti-hyaluronidase capacity of Tychonema sp. ethanolic extracts with an IC50 two times higher than Cyanobium sp., and even when compared to one of the most used anti-ageing products (green tea), the Cyanobium sp. extracts had an advantage in terms of the bioactive capacity, being the IC50 of the water extract about half of the green tea.
On the other hand, when it comes to the collagenase inhibition, the water extract from Cyanobium sp., showed a lower inhibition capacity, with an IC50 about five times higher than peptides from Arthrospira platensis [83].
With the demonstrated cosmetic potential, the extracts were formulated into ingredients, and further evaluated in a skin serum formulation. The stability was evaluated following European and International standards (ISO/TR 18811/2018) in terms of pigment content, colour change and bioactive capacity. The results showed that both ingredient and serum were stable in low to mild temperatures (up to 20 °C), although at high temperatures (40 °C) further formulation with preservatives would be required. Overall, both extracts can be potential ingredients for cosmetical uses (anti-ageing), with relatively simple formulation and storage. The stability of cyanobacterial pigments in cosmetic products has not been described, although a few studied on pigments have been found for food processing [84,85,86].
The overall constitution of a cosmetic contains an active component as well as excipients (such as a vehicle, thickening agents, and additives) [15]. Cyanobacteria can contain molecules that can be employed in cosmetic formulations for a variety of purposes: colourants in make-up, UV protectors in sunscreens and anti-aging creams, and moisturisers in body lotions are examples of active ingredients [15]. Due to the wide range of biological activities with interest in this industry, cyanobacteria compounds, such as pigments, polyphenols, fatty acids, mycosporine-like amino acids (MAAs), and polysaccharides are among the groups with greater potential to act as active ingredients in cosmetic formulations [87].
For carotenoids, the most extensively utilised molecules are β-carotene from Dunaliella salina and astaxanthin from Haematococcus pluvialis. In the case of cyanobacteria, organic extracts containing carotenoids have been extensively proposed for many applications. For example, Arthrospira platensis ethanolic extract have been suggested as potential tyrosinase inhibitors [88], while Singh et al. [89] evaluated the antioxidant capacity of four cyanobacteria methanolic extracts (Plectonema boryanum, Hapalosiphon intricatus, Anabaena doliolum and Oscillatoria acuta), with Hapalosiphon intricatus being the extract with highest potential. Thus, Lopes et al. [57] reported the antioxidant, anti-inflammatory, and antiproliferative potential of ethanolic and acetonic extracts (containing lutein, zeaxanthin, and β-carotene, among other carotenoids) from cyanobacteria (Leptolyngbya-like sp. and Nodosilinea antarctica) with potential application in psoriasis treatment.
Regarding phycobiliproteins, Arthrospira platensis is the most common source of phycobiliproteins for cosmetic usage; however, other cyanobacteria and microalgae have been suggested in more basic studies, including Aphanizomenon flos-aquae as a wound-healing ingredient [90] or as anti-inflammatory component [91] or Anacystis nidulans, as an anti-aging and sunscreen ingredient [92].
7. Cosmetic Ingredients Regulation
Cosmetic items are regulated and monitored worldwide to guarantee their safety and efficacy. Nevertheless, cosmetics legislation is far from being standardised, and rules differ widely between countries [93]. Companies that want to commercialise products containing microalgae or cyanobacteria (or their active ingredients) must follow the rules, which normally include applying for authorisation from a regional government, submitting scientific data, and performing health and safety evaluations [94].
Looking at the key markets, some aspects are common, such as: manufacturer responsibility for product safety, and risk/reaction notification procedure. Moreover, the attempt to have a more standardised industry leads many countries to follow the International Nomenclature of Cosmetic Ingredients (INCI) [93].
The Personal Care Products Council (US) created the INCI system in the early 1970s. INCI names are used to list ingredients on cosmetic product labels in the United States, the European Union, China, Japan, and many other countries. Companies can apply for an INCI name, and it takes roughly 3-6 months for an INCI name to be assigned. Of note, the inclusion of an ingredient in INCI does not imply that the ingredient has been reviewed in terms of safety. In the US, the Cosmetic Ingredient Review conducts this type of evaluation independently [95].
European Cosmetic Regulation (CE 1223/2009) on cosmetic products is the primary regulatory framework for completed cosmetic products placed on the European market, and it introduces the concept of a Responsible Person. This person is responsible for ensuring that every cosmetic product put on the EU market meets all of the Cosmetic Regulation’s (CE 1223/2009) standards.
Furthermore, in Europe, the assessment of the safety of a cosmetic component has shifted to an ethical approach exempt of animal testing [96], particularly in natural cosmetics. The main alternatives to animal tests are based on the use of human cells and tissue in vitro cultures, multiple high-throughput ‘omics’ technologies, and computational analytical methods [96].
8. Life Cycle Assessment and Scale-up Simulation
The majority of bioprocess optimization is performed in the laboratory, using small volumes in strictly controlled conditions, and scale-up is still a challenge. Patents and trade-secrets cover most large-scale processes, which are rarely available in the literature. Process modelling and simulation using software-based tools is a useful methodology for the evaluation of process feasibility at scales larger than laboratory-scale, such as demo and industrial scales [97]. For example, Lopes et al. [97] evaluated the direct production of ethanol using a genetically modified Synechocystis sp. using process modelling for a techno-economic analysis in a 100 m3 reactor. Process modelling is considered a useful tool for reporting the environmental assessment of a production scheme by using the Life Cycle Assessment (LCA) methodology. This is the approach used by some authors, such as Rodríguez et al. [98], which report the environmental performance of biogas production from Arthrospira maxima in continuous mode in a 12 L stirred reactor. Environmental LCA is an analytical method for measuring the environmental impacts associated with material and energy consumption across a product’s or process’s complete life cycle [99].
The goal of an LCA evaluation and scale-up modelling for Cyanobium sp. [49] was to identify environmental hotspots in pigment production from Cyanobium sp. and to provide specific information in decision-making for sustainable scale-up from 20 L to 100 m3. The results revealed that, from the laboratory to the industrial scale, the cultivation of cyanobacteria has the greatest impact, owing to temperature control and electricity consumption, as confirmed by some of the sensitivity analyses performed. Furthermore, the use of simulation tools has provided fundamental information on downstream processing and the potential impact caused, with results that are within the same range of values found in the literature. Finally, the scale-up process reduced the impact when allocated to pigment extracts, despite a 50% reduction in biomass productivity in the large-scale process. To summarise, this report could help on the strategy of cyanobacterial production with lower environmental impacts by identifying the critical factors that must be overcome.
9. The Remaining Challenges on Cyanobium Bioprocess
As previously stated, there are still limitations in the biological, engineering, and economic aspects of cyanobacteria-based processes. The high installation and operation costs are just a few of the major issues [16]
The cyanobacteria-based pigment production process has several associated costs, ranging from production to purification. Water, nutrients, CO2 source, and energy costs are the most pressing issues in culture growth. Furthermore, the energy demand is a critical factor in the process’s long-term viability [100]. Besides that, since pigments are intracellular components, biomass recovery and further downstream processing are required. Even so, there is no universal protocol, and each situation and strain require a unique approach.
In the case of Cyanobium sp., most of production and extraction concerns were addressed, although further studies on carbon source, and metabolism (e.g., mixotrophic) could be performed. Trabelsi et al. [101], for example, demonstrated that Arthrospira sp. could grow in photoautotrophic, heterotrophic, and mixotrophic metabolisms, with the highest biomass concentration under mixotrophic conditions, with the addition of 1.5 g L−1 of glucose.
Moreover, the scale-up was modelled in optimal and stable conditions, and it is fundamental to understand how conditions fluctuations will affect the final biomass and the final product, similar to what Pereira et al. [102] reported for the microalga Tetraselmis sp. by scaling-up from a 20 mL culture to 100 m3, considering temperature and light fluctuations for an extended period of time.
Additionally, employing a semi-continuous mode was suggested to limit the environmental impact on the upstream process, using a percentage of the end culture as pre-inoculum for the next one. On a laboratory scale, a distinct strain of Cyanobium sp. has been reported in semi-continuous mode for up to 10 cycles [60]. This might mean a lower environmental impact from reactor cleaning and pre-inoculation, which is only required once per ten batches.
Besides that, harvesting and drying were not covered during this optimization. Centrifugation is the preferred method of harvesting at an industrial scale, particularly in the production of high-value-added compounds, so the concentrates have a longer shelf life [103]. Centrifugation is the fastest method for harvesting, but it consumes a lot of energy and the settling characteristics of each strain morphology, the slurry’s residence time in the centrifuge, and the settling depth will have an influence in the degree of recovery and efficiency of the process [33,104].
Furthermore, one of the most important steps in determining the efficiency of the downstream process for cyanobacteria is drying, and the most common drying methods used in large-scale production are freezing, spray, and convection drying, with the feasibility of these methods varying depending on the desired end-use application [105]. Seghiri et al. [106] compared traditional convection drying, freeze-drying, and spray drying for phycobiliprotein extraction in Arthrospira platensis, ultimately deciding that convection drying (70 °C for 8 h) was the best method, despite the fact that freeze-drying produced a high purity final product. Pérez-López et al. [107] also suggested freeze-drying for the laboratory size and spray drying for the pilot scale to reduce time of operation. As no data is described for the Cyanobium sp. biomass, the use of freeze-drying is still recommended, although is the one with the highest environmental load, as it is the only method with available results.
10. Process Overview
The proposed bioprocess is based on the gathering of all optimization strategies proposed by Pagels et al. [42,43,44,45,46,47,48,49] and discussed above for Cyanobium sp., with the aim to maximise biomass utilisation and to reduce waste (Figure 3).
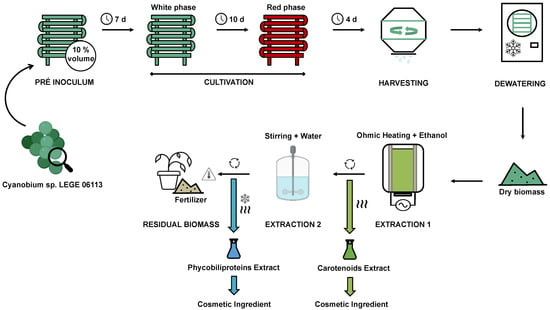
Figure 3.
Final proposed model for Cyanobium sp. bioprocess.
The process starts with a pre-inoculum, that should be produced in the same reactor of inoculation. With this approach, the need for a second cleaning stage for the reactor is avoided, leading to a reduction in water consumption. The reactor should be cleaned using ozone and filled with 10 % of final volume of BG11 medium supplemented with twice nitrates and phosphates (pH 9 and NaCl concentration of 10 g L−1) and sterilised though ozone sterilisation, prior to the addition of the Cyanobium sp. LEGE 06113 inoculum. The cyanobacterium culture is then incubated for 7 days under controlled conditions (20 ºC; continuous air supply; sun light for an average of 9 h and artificial illumination (40 W m−2, white LEDs) for 7 h per day to compensate for the reduced photoperiod.
Then, the cultivation is performed by adding the remaining 90 % of BG11 medium to the reactor and the culture is prolonged for 14 days (10 days on white LED plus 4 days on red LED) under the same conditions as the pre-inoculum; during the red phase, the reactor should be covered by a red filter (LEE 026 bright red). After cultivation, the biomass should be harvested by centrifugation and dewatered using freeze-dying. For the first extraction (carotenoids), the biomass should be submitted to ohmic heating technology (5 min, 70 °C, 20 kHz), using ethanol as a solvent at a ratio of 10:1 to biomass. After treatment, the mixture is centrifuged, and the ethanol is separated in a rotary-dryer, obtaining at this point the dry carotenoid extract (0.27 g g−1 of dry biomass).
For the extraction of phycobiliproteins, the remaining biomass is resuspended in water (same volume as ethanol) and shaken for 30 min. The solution is centrifuged and freeze-dried (48 h). At this point, the phycobiliproteins extract is obtained (0.25 g g−1 of dry biomass). Finally, the remaining biomass is dried in a drying oven, yielding a residual biomass product (0.48 g g−1 of dry biomass). The dry residual biomass is produced with the aim to be used as a fertiliser by-product due to its high nitrogen content (12.1 %).
The optimised process provides and integrates new knowledge about the production, extraction, and application of a cyanobacteria-based bioprocess, more specifically regarding Cyanobium sp., and providing considerable scientific advances on the pigments bioprocess, as the biorefinery concept rarely focused on these products, thereby contributing to facilitate and guide future optimisation strategies and process planning.
Author Contributions
Conceptualization, F.P., A.C.G., A.A.V. and V.V.; investigation, F.P.; writing—original draft preparation, F.P.; supervision, A.C.G., A.A.V. and V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was co-supported by a PhD fellowship granted to author F.P. [SFRH/BD/136767/ 2018] funded by Foundation for Science and Technology (FCT, Portugal) under the auspices of Operational Program Human Capital (POCH), supported by the European Social Fund and Portuguese funds (MECTES); as well as by the national funds through FCT, (UIDB/04423/2020 and UIDP/04423/2020) to CIIMAR and (UIDB/04469/2020) to CEB.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This paper and the research behind it would not have been possible without the exceptional support of all the researchers involved in this project: from University of Porto, Isabel Sousa Pinto, Helena Amaro, Graciliana Lopes, and Daniel Salvaterra; from University of Minho, Ricardo Pereira; from University of Málaga, Felix Figueroa, Roberto Abdala-Díaz and Julia Veiga; from Federal University of Santa Catarina, José Bonomi Barufi; from University of Santiago de Compostela, Maite Moreira and Ana Arias.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural Colorants from Plant Pigments and Their Encapsulation: An Emerging Window for the Food Industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from Microalgae. In Handbook of Microalgae-Based Processes and Products; Academc Press: Cambridge, MA, USA, 2020; pp. 465–492. [Google Scholar]
- Masojdek, J.; Koblek, M.; Torzillo, G. Photosynthesis in Microalgae. In Handbook of Microalgal Culture; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 20–39. ISBN 9780470673898. [Google Scholar]
- Stewart, S. Painted Faces: A Colourful History of Cosmetics; Amberley Publishing Limited: Stroud, UK, 2017; ISBN 9781445654003. [Google Scholar]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A.C. Cosmetic Applications of Microalgal and Cyanobacterial Pigments. In Algal Genetic Resources Cosmeceuticals, Nutraceuticals, and Pharmaceuticals from Algae; Sangeetha, J., Thangadurai, D., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2022; Volume 1. [Google Scholar]
- Scott, D.A. A Review of Ancient Egyptian Pigments and Cosmetics. Stud. Conserv. 2016, 61, 185–202. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of High Added-Value Compounds—A Brief Review of Recent Work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Bernal-Castillo, J.; Rozo, C.; Rodríguez, I. Sp/Rulina (Arthrospira) an Edible Microorganism a Review. Univ. Sci. 2003, 8, 7–24. [Google Scholar]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of Algae in Cosmetics: An Overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269–1278. [Google Scholar]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the Potential of Cyanobacteria in Cosmetics and Cosmeceuticals —A New Bioactive Approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Anunciato, T.P.; Rocha-Filho, P.A. Carotenoids and Polyphenols in Nutricosmetics, Nutraceuticals, and Cosmeceuticals. J. Cosmet. Derm. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Shegokar, R.; Mitri, K. Carotenoid Lutein: A Promising Candidate for Pharmaceutical and Nutraceutical Applications. J. Diet. Suppl. 2012, 9, 183–210. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from Marine Sources. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. ISBN 9783642539718. [Google Scholar]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production Cost of a Real Microalgae Production Plant and Strategies to Reduce It. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Microalgal Biotechnology at the Turn of the Millennium: A Personal View. J. Appl. Phycol. 2000, 12, 441–451. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the Potential of Microalgae for New Biotechnology Applications and beyond: A Review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Wang, Q. Ten Years of Algal Biofuel and Bioproducts: Gains and Pains. Planta 2019, 249, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Knoop, H.; Axmann, I.M.; Steuer, R. The Diversity of Cyanobacterial Metabolism: Genome Analysis of Multiple Phototrophic Microorganisms. BMC Genom. 2012, 13, 56. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Hayes, P.K.; Blank, C.E. Morphological and Habitat Evolution in the Cyanobacteria Using a Compartmentalization Approach. Geobiology 2005, 3, 145–165. [Google Scholar] [CrossRef]
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring Marine Cyanobacteria for Lead Compounds of Pharmaceutical Importance. Sci. World J. 2012, 2012, 179782. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Grewe, C.B.; Pulz, O. The Biotechnology of Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 9789400738553. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From Growth to Nutritional Product: A Review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Lackner, M.; Herwig, C. Bioprocess Engineering Aspects of Sustainable Polyhydroxyalkanoate Production in Cyanobacteria. Bioengineering 2018, 5, 111. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Anderson, L.K.; Rayner, M.C.; Sweet, R.M.; Eiserling, F.A. Regulation of Nostoc Sp. Phycobilisome Structure by Light and Temperature. J. Bacteriol. 1983, 155, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.E.; Graham, J.M.; Wilcox, L.W.; Cook, M.E. Algae, 3rd ed.; LJLM Press: Madison, WI, USA, 2016; ISBN 978-0321559654. [Google Scholar]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light Emitting Diodes (LEDs) Applied to Microalgal Production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Malcata, F.X. Enclosed “Non-Conventional” Photobioreactors for Microalga Production: A Review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Molina-Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- de Morais, M.G.; da Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from Microalgae: Properties, Extraction and Purification, with Some Recent Applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Bastiaens, L.; van Roy, S.; Thomassen, G.; Elst, K. Biorefinery of Algae: Technical and Economic Considerations. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 327–345. ISBN 9780081010273. [Google Scholar]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial Diversity Held in Microbial Biological Resource Centers as a Biotechnological Asset: The Case Study of the Newly Established LEGE Culture Collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef]
- Costa, M.; Sampaio-Dias, I.E.; Castelo-Branco, R.; Scharfenstein, H.; Rezende De Castro, R.; Silva, A.; Schneider, M.P.C.; Araújo, M.J.; Martins, R.; Domingues, V.F.; et al. Structure of Hierridin c, Synthesis of Hierridins b and c, and Evidence for Prevalent Alkylresorcinol Biosynthesis in Picocyanobacteria. J. Nat. Prod. 2019, 82, 393–402. [Google Scholar] [CrossRef]
- Freitas, S.; Martins, R.; Costa, M.; Leão, P.N.; Vitorino, R.; Vasconcelos, V.; Urbatzka, R. Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells. Mar. Drugs 2016, 14, 158. [Google Scholar] [CrossRef]
- Leão, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor Activity of Hierridin B, a Cyanobacterial Secondary Metabolite Found in Both Filamentous and Unicellular Marine Strains. PLoS ONE 2013, 8, e69562. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.N.; Martins, M.D.R.; Costa, M.; Vasconcelos, V.; Domingues, V.; Nogueira, F. Antimalarial Agent, Methods and Uses Thereof. Patent No. WO2016207869A1, 29 December 2016. [Google Scholar]
- Brandão, P.; Moreira, J.; Almeida, J.; Nazareth, N.; Sampaio-Dias, I.E.; Vasconcelos, V.; Martins, R.; Leão, P.; Pinto, M.; Saraíva, L.; et al. Norhierridin B, a New Hierridin B-Based Hydroquinone with Improved Antiproliferative Activity. Molecules 2020, 25, 1578. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Barufi, J.B.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light Regulating Metabolic Responses of Cyanobium Sp. (Cyanobacteria). Fundam. Appl. Limnol. 2020, 193, 285–297. [Google Scholar] [CrossRef]
- Pagels, F.; Bonomi-Barufi, J.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light Quality Triggers Biochemical Modulation of Cyanobium Sp.—Photobiology as Tool for Biotechnological Optimization. J. Appl. Phycol. 2020, 32, 2851–2861. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Lopes, G.; Sousa-Pinto, I.; Vasconcelos, V.; Guedes, A.C. Factorial Optimization of Upstream Process for Cyanobium Sp. Pigments Production. J. Appl. Phycol. 2020, 32, 3861–3872. [Google Scholar] [CrossRef]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A.C. White and Red LEDs as Two-Phase Batch for Cyanobacterial Pigments Production. Bioresour. Technol. 2020, 307, 123105. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Lopes, G.; Sousa-Pinto, I.; Vasconcelos, V.; Guedes, A.C. Bioactive Potential of Cyanobium Sp. Pigment-Rich Extracts. J. Appl. Phycol. 2020, 32, 3031–3040. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Amaro, H.M.; Vasconcelos, V.; Guedes, A.C.; Vicente, A.A. Continuous Pressurized Extraction versus Electric Fields-Assisted Extraction of Cyanobacterial Pigments. J. Biotechnol. 2021, 334, 35–42. [Google Scholar] [CrossRef]
- Pagels, F.; Almeida, C.; Vasconcelos, V.; Guedes, A.C. Cosmetic Potential of Pigments Extracts from the Marine Cyanobacterium Cyanobium Sp. Mar. Drugs 2022, 20, 481. [Google Scholar] [CrossRef]
- Pagels, F.; Arias, A.; Guedes, A.C.; Vicente, A.A.; Vasconcelos, V.; Moreira, M.T. Identifying Key Environmental Indicators in the Assessment of the Proof-of-Concept in Pigment Production from the Marine Cyanobacterium Cyanobium Sp. Appl. Sci. 2022, 12, 12999. [Google Scholar] [CrossRef]
- Rippka, R.; Cohen-Bazire, G. The Cyanobacteriales: A Legitimate Order Based on the Type Strain Cyanobacterium Stanieri? Ann. Inst. Pasteur Microbiol. 1983, 134, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How Many Species of Algae Are There? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.J.; Siqueira, A.S.; dos Santos, B.G.S.; da Silva, F.D.F.; Lima, C.P.; Cardoso, J.F.; Vianez, J.L.D.S.G.; Dall’Agnol, L.T.; McCulloch, J.A.; Nunes, M.R.T.; et al. Draft Genome Sequence of the Brazilian Cyanobium sp. Strain CACIAM 14. Genome Announc. 2014, 2, 669–714. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimura, Y.; Suzuki, S.; Yamagishi, T.; Tatarazako, N.; Kawachi, M. Complete Genome Sequence of Cyanobium Sp. NIES-981, a Marine Strain Potentially Useful for Ecotoxicological Bioassays. Genome Announc. 2016, 4, e00736-16. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Kopecký, J.; Cepák, V. Generic Characters of the Simplest Cyanoprokaryotes Cyanobium, Cyanobacterium and Synechococcus. Cryptogam. Algol. 1999, 20, 209–222. [Google Scholar] [CrossRef]
- Pérez-Portilla, P.; Araya, J.; Gallardo, K.; Aránguiz-Acuña, A. Potential of Arsenic Bioremediation by a Cyanobacterium Isolated from the Salado River in the Atacama Desert. J. Plankton Res. 2021, 43, 156–160. [Google Scholar] [CrossRef]
- Armstrong, L.; Vaz, M.G.M.V.; Genuário, D.B.; Fiore, M.F.; Debonsi, H.M. Volatile Compounds Produced by Cyanobacteria Isolated from Mangrove Environment. Curr. Microbiol. 2019, 76, 575–582. [Google Scholar] [CrossRef]
- Lopes, G.; Clarinha, D.; Vasconcelos, V. Carotenoids from Cyanobacteria: A Biotechnological Approach for the Topical Treatment of Psoriasis. Microorganisms 2020, 8, 302. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar. Drugs 2020, 18, 486. [Google Scholar] [CrossRef]
- Adriano, A.H.; Gabriel, M.D.R.; Luiza, M.; Michele, G.D.M.; Jorge, A.V.C. The Cultivation of Microalgae Cyanobium Sp. and Chlorella Sp. in Different Culture Media and Stirring Setting. Afr. J. Microbiol. Res. 2015, 9, 1431–1439. [Google Scholar] [CrossRef]
- Henrard, A.A.; de Morais, M.G.; Costa, J.A.V. Vertical Tubular Photobioreactor for Semicontinuous Culture of Cyanobium sp. Bioresour. Technol. 2011, 102, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Gutu, A.; Kehoe, D.M. Emerging Perspectives on the Mechanisms, Regulation, and Distribution of Light Color Acclimation in Cyanobacteria. Mol. Plant. 2012, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.L. Seeing New Light: Recent Insights into the Occurrence and Regulation of Chromatic Acclimation in Cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, L.B.; Kehoe, D.M. Diverse Light Responses of Cyanobacteria Mediated by Phytochrome Superfamily Photoreceptors. Nat. Rev. Microbiol. 2019, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Hemlata; Fatma, T. Screening of Cyanobacteria for Phycobiliproteins and Effect of Different Environmental Stress on Its Yield. Bull. Env. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Luna, L.D.; Bezerra, R.P.; Matsudo, M.C.; Sato, S.; Converti, A.; de Carvalho, J.C.M. Influence of PH, Temperature, and Urea Molar Flowrate on Arthrospira Platensis Fed-Batch Cultivation: A Kinetic and Thermodynamic Approach. Biotechnol. Bioeng. 2007, 96, 702–711. [Google Scholar] [CrossRef]
- Keithellakpam, O.S.; Nath, T.O.; Oinam, A.S.; Thingujam, I.; Oinam, G.; Dutt, S.G. Effect of External PH on Cyanobacterial Phycobiliproteins Production and Ammonium Excretion. J. Appl. Biol. Biotechnol. 2015, 3, 38–42. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Maurya, R.R.; Patidar, S.K.; Haldar, S.; Mishra, S. Effect of Light Quality on the C-Phycoerythrin Production in Marine Cyanobacteria Pseudanabaena sp. Isolated from Gujarat Coast, India. Protein Expr. Purif. 2012, 81, 5–10. [Google Scholar] [CrossRef]
- Khattar, J.I.S.; Kaur, S.; Kaushal, S.; Singh, Y.; Singh, D.P.; Rana, S.; Gulati, A. Hyperproduction of Phycobiliproteins by the Cyanobacterium Anabaena Fertilissima PUPCCC 410.5 under Optimized Culture Conditions. Algal Res. 2015, 12, 463–469. [Google Scholar] [CrossRef]
- Vijaya, V.; Anand, N. Blue light enhance the pigment synthesis in cyanobacterium Anabaena Ambigua Rao (NOSTACALES). ARPN J. Agric. Biol. Sci. 2009, 4, 36–43. [Google Scholar]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of Light Intensity and Quality on Phycobiliprotein Accumulation in the Cyanobacterium Nostoc Sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.S.; Choi, C.Y. Profiles of Photosynthetic Pigment Accumulation and Expression of Photosynthesis-Related Genes in the Marine Cyanobacteria Synechococcus Sp.: Effects of LED Wavelengths. Biotechnol. Bioprocess Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Kumar, M.; Kulshreshtha, J.; Singh, G.P. Growth and Biopigment Accumulation of Cyanobacterium Spirulina Platensis at Different Light Intensities and Temperature. Braz. J. Microbiol. 2011, 42, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhao, F.; Wei, W.; Wen, Z.; Qin, S. Carotenoid Biosynthesis in Cyanobacteria: Structural and Evolutionary Scenarios Based on Comparative Genomics. Int. J. Biol. Sci. 2006, 2, 197–207. [Google Scholar] [CrossRef]
- Lima, G.M.; Teixeira, P.C.N.; Teixeira, C.M.L.L.; Filócomo, D.; Lage, C.L.S. Influence of Spectral Light Quality on the Pigment Concentrations and Biomass Productivity of Arthrospira Platensis. Algal Res. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Olaizola, M.; Duerr, E.O. Effects of Light Intensity and Quality on the Growth Rate and Photosynthetic Pigment Content of Spirulina Platensis. J. Appl. Phycol. 1990, 2, 97–104. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Vambe, M.; Lovász, C.; Molnár, Z.; van Staden, J.; Ördög, V. Effect of Cell Disruption Methods on the Extraction of Bioactive Metabolites from Microalgal Biomass. J. Biotechnol. 2020, 307, 35–43. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Garcia-Perez, J.S.; Chandra, R.; Castillo-Zacarias, C.; Iqbal, H.M.N.; Parra-Saldívar, R. Methods for Extraction of Valuable Products from Microalgae Biomass. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 245–263. ISBN 9789811322648. [Google Scholar]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina Sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective Inhibition of Skin Cancer, Tyrosinase, and Antioxidative Properties by Astaxanthin and Astaxanthin Esters from the Green Alga Haematococcus Pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Pressurized Fluid Extraction of Bioactive Compounds from Phormidium Species. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-Phycocyanin Extraction Assisted by Pulsed Electric Field from Artrosphira Platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria and Microalgae Bioactive Compounds in Skin-Ageing: Potential to Restore Extracellular Matrix Filling and Overcome Hyperpigmentation. J. Enzym. Inhib. Med. Chem. 2021, 36, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, G.E.B.; Thomaz-Soccol, V.; Vandenberghe, L.P.S.; Carvalho, J.C.; Faulds, C.B.; Bertrand, E.; Prado, M.R.M.; Bonatto, S.J.R.; Soccol, C.R. Arthrospira Maxima OF15 Biomass Cultivation at Laboratory and Pilot Scale from Sugarcane Vinasse for Potential Biological New Peptides Production. Bioresour. Technol. 2019, 273, 103–113. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Galetović, A.; Dufossé, L. Phycobiliproteins as Food Additives. In Pigments from Microalgae Handbook; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Szterk, A.; Roszko, M.; Górnicka, E. Chemical Stability of the Lipid Phase in Concentrated Beverage Emulsions Colored with Natural β-Carotene. JAOCS J. Am. Oil Chem. Soc. 2013, 90, 483–491. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Microalgal Application in Cosmetics. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 317–323. ISBN 9780128114056. [Google Scholar]
- Sahin, S.C. The Potential of Arthrospira Platensis Extract as a Tyrosinase Inhibitor for Pharmaceutical or Cosmetic Applications. S. Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Meena, K.K.; Sharma, L.; Sharma, A.K. Induced Accumulation of Polyphenolics and Flavonoids in Cyanobacteria under Salt Stress Protects Organisms through Enhanced Antioxidant Activity. Am. J. Plant. Sci. 2014, 5, 726–735. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Catalán-Latorre, A.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-Encapsulating Hyalurosomes as Carrier for Skin Delivery and Protection from Oxidative Stress Damage. J. Mater. Sci. Mater. Med. 2016, 27, 75. [Google Scholar] [CrossRef]
- Scoglio, S.; Canestrari, F.; Benedetti, S.; Zolla, L. Extracts of Aphanizomenon Flos Aquae and Nutritional Cosmetic and Pharmaceutical Compositons Containing the Same. U.S. Patent US 2010/0021493 A1, 28 January 2010. [Google Scholar]
- Ruiz Canovas, E.; López Cerro, M.T.; Latil de Ros, D.G.; Durany Turk, O.; Segura De Yebra, J.; Mercadé Roca, J. Compositions for Protecting Skin Comprising DNA Repair Enzymes and Phycobiliprotein. U.S. Patent US 11071707, 20 July 2016. [Google Scholar]
- Dorato, S. General Concepts: Current Legislation on Cosmetics in Various Countries. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chsvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Nikitakis, J.; Lange, B. International Cosmetic Ingredient Dictionary and Handbook; Personal Care Products Council: Washington, DC, USA, 2016. [Google Scholar]
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S.; et al. Current EU Regulatory Requirements for the Assessment of Chemicals and Cosmetic Products: Challenges and Opportunities for Introducing New Approach Methodologies. Arch. Toxicol. 2021, 95, 1867–1897. [Google Scholar] [CrossRef]
- Lopes, T.F.; Cabanas, C.; Silva, A.; Fonseca, D.; Santos, E.; Guerra, L.T.; Sheahan, C.; Reis, A.; Gírio, F. Process Simulation and Techno-Economic Assessment for Direct Production of Advanced Bioethanol Using a Genetically Modified Synechocystis sp. Bioresour. Technol. Rep. 2019, 6, 113–122. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Moreno, J.; Vicente, G.; Bautista, L.F.; Morales, V.; Sánchez-Bayo, A.; Dufour, J. Environmental Analysis of Spirulina Cultivation and Biogas Production Using Experimental and Simulation Approach. Renew. Energy 2018, 129, 724–732. [Google Scholar] [CrossRef]
- Zhang, Y.I.; Singh, S.; Bakshi, B.R. Accounting for Ecosystem Sewices in Life Cycle Assessment Part I: A Critical Review. Env. Sci. Technol. 2010, 44, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M.; Barbosa, M.; Gouveia, L.; Sepúlveda, C.; Bazaes, J.; Arbib, Z. Economics of Microalgae Production. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 485–503. ISBN 9780081010273. [Google Scholar]
- Trabelsi, L.; ben Ouada, H.; Zili, F.; Mazhoud, N.; Ammar, J. Evaluation of Arthrospira Platensis Extracellular Polymeric Substances Production in Photoautotrophic, Heterotrophic and Mixotrophic Conditions. Folia Microbiol. 2013, 58, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Páramo, J.; Silva, J.; Marques, A.; Barros, A.; Maurício, D.; Santos, T.; Schulze, P.; Barros, R.; Gouveia, L.; et al. Scale-up and Large-Scale Production of Tetraselmis Sp. CTP4 (Chlorophyta) for CO2 Mitigation: From an Agar Plate to 100-M3 Industrial Photobioreactors. Sci. Rep. 2018, 8, 5112. [Google Scholar] [CrossRef]
- Heasman, M.; Diemar, J.; O’Connor, W.; Sushames, T.; Foulkes, L. Development of Extended Shelf-Life Microalgae Concentrate Diets Harvested by Centrifugation for Bivalve Molluscs—A Summary. Aquac. Res. 2000, 31, 637–659. [Google Scholar] [CrossRef]
- Guedes, A.C.; Katkam, N.G.; Varela, J.; Malcata, F.X. Photobioreactors for Cyanobacterial Culturing. In Cyanobacteria: An Economic Perspective; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 270–292. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Danquah, M.K. Chapter 9—Dewatering and Drying of Algal Cultures. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 207–224. ISBN 978-0-12-818536-0. [Google Scholar]
- Seghiri, R.; Legrand, J.; Hsissou, R.; Essamri, A. Comparative Study of the Impact of Conventional and Unconventional Drying Processes on Phycobiliproteins from Arthrospira Platensis. Algal Res. 2021, 53, 102165. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of the Red Antioxidant Carotenoid Astaxanthin by Microalgae: From Lab to Pilot Scale. J. Clean Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).