Sociodemographic Associations of Dementia Literacy in Older Australians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population and Recruitment
2.3. Procedure
2.4. Measures
2.5. Statistical Analysis
3. Results
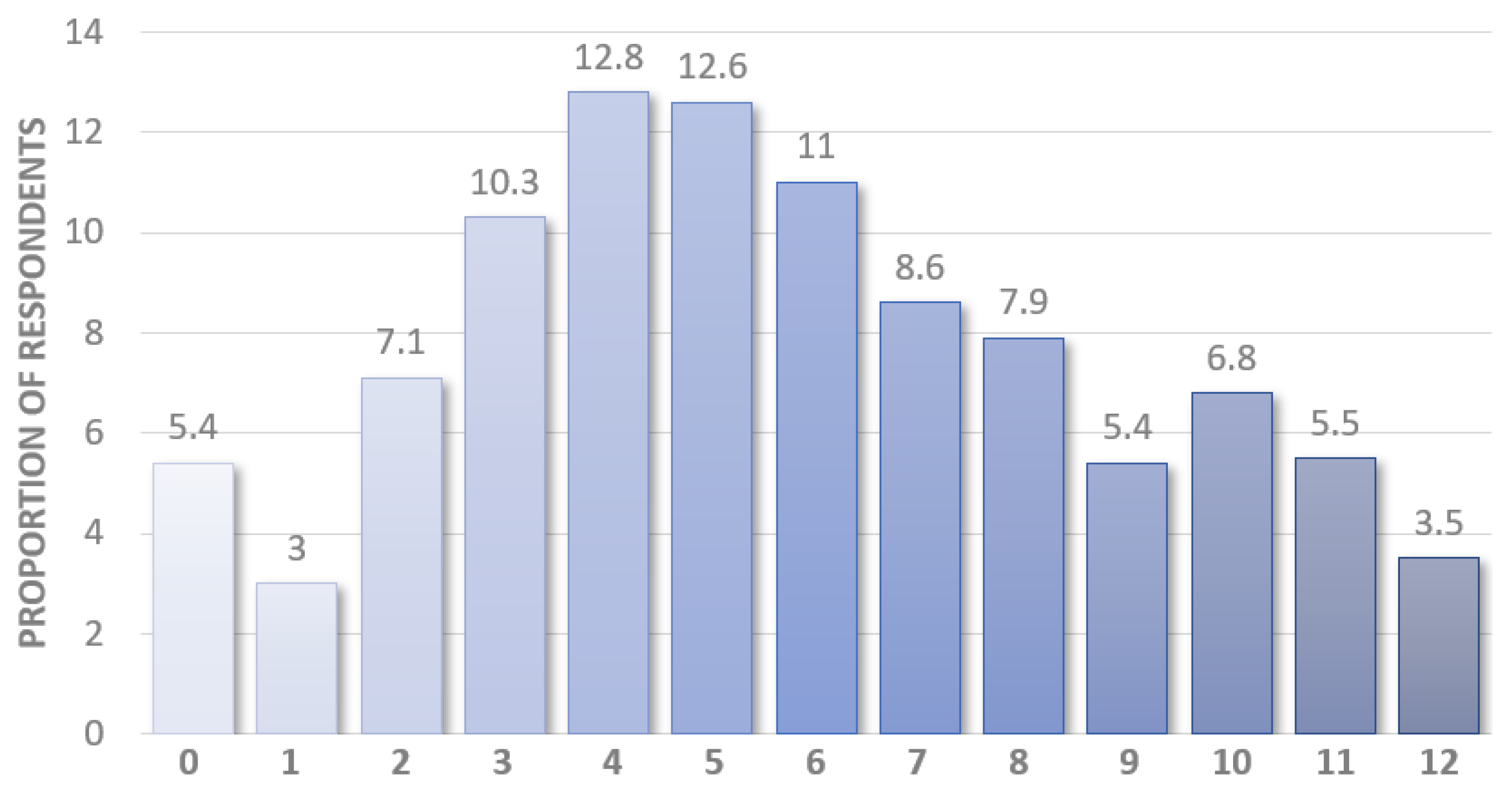
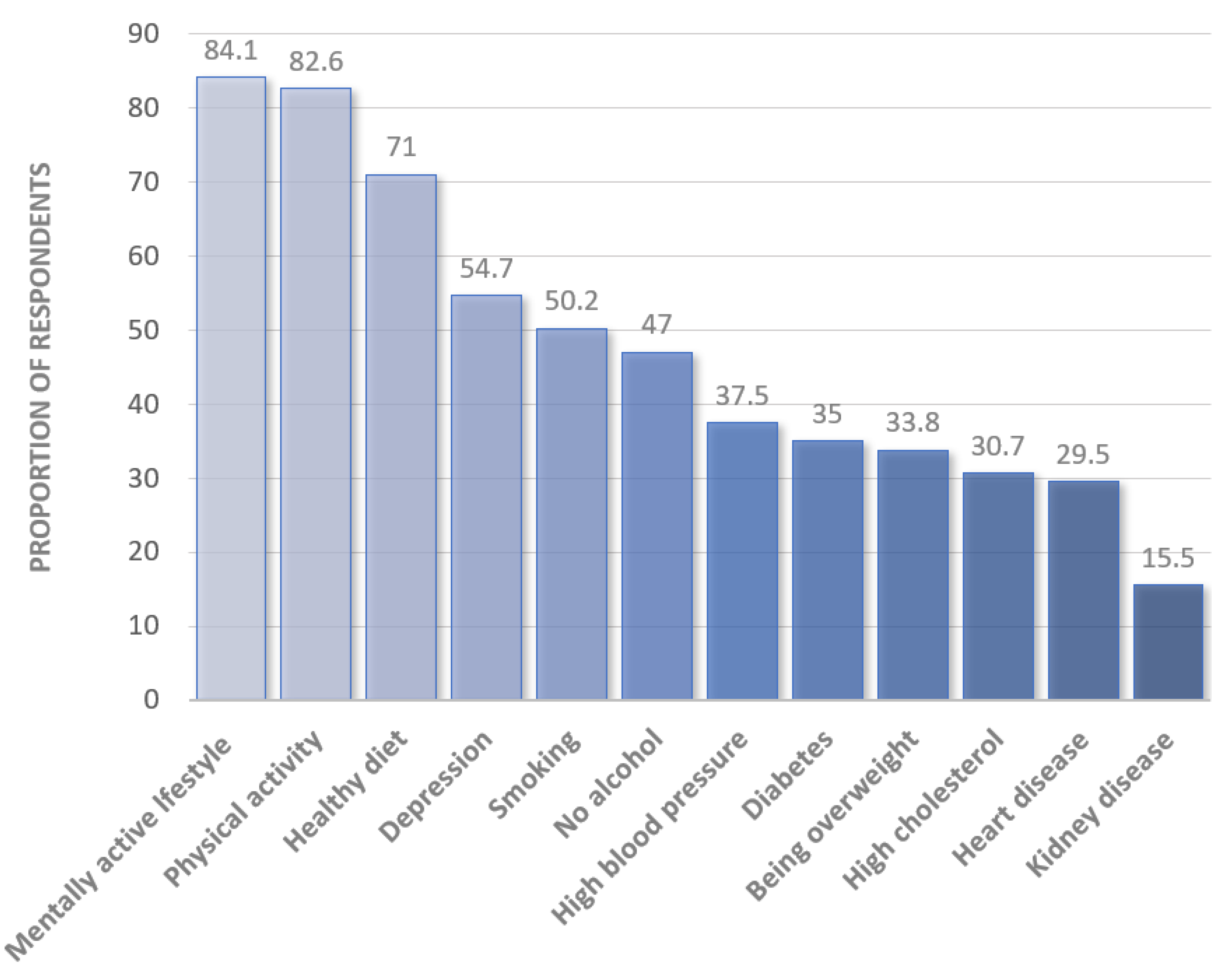
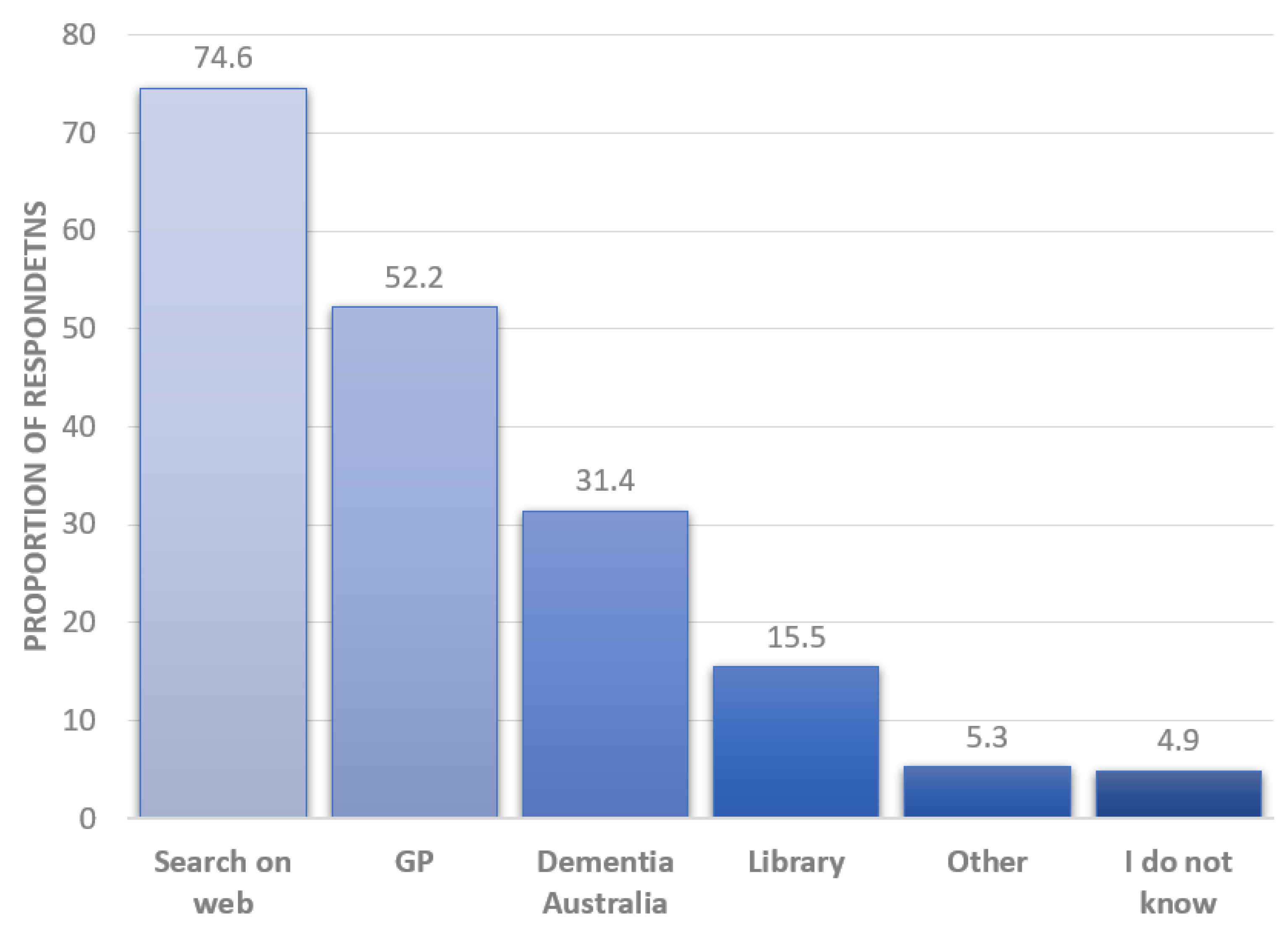
3.1. Dementia Literacy
3.2. Association of Identified Dementia Risk and Protective Factors with Demographic Variables
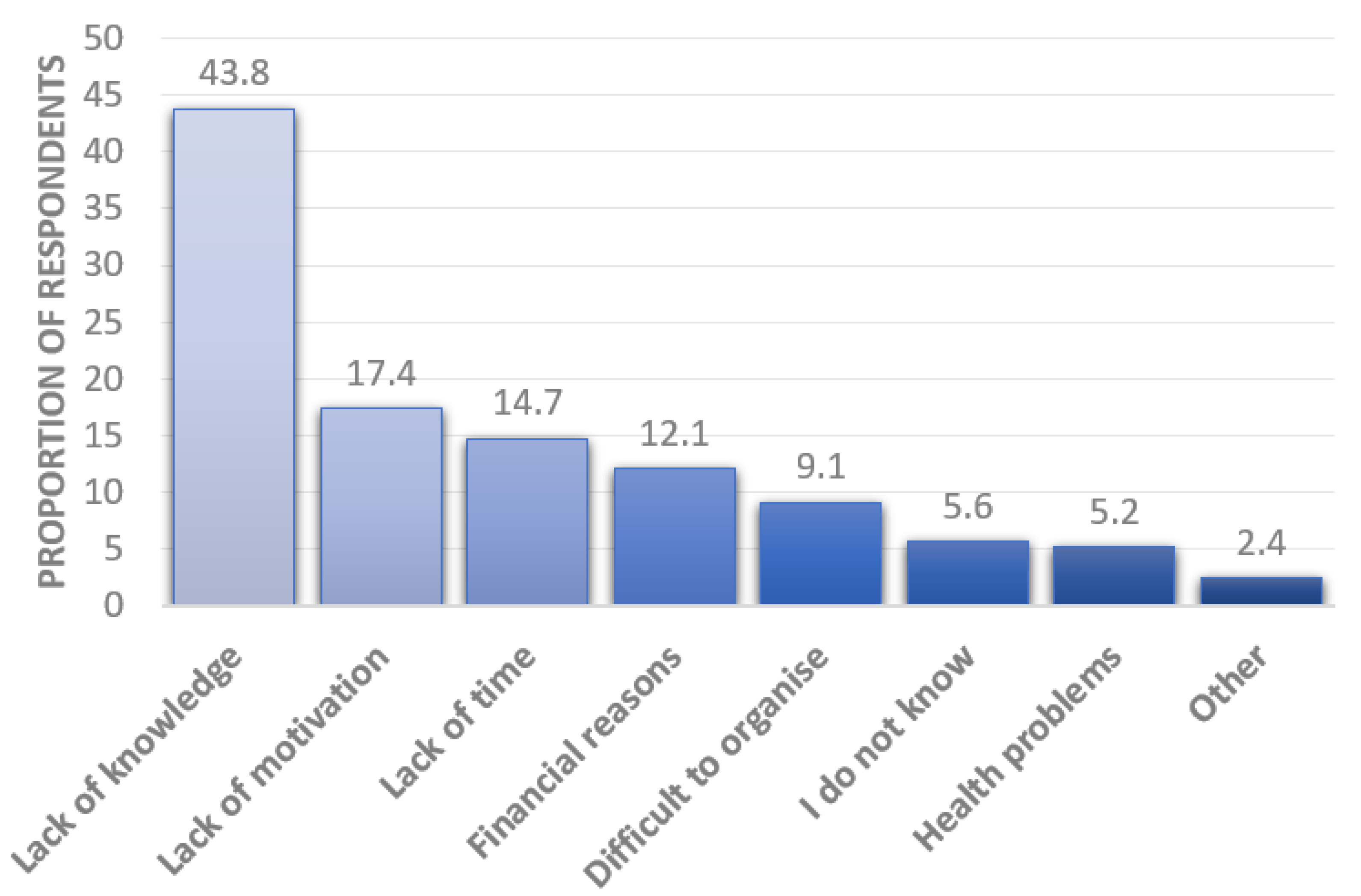
3.3. Barriers to Dementia Risk Reduction
4. Discussion
4.1. Dementia Literacy
4.2. Barriers to Dementia Risk Literacy and Reduction
4.3. Limitations
4.4. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia. 2021. Available online: who.int/news-room/fact-sheets/detail/dementia (accessed on 10 August 2022).
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Alzheimer’s Research UK. Dementia Statistics Hub: Global Prevalence UK. 2018. Available online: https://www.dementiastatistics.org/statistics/global-prevalence/ (accessed on 10 August 2022).
- Dementia Australia, What is Dementia. 2022. Available online: https://www.dementia.org.au/about-dementia/what-is-dementia (accessed on 10 August 2022).
- Royal Commission into Aged Care Quality and Safety, Dementia in Australia: Nature, Prevalence and Care. 2019; pp. 1–25. Available online: https://agedcare.royalcommission.gov.au/sites/default/files/2019-12/background-paper-3.pdf (accessed on 10 August 2022).
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The Influence of Vicarious Fear-Learning in “Infecting” Reactive Action Inhibition. Front. Behav. Neurosci. 2022, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Poirier, G.; Ohayon, A.; Juranville, A.; Mourey, F.; Gaveau, J. Deterioration, Compensation and Motor Control Processes in Healthy Aging, Mild Cognitive Impairment and Alzheimer’s Disease. Geriatrics 2021, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Ashby-Mitchell, K.; Burns, R.; Shaw, J.; Anstey, K.J. Proportion of dementia in Australia explained by common modifiable risk factors. Alzheimer’s Res. Ther. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- O’Donnell, C.A.; Browne, S.; Pierce, M.; McConnachie, A.; Deckers, K.; Van Boxtel, M.P.J.; Manera, V.; Köhler, S.; Redmond, M.; Verhey, F.R.J.; et al. Reducing dementia risk by targeting modifiable risk factors in mid-life: Study protocol for the Innovative Midlife Intervention for Dementia Deterrence (In-MINDD) randomised controlled feasibility trial. Pilot Feasibility Stud. 2015, 1, 40. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Anstey, K.J.; Peters, R.; Clare, L.; Lautenschlager, N.T.; Dodge, H.H.; Barnes, D.E.; Shahar, S.; Brodaty, H.; Rees, G. Joining forces to prevent dementia: The International Research Network On Dementia Prevention (IRNDP). Int. Psychogeriatr. 2017, 29, 1757–1760. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Qiu, C. Prevention of cognitive decline in ageing: Dementia as the target, delayed onset as the goal. Lancet Neurol. 2011, 10, 778–779. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Furie, K.L.; Iadecola, C.; Smith, E.E.; Waddy, S.P.; Lloyd-Jones, D.M.; Bae, H.-J.; Bauman, M.A.; Dichgans, M.; Duncan, P.W.; et al. Defining Optimal Brain Health in Adults: A Presidential Advisory from the American Heart Association/American Stroke Association. Stroke 2017, 48, e284–e303. [Google Scholar] [CrossRef] [PubMed]
- Curran, E.; Chong, T.W.H.; Godbee, K.; Abraham, C.; Lautenschlager, N.T.; Palmer, V.J. General population perspectives of dementia risk reduction and the implications for intervention: A systematic review and thematic synthesis of qualitative evidence. PLoS ONE 2021, 16, e0257540. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, P.; Fenton, K.; Alessi, C.; Prince, M.; Brayne, C.; Wortmann, M.; Patel, K.; Deanfield, J.; Mwatsama, M. The Blackfriars Consensus on brain health and dementia. Lancet 2014, 383, 1805–1806. [Google Scholar] [CrossRef]
- Rosato, M.; Leavey, G.; Cooper, J.; de Cock, P.; Devine, P. Factors associated with public knowledge of and attitudes to dementia: A cross-sectional study. PLoS ONE 2019, 14, e0210543. [Google Scholar] [CrossRef]
- Şahin, H.A.; Gurvit, I.H.; Emre, M.; Hanagasi, H.A.; Bilgic, B.; Harmanci, H. The attitude of elderly lay people towards the symptoms of dementia. Int. Psychogeriatr. 2006, 18, 251–258. [Google Scholar] [CrossRef]
- Loi, S.M.; Lautenschlager, N.T. Dementia literacy in older adults. Asia-Pac. Psychiatry 2014, 7, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.; Pierce, M.; Werner, P.; Darley, A.; Bobersky, A. A Systematic Review of the Public’s Knowledge and Understanding of Alzheimer’s Disease and Dementia. Alzheimer Dis. Assoc. Disord. 2015, 29, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, E.; Wojciechowska, M.; Stave, C.; Sganga, A.; O’Connell, B. Implications of the Facing Dementia Survey for the general population, patients and caregivers across Europe. Int. J. Clin. Pract. 2005, 59, 17–24. [Google Scholar] [CrossRef]
- Yeo, L.J.; Horan, M.A.; Jones, M.; Pendleton, N. Perceptions of Risk and Prevention of Dementia in the Healthy Elderly. Dement. Geriatr. Cogn. Disord. 2007, 23, 368–371. [Google Scholar] [CrossRef]
- Kim, S.; Sargent-Cox, K.; Anstey, K. A qualitative study of older and middle-aged adults’ perception and attitudes towards dementia and dementia risk reduction. J. Adv. Nurs. 2015, 71, 1694–1703. [Google Scholar] [CrossRef]
- Ayalon, L.; Areán, P.A. Knowledge of Alzheimer’s disease in four ethnic groups of older adults. Int. J. Geriatr. Psychiatry 2004, 19, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Low, L.-F.; Anstey, K.J. Dementia literacy: Recognition and beliefs on dementia of the Australian public. Alzheimer’s Dement. 2009, 5, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Ali, S.; Quach, H. Public knowledge and beliefs about dementia risk reduction: A national survey of Australians. BMC Public Health 2014, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.Y.M.; Leung, S.F.; Ho, G.W.; Bressington, D.; Molassiotis, A.; Lam, C.; Ligot, J.; Valdez, L.P.; Lazalita, L.A.; Chhit, S.; et al. Dementia literacy in Western Pacific countries: A mixed methods study. Int. J. Geriatr. Psychiatry 2019, 34, 1815–1825. [Google Scholar] [CrossRef]
- Barak, Y.; Rapsey, C.; Scott, K.M. Clusters of Dementia Literacy: Implications from a Survey of Older Adults. J. Prev. Alzheimer’s Dis. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Loi, S.M.; Zhou, S.; Zhao, M.; Lv, X.; Wang, J.; Wang, X.; Lautenschlager, N.; Yu, X.; Wang, H. Dementia Literacy among Community-Dwelling Older Adults in Urban China: A Cross-sectional Study. Front. Public Health 2017, 5, 124. [Google Scholar] [CrossRef]
- Siette, J. Brain Bootcamp, Registration. 2022. Available online: https://www.brainbootcamp.com.au/registration/ (accessed on 10 August 2022).
- Department of Health and Aged Care. Measuring Remoteness: Accessibility/Remoteness Index of Australia (ARIA) Australia; NKCfSAoGIS, Care DoHA, Eds.; Commonwealth Government of Australia: Canberra, Australia, 2001. [Google Scholar]
- Australian Bureau of Statistics. 2033.0.55.001—Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA) Canberra: Commonwealth Government of Australia. 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2033.0.55.001~2016~Main%20Features~IRSAD~20#:~:text=The%20Index%20of%20Relative%20Socio,relative%20advantage%20and%20disadvantage%20measures (accessed on 10 August 2022).
- Marcinkiewicz, A.; Reid, S. Attitudes to Dementia: Findings from the 2015 British Social Attitudes Survey; Public Health England, Nat Gen Social Research: London, UK, 2016. [Google Scholar]
- Heger, I.; Deckers, K.; Van Boxtel, M.; De Vugt, M.; Hajema, K.; Verhey, F.; Köhler, S. Dementia awareness and risk perception in middle-aged and older individuals: Baseline results of the MijnBreincoach survey on the association between lifestyle and brain health. BMC Public Health 2019, 19, 678. [Google Scholar] [CrossRef]
- Vos, S.J.; van Boxtel, M.P.; Schiepers, O.J.; Deckers, K.; de Vugt, M.; Carrière, I.; Dartigues, J.-F.; Peres, K.; Artero, S.; Ritchie, K.; et al. Modifiable Risk Factors for Prevention of Dementia in Midlife, Late Life and the Oldest-Old: Validation of the LIBRA Index. J. Alzheimer’s Dis. 2017, 58, 537–547. [Google Scholar] [CrossRef]
- Deckers, K.; Köhler, S.; Van Boxtel, M.; Verhey, F.; Brayne, C.; Fleming, J. Lack of associations between modifiable risk factors and dementia in the very old: Findings from the Cambridge City over-75s cohort study. Aging Ment. Health 2017, 22, 1272–1278. [Google Scholar] [CrossRef]
- Maust, D.T.; Solway, E.; Langa, K.M.; Kullgren, J.T.; Kirch, M.; Singer, D.C.; Malani, P. Perception of Dementia Risk and Preventive Actions Among US Adults Aged 50 to 64 Years. JAMA Neurol. 2020, 77, 259. [Google Scholar] [CrossRef]
- Van Asbroeck, S.; van Boxtel, M.P.; Steyaert, J.; Köhler, S.; Heger, I.; de Vugt, M.; Verhey, F.; Deckers, K. Increasing knowledge on dementia risk reduction in the general population: Results of a public awareness campaign. Prev. Med. 2021, 147, 106522. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Gray, A.R.; Rapsey, C.; Scott, K. The Dunedin dementia risk awareness project: Pilot study in older adults. Int. Psychogeriatr. 2019, 32, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, K.; Shen, Y.; Gao, X.; Prieto, L.R. Dementia literacy and worry among older Chinese Americans in Arizona: A comparison between 2013 and 2017. Int. Psychogeriatr. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Farrow, M. Review of Public Knowledge of Dementia Risk Reduction. JMIR Research Protocols 2008, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Sharp, E.S.; Gatz, M. Relationship Between Education and Dementia: An updated systematic review. Alzheimer Dis. Assoc. Disord. 2011, 25, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Etgen, T.; Chonchol, M.; Förstl, H.; Sander, D. Chronic Kidney Disease and Cognitive Impairment: A Systematic Review and Meta-Analysis. Am. J. Nephrol. 2012, 35, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Barber, P.A. Dementia risk and prevention by targeting modifiable vascular risk factors. J. Neurochem. 2017, 144, 565–581. [Google Scholar] [CrossRef]
- Santos, C.Y.; Snyder, P.J.; Wu, W.-C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef]
- Deal, J.A.; Power, M.C.; Palta, P.; Alonso, A.; Schneider, A.L.; Perryman, K.; Bandeen-Roche, K.; Sharrett, A.R. Relationship of Cigarette Smoking and Time of Quitting with Incident Dementia and Cognitive Decline. J. Am. Geriatr. Soc. 2019, 68, 337–345. [Google Scholar] [CrossRef]
- Horst, B.R.; Furlano, J.A.; Wong, M.Y.S.; Ford, S.D.; Han, B.B.; Nagamatsu, L.S. Identification of Demographic Variables Influencing Dementia Literacy and Risk Perception Through a Global Survey. Front. Public Health 2021, 9, 660600. [Google Scholar] [CrossRef]
- Smith, G.D.; Ho, K.H.M.; Poon, S.; Chan, S.W. Beyond the tip of the iceberg: Health literacy in older people. J. Clin. Nurs. 2021, 31, E3–E5. [Google Scholar] [CrossRef] [PubMed]
- Liljas, A.E.M.; Walters, K.; Jovicic, A.; Iliffe, S.; Manthorpe, J.; Goodman, C.; Kharicha, K. Strategies to improve engagement of ‘hard to reach’ older people in research on health promotion: A systematic review. BMC Public Health 2017, 17, 349. [Google Scholar] [CrossRef] [PubMed]
- Aihara, Y.; Maeda, K. Dementia Literacy and Willingness to Dementia Screening. Int. J. Environ. Res. Public Health 2020, 17, 8134. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Jones, K.A.; Di Lorito, C.; Stephan, B.C.M.; Orrell, M.; Oliveira, D. Changing lifestyle for dementia risk reduction: Inductive content analysis of a national UK survey. PLoS ONE 2020, 15, e0233039. [Google Scholar] [CrossRef]
- Millard, F.B.; Kennedy, R.L.; Baune, B.T. Dementia: Opportunities for risk reduction and early detection in general practice. Aust. J. Prim. Health 2011, 17, 89–94. [Google Scholar] [CrossRef]
- Glymour, M.M.; Whitmer, R.A. Using Cross-Cultural Studies to Improve Evidence on Dementia Prevention: Lessons from the Special Issue Sponsored by the International Research Network on Dementia Prevention (IRNDP). J. Alzheimer’s Dis. 2019, 70, S5–S10. [Google Scholar] [CrossRef]
- Johnston, K.; Preston, R.; Strivens, E.; Qaloewai, S.; Larkins, S. Understandings of dementia in low and middle income countries and amongst indigenous peoples: A systematic review and qualitative meta-synthesis. Aging Ment. Health 2019, 24, 1183–1195. [Google Scholar] [CrossRef]
- Garvey, G.; Simmonds, D.; Clements, V.; O’Rourke, P.; Sullivan, K.; Gorman, D.; Curnow, V.; Wise, S.; Beattie, E. Making sense of dementia: Understanding amongst indigenous Australians. Int. J. Geriatr. Psychiatry 2010, 26, 649–656. [Google Scholar] [CrossRef]
- Stephan, B.C.M.; Siervo, M.; Brayne, C. How can population-based studies best be utilized to reduce the global impact of dementia? Recommendations for researchers, funders, and policymakers. Alzheimer’s Dement. 2020, 16, 1448–1456. [Google Scholar] [CrossRef]
- Siette, J.; Taylor, N.; Deckers, K.; Köhler, S.; Braithwaite, J.; Valenzuela, M.; Armitage, C.J. Advancing Australian public health initiatives targeting dementia risk reduction. Australas. J. Ageing 2022, 41, e190–e195. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Sanches, C.; Stengel, C.; Godard, J.; Mertz, J.; Teichmann, M.; Migliaccio, R.; Valero-Cabré, A. Past, Present, and Future of Non-invasive Brain Stimulation Approaches to Treat Cognitive Impairment in Neurodegenerative Diseases: Time for a Comprehensive Critical Review. Front. Aging Neurosci. 2021, 12, 578339. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lane, H.-Y.; Lin, C.-H. Brain Stimulation in Alzheimer’s Disease. Front. Psychiatry 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Phan, H.T.; Terry, D.; Doherty, K.; McInerney, F. Impact of dementia literacy interventions for non-health-professionals: Systematic review and meta-analysis. Aging Ment. Health 2021, 26, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Montayre, J.; Karamacoska, D.; Siette, J. Progressing dementia risk reduction initiatives for culturally and linguistically diverse older adults in Australia. Australas. J. Ageing 2022, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Low, L.-F.; Barcenilla-Wong, A.L.; Brijnath, B. Including ethnic and cultural diversity in dementia research. Med. J. Aust. 2019, 211, 345–346.e1. [Google Scholar] [CrossRef] [PubMed]
- Strecher, V.J.; Rosenstock, I.M. The health belief model. Camb. Handb. Psychol. Health Med. 1997, 25, 113–117. [Google Scholar]
- Rivadeneira, M.F.; Mendieta, M.J.; Villavicencio, J.; Caicedo-Gallardo, J.; Buendía, P. A multidimensional model of healthy ageing: Proposal and evaluation of determinants based on a population survey in Ecuador. BMC Geriatr. 2021, 21, 615. [Google Scholar] [CrossRef]
| Characteristic | n (%) |
|---|---|
| Gender | |
| Female | 597 (70.0) |
| Male | 256 (30.0) |
| Age (Mean (SD), range) | 73.4 (6.2), 65–94 |
| 65–69 | 276 (32.2) |
| 70–79 | 444 (51.9) |
| 80+ | 136 (15.9) |
| Education | |
| Low | 293 (34.6) |
| Middle | 176 (20.8) |
| High | 377 (44.6) |
| Country of birth | |
| English-speaking country | 719 (84.1) |
| Non-English-speaking country | 136 (15.9) |
| Socioeconomic Status | |
| 1 (lowest) | 43 (5.4) |
| 2 | 125 (15.8) |
| 3 | 138 (17.4) |
| 4 | 95 (12.0) |
| 5 (highest) | 391 (49.4) |
| Locality | |
| Metropolitan | 627 (77.7) |
| Regional/Remote/Rural | 180 (22.3) |
| Interest in receiving information to improve your brain health | |
| Yes | 820 (98.3) |
| No | 11 (1.3) |
| Statement | Agree | Neutral | Disagree |
|---|---|---|---|
| There is nothing anyone can do to reduce their risk of dementia | 106 (12.8) | 150 (18.1) | 572 (69.1) |
| High blood pressure contributes to dementia risk | 313 (37.8) | 435 (52.5) | 81 (9.8) |
| Smoking increases your chances of getting dementia | 419 (50.7) | 320 (38.7) | 88 (10.6) |
| No or moderate alcohol use lowers your chances of getting dementia | 392 (47.6) | 302 (36.7) | 129 (15.7) |
| Regular physical activity lowers your chances of getting dementia | 689 (83.4) | 81 (9.8) | 56 (6.8) |
| Depression increases the chances of getting dementia | 456 (55.0) | 296 (32.7) | 77 (9.3) |
| Diabetes increases the chances of getting dementia | 292 (35.4) | 448 (54.2) | 86 (10.4) |
| A mentally active lifestyle lowers the chances of dementia | 701 (85.0) | 75 (9.1) | 49 (5.9) |
| Heart disease increases the chances of getting dementia | 246 (29.8) | 475 (57.5) | 105 (12.7) |
| Kidney disease increases the chances of getting dementia | 129 (15.5) | 576 (69.4) | 125 (15.1) |
| High cholesterol increases the chances of getting dementia | 256 (31.0) | 444 (53.7) | 127 (15.4) |
| A healthy diet lowers the chances of getting dementia | 592 (71.8) | 180 (21.8) | 53 (6.4) |
| Characteristic | Agree | Neutral | Disagree | χ2/F | p-Value * |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 70 (11.8) | 113 (19.1) | 409 (69.1) | 0.859 | 0.651 |
| Male | 36 (14.1) | 47 (18.4) | 172 (67.5) | ||
| Age (Mean (SD), range) | 73.3 (6.4) | 73.8 (6.7) | 73.1 (5.8) | 1.688 | 0.168 |
| 65–69 | 38 (13.8) | 53 (19.2) | 185 (67.0) | 9.140 | 0.010 |
| 70–79 | 49 (11.0) | 75 (16.9) | 320 (72.1) | ||
| 80+ | 20 (15.4) | 32 (24.6) | 78 (60.0) | ||
| Education | |||||
| Low | 39 (13.5) | 62 (21.5) | 187 (64.9) | 6.421 | 0.040 |
| Middle | 25 (14.2) | 36 (20.5) | 115 (65.3) | ||
| High | 42 (11.2) | 60 (16.0) | 274 (72.9) | ||
| Country of birth | |||||
| English-speaking country | 86 (12.0) | 131 (18.3) | 497 (69.6) | 2.034 | 0.362 |
| Non-English-speaking country | 21 (15.6) | 28 (20.7) | 86 (63.7) | ||
| Socioeconomic Status | |||||
| 1 (lowest) | 4 (9.3) | 7 (16.3) | 32 (74.4) | 9.248 | 0.322 |
| 2 | 12 (9.6) | 25 (20.0) | 88 (70.4) | ||
| 3 | 10 (7.2) | 32 (23.2) | 96 (69.6) | ||
| 4 | 15 (15.8) | 18 (18.9) | 62 (65.3) | ||
| 5 (highest) | 56 (14.3) | 65 (16.6) | 270 (69.1) | ||
| Locality | |||||
| Metropolitan | 82 (13.2) | 120 (19.3) | 420 (67.5) | 4.800 | 0.028 |
| Regional/Remote/Rural | 16 (8.9) | 31 (17.3) | 132 (73.7) |
| Independent Variable | Odds Ratio | 95% CI | p-Value * |
|---|---|---|---|
| Gender (reference category: male) | 0.762 | 0.546–1.064 | 0.111 |
| Age (reference category: 65–69 years) | 0.955 | 0.752–1.213 | 0.705 |
| Education (reference category: <12 years) | 1.228 | 1.023–1.474 | 0.028 |
| Country of birth (reference category: non-English-speaking country) | 0.805 | 0.530–1.222 | 0.308 |
| Socioeconomic Status (reference category: lowest) | 0.996 | 0.996–0.857 | 0.960 |
| Locality (reference category: regional) | 0.123 | 1.478–0.899 | 0.123 |
| Variable | B | SE | β | 95% CI | p-Value * |
|---|---|---|---|---|---|
| Constant | 4.896 | 1.026 | 2.881–6.910 | ||
| Gender | −0.201 | 0.242 | −0.03 | −0.675–0.273 | 0.406 |
| Age | −0.400 | 0.174 | −0.086 | −0.736–−0.065 | 0.019 |
| Education | 0.167 | 0.309 | 0.020 | −0.440–0.775 | 0.588 |
| Country of birth | 0.458 | 0.343 | 0.062 | −0.215–1.131 | 0.182 |
| Socioeconomic Status | 0.208 | 0.108 | 0.088 | −0.003–0.419 | 0.054 |
| Locality | 0.141 | 0.131 | 0.040 | −0.116–0.397 | 0.282 |
| R2 | 0.017 | ||||
| F | 2.112 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siette, J.; Dodds, L. Sociodemographic Associations of Dementia Literacy in Older Australians. J. Ageing Longev. 2022, 2, 252-265. https://doi.org/10.3390/jal2040021
Siette J, Dodds L. Sociodemographic Associations of Dementia Literacy in Older Australians. Journal of Ageing and Longevity. 2022; 2(4):252-265. https://doi.org/10.3390/jal2040021
Chicago/Turabian StyleSiette, Joyce, and Laura Dodds. 2022. "Sociodemographic Associations of Dementia Literacy in Older Australians" Journal of Ageing and Longevity 2, no. 4: 252-265. https://doi.org/10.3390/jal2040021
APA StyleSiette, J., & Dodds, L. (2022). Sociodemographic Associations of Dementia Literacy in Older Australians. Journal of Ageing and Longevity, 2(4), 252-265. https://doi.org/10.3390/jal2040021






