Effect of Physical Exercise on Sleep Quality in Elderly Adults: A Systematic Review with a Meta-Analysis of Controlled and Randomized Studies
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Data Source and Search Strategies
2.3. Selection of Clinical Trials
2.4. Data Collection
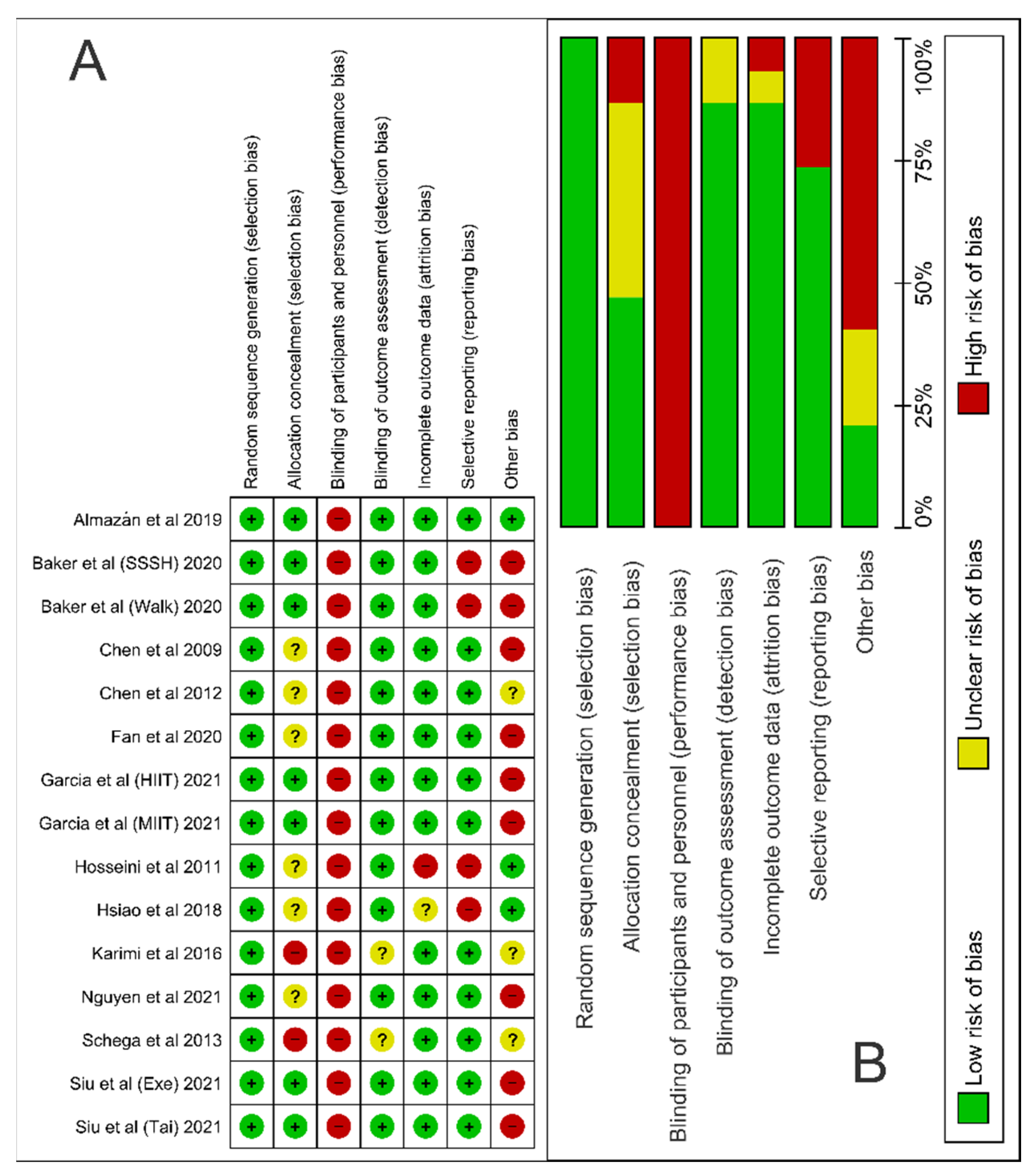
2.5. Assessment of Risk of Bias
2.6. Data Analysis
3. Results
3.1. Article Selection and Bias Analysis
3.2. Study Characteristics
3.3. Effect of Physical Exercise on Sleep Quality
3.4. Publication Bias, Sensitivity Analysis, Statistical Power and Quality of Evidence
3.5. Difference in Dropout between Groups
4. Discussion
4.1. This Work
4.2. Limitations
4.3. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-Derived Neurotrophic Factor |
| GABA | Grading of Recommendations, Assessment, Development and Evaluation |
| HIIT | High-Intensity Interval Training Program |
| MIIT | Moderate-Intensity Interval Training Program |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | Prospective Register of Systematic Reviews |
| PSQI | Pittsburgh Sleep Quality Index |
| RCT | Randomized Controlled Trial |
| SSSH | Stay Strong, Stay Healthy training program |
| SMD | Standardized Mean Difference |
| RoB 2 | Cochrane Risk of Bias Tool 2 |
| REM | Rapid eye Movement |
| VIP | Vasoactive intestinal peptide |
References
- Vanderlinden, J.; Boen, F.; van Uffelen, J.G.Z. Effects of physical activity programs on sleep outcomes in older adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [PubMed]
- Panagiotou, M.; Michel, S.; Meijer, J.H.; Deboer, T. The aging brain: Sleep, the circadian clock and exercise. Biochem. Pharmacol. 2021, 191, 114563. [Google Scholar] [CrossRef] [PubMed]
- Vinke, E.J.; de Groot, M.; Venkatraghavan, V.; Klein, S.; Niessen, W.J.; Ikram, M.A.; Vernooij, M.W. Trajectories of imaging markers in brain aging: The Rotterdam Study. Neurobiol. Aging 2018, 71, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Monjan, A.A. Perspective on sleep and aging. Front. Neurol. 2010, 1, 124. [Google Scholar] [CrossRef] [Green Version]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [Green Version]
- Wetterberg, L.; Bratlid, T.; von Knorring, L.; Eberhard, G.; Yuwiler, A. A multinational study of the relationships between nighttime urinary melatonina production, age, gender, body size, and latitude. Eur. Arch. Psychiatry Clin. Neurosci. 1999, 249, 256–262. [Google Scholar] [CrossRef]
- Stewart, R.; Besset, A.; Bebbington, P.; Brugha, T.; Lindesay, J.; Jenkins, R.; Singleton, N.; Meltzer, H. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep 2006, 29, 1391–1397. [Google Scholar] [CrossRef] [Green Version]
- Matheson, E.; Hainer, B.L. Insomnia: Pharmacologic Therapy. Am. Fam. Physician 2017, 96, 29–35. [Google Scholar]
- Léger, D.; Poursain, B.; Neubauer, D.; Uchiyama, M. An international survey of sleeping problems in the general population. Curr. Med. Res. Opin. 2008, 24, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Bertolazi, A.N.; Fagondes, S.C.; Hoff, L.S.; Dartora, E.G.; Miozzo, I.C.; de Barba, M.E.; Barreto, S.S. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011, 12, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banno, M.; Harada, Y.; Taniguchi, M.; Tobita, R.; Tsujimoto, H.; Tsujimoto, Y.; Kataoka, Y.; Noda, A. Exercise can improve sleep quality: A systematic review and meta-analysis. PeerJ 2018, 6, e5172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, G.A.; Kelley, K.S. Exercise and sleep: A systematic review of previous meta-analyses. J. Evid. Based Med. 2017, 10, 26–36. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.S.; Weitzel, K.J.; Royse, L.A.; Miller, K.; Guess, T.M.; Ball, S.D.; Duren, D.L. Efficacy of an 8-Week Resistance Training Program in Older Adults: A Randomized Controlled Trial. J. Aging Phys. Act. 2020, 29, 121–129. [Google Scholar] [CrossRef]
- Jiménez-García, J.D.; Hita-Contreras, F.; de la Torre-Cruz, M.J.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Fábrega-Cuadros, R.; Martínez-Amat, A. Effects of HIIT and MIIT Suspension Training Programs on Sleep Quality and Fatigue in Older Adults: Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 1211. [Google Scholar] [CrossRef]
- Siu, P.M.; Yu, A.P.; Tam, B.T.; Chin, E.C.; Yu, D.S.; Chung, K.F.; Hui, S.S.; Woo, J.; Fong, D.Y.; Lee, P.H.; et al. Effects of Tai Chi or Exercise on Sleep in Older Adults With Insomnia: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2037199. [Google Scholar] [CrossRef]
- Aibar-Almazán, A.; Hita-Contreras, F.; Cruz-Díaz, D.; de la Torre-Cruz, M.; Jiménez-García, J.D.; Martínez-Amat, A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: A randomized controlled trial. Maturitas 2019, 124, 62–67. [Google Scholar] [CrossRef]
- Chen, K.M.; Chen, M.H.; Chao, H.C.; Hung, H.M.; Lin, H.S.; Li, C.H. Sleep quality, depression state, and health status of older adults after silver yoga exercises: Cluster randomized trial. Int. J. Nurs. Stud. 2009, 46, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Liu, H.E.; Huang, H.Y.; Chiou, A.F. The effect of a simple traditional exercise programme (Baduanjin exercise) on sleep quality of older adults: A randomized controlled trial. Int. J. Nurs. Stud. 2012, 49, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Song, W.; Zhang, J.; Er, Y.; Xie, B.; Zhang, H.; Liao, Y.; Wang, C.; Hu, X.; Mcintyre, R.; et al. The efficacy of mind-body (Baduanjin) exercise on self-reported sleep quality and quality of life in elderly subjects with sleep disturbances: A randomized controlled trial. Sleep Breath 2020, 24, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Esfirizi, M.F.; Marandi, S.M.; Rezaei, A. The effect of Ti Chi exercise on the sleep quality of the elderly residents in Isfahan, Sadeghieh elderly home. Iran J. Nurs. Midwifery Res. 2011, 16, 55–60. [Google Scholar]
- Hsiao, C.Y.; Chen, K.M.; Tsai, H.Y.; Huang, H.T.; Cheng, Y.Y.; Tsai, A.Y. Self-Perceived Health and Sleep Quality of Community Older Adults after Acupunch Exercises. Am. J. Geriatr. Psychiatry 2018, 26, 511–520. [Google Scholar] [CrossRef]
- Karimi, S.; Soroush, A.; Towhidi, F.; Makhsosi, B.R.; Karimi, M.; Jamehshorani, S.; Akhgar, A.; Fakhri, M.; Abdi, A. Surveying the effects of an exercise program on the sleep quality of elderly males. Clin. Interv. Aging 2016, 11, 997–1002. [Google Scholar]
- Nguyen, M.H.; Kruse, A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin. Interv. Aging 2012, 7, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Schega, L.; Peter, B.; Törpel, A.; Mutschler, H.; Isermann, B.; Hamacher, D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: A pilot study. Gerontology 2013, 59, 316–323. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2005, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Pigott, T.D. Advances in Meta-Analysis. In Springer New York Dordrecht Heidelberg London; Statistics for Social and Behavioral Sciences: New York, NY, USA, 2012; pp. 35–54. [Google Scholar]
- Muncer, S.; Taylor, S.; Craigie, M. Power dressing and meta-analysis: Incorporating power analysis into meta-analysis. J. Adv. Nurs. 2002, 38, 274–280. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines:1.Introduction-GRADEevidenceprofiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.; Park, I.; Kokudo, C.; Zhang, S.; Suzuki, C.; Yajima, K.; Satoh, M.; Tokuyama, K.; Okura, T. Distinct effects of low-intensity physical activity in the evening on sleep quality in older women: A comparison of exercise and housework. Exp. Gerontol. 2021, 143, 111–165. [Google Scholar] [CrossRef] [PubMed]
- Awick, E.A.; Ehlers, D.K.; Aguiñaga, S.; Daugherty, A.M.; Kramer, A.F.; McAuley, E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen. Hosp. Psychiatry 2017, 49, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bruseghini, P.; Tam, E.; Calabria, E.; Milanese, C.; Capelli, C.; Galvani, C. High Intensity Interval Training Does Not Have Compensatory Effects on Physical Activity Levels in Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, T.E.; Anderson, M.L.; Renz, A.D.; Greenwood-Hickman, M.A.; McClure, J.B.; Rosenberg, D.E. Changes in Self-Reported Health and Psychosocial Outcomes in Older Adults Enrolled in Sedentary Behavior Intervention Study. Am. J. Health Promot. 2019, 33, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fisher, K.J.; Harmer, P.; Irbe, D.; Tearse, R.G.; Weimer, C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: A randomized controlled trial. J. Am. Geriatr. Soc. 2004, 52, 892–900. [Google Scholar] [CrossRef]
- Rubio-Arias, J.; Marín-Cascales, E.; Ramos-Campo, D.J.; Hernandez, A.V.; Pérez-López, F.R. Effect of exercise on sleep quality and insomnia in middle-aged women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2017, 100, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Liu, S.; Chen, X.J.; Yu, H.H.; Yang, Y.; Wang, W. Effects of Exercise on Sleep Quality and Insomnia in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Psychiatry 2021, 12, 664499. [Google Scholar] [CrossRef]
- Hughes, A.T.L.; Samuels, R.E.; Baño-Otálora, B.; Belle, M.D.C.; Wegner, S.; Guilding, C.; Northeast, R.C.; Loudon, A.S.I.; Gigg, J.; Piggins, H.D. Timed daily exercise remodels circadian rhythms in mice. Commun. Biol. 2021, 4, 761. [Google Scholar] [CrossRef] [PubMed]
- Theron, J.J.; Oosthuizen, J.M.; Rautenbach, M.M. Effect of physical exercise on plasma melatonin levels in normal volunteers. S. Afr. Med. J. 1984, 66, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Maj, M.; Fusco, M.; Orazzo, C.; Kemali, D. Physical exercise at night blunts the nocturnal increase of plasma melatonin levels in healthy humans. Life Sci. 1990, 47, 1989–1995. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hashimoto, S.; Masubuchi, S.; Honma, S.; Honma, K.I. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Shioda, K.; Morita, Y.; Kubota, C.; Ganeko, M.; Takeda, N. Exercise Effects on Sleep Physiology. Front. Neurol. 2012, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; van Egmond, L.T.; Cedernaes, J.; Benedict, C. The role of exercise-induced peripheral factors in sleep regulation. Mol. Metab. 2020, 42, 101096. [Google Scholar] [CrossRef]
- Dundar, A.; Kocahan, S.; Sahin, L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch. Physiol. Biochem. 2019, 127, 291–295. [Google Scholar] [CrossRef]
- Zhu, B.; Shi, C.; Park, C.G.; Zhao, X.; Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2019, 45, 18–39. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Aerobic exercise modulates cytokine profile and sleep quality in elderly. Afr. Health Sci. 2019, 19, 2198–2207. [Google Scholar] [CrossRef] [Green Version]
- Sewell, K.R.; Erickson, K.I.; Rainey-Smith, S.R.; Peiffer, J.J.; Sohrabi, H.R.; Brown, B.M. Relationships between physical activity, sleep and cognitive function: A narrative review. Neurosci. Biobehav. Rev. 2021, 130, 369–378. [Google Scholar] [CrossRef]
- Sato, M.; Betriana, F.; Tanioka, R.; Osaka, K.; Tanioka, T.; Schoenhofer, S. Balance of Autonomic Nervous Activity, Exercise, and Sleep Status in Older Adults: A Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 12896. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hippel, P.T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [Green Version]



| Study (Year) Location | Population Condition | Sample Size (n) E/C | Sex (Male, %) | Age Mean (SD) | Intervention Group | Control Group | Duration (Weeks) | Results * |
|---|---|---|---|---|---|---|---|---|
| Almazán [20] (2019) Spain | Healthy postmenopausal woman | E: 55 C: 55 | E: 0% C: 0% | E: 69.98 (7.8) C: 66.8 (10.1) | Pilates (1 h, 2 times a week) | No intervention | 12 | E > C ** |
| Baker [17] (2020) USA SSSH | Healthy older adults | E: 20 C: 20 | 76% | E: 68.2 (6.7) C: 67.6 (6.9) | SSSH (1 h, 2 times a week) | No intervention | 8 | E > C |
| Baker [17] (2020) USA Walk | Healthy older adults | E: 20 C: 20 | 76% | E: 68.6 (8.7) C: 67.6 (6.9) | Walking (1 h, 2 times a week) | No intervention | 8 | E > C |
| Chen [21] (2009) Taiwan | Healthy older adults | E: 62 C: 66 | E: 83.87% C: 62.12% | E: 65.77 (4.3) C: 72.42 (6.0) | Yoga (70 min, 3 times a week) | No intervention | 24 (6 months) | E > C |
| Chen [22] (2012) Taiwan | Healthy older adults | E: 27 C: 28 | E: 37% C: 32.1% | E: 70.5 (7.9) C: 73.0 (8.3) | Baduanjin exercise (30 min, 3 times a week) | No intervention | 12 | E > C |
| Fan [23] (2020) China | Healthy older adults with PSQI ≥ 5 and insomnia | E: 67 C: 72 | E: 17.9% C: 30.6% | E: 70.3 (5.7) C: 71.8 (6.7) | Baduanjin exercise (45 min, 5 times a week) | Usual lifestyle behavior | 24 | E > C |
| García [18] (2021) Spain HIIT | Healthy older adults | E: 28 C: 27 | E: 11.8% C: 47.1% | E: 68.2 (3.0) C: 68.5 (6.3) | HIIT (1 h/day, 2 times a week) | Daily activities | 12 | E > C |
| García [18] (2021) Spain MIIT | Healthy older adults | E: 27 C: 27 | E: 41.2% C: 47.1% | E: 68.7 (6.0) C: 68.5 (6.3) | MIIT (1 h/day, 2 times a week) | Daily activities | 12 | No significant difference |
| Hosseini [24] (2011) Iran | Healthy older adults | E: 31 C: 31 | E: 51.6% C: 45.1% | E: 68.7 (5.5) C: 69.4 (5.3) | Tai chi (5–25 min, 3 times a week) | No intervention | 12 | E > C |
| Hsiao [25] (2018) Taiwan | Healthy older adults | E: 106 C: 114 | E: 17.7% C: 25.2% | E: 74.6 (6.0) C: 73.9 (5.4) | Healthy Beat Acupunch (40 min, 3 times a week) | Daily activities | 52 (12 months) | E > C |
| Karimi [26] (2016) Iran | Older adults with primary insomnia | E: 23 C: 23 | E: 100% C: 100% | E: 66.8 (3.8) C: 67.5 (4.3) | Walking (30 min, 3 times a week) | Usual lifestyle behavior | 8 | E > C |
| Nguyen [27] (2012) Vietnam | Healthy older adults | E: 48 C: 48 | E: 50% C: 50% | E: 69.3 (5.3) C: 68.7 (4.9) | Tai chi (1 h/day, 2 times a week) | Daily activities | 26 (6 months) | E > C |
| Schega [28] (2013) Germany | Healthy older adults | E: 17 C: 17 | E: 30.76% C: 30.76% | E: 63.7 (3.4) C: 63.6 (3.2) | Hypoxia and resistance training (45 min protocol, 3 times a week) | Placebo air mixture | 6 | E > C |
| Siu [19] (2021) China Conventional exercise | Older adults with insomnia | E: 105 C: 100 | E: 20% C: 20% | E: 67.3 (5.7) C: 68.0 (8.2) | Brisk walking andmuscle-strengthening exercises (1 h/day, 3 times a week) | No intervention | 12 | E > C |
| Siu [19] (2021) China Tai chi | Older adults with insomnia | E: 105 C: 100 | E: 20% C: 20% | E: 67.3 (5.7) C: 68.0 (8.2) | Tai chi (1 h/day, 3 times a week) | No intervention | 12 | E > C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.P.O.; Silva, M.P.O.; Silva, V.L.d.S.; Mantovani, D.B.C.; Mittelmann, J.V.; Oliveira, J.V.V.; Pessoa, J.P.d.L.; Chaves, Y.L.; Haddad, M.P.; Andrielli, O.; et al. Effect of Physical Exercise on Sleep Quality in Elderly Adults: A Systematic Review with a Meta-Analysis of Controlled and Randomized Studies. J. Ageing Longev. 2022, 2, 85-97. https://doi.org/10.3390/jal2020008
Silva VPO, Silva MPO, Silva VLdS, Mantovani DBC, Mittelmann JV, Oliveira JVV, Pessoa JPdL, Chaves YL, Haddad MP, Andrielli O, et al. Effect of Physical Exercise on Sleep Quality in Elderly Adults: A Systematic Review with a Meta-Analysis of Controlled and Randomized Studies. Journal of Ageing and Longevity. 2022; 2(2):85-97. https://doi.org/10.3390/jal2020008
Chicago/Turabian StyleSilva, Vitor P. O., Marcelo P. O. Silva, Vitor L. de S. Silva, David B. C. Mantovani, João V. Mittelmann, João V. V. Oliveira, João P. de L. Pessoa, Yuri L. Chaves, Mikhail P. Haddad, Otávio Andrielli, and et al. 2022. "Effect of Physical Exercise on Sleep Quality in Elderly Adults: A Systematic Review with a Meta-Analysis of Controlled and Randomized Studies" Journal of Ageing and Longevity 2, no. 2: 85-97. https://doi.org/10.3390/jal2020008
APA StyleSilva, V. P. O., Silva, M. P. O., Silva, V. L. d. S., Mantovani, D. B. C., Mittelmann, J. V., Oliveira, J. V. V., Pessoa, J. P. d. L., Chaves, Y. L., Haddad, M. P., Andrielli, O., Bento, V. L., Dourado, M. L. C., & Melo, H. M. d. A. (2022). Effect of Physical Exercise on Sleep Quality in Elderly Adults: A Systematic Review with a Meta-Analysis of Controlled and Randomized Studies. Journal of Ageing and Longevity, 2(2), 85-97. https://doi.org/10.3390/jal2020008