Digital Health Solutions for Chronic Illnesses: A Systematic Review of Mobile Health Apps and Quality Analysis with Mobile App Rating Scale
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Evaluation
2.4. Statistical Analysis
3. Results
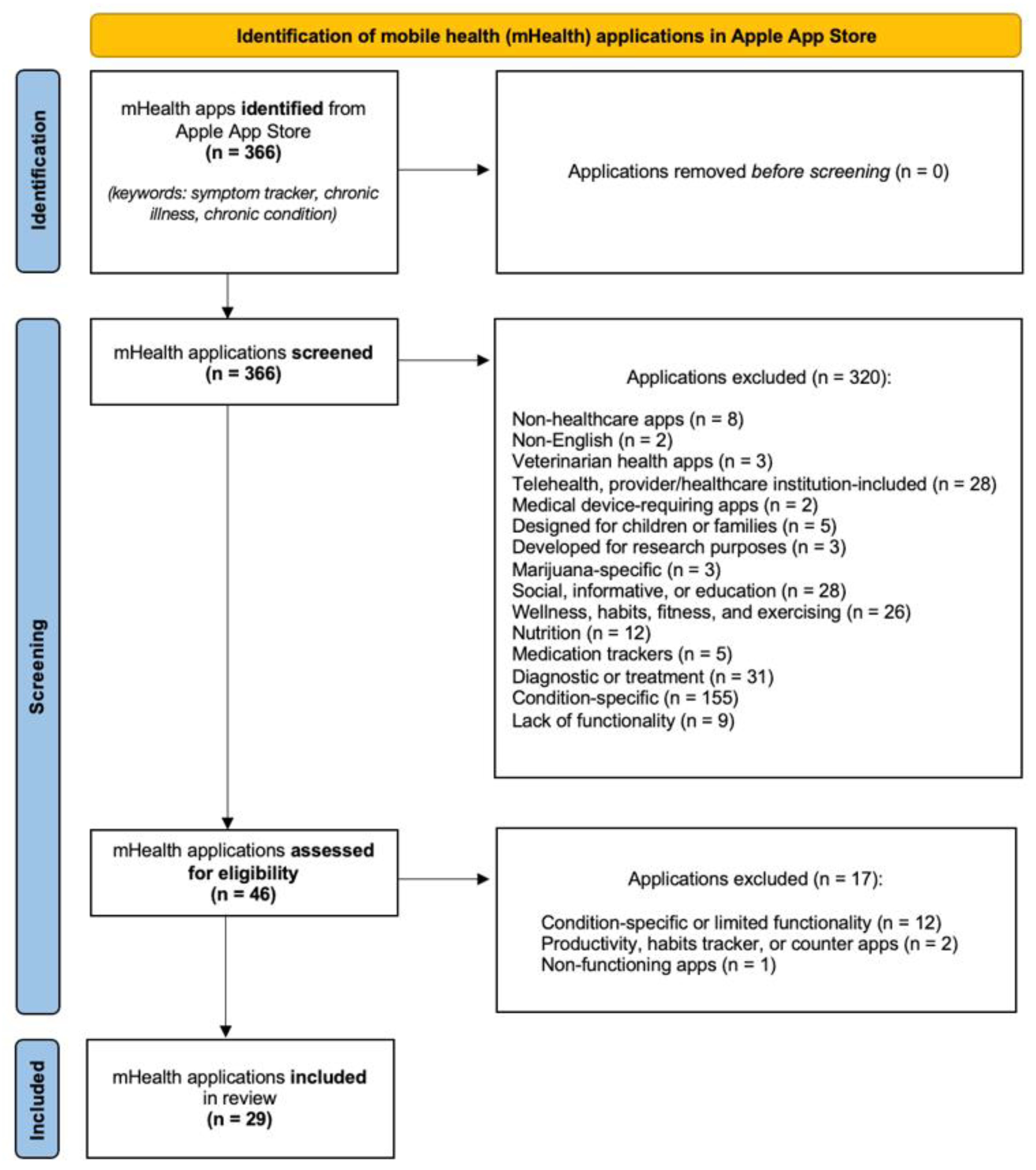
3.1. Eligibility
3.2. Overview of the mHealth Apps
3.3. Symptom and Medication Tracking Functionality
3.4. Mhealth Apps MARS Quality Score
3.5. Quality Comparison by Different Characteristics
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef]
- Yach, D.; Hawkes, C.; Gould, C.L.; Hofman, K.J. The Global Burden of Chronic Diseases: Overcoming Impediments to Prevention and Control. JAMA 2004, 291, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- WHO. United Nations High-Level Meeting on Noncommunicable Disease Prevention and Control. 2011. Available online: https://www.un.org/en/ga/ncdmeeting2011/ (accessed on 15 March 2022).
- United-Nations-General-Assembly. Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. 2012. Available online: https://www.who.int/nmh/events/un_ncd_summit2011/political_declaration_en.pdf (accessed on 15 March 2022).
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond. Engl. 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, J.W.; Villavicencio, F.; Bergeron-Boucher, M.P. Demographic perspectives on the rise of longevity. Proc. Natl. Acad. Sci. USA 2022, 118, e2019536118. [Google Scholar] [CrossRef]
- Meyer, A.C.; Drefahl, S.; Ahlbom, A.; Lambe, M.; Modig, K. Trends in life expectancy: Did the gap between the healthy and the ill widen or close? BMC Med. 2020, 18, 41. [Google Scholar] [CrossRef]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Boersma, P.; Black, L.I.; Ward, B.W. Prevalence of Multiple Chronic Conditions among US Adults, 2018. Prev. Chronic Dis. 2020, 17, E106. [Google Scholar] [CrossRef]
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef]
- Cheng, C.; Inder, K.; Chan, S.W. Coping with multiple chronic conditions: An integrative review. Nurs. Health Sci. 2020, 22, 486–497. [Google Scholar] [CrossRef]
- WHO. Noncommunicable Diseases. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=Noncommunicable%20diseases%20(NCDs)%20kill%2041,%2D%20and%20middle%2Dincome%20countries (accessed on 16 March 2022).
- The Lancet Global Health. Mental health matters. Lancet Glob. Health 2020, 8, e1352. [Google Scholar] [CrossRef]
- Nochaiwong, S.; Ruengorn, C.; Thavorn, K.; Hutton, B.; Awiphan, R.; Phosuya, C.; Ruanta, Y.; Wongpakaran, N.; Wongpakaran, T. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10173. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Servais, J.; Martin, C.B.; Kohen, D. Prescription Drug Use among Adults Aged 40–79 in the United States and Canada. 2019. Available online: https://www.cdc.gov/nchs/products/databriefs/db347.htm (accessed on 16 March 2022).
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 2018, 8, e016982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.T.; Bussell, J.K. Medication Adherence: WHO Cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, P.E.; Washington, S.L., III; Branagan, L.; Gleason, N.; Pruthi, R.S.; Carroll, P.R.; Odisho, A.Y. Rapid Utilization of Telehealth in a Comprehensive Cancer Center as a Response to COVID-19: Cross-Sectional Analysis. J. Med. Internet Res. 2020, 22, e19322. [Google Scholar] [CrossRef]
- Webster, P. Virtual health care in the era of COVID-19. Lancet 2020, 395, 1180–1181. [Google Scholar] [CrossRef]
- Shachar, C.; Engel, J.; Elwyn, G. Implications for Telehealth in a Postpandemic Future. JAMA 2020, 323, 2375–2376. [Google Scholar] [CrossRef]
- Innovatemedtec. What is mHealth? Available online: https://innovatemedtec.com/digital-health/mhealth/ (accessed on 16 March 2022).
- Mittermaier, M.; Sina, C.; Richter, J.G.; Raspe, M.; Stais, P.; Vehreschild, J.; Wolfrum, S.; Anthes, C.; Möckel, M.; AG DiGA und KI in Leitlinien der Kommission Digitale Transformation der DGIM. Praktische Anwendung digitaler Gesundheitsanwendungen (DiGA) in der Inneren Medizin. Internist 2022, 63, 245–254. [Google Scholar] [CrossRef]
- Prescribable Digital Healthcare Applications in Germany. Available online: https://diga.bfarm.de/de/verzeichnis (accessed on 16 March 2022).
- Dittrich, F.; Albrecht, U.V.; von Jan, U.; Malinka, C.; Ansorg, J.; Jung, J.; Back, D.A.; Digitalisierung, A. The Digital Healthcare Act—A Turning Point in the German Digitisation Strategy? Z. Orthopädie Unf. 2021, 159, 259–265. [Google Scholar] [CrossRef]
- Olesch, A. A Year with Apps On Prescription in Germany. 2021. Available online: https://sidekickhealth.com/news/a-year-with-apps-on-prescription-in-germany/ (accessed on 16 March 2022).
- Domhardt, M.; Messner, E.M.; Eder, A.S.; Engler, S.; Sander, L.B.; Baumeister, H.; Terhorst, Y. Mobile-based interventions for common mental disorders in youth: A systematic evaluation of pediatric health apps. Child Adolesc. Psychiatry Ment. Health 2021, 15, 49. [Google Scholar] [CrossRef]
- McGee-Vincent, P.; Juhasz, K.; Jamison, A.L.; Avery, T.J.; Owen, J.E.; Jaworski, B.K.; Blonigen, D.M. Mobile Mental Health Apps from the National Center for PTSD: Digital Self-Management Tools for Co-Occurring Disorders. J. Dual Diagn. 2021, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, B.; Chemi, C.; Ruiz-Valcarcel, J.; Hein, P.; Hu, L. Smartphone Apps Targeting Alcohol and Illicit Substance Use: Systematic Search in Commercial App Stores and Critical Content Analysis. JMIR mHealth uHealth 2019, 7, e11831. [Google Scholar] [CrossRef] [PubMed]
- Colbert, S.; Thornton, L.; Richmond, R. Smartphone apps for managing alcohol consumption: A literature review. Addict. Sci. Clin. Pract. 2020, 15, 17. [Google Scholar] [CrossRef]
- Vilardaga, R.; Casellas-Pujol, E.; McClernon, J.F.; Garrison, K.A. Mobile Applications for the Treatment of Tobacco Use and Dependence. Curr. Addict. Rep. 2019, 6, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, R.; Fisher, T.; Palenski, P.E.; Kumaresan, V.; Mannelli, P.; Sweitzer, M.M.; McClernon, F.J.; Engelhard, M.M.; Sabo, P.L.; Garrison, K.A. Review of Popularity and Quality Standards of Opioid-Related Smartphone Apps. Curr. Addict. Rep. 2020, 7, 486–496. [Google Scholar] [CrossRef]
- Staiger, P.K.; O’Donnell, R.; Liknaitzky, P.; Bush, R.; Milward, J. Mobile Apps to Reduce Tobacco, Alcohol, and Illicit Drug Use: Systematic Review of the First Decade. J. Med. Internet Res. 2020, 22, e17156. [Google Scholar] [CrossRef]
- Cucciniello, M.; Petracca, F.; Ciani, O.; Tarricone, R. Development features and study characteristics of mobile health apps in the management of chronic conditions: A systematic review of randomised trials. NPJ Digit. Med. 2021, 4, 144. [Google Scholar] [CrossRef]
- Janjua, S.; Banchoff, E.; Threapleton, C.J.; Prigmore, S.; Fletcher, J.; Disler, R.T. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2021, 2021, CD013246. [Google Scholar] [CrossRef]
- Huang, Z.; Soljak, M.; Boehm, B.O.; Car, J. Clinical relevance of smartphone apps for diabetes management: A global overview. Diabetes Metab. Res. Rev. 2018, 34, e2990. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, H.; Huang, Y.; Huang, L.; Zhang, D. A Systematic Review of Application and Effectiveness of mHealth Interventions for Obesity and Diabetes Treatment and Self-Management. Adv. Nutr. Int. Rev. J. 2017, 8, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Eberle, C.; Löhnert, M.; Stichling, S. Effectiveness of Disease-Specific mHealth Apps in Patients with Diabetes Mellitus: Scoping Review. JMIR mHealth uHealth 2021, 9, e23477. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.C.; Uddin, R.; Schröder-Pfeifer, P.; Holl, F.; Swoboda, W.; Schiltenwolf, M. Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness. J. Clin. Med. 2020, 9, 3557. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, G.E.; Brittain, E.L. mHealth Technologies in Cardiopulmonary Disease. Chest 2020, 157, 654–664. [Google Scholar] [CrossRef] [PubMed]
- National-Headache-Foundation. Migraine Apps. Available online: https://headaches.org/resources/migraine-monitor-app/ (accessed on 16 March 2022).
- Pérez-Jover, V.; Sala-González, M.; Guilabert, M.; Mira, J.J. Mobile Apps for Increasing Treatment Adherence: Systematic Review. J. Med. Internet Res. 2019, 21, e12505. [Google Scholar] [CrossRef] [Green Version]
- Böhme, C.; Osthoff, M.B.; von Frey, K.; Hübner, J. Qualitative evaluation of mobile cancer apps with particular attention to the target group, content, and advertising. J. Cancer Res. Clin. 2017, 144, 173–181. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR mHealth uHealth 2015, 3, e27. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Research2guidance. How to Get Your Digital Health App Reimbursed in Europe? Available online: https://research2guidance.com/how-to-get-your-digital-health-app-reimbursed-in-europe-start-with-germany-belgium-and-france/ (accessed on 21 March 2022).
- Nichol, J.R.; Sundjaja, J.H.; Nelson, G. Medical History. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534249/ (accessed on 23 March 2022).
- Rich, E.C.; Crowson, T.W.; Harris, I.B. The Diagnostic Value of the Medical History: Perceptions of Internal Medicine Physicians. Arch. Intern. Med. 1987, 147, 1957–1960. [Google Scholar] [CrossRef]
- Jimmy, B.; Jose, J. Patient Medication Adherence: Measures in Daily Practice. Oman Med. J. 2011, 26, 155–159. [Google Scholar] [CrossRef]
- GlobalStats. Mobile Operating System Market Share United States of America—May 2022. 2022. Available online: https://gs.statcounter.com/os-market-share/mobile/united-states-of-america (accessed on 5 June 2022).
- GlobalStats. Mobile Operating System Market Share Worldwide—February 2022. 2022. Available online: https://gs.statcounter.com/os-market-share/mobile/worldwide (accessed on 23 March 2022).
- Grand View Research. mHealth Apps Market Size and Share Report, 2022–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/mhealth-app-market (accessed on 5 June 2022).
| Characteristics (n = 29) | ||
|---|---|---|
| Apple App Store category, n (%) | ||
| Health and Fitness | 19 (65.2) | |
| Medical | 10 (34.5) | |
| Time of the last update, n (%) | ||
| 1 week—9 months ago | 21 (72.4) | |
| 2 years—4 years ago | 7 (24.1) | |
| Never updated since the release | 1 (3.4) | |
| Average App Store rating (max. 5) | 4.17 | |
| Registration, n (%) | ||
| Required | 22 (75.9) | |
| Not required | 7 (24.1) | |
| Cost, n (%) | ||
| Free, no premium features | 11 (37.9) | |
| Free, one-time payment for premium features | 2 (6.9) | |
| Free, subscription for premium features (monthly or annual) | 10 (34.5) | |
| Paid, one-time payment | 1 (3.4) | |
| Paid, subscription required (monthly or annual) | 5 (17.2) | |
| Price, median (IQR) | ||
| One-time payment for premium | USD 6.99 | - |
| Monthly premium subscription | USD 4.99 | [4.24–6.99] |
| One-time payment | USD 4.99 | - |
| Monthly subscription required | USD 4.99 | [2.99–8.49] |
| Duration of the trial version, days median (IQR) | ||
| Free, with premium features | 3 (0–7) | |
| Paid | 7 (0–18.5) | |
| mHealth App Name | Symptom Tracking Function | |||||||
|---|---|---|---|---|---|---|---|---|
| Symptoms Listed | Possibility to Add New Symptoms | Symptoms Severity | Possibility to Add Notes | Graphical Summary | Data Export | Medication Tracking | Medication Reminders | |
| Effecto Symptom Tracker | • | • | • | • | • | • | • | • |
| Wave: health and symptom tracker | • | • | • | • | • | • | • | • |
| CareClinic—Tracker, Reminder | • | • | • | • | • | • | • | • |
| Healthily: Self-care and Tracker | • | • | • | • | • | • | - | - |
| Moodflow | - | • | • | • | • | • | • | - |
| Symptom and Mood Tracker | • | • | • | • | • | • | • | • |
| Avanti | • | • | • | • | - | • | • | - |
| Journal My Health | • | • | • | • | • | • | • | - |
| itFeels | • | • | • | • | • | • | - | - |
| Crystal™ | • | • | • | • | • | • | • | • |
| Folia Health | • | - | • | • | • | • | • | - |
| Wanngi Health tracker | • | • | • | • | • | • | • | • |
| Medication Reminder—Care | • | • | - | • | • | • | • | • |
| Health Storylines | • | • | • | • | • | • | • | • |
| Opencare—Track symptoms | - | • | • | • | • | • | • | • |
| #trackit: Track Health and Pain | - | • | • | • | • | - | - | - |
| Chronic insights | • | • | • | • | • | • | - | - |
| MDHealthTrak—Symptom Tracker | • | • | • | • | • | • | - | - |
| Symptomator | - | • | • | • | • | - | - | - |
| CoVstat | • | • | • | • | • | • | - | - |
| Chronic illness Monitor | • | • | • | • | • | • | • | • |
| Metriport—Tracker and Lifelog | • | • | • | • | • | • | • | • |
| Symple Symptom Tracker | • | • | • | - | - | • | - | - |
| Flaredown for Chronic Illness | • | • | • | • | • | • | - | - |
| Symptom Tracker | - | • | • | • | • | • | • | - |
| Wellth Health Tracker | • | • | • | - | • | • | • | • |
| TracknShare LITE | - | • | • | - | • | • | • | - |
| PeopleWith—Symptoms and Health | • | - | • | • | • | - | • | • |
| Healthmatica | - | • | • | • | • | • | - | - |
| APP NAME | Engagement | Functionality | Esthetics | Information | Subjective Quality | Overall † | Overall-Excluded ‡ |
|---|---|---|---|---|---|---|---|
| Effecto Symptom Tracker | 4.60 | 4.50 | 4.67 | 4.00 | 4.75 | 4.50 | 4.44 |
| Wave: health and symptom tracker | 4.20 | 4.75 | 4.33 | 4.14 | 4.75 | 4.44 | 4.36 |
| CareClinic—Tracker, Reminder | 4.60 | 3.75 | 4.33 | 4.29 | 4.50 | 4.29 | 4.24 |
| Healthily: Self-care and Tracker | 4.60 | 3.75 | 4.33 | 3.57 | 5.00 | 4.25 | 4.06 |
| Moodflow | 4.80 | 3.75 | 4.33 | 3.71 | 4.50 | 4.22 | 4.15 |
| Symptom and Mood Tracker | 4.40 | 4.50 | 4.00 | 3.57 | 4.25 | 4.14 | 4.12 |
| Avanti | 3.40 | 4.00 | 4.00 | 3.71 | 4.25 | 3.87 | 3.78 |
| Journal My Health | 3.40 | 4.50 | 4.00 | 3.57 | 3.25 | 3.74 | 3.87 |
| itFeels | 3.20 | 4.75 | 3.67 | 3.14 | 3.75 | 3.70 | 3.69 |
| Crystal™ | 3.20 | 4.50 | 4.00 | 2.86 | 3.75 | 3.66 | 3.64 |
| Folia Health | 3.80 | 3.50 | 4.00 | 3.14 | 3.75 | 3.64 | 3.61 |
| Wanngi Health tracker | 3.40 | 3.75 | 4.00 | 2.86 | 4.00 | 3.60 | 3.50 |
| Medication Reminder—Care | 3.20 | 3.50 | 4.33 | 3.00 | 3.75 | 3.56 | 3.51 |
| Health Storylines | 3.40 | 3.25 | 4.00 | 3.14 | 3.75 | 3.51 | 3.45 |
| Opencare—Track symptoms | 3.60 | 3.75 | 3.33 | 3.00 | 3.75 | 3.49 | 3.42 |
| #trackit: Track Health and Pain | 2.80 | 4.50 | 4.00 | 3.14 | 2.50 | 3.39 | 3.61 |
| Chronic insights | 3.20 | 3.25 | 3.67 | 3.00 | 3.75 | 3.37 | 3.28 |
| MDHealthTrak—Symptom Tracker | 3.20 | 3.75 | 3.67 | 3.14 | 2.75 | 3.30 | 3.44 |
| Symptomator | 2.20 | 4.50 | 3.67 | 2.57 | 3.50 | 3.29 | 3.23 |
| CoVstat | 3.20 | 3.50 | 3.33 | 3.00 | 3.25 | 3.26 | 3.26 |
| Chronic iIllness Monitor | 3.20 | 3.50 | 3.33 | 2.86 | 2.75 | 3.13 | 3.22 |
| Metriport—Tracker and Lifelog | 3.20 | 3.50 | 3.33 | 2.43 | 3.00 | 3.09 | 3.12 |
| Symple Symptom Tracker | 3.20 | 3.25 | 3.00 | 2.43 | 3.25 | 3.03 | 2.97 |
| Below average # | |||||||
| Flaredown for Chronic Illness | 3.00 | 2.75 | 3.33 | 2.86 | 3.00 | 2.99 | 2.99 |
| Symptom Tracker | 2.60 | 4.00 | 2.67 | 2.00 | 3.00 | 2.85 | 2.82 |
| Wellth Health Tracker | 2.60 | 3.25 | 3.33 | 2.71 | 2.00 | 2.78 | 2.97 |
| TracknShare LITE | 2.40 | 3.00 | 2.67 | 2.14 | 2.50 | 2.54 | 2.55 |
| PeopleWith—Symptoms and Health | 2.40 | 3.00 | 2.67 | 2.14 | 1.75 | 2.39 | 2.55 |
| Healthmatica | 1.60 | 2.50 | 2.33 | 2.00 | 1.75 | 2.04 | 2.11 |
| Mean quality score | 3.33 | 3.75 | 3.67 | 3.04 | 3.47 | 3.45 | 3.45 |
| Category | Registration, Median (IQR) | Cost, Median (IQR) | ||||
|---|---|---|---|---|---|---|
| Required (n = 22) | Not Required (n = 7) | p Value | Free (n = 23) † | Paid (n = 6) ‡ | p Value | |
| Engagement | 3.40 (3.2–4.25) | 2.80 (2.4–3.2) | 0.013 | 3.20 (2.60–3.60) | 3.30 (3.20–4.60) | 0.232 |
| Functionality | 3.63 (3.25–3.81) | 4.5 (3.25–4.5) | 0.165 | 3.75 (3.25–4.50) | 3.75 (3.50–4.50) | 0.477 |
| Aesthetics | 4.00 (3.33–4.33) | 3.67 (2.67–4.00) | 0.165 | 3.67 (3.30–4.00) | 4.00 (3.33–4.42) | 0.254 |
| Information | 3.07 (2.86–3.61) | 2.57 (2.13–3.14) | 0.048 | 3.00 (2.43–3.57) | 2.93 (2.86–3.68) | 0.773 |
| Subjective quality | 3.75 (2.94–4.31) | 3.25 (2.5–3.75) | 0.217 | 3.50 (2.75–3.75) | 3.88 (3.13–4.81) | 0.192 |
| Overall | 3.54 (3.12–4.16) | 3.29 (2.85–3.66) | 0.237 | 3.39 (2.99–3.74) | 3.63 (3.23–4.31) | 0.254 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaitkienė, G.; Kuzborska, Z.; Žukauskienė, M. Digital Health Solutions for Chronic Illnesses: A Systematic Review of Mobile Health Apps and Quality Analysis with Mobile App Rating Scale. J. Ageing Longev. 2022, 2, 193-205. https://doi.org/10.3390/jal2030016
Vaitkienė G, Kuzborska Z, Žukauskienė M. Digital Health Solutions for Chronic Illnesses: A Systematic Review of Mobile Health Apps and Quality Analysis with Mobile App Rating Scale. Journal of Ageing and Longevity. 2022; 2(3):193-205. https://doi.org/10.3390/jal2030016
Chicago/Turabian StyleVaitkienė, Gintarė, Zyta Kuzborska, and Milda Žukauskienė. 2022. "Digital Health Solutions for Chronic Illnesses: A Systematic Review of Mobile Health Apps and Quality Analysis with Mobile App Rating Scale" Journal of Ageing and Longevity 2, no. 3: 193-205. https://doi.org/10.3390/jal2030016
APA StyleVaitkienė, G., Kuzborska, Z., & Žukauskienė, M. (2022). Digital Health Solutions for Chronic Illnesses: A Systematic Review of Mobile Health Apps and Quality Analysis with Mobile App Rating Scale. Journal of Ageing and Longevity, 2(3), 193-205. https://doi.org/10.3390/jal2030016





