Preclinical and Prodromal Frontotemporal Dementia: Challenges and Opportunities
Abstract
1. Introduction
2. Complexity of the FTD Spectrum
2.1. Complexity of the FTD Spectrum: A Multiform Phenotype
- (I)
- Behavioural variant FTD (bvFTD), characterized by progressive deterioration in social conduct and personality, including apathy or disinhibition, loss of empathy, poor judgment and compulsive or repetitive behaviours [9]. This variant primarily involves the bilateral frontal cortex (dorsolateral prefrontal cortex which mediates executive functions; orbitofrontal cortex, regulating social behaviour, inhibition, and emotional responses; and medial prefrontal cortex, important for motivation and social cognition) and anterior temporal regions (including the amygdala and temporal pole, involved in emotional processing, social understanding, and semantic memory).
- (II)
- a.
- Semantic variant (svPPA), characterized by progressive loss of semantic knowledge and naming, with involvement of the anterior temporal region.
- b.
- Non fluent/agrammatic variant (nfvPPA), characterized by impaired grammar and motor speech deficits, involving left posterior frontal and insular regions.
- (III)
- Right temporal variant FTD (rtvFTD), characterized by loss of empathy, behavioural changes (disinhibition or apathy, compulsive or ritualistic behaviour), mood changes (irritability, anxiety, or suspiciousness), and prosopagnosia, associated with degeneration in the right anterior temporal lobe, potentially extending into the right orbitofrontal cortex [43,44].
- (IV)
- Extrapyramidal motor syndromes, including:
- a.
- Progressive supranuclear palsy–Richardson syndrome (PSP-RS) characterized by supranuclear gaze palsy, postural instability, axial rigidity, bradykinesia, dysarthria and dysphagia, cognitive impairment (executive dysfunction, apathy, and reduced verbal fluency), facial expression changes with a “staring” facial appearance due to reduced blinking and facial rigidity. PSP-RS primarily involves the tectum and tegmentum of the midbrain (causing vertical gaze palsy and postural instability), the subthalamic nucleus, globus pallidus, dentate nucleus of the cerebellum, and substantia nigra (motor symptoms), as well as the frontal cortex (executive dysfunction and apathy) [17,45].
- b.
- Corticobasal syndrome (CBS), characterized by cortical signs (limb apraxia, cortical sensory loss, alien limb), motor signs (asymmetric rigidity, apraxia, dystonia, myoclonus) and cognitive/behavioural signs (executive dysfunction; aphasia; visuospatial dysfunction; behavioural changes). CBS typically involves the frontoparietal lobes asymmetrically [18].
- (V)
- Pyramidal motor disorders, most notably amyotrophic lateral sclerosis (ALS) [19]. Traditionally, ALS and FTD are wider considered the two phenotypic extremes on the so called motor neuron disease–FTD continuum, where (i) about half of all patients exhibit pure motor involvement with preserved cognition during the disease course (classical ALS), (ii) up to half of ALS patients display some degree of cognitive impairment or behavioural changes, without fulfilling the diagnostic criteria for FTD (ALS with executive dysfunction [ALS-eci], ALS with no executive dysfunction but impairment in other cognitive domains [ALS-neci], e.g., memory, or ALS with behavioural changes [ALS-bi]), and (iii) about 5–10% of patients firstly presenting FTD (most frequently bvFTD, occasionally PPA) while developing motor neuron involvement without full ALS manifestation [46]. Pathologically, ALS-FTD involves the primary motor cortex, corticospinal tracts, and brainstem motor nuclei (motor dysfunction), as well as the frontal lobes (orbitofrontal, medial prefrontal, anterior cingulate), anterior temporal lobes, insular cortex (cognitive/behavioural impairment), and occasionally the striatum and hippocampus [47,48].
2.2. Complexity of the FTD Spectrum: Underpinning Neuropathology
2.2.1. Tau Pathology
- a.
- Pick’s disease (PiD), with 3R >> 4R tau, forming Pick bodies (intracytoplasmic, eosinophilic, round or oval, strongly argyrophilic neuronal inclusions) in association with ubiquitin. They mainly affect layers II–III of the frontal and temporal cortices and the hippocampus, with a phenotype mostly corresponding to bvFTD and PPA [52,53].
- b.
- Corticobasal degeneration (CBD) with 4R tau aggregating in neurons and glia (astrocytes and oligodendrocytes). 4R tau forms astrocytic plaques, coiled bodies (cytoplasmic inclusions within oligodendrocytes), tau-positive threads (thin, thread-like structures in neurites and neuropil) in grey matter (GM) and white matter (WM), and neuronal cytoplasmic tau inclusions. They mainly aggregate in layers V–VI of the frontal and parietal cortices, particularly in motor and premotor cortices, but also in the basal ganglia (especially the subthalamic nucleus and globus pallidus) and subcortical WM [18,54]. Typical phenotype corresponds to CBS, PSP-RS, nfvPPA, frontobehavioural spatial syndrome (FBS) [18].
- c.
- Progressive supranuclear palsy (PSP), with 4R tau forming tufted astrocytes (star-like inclusions in proximal astrocytic processes, pathognomonic for PSP), coiled bodies, globose neurofibrillary tangles (NFTs) (round/oval, eosinophilic, silver-positive, intraneuronal inclusions), and tau-positive threads [55,56]. Cortical involvement mainly affects layers V–VI of the frontal and parietal cortices. Typical phenotype corresponds to PSP-RS, PSP-Parkinson’s (PSP-P), Pure akinesia with gait freezing (PAGF), CBS, nfvPPA, bvFTD [17].
- d.
- Globular glial tauopathy (GGT), with 4R tau forming globular oligodendroglial inclusions (GOIs), globular astrocytic inclusions (GAIs), and tau-positive threads in WM, primarily in the frontotemporal cortex, basal ganglia, and brainstem. The clinical presentation often includes bvFTD, parkinsonism, or pyramidal motor disorders [57,58].
- e.
- FTLD-tau/MAPT, with 3R or 4R tau preferentially localizing in von Economo neurons and fork cells, aggregating in globose NFTs. FTLD-tau/MAPT is linked to genetic causes (familial cases) and presents features resembling PSP, CBD, or Pick’s disease [59].
2.2.2. TDP-43 Pathology
- a.
- FTLD-TDP subtype A, characterized by moderate to numerous NCIs, numerous short DNs, and occasional NIIs, mainly in upper cortical layers II/III. It also has thread pathology in the subcortical WM, delicate wispy threads in hippocampal CA1, and a predominance of DN and occasional NII in striatum and other subcortical GM regions. Subtype A is often associated with bvFTD (apathy and social withdrawal) and nfvPPA. Executive dysfunction, some degree of memory impairment, and neuropsychiatric manifestations (delusions, hallucinations or obsessive behaviours) are not uncommon.
- b.
- FTLD-TDP subtype B, with moderate to numerous NCIs, sparse DNs, and rare NIIs across all cortical layers. It is also characterized by the presence of glial cytoplasmic inclusions in the subcortical WM, a predominance of diffuse NCIs in subcortical GM, and NCI in lower motor neurons of the medulla and spinal cord. Subtype B is typically linked to the FTD-MND spectrum and to a lesser extent to bvFTD, nfvPPA. Neuropsychiatric manifestation (psychosis) is particularly frequent.
- c.
- FTLD-TDP subtype C, with long, tortuous DNs and infrequent NCIs, primarily in upper cortices. It also has compact “Pick body-like” NCI in dentate granule cells of the hippocampus and striatum. Subtype C is frequently seen in svPPA and sometimes in rtvFTD.
- d.
- FTLD-TDP subtype D, with numerous NIIs, fewer NCIs and DNs concentrated in superficial laminae. Modest numbers of DN and NII are also present in the amygdala, basal ganglia, nucleus basalis, thalamus and midbrain. It is exclusively seen in inclusion body myopathy with Paget disease of bone and frontotemporal dementia (IBMPFD) and FTD-MND spectrum linked to valosin-containing protein (VCP) gene mutations [70].
- e.
- FTLD-TDP subtype E, with weakly staining granulofilamentous neuronal cytoplasmic inclusions (GFNI) set in a background of very fine grain-like deposits throughout the neocortex sparing only of the occipital neocortex and cerebellum [68]. Motor neuron involvement was a typical feature, although only sometimes associated with clinical features of ALS. FTLD-TDP type E is consistently associated with a rapid clinical course of one to three years duration.
2.2.3. FET Family Pathology
- a.
- Basophilic inclusion body disease (BIBD), characterized by basophilic NCIs, dense, round-to-oval accumulations of FET proteins (mainly FUS) and ubiquitinated proteins within neuronal cytoplasm.
- b.
- Atypical FTLD (aFTLD-U), characterized by FUS positive NCIs, DNs, and rarely NIIs.
- c.
- Neuronal intermediate filament inclusion disease (NIFID/NIBD), characterized by neuronal intermediate filament aggregates, forming NCIs, NIIs, and DNs inclusions.
- d.
- FUS not otherwise specified (FUS NOS), with variably shaped FUS-positive NCIs, NIIs, and DNs.
2.2.4. DPR Pathology
2.2.5. UPS Pathology
- a.
- FTLD-UPS (FTLD-3 or CHMP2B), characterized by ubiquitin inclusions, mainly localized in the hippocampal dentate gyrus and frontal cortex.
- b.
- FTLD-UPS not otherwise specified (FTLD-UPS NOS), with ubiquitin-immunoreactive NCIs and DNs localized in the frontal and temporal cortices.
2.3. Complexity of the FTD Spectrum: Genetic Underpinnings
- (I)
- MAPT is essential for neuronal integrity and axonal transport. Mutations in MAPT, including both missense and splicing mutations, alter tau function, resulting in either a loss of function, via reduced microtubule-binding affinity or impaired microtubule assembly, or a toxic gain of function, characterized by increased aggregation propensity. These mechanisms ultimately lead to aberrant tau aggregation and seeding [89,90]. Nearly 100 MAPT variants have been linked to FTLD-spectrum disorders, accounting for up to 20% of familial cases (Alzforum database, 08/2025) [91,92,93]. Common genetic variants within the major MAPT haplotypes (H1 and H2) are also associated with an increased risk of sporadic tauopathies [94]. These mutations are frequently associated with PSP and CBD pathology [95,96,97], and genotype–phenotype correlations indicate that MAPT mutation carriers predominantly present with bvFTD, PPA, or FTD with parkinsonism [98]. The median age at onset is approximately 50 years, indicating an early onset and rapid progression [99].
- (II)
- Over 70 pathogenic mutations in GRN have been identified, most of which result in loss of function due to defective transcription or translational inhibition, leading to GRN haploinsufficiency and a reduction in progranulin levels in serum, blood and CSF to less than 50% of normal [100]. Loss of progranulin, particularly in lysosomes, results in lysosomal storage disorders characterized by neuronal ceroid lipofuscinosis [101]. Autosomal dominant loss-of-function mutations in GRN account for 10–15% of FTLD-TDP cases and are specifically associated with FTLD-TDP type A pathology [31,86,102]. Clinically, GRN mutation carriers often exhibit a bvFTD phenotype, although PPA has also been observed [98]. The median age at onset is about 61 years, reflecting an intermediate onset, incomplete penetrance, and rapid clinical deterioration once symptoms emerge [99].
- (III)
- The C9orf72 gene product plays several critical roles: (i) it is involved in RNA metabolism; (ii) it facilitates the accumulation of sense and antisense RNA transcripts of expanded repeats, which serve as templates for the synthesis of dipeptide repeat proteins (DPRs) through repeat-associated non-ATG translation; and (iii) it participates in the autophagy–lysosome pathway. The hexanucleotide repeat expansion (GGGGCC) in the non-coding region of C9orf72 causes disease via multiple mechanisms: (i) reduced gene expression, which disrupts endosomal trafficking and autophagy [103]; (ii) (ii) toxicity from RNA foci and DPRs generated through non-canonical translation [104]; (iii) nucleolar stress; (iv) RNA dysregulation; and (v) defects in nucleocytoplasmic transport and protein degradation [105]. Repeat expansions in C9orf72 account for about 25% of FTLD-TDP cases, primarily involving FTLD-TDP type A and B pathology, and occasionally type C pathology [70,96,97]. Although C9orf72 repeat expansions are typically inherited, they are detected in up to 10% of individuals with apparently sporadic disease. These expansions are twice as common in familial ALS compared to SOD1 mutations, the first gene linked to ALS [32]. Clinically, C9orf72 expansion carriers with FTD commonly present with bvFTD, and in some cases, with PPA [98]. The median age at onset is approximately 58 years, and disease expression is marked by wide variability and a prolonged preclinical phase [99].
- (IV)
- The VCP gene encodes the valosin-containing protein, an enzyme involved in diverse cellular processes such as intracellular trafficking, proteasomal degradation, and programmed cell death. VCP mutations: (i) alter TDP-43 localization between the nucleus and cytoplasm; (ii) impair proteasome activity; (iii) induce endoplasmic reticulum stress; (iv) increase apoptotic markers; and (v) reduce cell viability. These mutations are associated with FTLD-TDP type D pathology.
3. Pathophysiological Mechanisms
4. Defining Preclinical and Prodromal FTD
5. Clinical Characterization and Early Symptomatology
6. Biomarkers for Preclinical and Prodromal FTD
6.1. Fluid Biomarkers (Figure 1C)
6.1.1. Tauopathy
6.1.2. TDP-43 Proteinopathy
6.1.3. FET Proteinopathy
6.1.4. DPR Proteinopathy
6.2. Neuroimaging Biomarkers
6.2.1. Magnetic Resonance Imaging (MRI)
6.2.2. Positron Emission Tomography (PET) Imaging
7. Innovative Interventional Approaches in Early Disease Stages
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A-IADL-Q | Amsterdam instrumental activities of daily living questionnaire |
| AD | Alzheimer’s disease |
| aFTLD-U | atypical frontotemporal lobar degeneration with ubiquitin-positive inclusions |
| ALS | amyotrophic lateral sclerosis |
| ALS-CBS | ALS cognitive behavioural screen |
| AQP4 | aquaporin-4 |
| ASO | antisense oligonucleotide |
| BIBD | basophilic inclusion body disease |
| bvFTD | behavioural variant frontotemporal dementia |
| C9orf72 | chromosome 9 open reading frame 72 |
| CBD | corticobasal degeneration |
| CBS | corticobasal syndrome |
| CDR | clinical dementia rating |
| CHIT-1 | chitinase 1 |
| CSF | cerebrospinal fluid |
| DaTScan | dopamine transporter scan |
| DLPFC | dorsolateral prefrontal cortex |
| DNs | dystrophic neurites |
| DPR | dipeptide repeat proteins |
| DTI | diffusion tensor imaging |
| ECAS | Edinburgh cognitive and behavioural ALS screen |
| FBS | frontobehavioural spatial syndrome |
| FDG-PET | fluorodeoxyglucose positron emission tomography |
| FTD | frontotemporal dementia |
| FTLD | frontotemporal lobar degeneration |
| FUS | fused in sarcoma |
| GAF | global assessment of functioning |
| GAIs | globular astrocytic inclusions |
| GCIs | glial cytoplasmic inclusions |
| GENFI | GENetic Frontotemporal dementia Initiative |
| GFAP | glial fibrillary acidic protein |
| GGT | globular glial tauopathy |
| GM | grey matter |
| GOIs | globular oligodendroglial inclusions |
| GRN | progranulin |
| ICF | intracortical facilitation |
| LICI | long-interval intracortical inhibition |
| lvPPA | logopenic variant primary progressive aphasia |
| MAPT | microtubule-associated protein tau |
| MBI | mild behavioural impairment |
| MCBMI | mild cognitive and/or behavioural and/or motor impairment |
| MRI | magnetic resonance imaging |
| MTL | medial temporal lobe |
| NACC | national Alzheimer’s coordinating centre |
| NCIs | neuronal cytoplasmic inclusions |
| NfL | neurofilament light chain |
| NFTs | neurofibrillary tangles |
| nfvPPA | non-fluent/agrammatic variant primary progressive aphasia |
| NIFID | neuronal intermediate filament inclusion disease |
| NOS | FUS not otherwise specified |
| NIIs | neuronal intranuclear inclusions |
| PET | positron emission tomography |
| PiD | Pick’s disease |
| PGRN | progranulin |
| PSP | progressive supranuclear palsy |
| rtvFTD | right temporal variant frontotemporal dementia |
| SAA | seed amplification assay |
| sAPPβ | soluble amyloid precursor protein beta |
| SICF | short-interval intracortical facilitation |
| SICI | short-interval intracortical inhibition |
| SPECT | single-photon emission computed tomography |
| svPPA | semantic variant primary progressive aphasia |
| tACS | transcranial alternating current stimulation |
| tDCS | transcranial direct current stimulation |
| TDP-43 | TAR DNA-binding protein 43 |
| TMS | transcranial magnetic stimulation |
| UPS | ubiquitin proteasome system |
| VCP | valosin-containing protein |
| WM | White matter |
References
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef]
- Russell, L.L.; Rohrer, J.D. Defining the presymptomatic phase of frontotemporal dementia. Curr. Opin. Neurol. 2023, 36, 276–282. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Logroscino, G.; Piccininni, M.; Binetti, G.; Zecca, C.; Turrone, R.; Capozzo, R.; Tortelli, R.; Battista, P.; Bagoj, E.; Barone, R.; et al. Incidence of frontotemporal lobar degeneration in Italy: The Salento-Brescia Registry study. Neurology 2019, 92, e2355–e2363, Erratum in Neurology 2019, 93, 608. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Boxer, A.L.; Kumfor, F.; Pijnenburg, Y.; Rohrer, J.D. Advances and controversies in frontotemporal dementia: Diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. 2022, 21, 258–272. [Google Scholar] [CrossRef]
- Benussi, A.; Padovani, A.; Borroni, B. Phenotypic Heterogeneity of Monogenic Frontotemporal Dementia. Front. Aging Neurosci. 2015, 7, 171. [Google Scholar] [CrossRef]
- Borroni, B.; Benussi, A. Recent advances in understanding frontotemporal degeneration. F1000Research 2019, 8, 2098. [Google Scholar] [CrossRef]
- Josephs, K.A.; Hodges, J.R.; Snowden, J.S.; Mackenzie, I.R.; Neumann, M.; Mann, D.M.; Dickson, D.W. Neuropathological background of phenotypical variability in frontotemporal dementia Transactive response DNA binding protein of 43 kD FUS Fused in sarcoma. Acta Neuropathol. 2011, 122, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Chan, D.; Anderson, V.; Pijnenburg, Y.; Whitwell, J.; Barnes, J.; Scahill, R.; Stevens, J.M.; Barkhof, F.; Scheltens, P.; Rossor, M.N.; et al. The clinical profile of right temporal lobe atrophy. Brain 2009, 132, 1287–1298. [Google Scholar] [CrossRef]
- Edwards-Lee, T.; Miller, B.L.; Benson, D.F.; Cummings, J.L.; Russell, G.L.; Boone, K.; Mena, I. The temporal variant of frontotemporal dementia. Brain A J. Neurol. 1997, 120, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.A.; Patterson, K.; Hodges, J.R. Left/right asymmetry of atrophy in semantic dementia: Behavioral-cognitive implications. Neurology 2003, 61, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Whitwell, J.L.; Knopman, D.S.; Boeve, B.F.; Vemuri, P.; Senjem, M.L.; Parisi, J.E.; Ivnik, R.J.; Dickson, D.W.; Petersen, R.C.; et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 2009, 73, 1443–1450. [Google Scholar] [CrossRef]
- Seeley, W.W.; Bauer, A.M.; Miller, B.L.; Gorno-Tempini, M.L.; Kramer, J.H.; Weiner, M.; Rosen, H.J. The natural history of temporal variant frontotemporal dementia. Neurology 2005, 64, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L.; Chang, L.; Mena, I.; Boone, K.; Lesser, I.M. Progressive right frontotemporal degeneration: Clinical, neuropsychological and SPECT characteristics. Dementia 1993, 4, 204–213. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; Mclaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; HortobáGyi, T.; et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef]
- Balasa, M.; Gelpi, E.; Martín, I.; Antonell, A.; Rey, M.J.; Grau-Rivera, O.; Molinuevo, J.L.; Sánchez-Valle, R.; Lladó, A.; Catalan collaborative Study Group for FTLD. Diagnostic accuracy of behavioral variant frontotemporal dementia consortium criteria (FTDC) in a clinicopathological cohort. Neuropathol. Appl. Neurobiol. 2015, 41, 882–892. [Google Scholar] [CrossRef]
- Lanata, S.C.; Miller, B.L. The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J. Neurol. Neurosurg. Psychiatry 2016, 87, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.D.; Khan, B.K.; Murthy, N.K.; Miller, B.L.; Rankin, K.P. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J. Clin. Psychiatry 2011, 72, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Misirocchi, F.; Zilioli, A.; Benussi, A.; Cappellari, S.; Mutti, C.; Florindo, I.; Spallazzi, M.; Parrino, L. A Novel CSF1R Mutation Mimicking Frontotemporal Dementia: A Glimpse into a Microgliopathy. Can. J. Neurol. Sci. 2023, 50, 642–644. [Google Scholar] [CrossRef]
- Riedl, L.; Mackenzie, I.R.; Förstl, H.; Kurz, A.; Diehl-Schmid, J. Frontotemporal lobar degeneration: Current perspectives. Neuropsychiatr. Dis. Treat. 2014, 10, 297–310. [Google Scholar] [CrossRef]
- Robinson, J.L.; Lee, E.B.; Xie, S.X.; Rennert, L.; Suh, E.; Bredenberg, C.; Caswell, C.; Van Deerlin, V.M.; Yan, N.; Yousef, A.; et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018, 141, 2181–2193. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Baborie, A.; Pickering-Brown, S.; Plessis DDu Jaros, E.; Perry, R.H.; Neary, D.; Snowden, J.S.; Mann, D.M. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: Classification and relation to clinical phenotype. Acta Neuropathol. 2006, 112, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Neumann, M.; Baborie, A.; Sampathu, D.M.; Du Plessis, D.; Jaros, E.; Perry, R.H.; Trojanowski, J.Q.; Mann, D.M.; Lee, V.M.; et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011, 122, 111–113. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Neumann, M. Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol. 2017, 134, 79–96. [Google Scholar] [CrossRef]
- Borroni, B.; Padovani, A. Dementia: A new algorithm for molecular diagnostics in FTLD. Nat. Rev. Neurol. 2013, 9, 241–242. [Google Scholar] [CrossRef]
- Grover, A.; Houlden, H.; Baker, M.; Adamson, J.; Lewis, J.; Prihar, G.; Pickering-Brown, S.; Duff, K.; Hutton, M. 5’ splice site mutations in tau associated with the inherited dementia FTDP-17 affect a stem-loop structure that regulates alternative splicing of exon 10. J. Biol. Chem. 1999, 274, 15134–15143. [Google Scholar] [CrossRef]
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Pengo, M.; Alberici, A.; Libri, I.; Benussi, A.; Gadola, Y.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Borroni, B. Sex influences clinical phenotype in frontotemporal dementia. Neurol. Sci. 2022, 43, 5281–5287. [Google Scholar] [CrossRef]
- Falconer, D.S. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann. Hum. Genet. 1965, 29, 51. [Google Scholar] [CrossRef]
- Benussi, A.; Alberici, A.; Samra, K.; Russell, L.L.; Greaves, C.V.; Bocchetta, M.; Ducharme, S.; Finger, E.; Fumagalli, G.; Galimberti, D.; et al. Conceptual framework for the definition of preclinical and prodromal frontotemporal dementia. Alzheimers Dement. 2022, 18, 1408–1423. [Google Scholar] [CrossRef]
- Whiteside, D.J.; Malpetti, M.; Jones, P.S.; Ghosh, B.C.P.; Coyle-Gilchrist, I.; van Swieten, J.C.; Seelaar, H.; Jiskoot, L.; Borroni, B.; Sanchez-Valle, R.; et al. Temporal dynamics predict symptom onset and cognitive decline in familial frontotemporal dementia. Alzheimers Dement. 2023, 19, 1947–1962. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, J.; Blommaert, J.; Devrome, M.; Radwan, A.; Van Weehaeghe, D.; De Schaepdryver, M.; Ceccarini, J.; Rezaei, A.; Schramm, G.; van Aalst, J.; et al. Use of multimodal imaging and clinical biomarkers in presymptomatic carriers of C9orf72 repeat expansion. JAMA Neurol. 2020, 77, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ende, E.L.; Bron, E.E.; Poos, J.M.; Jiskoot, L.C.; Panman, J.L.; Papma, J.M.; Meeter, L.H.; Dopper, E.G.P.; Wilke, C.; Synofzik, M.; et al. A data-driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain 2022, 145, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Premi, E.; Grassi, M.; Alberici, A.; Cantoni, V.; Gazzina, S.; Archetti, S.; Gasparotti, R.; Fumagalli, G.G.; Bouzigues, A.; et al. Diagnostic accuracy of research criteria for prodromal frontotemporal dementia. Alzheimers Res. Ther. 2024, 16, 10. [Google Scholar] [CrossRef]
- Barker, M.S.; Gottesman, R.T.; Manoochehri, M.; Chapman, S.; Appleby, B.S.; Brushaber, D.; Devick, K.L.; Dickerson, B.C.; Domoto-Reilly, K.; Fields, J.A.; et al. Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain 2022, 145, 1079–1097. [Google Scholar] [CrossRef]
- Wilson, S.M.; Ogar, J.M.; Laluz, V.; Growdon, M.; Jang, J.; Glenn, S.; Miller, B.L.; Weiner, M.W.; Gorno-Tempini, M.L. Automated MRI-based classification of primary progressive aphasia variants. Neuroimage 2009, 47, 1558–1567. [Google Scholar] [CrossRef]
- Younes, K.; Borghesani, V.; Montembeault, M.; Spina, S.; Mandelli, M.L.; Welch, A.E.; Weis, E.; Callahan, P.; Elahi, F.M.; Hua, A.Y.; et al. Right temporal degeneration and socioemotional semantics: Semantic behavioural variant frontotemporal dementia. Brain 2022, 145, 4080–4096. [Google Scholar] [CrossRef]
- Ulugut Erkoyun, H.; Groot, C.; Heilbron, R.; Nelissen, A.; van Rossum, J.; Jutten, R.; Koene, T.; Van der Flier, W.M.; Wattjes, M.P.; Scheltens, P.; et al. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain 2020, 143, 2831–2843. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Lillo, P.; Yew, B.; Hsieh, S.; Savage, S.; Hodges, J.R.; Kiernan, M.C.; Hornberger, M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 2013, 80, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Hu, N.; Xiao, Y.; Zhang, W.; Gong, Q.; Lui, S. Comparison of Gray Matter Atrophy in Behavioral Variant Frontal Temporal Dementia and Amyotrophic Lateral Sclerosis: A Coordinate-Based Meta-Analysis. Front. Aging Neurosci. 2020, 12, 500359. [Google Scholar] [CrossRef]
- Lee Virginia, M.Y.; Zhukareva, V.; Vogelsberg-Ragaglia, V.; Wszolek, Z.; Reed, L.; Miller, B.I.; Geschwind, D.H.; Bird, T.D.; McKeel, D.; Goate, A.; et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282, 1914–1917. [Google Scholar] [CrossRef]
- D’Souza, I.; Poorkaj, P.; Hong, M.; Nochlin, D.; Lee, V.M.Y.; Bird, T.D.; Schellenberg, G.D. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 1999, 96, 5598. [Google Scholar] [CrossRef] [PubMed]
- Von Bergen, M.; Barghorn, S.; Li, L.; Marx, A.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Mutations of Tau Protein in Frontotemporal Dementia Promote Aggregation of Paired Helical Filaments by Enhancing Local β-Structure. J. Biol. Chem. 2001, 276, 48165–48174. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Kishimoto, Y.; Yokota, O. Pick’s disease. Adv. Exp. Med. Biol. 2012, 724, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathology of Pick’s disease. Neurology 2001, 56 (Suppl. S4), S16–S20. [Google Scholar] [CrossRef]
- Ling, H.; O’Sullivan, S.S.; Holton, J.L.; Revesz, T.; Massey, L.A.; Williams, D.R.; Paviour, D.C.; Lees, A.J. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 2010, 133, 2045–2057. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Lees, A.J. Progressive supranuclear palsy: Clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009, 8, 270–279. [Google Scholar] [CrossRef]
- Ahmed, Z.; Bigio, E.H.; Budka, H.; Dickson, D.W.; Ferrer, I.; Ghetti, B.; Giaccone, G.; Hatanpaa, K.J.; Holton, J.L.; Josephs, K.A.; et al. Globular glial tauopathies (GGT): Consensus recommendations. Acta Neuropathol. 2013, 126, 537–544. [Google Scholar] [CrossRef]
- Chung, D.E.C.; Carlomagno, Y.; Cook, C.N.; Jansen-West, K.; Daughrity, L.; Lewis-Tuffin, L.J.; Castanedes-Casey, M.; DeTure, M.; Dickson, D.W.; Petrucelli, L.; et al. Tau exhibits unique seeding properties in globular glial tauopathy. Acta Neuropathol. Commun. 2019, 7, 36. [Google Scholar] [CrossRef]
- Lin, L.C.; Nana, A.L.; Hepker, M.; Hwang, J.H.L.; Gaus, S.E.; Spina, S.; Cosme, C.G.; Gan, L.; Grinberg, L.T.; Geschwind, D.H.; et al. Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathol. Commun. 2019, 7, 159. [Google Scholar] [CrossRef]
- Boeve, B.F. Links between frontotemporal lobar degeneration, corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis. Assoc. Disord. 2007, 21, S31–S38. [Google Scholar] [CrossRef]
- Janssens, J.; Van Broeckhoven, C. Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD–ALS spectrum disorders. Hum. Mol. Genet. 2013, 22, R77–R87. [Google Scholar] [CrossRef]
- Buratti, E.; Baralle, F.E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008, 13, 867–878. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, T.; Nonaka, T.; Kametani, F.; Yoshida, M.; Hashizume, Y.; Beach, T.G.; Buratti, E.; Baralle, F.; Morita, M.; et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and ALS. Ann. Neurol. 2008, 64, 60. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, N.T.; Gozal, Y.M.; Dammer, E.B.; Xia, Q.; Duong, D.M.; Cheng, D.; Lah, J.J.; Levey, A.I.; Peng, J. Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains. Mol. Cell. Proteom. 2010, 9, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Hwang, A.W.; Unger, T.; Trojanowski, J.Q.; Lee, V.M.Y. Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J. 2012, 31, 1241–1252. [Google Scholar] [CrossRef]
- Buratti, E. TDP-43 post-translational modifications in health and disease. Expert. Opin. Ther. Targets 2018, 22, 279–293. [Google Scholar] [CrossRef]
- Lee, E.B.; Porta, S.; Michael Baer, G.; Xu, Y.; Suh, E.R.; Kwong, L.K.; Elman, L.; Grossman, M.; Lee, V.M.; Irwin, D.J.; et al. Expansion of the classification of FTLD-TDP: Distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol. 2017, 134, 65–78. [Google Scholar] [CrossRef]
- Neumann, M.; Lee, E.B.; Mackenzie, I.R. FTLD-TDP pathological subtypes: Clinical and mechanistic significance. Adv. Exp. Med. Biol. 2021, 1281, 201. [Google Scholar] [CrossRef]
- Gitcho, M.A.; Strider, J.; Carter, D.; Taylor-Reinwald, L.; Forman, M.S.; Goate, A.M.; Cairns, N.J. VCP Mutations Causing Frontotemporal Lobar Degeneration Disrupt Localization of TDP-43 and Induce Cell Death. J. Biol. Chem. 2009, 284, 12384–12398. [Google Scholar] [CrossRef]
- Forman, M.S.; Farmer, J.; Johnson, J.K.; Clark, C.M.; Arnold, S.E.; Coslett, H.B.; Chatterjee, A.; Hurtig, H.I.; Karlawish, J.H.; Rosen, H.J.; et al. Frontotemporal dementia: Clinicopathological correlations. Ann. Neurol. 2006, 59, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Scarioni, M.; Gami-Patel, P.; Timar, Y.; Seelaar, H.; van Swieten, J.C.; Rozemuller, A.J.M.; Dols, A.; Scarpini, E.; Galimberti, D.; Netherlands Brain Bank; et al. Frontotemporal Dementia: Correlations Between Psychiatric Symptoms and Pathology. Ann. Neurol. 2020, 87, 950. [Google Scholar] [CrossRef]
- Chornenka, K.; Hirsch-Reinshagen, V.; Perez-Rosendahl, M.; Feldman, H.; Segal-Gidan, F.; Vinters, H.V.; Mackenzie, I.R. Expanding the Phenotype of Frontotemporal Lobar Degeneration with FUS-Positive Pathology (FTLD-FUS). J. Neuropathol. Exp. Neurol. 2020, 79, 809–812. [Google Scholar] [CrossRef]
- Schmitz, A.; Pinheiro Marques, J.; Oertig, I.; Maharjan, N.; Saxena, S. Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD. Front. Cell. Neurosci. 2021, 15, 637548. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.; Puangmalai, N.; Sengupta, U.; Jerez, C.; Kidd, M.; Gandhi, S.; Kayed, R. C9orf72-associated dipeptide protein repeats form A11-positive oligomers in amyotrophic lateral sclerosis and frontotemporal dementia. J. Biol. Chem. 2024, 300, 105628. [Google Scholar] [CrossRef]
- Liu, F.; Morderer, D.; Wren, M.C.; Vettleson-Trutza, S.A.; Wang, Y.; Rabichow, B.E.; Salemi, M.R.; Phinney, B.S.; Oskarsson, B.; Dickson, D.W.; et al. Proximity proteomics of C9orf72 dipeptide repeat proteins identifies molecular chaperones as modifiers of poly-GA aggregation. Acta Neuropathol. Commun. 2022, 10, 22. [Google Scholar] [CrossRef]
- Krishnan, G.; Raitcheva, D.; Bartlett, D.; Prudencio, M.; McKenna-Yasek, D.M.; Douthwright, C.; Oskarsson, B.E.; Ladha, S.; King, O.D.; Barmada, S.J.; et al. Poly(GR) and poly(GA) in cerebrospinal fluid as potential biomarkers for C9ORF72-ALS/FTD. Nat. Commun. 2022, 13, 2799. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.; Vercruysse, T.; Boeynaems, S.; Sicart, A.; Van Damme, P.; Daelemans, D.; Van Den Bosch, L. C9orf72-generated poly-GR and poly-PR do not directly interfere with nucleocytoplasmic transport. Sci. Rep. 2019, 9, 15728. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A. The role of dipeptide-repeat protein pathology in C9orf72 mutation cases. Neuropathol. Appl. Neurobiol. 2016, 42, 217–219. [Google Scholar] [CrossRef]
- Gendron, T.F.; Chew, J.; Stankowski, J.N.; Hayes, L.R.; Zhang, Y.J.; Prudencio, M.; Carlomagno, Y.; Daughrity, L.M.; Jansen-West, K.; Perkerson, E.A.; et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci. Transl. Med. 2017, 9, eaai7866. [Google Scholar] [CrossRef]
- Rubio-Guerra, S.; Bernal, S.; Almenta, D.; Pérez-Blanco, J.; Camacho, V.; Sala, I.; Sánchez-Saudinós, M.B.; García Castro, J.; Selma-González, J.; Santos-Santos, M.Á.; et al. A Novel CHMP2B Splicing Variant in Atypical Presentation of Familial Frontotemporal Lobar Degeneration. Ann. Clin. Transl. Neurol. 2025, 12, 1894–1900. [Google Scholar] [CrossRef]
- Rizzu, P.; Van Mil, S.E.; Anar, B.; Rosso, S.M.; Kaat, L.D.; Heutink, P.; Van Swieten, J.C. CHMP2B mutations are not a cause of dementia in Dutch patients with familial and sporadic frontotemporal dementia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Carbayo, Á.; Borrego-Écija, S.; Turon-Sans, J.; Cortés-Vicente, E.; Molina-Porcel, L.; Gascón-Bayarri, J.; Rubio, M.Á.; Povedano, M.; Gámez, J.; Sotoca, J.; et al. Clinicopathological correlates in the frontotemporal lobar degeneration-motor neuron disease spectrum. Brain 2024, 147, 2357–2367. [Google Scholar] [CrossRef]
- Belder, C.R.S.; Marshall, C.R.; Jiang, J.; Mazzeo, S.; Chokesuwattanaskul, A.; Rohrer, J.D.; Volkmer, A.; Hardy, C.J.D.; Warren, J.D. Primary progressive aphasia: Six questions in search of an answer. J. Neurol. 2024, 271, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Cruts, M.; Gijselinck, I.; Van Der Zee, J.; Engelborghs, S.; Wils, H.; Pirici, D.; Rademakers, R.; Vandenberghe, R.; Dermaut, B.; Martin, J.J.; et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442, 920–924. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Guerreiro, R.; Vandrovcova, J.; Uphill, J.; Reiman, D.; Beck, J.; Isaacs, A.M.; Authier, A.; Ferrari, R.; Fox, N.C.; et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology 2009, 73, 1451–1456. [Google Scholar] [CrossRef]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Strang, K.H.; Croft, C.L.; Sorrentino, Z.A.; Chakrabarty, P.; Golde, T.E.; Giasson, B.I. Distinct differences in prion-like seeding and aggregation between Tau protein variants provide mechanistic insights into tauopathies. J. Biol. Chem. 2018, 293, 2408–2421, Erratum in J. Biol. Chem. 2018, 293, 4579. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Non-Alzheimer’s dementia 1: Frontotemporal dementia. Lancet 2015, 386, 1672. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Ghetti, B.; Goedert, M. Classification of Diseases with Accumulation of Tau Protein. Neuropathol. Appl. Neurobiol. 2022, 48, e12792, Erratum in Neuropathol. Appl. Neurobiol. 2022, 48, e12821. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, J.S.; Karch, C.M.; Fan, C.C.; Bonham, L.W.; Kouri, N.; Ross, O.A.; Rademakers, R.; Kim, J.; Wang, Y.; Höglinger, G.U.; et al. Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol. 2017, 133, 825–837. [Google Scholar] [CrossRef]
- Irwin, D.J.; Cairns, N.J.; Grossman, M.; McMillan, C.T.; Lee, E.B.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q. Frontotemporal Lobar Degeneration: Defining Phenotypic Diversity Through Personalized Medicine. Acta Neuropathol. 2014, 129, 469. [Google Scholar] [CrossRef] [PubMed]
- Kouri, N.; Ross, O.A.; Dombroski, B.; Younkin, C.S.; Serie, D.J.; Soto-Ortolaza, A.; Baker, M.; Finch, N.C.A.; Yoon, H.; Kim, J.; et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat. Commun. 2015, 6, 7247. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Melhem, N.M.; Dickson, D.W.; Sleiman, P.M.A.; Wang, L.S.; Klei, L.; Rademakers, R.; de Silva, R.; Litvan, I.; Riley, D.E.; et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 2011, 43, 699–705. [Google Scholar] [CrossRef]
- Gossye, H.; Van Broeckhoven, C.; Engelborghs, S. The Use of Biomarkers and Genetic Screening to Diagnose Frontotemporal Dementia: Evidence and Clinical Implications. Front. Neurosci. 2019, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Nicholas, J.; Grossman, M.; McMillan, C.T.; Irwin, D.J.; Massimo, L.; Van Deerlin, V.M.; Warren, J.D.; Fox, N.C.; Rossor, M.N.; et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020, 19, 145–156, Erratum in Lancet Neurol. 2020, 19, 12. [Google Scholar] [CrossRef]
- Finch, N.; Baker, M.; Crook, R.; Swanson, K.; Kuntz, K.; Surtees, R.; Bisceglio, G.; Rovelet-Lecrux, A.; Boeve, B.; Petersen, R.C.; et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 2009, 132, 583–591. [Google Scholar] [CrossRef]
- Smith, K.R.; Damiano, J.; Franceschetti, S.; Carpenter, S.; Canafoglia, L.; Morbin, M.; Rossi, G.; Pareyson, D.; Mole, S.E.; Staropoli, J.F.; et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 2012, 90, 1102–1107. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Baker, M.; Pickering-Brown, S.; Hsiung, G.Y.R.; Lindholm, C.; Dwosh, E.; Gass, J.; Cannon, A.; Rademakers, R.; Hutton, M.; et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 2006, 129, 3081–3090. [Google Scholar] [CrossRef]
- Farg, M.A.; Sundaramoorthy, V.; Sultana, J.M.; Yang, S.; Atkinson, R.A.K.; Levina, V.; Halloran, M.A.; Gleeson, P.A.; Blair, I.P.; Soo, K.Y.; et al. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 2014, 23, 3579–3595. [Google Scholar] [CrossRef]
- Van Blitterswijk, M.; Dejesus-Hernandez, M.; Rademakers, R. How do C9ORF72 repeat expansions cause amyotrophic lateral sclerosis and frontotemporal dementia: Can we learn from other noncoding repeat expansion disorders? Curr. Opin. Neurol. 2012, 25, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Petrucelli, L. Disease mechanisms of c9orf72 repeat expansions. Cold Spring Harb. Perspect. Med. 2017, 8, a024224. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Libri, I.; Premi, E.; Alberici, A.; Cantoni, V.; Gadola, Y.; Rivolta, G.; Pengo, M.; Gazzina, S.; Calhoun, V.D.; et al. Differences and similarities between familial and sporadic frontotemporal dementia: An Italian single-center cohort study. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12326. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Küçükali, F.; Baker, M.; Batzler, A.; Jenkins, G.D.; van Blitterswijk, M.; Vicente, C.T.; De Coster, W.; Wynants, S.; Van de Walle, P.; et al. Deciphering distinct genetic risk factors for FTLD-TDP pathological subtypes via whole-genome sequencing. Nat. Commun. 2025, 16, 3914. [Google Scholar] [CrossRef]
- van der Ende, E.L.; Heller, C.; Sogorb-Esteve, A.; Swift, I.J.; McFall, D.; Peakman, G.; Bouzigues, A.; Poos, J.M.; Jiskoot, L.C.; Panman, J.L.; et al. Elevated CSF and plasma complement proteins in genetic frontotemporal dementia: Results from the GENFI study. J. Neuroinflamm. 2022, 19, 217. [Google Scholar] [CrossRef]
- Pottier, C.; Ravenscroft, T.A.; Sanchez-Contreras, M.; Rademakers, R. Genetics of FTLD: Overview and what else we can expect from genetic studies. J. Neurochem. 2016, 138 (Suppl. S1), 32–53. [Google Scholar] [CrossRef]
- Müller, U.; Höglinger, G.; Dickson, D.W. Multifactorial etiology of progressive supranuclear palsy (PSP): The genetic component. Acta Neuropathol. 2025, 149, 58. [Google Scholar] [CrossRef]
- Van Langenhove, T.; Van Der Zee, J.; Gijselinck, I.; Engelborghs, S.; Vandenberghe, R.; Vandenbulcke, M.; De Bleecker, J.; Sieben, A.; Versijpt, J.; Ivanoiu, A.; et al. Distinct Clinical Characteristics of C9orf72 Expansion Carriers Compared with GRN, MAPT, and Nonmutation Carriers in a Flanders-Belgian FTLD Cohort. JAMA Neurol. 2013, 70, 365–373. [Google Scholar] [CrossRef]
- Pottier, C.; Ren, Y.; Perkerson, R.B.; Baker, M.; Jenkins, G.D.; van Blitterswijk, M.; DeJesus-Hernandez, M.; van Rooij, J.G.J.; Murray, M.E.; Christopher, E.; et al. Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 2019, 137, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Van Deerlin, V.M.; Sleiman, P.M.A.; Martinez-Lage, M.; Chen-Plotkin, A.; Wang, L.S.; Graff-Radford, N.R.; Dickson, D.W.; Rademakers, R.; Boeve, B.F.; Grossman, M.; et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat. Genet. 2010, 42, 234–239. [Google Scholar] [CrossRef]
- Premi, E.; Grassi, M.; Van Swieten, J.; Galimberti, D.; Graff, C.; Masellis, M.; Tartaglia, C.; Tagliavini, F.; Rowe, J.B.; Laforce, R., Jr.; et al. Cognitive reserve and TMEM106B genotype modulate brain damage in presymptomatic frontotemporal dementia: A GENFI study. Brain 2017, 140, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Formenti, A.; Gazzina, S.; Archetti, S.; Gasparotti, R.; Padovani, A.; Borroni, B. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 2014, 71, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.I.; Unger, T.L.; Jain, N.; Skrinak, R.T.; Charan, R.A.; Chen-Plotkin, A.S. Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes. Hum. Mol. Genet. 2016, 25, 2681–2697. [Google Scholar] [CrossRef]
- Van Blitterswijk, M.; Mullen, B.; Nicholson, A.M.; Bieniek, K.F.; Heckman, M.G.; Baker, M.C.; DeJesus-Hernandez, M.; Finch, N.A.; Brown, P.H.; Murray, M.E.; et al. TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol. 2014, 127, 397–406. [Google Scholar] [CrossRef]
- Soppela, H.; Katisko, K.; Gadola, Y.; Krüger, J.; Hartikainen, P.; Alberici, A.; Benussi, A.; Koivisto, A.; Haapasalo, A.; Remes, A.M.; et al. Modifiable potential risk factors in familial and sporadic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 1195–1205. [Google Scholar] [CrossRef]
- Gazzina, S.; Grassi, M.; Premi, E.; Cosseddu, M.; Alberici, A.; Archetti, S.; Gasparotti, R.; Van Swieten, J.; Galimberti, D.; Sanchez-Valle, R.; et al. Education modulates brain maintenance in presymptomatic frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1124–1130. [Google Scholar] [CrossRef]
- Alladi, S.; Bak, T.H.; Shailaja, M.; Gollahalli, D.; Rajan, A.; Surampudi, B.; Hornberger, M.; Duggirala, V.; Chaudhuri, J.R.; Kaul, S. Bilingualism delays the onset of behavioral but not aphasic forms of frontotemporal dementia. Neuropsychologia 2017, 99, 207–212. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Staffaroni, A.M.; Wolf, A.; Appleby, B.; Brushaber, D.; Coppola, G.; Dickerson, B.; Domoto-Reilly, K.; Elahi, F.M.; Fields, J.; et al. Active lifestyles moderate clinical outcomes in autosomal dominant frontotemporal degeneration. Alzheimers Dement. 2020, 16, 91–105. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Shafiei, G.; Bazinet, V.; Dadar, M.; Manera, A.L.; Collins, D.L.; Dagher, A.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; et al. Network structure and transcriptomic vulnerability shape atrophy in frontotemporal dementia. Brain 2023, 146, 321–336. [Google Scholar] [CrossRef]
- Franciotti, R.; Moretti, D.V.; Benussi, A.; Ferri, L.; Russo, M.; Carrarini, C.; Barbone, F.; Arnaldi, D.; Falasca, N.W.; Koch, G. Cortical network modularity changes along the course of frontotemporal and Alzheimer’s dementing diseases. J. Neurol. Sci. 2021, 429, 118988. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Fatima, M.; Tan, R.; Halliday, G.M.; Kril, J.J. Spread of pathology in amyotrophic lateral sclerosis: Assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol. Commun. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Rademakers, R.; Roeber, S.; Baker, M.; Kretzschmar, H.A.; MacKenzie, I.R.A. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 2009, 132, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Munoz, D.G.; Neumann, M.; Kusaka, H.; Yokota, O.; Ishihara, K.; Terada, S.; Kuroda, S.; Mackenzie, I.R. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009, 118, 617–627. [Google Scholar] [CrossRef]
- Schludi, M.H.; May, S.; Grässer, F.A.; Rentzsch, K.; Kremmer, E.; Küpper, C.; Klopstock, T.; German Consortium for Frontotemporal Lobar Degeneration; Bavarian Brain Banking Alliance; Arzberger, T.; et al. Distribution of dipeptide repeat proteins in cellular models and C9orf72 mutation cases suggests link to transcriptional silencing. Acta Neuropathol. 2015, 130, 537–555, Erratum in Acta Neuropathol. 2015, 130, 557–558. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, I.R.; Arzberger, T.; Kremmer, E.; Troost, D.; Lorenzl, S.; Mori, K.; Weng, S.M.; Haass, C.; Kretzschmar, H.A.; Edbauer, D.; et al. Dipeptide repeat protein pathology in C9ORF72 mutation cases: Clinico-pathological correlations. Acta Neuropathol. 2013, 126, 859–879. [Google Scholar] [CrossRef]
- Quaegebeur, A.; Glaria, I.; Lashley, T.; Isaacs, A.M. Soluble and insoluble dipeptide repeat protein measurements in C9orf72-frontotemporal dementia brains show regional differential solubility and correlation of poly-GR with clinical severity. Acta Neuropathol. Commun. 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, I.R.A.; Neumann, M.; Bigio, E.H.; Cairns, N.J.; Alafuzoff, I.; Kril, J.; Kovacs, G.G.; Ghetti, B.; Halliday, G.; Holm, I.E.; et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Götzl, J.K.; Lang, C.M.; Haass, C.; Capell, A. Impaired protein degradation in FTLD and related disorders. Ageing Res. Rev. 2016, 32, 122–139. [Google Scholar] [CrossRef]
- Finger, E.; Malik, R.; Bocchetta, M.; Coleman, K.; Graff, C.; Borroni, B.; Masellis, M.; Laforce, R.; Greaves, C.V.; Russell, L.L.; et al. Neurodevelopmental effects of genetic frontotemporal dementia in young adult mutation carriers. Brain 2022, 146, 2120–2131. [Google Scholar] [CrossRef]
- Hendricks, E.; Quihuis, A.M.; Hung, S.T.; Chang, J.; Dorjsuren, N.; Der, B.; Staats, K.A.; Shi, Y.; Sta Maria, N.S.; Jacobs, R.E. The C9ORF72 repeat expansion alters neurodevelopment. Cell Rep. 2023, 42, 112983. [Google Scholar] [CrossRef]
- Longhena, F.; Zaltieri, M.; Grigoletto, J.; Faustini, G.; La Via, L.; Ghidoni, R.; Benussi, L.; Missale, C.; Spano, P.; Bellucci, A. Depletion of Progranulin Reduces GluN2B-Containing NMDA Receptor Density, Tau Phosphorylation, and Dendritic Arborization in Mouse Primary Cortical Neurons. J. Pharmacol. Exp. Ther. 2017, 363, 164–175. [Google Scholar] [CrossRef]
- Hefti, M.M.; Farrell, K.; Kim, S.H.; Bowles, K.R.; Fowkes, M.E.; Raj, T.; Crary, J.F. High-resolution temporal and regional mapping of MAPT expression and splicing in human brain development. PLoS ONE 2018, 13, e0195771. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. Continuum 2016, 22, 404–418. [Google Scholar] [CrossRef]
- Petersen, R.C. Clinical practice. Mild cognitive impairment. N. Engl. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Negash, S. Mild cognitive impairment: An overview. CNS Spectr. 2008, 13, 45–53. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild cognitive impairment: Ten years later. Arch. Neurol. 2009, 66, 1447. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Smith, E.E.; Geda, Y.; Sultzer, D.; Brodaty, H.; Smith, G.; Agüera-Ortiz, L.; Sweet, R.; Miller, D.; Lyketsos, C.G.; et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016, 12, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Huey, E.D.; McMillan, C.T.; Petersen, R.C.; Postuma, R.; McHutchison, C.; Dratch, L.; Arias, J.J.; Crawley, A.; et al. The Miami Framework for ALS and related neurodegenerative disorders: An integrated view of phenotype and biology. Nat. Rev. Neurol. 2024, 20, 364–376, Erratum in Nat. Rev. Neurol. 2024, 20, 377. [Google Scholar] [CrossRef]
- Franklin, H.D.; Russell, L.L.; Peakman, G.; Greaves, C.V.; Bocchetta, M.; Nicholas, J.; Poos, J.; Convery, R.S.; Cash, D.M.; van Swieten, J.; et al. The Revised Self-Monitoring Scale detects early impairment of social cognition in genetic frontotemporal dementia within the GENFI cohort. Alzheimers Res. Ther. 2021, 13, 127. [Google Scholar] [CrossRef]
- Lule, D.E.; Müller, H.P.; Finsel, J.; Weydt, P.; Knehr, A.; Winroth, I.; Andersen, P.; Weishaupt, J.; Uttner, I.; Kassubek, J.; et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers—A developmental disorder. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.S.; Manoochehri, M.; Rizer, S.J.; Appleby, B.S.; Brushaber, D.; Dev, S.I.; Devick, K.L.; Dickerson, B.C.; Fields, J.A.; Foroud, T.M.; et al. Recognition memory and divergent cognitive profiles in prodromal genetic frontotemporal dementia. Cortex 2021, 139, 99–115. [Google Scholar] [CrossRef]
- Poos, J.M.; Russell, L.L.; Peakman, G.; Bocchetta, M.; Greaves, C.V.; Jiskoot, L.C.; van der Ende, E.L.; Seelaar, H.; Papma, J.M.; van den Berg, E.; et al. Impairment of episodic memory in genetic frontotemporal dementia: A genfi study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12185. [Google Scholar] [CrossRef]
- Samra, K.; MacDougall, A.M.; Bouzigues, A.; Bocchetta, M.; Cash, D.M.; Greaves, C.V.; Convery, R.S.; van Swieten, J.C.; Jiskoot, L.; Seelaar, H.; et al. Prodromal language impairment in genetic frontotemporal dementia within the GENFI cohort. J. Neurol. Sci. 2023, 451, 120711. [Google Scholar] [CrossRef]
- Bouzigues, A.; Russell, L.L.; Peakman, G.; Bocchetta, M.; Greaves, C.V.; Convery, R.S.; Todd, E.; Rowe, J.B.; Borroni, B.; Galimberti, D.; et al. Anomia is present pre-symptomatically in frontotemporal dementia due to MAPT mutations. J. Neurol. 2022, 269, 4322–4332. [Google Scholar] [CrossRef]
- Russell, L.L.; Bouzigues, A.; Convery, R.S.; Foster, P.H.; Ferry-Bolder, E.; Cash, D.M.; Van Swieten, J.C.; Jiskoot, L.C.; Seelaar, H.; Moreno, F.; et al. Executive Function Deficits in Genetic Frontotemporal Dementia. Neurol. Genet. 2025, 11, e200248. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Nicholas, J.M.; Cash, D.M.; van Swieten, J.; Dopper, E.; Jiskoot, L.; van Minkelen, R.; Rombouts, S.A.; Cardoso, M.J.; Clegg, S.; et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: A cross-sectional analysis. Lancet Neurol. 2015, 14, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; et al. Apathy in presymptomatic genetic frontotemporal dementia predicts cognitive decline and is driven by structural brain changes. Alzheimers Dement. 2021, 17, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.P.; Mitchell, D.G.V.; Coleman, K.K.L.; Coleman, B.L.; Shoesmith, C.L.; Butler, C.R.; Santana, I.; Danek, A.; Gerhard, A.; de Mendonca, A.; et al. Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 2020, 91, 975–984, Correction in J. Neurol. Neurosurg. Psychiatry 2020, 91, e3. [Google Scholar] [CrossRef]
- Nelson, A.; Russell, L.L.; Peakman, G.; Convery, R.S.; Bouzigues, A.; Greaves, C.V.; Bocchetta, M.; Cash, D.M.; van Swieten, J.C.; Jiskoot, L.; et al. The CBI-R detects early behavioural impairment in genetic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.H.; Russell, L.L.; Peakman, G.; Convery, R.S.; Bouzigues, A.; Greaves, C.V.; Bocchetta, M.; Cash, D.M.; van Swieten, J.C.; Jiskoot, L.C.; et al. Examining empathy deficits across familial forms of frontotemporal dementia within the GENFI cohort. Cortex 2022, 150, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Brun, A.; Englund, B.; Gustafson, L.; Passant, U.; Mann, D.M.A.; Snowden, J.S. Clinical and neuropathological criteria for frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 1994, 57, 416–418. [Google Scholar] [CrossRef]
- Cosseddu, M.; Benussi, A.; Gazzina, S.; Alberici, A.; Dell’Era, V.; Manes, M.; Cristillo, V.; Borroni, B.; Padovani, A. Progression of behavioural disturbances in frontotemporal dementia: A longitudinal observational study. Eur. J. Neurol. 2020, 27, 265–272. [Google Scholar] [CrossRef]
- Wylie, M.A.; Shnall, A.; Onyike, C.U.; Huey, E.D. Management of frontotemporal dementia in mental health and multidisciplinary settings. Int. Rev. Psychiatry 2013, 25, 230–236. [Google Scholar] [CrossRef]
- Sha, S.J.; Takada, L.T.; Rankin, K.P.; Yokoyama, J.S.; Rutherford, N.J.; Fong, J.C.; Khan, B.; Karydas, A.; Baker, M.C.; DeJesus-Hernandez, M.; et al. Frontotemporal dementia due To C9ORF72 mutations clinical and imaging features. Neurology 2012, 79, 1002–1011. [Google Scholar] [CrossRef]
- Benussi, A.; Borroni, B. Advances in the treatment and management of frontotemporal dementia. Expert. Rev. Neurother. 2023, 23, 621–639. [Google Scholar] [CrossRef]
- Devenney, E.M.; Ahmed, R.M.; Halliday, G.; Piguet, O.; Kiernan, M.C.; Hodges, J.R. Psychiatric disorders in C9orf72 kindreds study of 1,414 family members. Neurology 2018, 91, e1498–e1507, Correction in Neurology 2019, 93, 1022. [Google Scholar] [CrossRef]
- Benussi, A.; Premi, E.; Gazzina, S.; Brattini, C.; Bonomi, E.; Alberici, A.; Jiskoot, L.; van Swieten, J.C.; Sanchez-Valle, R.; Moreno, F.; et al. Progression of Behavioral Disturbances and Neuropsychiatric Symptoms in Patients with Genetic Frontotemporal Dementia. JAMA Netw. Open 2021, 4, e2030194. [Google Scholar] [CrossRef]
- Silvestri, C.; Almici, V.; Libri, I.; Mattioli, I.; Cosseddu, M.; Turrone, R.; Rivolta, J.; Grassini, C.; Caratozzolo, S.; Alberici, A.; et al. Sex Differences in the Severity and Progression of Neuropsychiatric Symptoms Across Different Dementia Types. Neurol. Clin. Pract. 2024, 14, e200299. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Ashton, N.J.; Karikari, T.K.; Alberici, A.; Saraceno, C.; Ghidoni, R.; Benussi, L.; Zetterberg, H.; Blennow, K.; Borroni, B.; et al. Prodromal frontotemporal dementia: Clinical features and predictors of progression. Alzheimers Res. Ther. 2021, 13, 188. [Google Scholar] [CrossRef]
- Samra, K.; MacDougall, A.M.; Peakman, G.; Bouzigues, A.; Bocchetta, M.; Cash, D.M.; Greaves, C.V.; Convery, R.S.; van Swieten, J.C.; Jiskoot, L.; et al. Motor symptoms in genetic frontotemporal dementia: Developing a new module for clinical rating scales. J. Neurol. 2022, 270, 1466. [Google Scholar] [CrossRef]
- Peakman, G.; Russell, L.L.; Convery, R.S.; Nicholas, J.M.; van Swieten, J.C.; Jiskoot, L.C.; Moreno, F.; Sanchez-Valle, R.; Laforce, R.; Graff, C.; et al. Comparison of clinical rating scales in genetic frontotemporal dementia within the GENFI cohort. J. Neurol. Neurosurg. Psychiatry 2021, 93, 158–168. [Google Scholar] [CrossRef]
- Öijerstedt, L.; Andersson, C.; Jelic, V.; van Swieten, J.C.; Jiskoot, L.C.; Seelaar, H.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R., Jr.; et al. Practice effects in genetic frontotemporal dementia and at-risk individuals: A GENFI study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Poos, J.M.; Moore, K.M.; Nicholas, J.; Russell, L.L.; Peakman, G.; Convery, R.S.; Jiskoot, L.C.; van der Ende, E.; van den Berg, E.; Papma, J.M.; et al. Cognitive composites for genetic frontotemporal dementia: GENFI-Cog. Alzheimers Res. Ther. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Brushaber, D.; Syrjanen, J.; Kremers, W.; Fields, J.; Forsberg, L.K.; Heuer, H.W.; Knopman, D.; Kornak, J.; Boxer, A.; et al. Use of the CDR® plus NACC FTLD in mild FTLD: Data from the ARTFL/LEFFTDS consortium. Alzheimers Dement. 2020, 16, 79–90. [Google Scholar] [CrossRef]
- Knopman, D.S.; Kramer, J.H.; Boeve, B.F.; Caselli, R.J.; Graff-Radford, N.R.; Mendez, M.F.; Miller, B.L.; Mercaldo, N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008, 131, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Samra, K.; Peakman, G.; MacDougall, A.M.; Bouzigues, A.; Greaves, C.V.; Convery, R.S.; Van Swieten, J.C.; Jiskoot, L.; Seelaar, H.; Moreno, F.; et al. Extending the phenotypic spectrum assessed by the CDR plus NACC FTLD in genetic frontotemporal dementia. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2024, 16, e12571. [Google Scholar] [CrossRef]
- Sikkes, S.A.M.; Knol, D.L.; Pijnenburg, Y.A.L.; De Lange-De Klerk, E.S.M.; Uitdehaag, B.M.J.; Scheltens, P. Validation of the amsterdam IADL questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology 2013, 41, 35–41. [Google Scholar] [CrossRef]
- Aas, I.M. Global Assessment of Functioning (GAF): Properties and frontier of current knowledge. Ann. Gen. Psychiatry 2010, 9, 20. [Google Scholar] [CrossRef]
- Tanguy, D.; Batrancourt, B.; Estudillo-Romero, A.; Baxter, J.S.H.; Le Ber, I.; Bouzigues, A.; Godefroy, V.; Funkiewiez, A.; Chamayou, C.; Volle, E.; et al. An ecological approach to identify distinct neural correlates of disinhibition in frontotemporal dementia. Neuroimage Clin. 2022, 35, 103079. [Google Scholar] [CrossRef]
- Grossman, M.; Seeley, W.W.; Boxer, A.L.; Hillis, A.E.; Knopman, D.S.; Ljubenov, P.A.; Miller, B.; Piguet, O.; Rademakers, R.; Whitwell, J.L.; et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Primers 2023, 9, 40. [Google Scholar] [CrossRef]
- Crawford, J.R.; Allan, K.M.; Stephen, D.W.; Parker, D.M.; Besson, J.A.O. The Wechsler Adult Intelligence Scale-Revised (WAIS-R): Factor structure in a UK sample. Personal. Individ. Differ. 1989, 10, 1209–1212. [Google Scholar] [CrossRef]
- Ryan, J.J.; Lopez, S.J. Wechsler Adult Intelligence Scale-III. In Understanding Psychological Assessment; Springer: Boston, MA, USA, 2001; pp. 19–42. [Google Scholar] [CrossRef]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. Delis-Kaplan Executive Function System; PsycTESTS Dataset; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. 9/geriatric depression scale (Gds) recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983, 44, 113–126. [Google Scholar] [CrossRef]
- Vijverberg, E.G.B.; Dols, A.; Krudop, W.A.; Del Campo Milan, M.; Kerssens, C.J.; Gossink, F.; Prins, N.D.; Stek, M.L.; Scheltens, P.; Teunissen, C.E.; et al. Cerebrospinal fluid biomarker examination as a tool to discriminate behavioral variant frontotemporal dementia from primary psychiatric disorders. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lleó, A.; Xie, S.X.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. Ante mortem CSF tau levels correlate with post mortem tau pathology in FTLD. Ann. Neurol. 2017, 82, 247. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Ashton, N.J.; Karikari, T.K.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Binetti, G.; et al. Serum Glial Fibrillary Acidic Protein (GFAP) Is a Marker of Disease Severity in Frontotemporal Lobar Degeneration. J. Alzheimers Dis. 2020, 77, 1129–1141. [Google Scholar] [CrossRef]
- Benussi, A.; Karikari, T.K.; Ashton, N.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Simrén, J.; et al. Diagnostic and prognostic value of serum NfL and p-Tau 181 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 2020, 91, 960–967. [Google Scholar] [CrossRef]
- Benussi, A.; Huber, H.; Tan, K.; Cantoni, V.; Rivolta, J.; Cotelli, M.S.; Benedet, A.L.; Blennow, K.; Zetterberg, H.; Ashton, N.J.; et al. Plasma p-tau217 and neurofilament/p-tau217 ratio in differentiating Alzheimer’s disease from syndromes associated with frontotemporal lobar degeneration. Alzheimers Dement. 2025, 21, e14482. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Rivolta, J.; Archetti, S.; Micheli, A.; Ashton, N.; Zetterberg, H.; Blennow, K.; Borroni, B. Classification accuracy of blood-based and neurophysiological markers in the differential diagnosis of Alzheimer’s disease and frontotemporal lobar degeneration. Alzheimers Res. Ther. 2022, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Schöll, M.; Maass, A.; Mattsson, N.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Jagust, W. Biomarkers for tau pathology. Mol. Cell Neurosci. 2019, 97, 18. [Google Scholar] [CrossRef]
- Borroni, B.; Benussi, A.; Cosseddu, M.; Archetti, S.; Padovani, A. Cerebrospinal fluid tau levels predict prognosis in non-inherited frontotemporal dementia. Neurodegener. Dis. 2014, 13, 224–229. [Google Scholar] [CrossRef]
- Norise, C.; Ungrady, M.; Halpin, A.; Jester, C.; McMillan, C.T.; Irwin, D.J.; Cousins, K.A.; Grossman, M. Clinical Correlates of Alzheimer’s Disease Cerebrospinal Fluid Analytes in Primary Progressive Aphasia. Front. Neurol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Townley, R.A.; Graff-Radford, J.; Mantyh, W.G.; Botha, H.; Polsinelli, A.J.; Przybelski, S.A.; Machulda, M.M.; Makhlouf, A.T.; Senjem, M.L.; Murray, M.E.; et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: A description of 55 cases and comparison to other phenotypes. Brain Commun. 2020, 2, fcaa068. [Google Scholar] [CrossRef]
- Paterson, R.W.; Slattery, C.F.; Poole, T.; Nicholas, J.M.; Magdalinou, N.K.; Toombs, J.; Chapman, M.D.; Lunn, M.P.; Heslegrave, A.J.; Foiani, M.S.; et al. Cerebrospinal fluid in the differential diagnosis of Alzheimer’s disease: Clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res. Ther. 2018, 10, 32. [Google Scholar] [CrossRef]
- Illán-Gala, I.; Pegueroles, J.; Montal, V.; Alcolea, D.; Vilaplana, E.; Bejanin, A.; Borrego-Écija, S.; Sampedro, F.; Subirana, A.; Sánchez-Saudinós, M.B.; et al. APP-derived peptides reflect neurodegeneration in frontotemporal dementia. Ann. Clin. Transl. Neurol. 2019, 6, 2518–2530. [Google Scholar] [CrossRef]
- Gabelle, A.; Roche, S.; Gény, C.; Bennys, K.; Labauge, P.; Tholance, Y.; Quadrio, I.; Tiers, L.; Gor, B.; Boulanghien, J.; et al. Decreased sAβPPβ, Aβ38, and Aβ40 cerebrospinal fluid levels in frontotemporal dementia. J. Alzheimers Dis. 2011, 26, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Lleó, A.; Irwin, D.J.; Illán-Gala, I.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. A 2-Step Cerebrospinal Algorithm for the Selection of Frontotemporal Lobar Degeneration Subtypes. JAMA Neurol. 2018, 75, 738. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Galimberti, D.; Elias, N.; Boonkamp, L.; Pijnenburg, Y.A.; van Swieten, J.C.; Watts, K.; Paciotti, S.; Beccari, T.; Hu, W.; et al. Novel CSF biomarkers to discriminate FTLD and its pathological subtypes. Ann. Clin. Transl. Neurol. 2018, 5, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Benussi, A.; Archetti, S.; Galimberti, D.; Parnetti, L.; Nacmias, B.; Sorbi, S.; Scarpini, E.; Padovani, A. Csf p-tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 86–91. [Google Scholar] [CrossRef]
- Hu, W.T.; Watts, K.; Grossman, M.; Glass, J.; Lah, J.J.; Hales, C.; Shelnutt, M.; Van Deerlin, V.; Trojanowski, J.Q.; Levey, A.I. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology 2013, 81, 1945. [Google Scholar] [CrossRef]
- Meeter, L.H.H.; Vijverberg, E.G.; Del Campo, M.; Rozemuller, A.J.M.; Donker Kaat, L.; de Jong, F.J.; van der Flier, W.M.; Teunissen, C.E.; van Swieten, J.C.; Pijnenburg, Y.A.L. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 2018, 90, e1231–e1239. [Google Scholar] [CrossRef]
- Pijnenburg, Y.A.L.; Verwey, N.A.; van der Flier, W.M.; Scheltens, P.; Teunissen, C.E. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2015, 1, 505. [Google Scholar] [CrossRef]
- Horie, K.; Barthélemy, N.R.; Spina, S.; VandeVrede, L.; He, Y.; Paterson, R.W.; Wright, B.A.; Day, G.S.; Davis, A.A.; Karch, C.M.; et al. CSF tau microtubule-binding region identifies pathological changes in primary tauopathies. Nat. Med. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Strom, A.; Fonseca, C.; Iaccarino, L.; Wolf, A.; Spina, S.; Allen, I.E.; Cobigo, Y.; Heuer, H.; et al. Association of Plasma P-tau217 and P-tau181 with clinical phenotype, neuropathology, and imaging markers in Alzheimer’s disease and frontotemporal lobar degeneration: A retrospective diagnostic performance study. Lancet Neurol. 2021, 20, 739, Erratum in Lancet Neurol. 2021, 20, e6. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Cousins, K.A.Q.; Shaw, L.M.; Shellikeri, S.; Dratch, L.; Rosario, L.; Elman, L.B.; Quinn, C.; Amado, D.A.; Wolk, D.A.; Tropea, T.F.; et al. Elevated Plasma Phosphorylated Tau 181 in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2022, 92, 807–818. [Google Scholar] [CrossRef]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Baiardi, S.; Avoni, P.; Polischi, B.; Capellari, S.; Salvi, F.; Liguori, R.; Parchi, P.; et al. Elevated plasma p-tau181 levels unrelated to Alzheimer’s disease pathology in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 428–435. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Scholle, L.; Mensch, A.; Großkopf, H.; Ratti, A.; Kölsch, A.; Stoltenburg-Didinger, G.; Conrad, J.; De Gobbi, A.; Barba, L.; et al. Phosphorylated tau 181 and 217 are elevated in serum and muscle of patients with amyotrophic lateral sclerosis. Nat. Commun. 2025, 16, 2019. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Tartaglia, M.C.; Fox, S.H.; Lang, A.E.; Kovacs, G.G. Four-Repeat Tau Seeding in the Skin of Patients with Progressive Supranuclear Palsy. JAMA Neurol. 2024, 81, 1228–1230. [Google Scholar] [CrossRef]
- Dellarole, I.L.; Vacchi, E.; Ruiz-Barrio, I.; Pinton, S.; Raimondi, A.; Rossi, S.; Morandi, S.; Bianco, G.; Begum Bacinoglu, M.; Lombardo, A.; et al. Tau seeding activity in skin biopsy differentiates tauopathies from synucleinopathies. NPJ Park. Dis. 2024, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Vanmechelen, E.; Trojanowski, J.Q.; Lee, V.M.Y.; Van Broeckhoven, C.; van der Zee, J.; Engelborghs, S. TDP-43 as a possible biomarker for frontotemporal lobar degeneration: A systematic review of existing antibodies. Acta Neuropathol. Commun. 2015, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Scialò, C.; Tran, T.H.; Salzano, G.; Novi, G.; Caponnetto, C.; Chiò, A.; Calvo, A.; Canosa, A.; Moda, F.; Caroppo, P.; et al. TDP-43 real-Time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2020, 2, fcaa142. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Dols-Icardo, O.; Lladó, A.; Sánchez-Valle, R.; Hernández, I.; Amer, G.; Antón-Aguirre, S.; Alcolea, D.; Fortea, J.; Ferrer, I.; et al. Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J. Neurol. Neurosurg. Psychiatry 2014, 85, 684–691. [Google Scholar] [CrossRef]
- Fontana, E.; Bongianni, M.; Benussi, A.; Bronzato, E.; Scialo, C.; Sacchetto, L.; Cagnin, A.; Castriciano, S.; Buratti, E.; Gardoni, F.; et al. Detection of TDP-43 seeding activity in the olfactory mucosa from patients with frontotemporal dementia. Alzheimers Dement. 2023, 20, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Vizziello, M.; Dellarole, I.L.; Ciullini, A.; Pascuzzo, R.; Lombardo, A.; Bellandi, F.; Celauro, L.; Battipaglia, C.; Ciusani, E.; Rizzo, A.; et al. TDP-43 seeding activity in the olfactory mucosa of patients with amyotrophic lateral sclerosis. Mol. Neurodegener. 2025, 20, 49. [Google Scholar] [CrossRef]
- Goossens, J.; Bjerke, M.; Van Mossevelde, S.; Van Den Bossche, T.; Goeman, J.; De Vil, B.; Sieben, A.; Martin, J.J.; Cras, P.; De Deyn, P.P.; et al. Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration. Alzheimers Res. Ther. 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Huin, V.; Barbier, M.; Bottani, A.; Lobrinus, J.A.; Clot, F.; Lamari, F.; Chat, L.; Rucheton, B.; Fluchère, F.; Auvin, S.; et al. Homozygous GRN mutations: New phenotypes and new insights into pathological and molecular mechanisms. Brain 2020, 143, 303–319. [Google Scholar] [CrossRef]
- Meeter, L.H.H.; Gendron, T.F.; Sias, A.C.; Jiskoot, L.C.; Russo, S.P.; Donker Kaat, L.; Papma, J.M.; Panman, J.L.; van der Ende, E.L.; Dopper, E.G.; et al. Poly(GP), neurofilament and grey matter deficits in C9orf72 expansion carriers. Ann. Clin. Transl. Neurol. 2018, 5, 583–597. [Google Scholar] [CrossRef]
- del Campo, M.; Zetterberg, H.; Gandy, S.; Onyike, C.U.; Oliveira, F.; Udeh-Momoh, C.; Lleó, A.; Teunissen, C.E.; Pijnenburg, Y. New developments of biofluid-based biomarkers for routine diagnosis and disease trajectories in frontotemporal dementia. Alzheimers Dement. 2022, 18, 2292–2307. [Google Scholar] [CrossRef]
- Seddighi, S.; Qi, Y.A.; Brown, A.L.; Wilkins, O.G.; Bereda, C.; Belair, C.; Zhang, Y.J.; Prudencio, M.; Keuss, M.J.; Khandeshi, A.; et al. Mis-spliced transcripts generate de novo proteins in TDP-43–related ALS/FTD. Sci. Transl. Med. 2024, 16, eadg7162. [Google Scholar] [CrossRef]
- Deng, H.; Gao, K.; Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 2014, 10, 337–348. [Google Scholar] [CrossRef]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef]
- Lehmer, C.; Oeckl, P.; Weishaupt, J.H.; Volk, A.E.; Diehl-Schmid, J.; Schroeter, M.L.; Lauer, M.; Kornhuber, J.; Levin, J.; Fassbender, K.; et al. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol. Med. 2017, 9, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, S.E.; Maltos, A.M.; Gill, K.; Aladesuyi Arogundade, O.; Brown, K.A.; Sachdev, A.; Sckaff, M.; Lam, K.J.K.; Fisher, I.J.; Chouhan, R.S.; et al. Validated assays for the quantification of C9orf72 human pathology. Sci. Rep. 2024, 14, 828. [Google Scholar] [CrossRef]
- Bridel, C.; Van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035. [Google Scholar] [CrossRef]
- Wilke, C.; Reich, S.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; Galimberti, D.; Rowe, J.B.; et al. Stratifying the Presymptomatic Phase of Genetic Frontotemporal Dementia by Serum NfL and pNfH: A Longitudinal Multicentre Study. Ann. Neurol. 2022, 91, 33–47. [Google Scholar] [CrossRef]
- Liu, E.; Jones, S.L.; Light, V.; Teunissen, C.; Bouzigues, A.; Russell, L.L.; Foster, P.; Ferry-Bolder, E.; van Swieten, J.; Jiskoot, L.; et al. Accuracy of blood-based neurofilament light to different genetic frontotemporal dementia from primary psychiatric disorders. J. Alzheimers Dis. 2025, 106, 1337–1354. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, I.O.C.; Swift, I.J.; Sogorb-Esteve, A.; Heller, C.; Knowles, K.; Bouzigues, A.; Russell, L.L.; Peakman, G.; Greaves, C.V.; Convery, R.; et al. CSF glial markers are elevated in a subset of patients with genetic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, D.; Vilaplana, E.; Suárez-Calvet, M.; Illán-Gala, I.; Blesa, R.; Clarimón, J.; Lladó, A.; Sánchez-Valle, R.; Molinuevo, J.L.; García-Ribas, G.; et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 2017, 89, 178–188. [Google Scholar] [CrossRef]
- Bergström, S.; Öijerstedt, L.; Remnestål, J.; Olofsson, J.; Ullgren, A.; Seelaar, H.; van Swieten, J.C.; Synofzik, M.; Sanchez-Valle, R.; Moreno, F.; et al. A panel of CSF proteins separates genetic frontotemporal dementia from presymptomatic mutation carriers: A GENFI study. Mol. Neurodegener. 2021, 16, 79. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Perrin, R.J.; Roe, C.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef]
- Alcolea, D.; Irwin, D.J.; Illán-Gala, I.; Muñoz, L.; Clarimón, J.; McMillan, C.T.; Fortea, J.; Blesa, R.; Lee, E.B.; Trojanowski, J.Q.; et al. Elevated YKL-40 and low sAPPβ:YKL-40 ratio in antemortem cerebrospinal fluid of patients with pathologically confirmed FTLD. J. Neurol. Neurosurg. Psychiatry 2018, 90, 180. [Google Scholar] [CrossRef]
- Sogorb-Esteve, A.; Weiner, S.; Simrén, J.; Swift, I.J.; Bocchetta, M.; Todd, E.G.; Cash, D.M.; Bouzigues, A.; Russell, L.L.; Foster, P.H.; et al. Proteomic analysis reveals distinct cerebrospinal fluid signatures across genetic frontotemporal dementia subtypes. Sci. Transl. Med. 2025, 17, eadm9654. [Google Scholar] [CrossRef]
- Sogorb-Esteve, A.; Nilsson, J.; Swift, I.J.; Heller, C.; Bocchetta, M.; Russell, L.L.; Peakman, G.; Convery, R.S.; van Swieten, J.C.; Seelaar, H.; et al. Differential impairment of cerebrospinal fluid synaptic biomarkers in the genetic forms of frontotemporal dementia. Alzheimers Res. Ther. 2022, 14, 118. [Google Scholar] [CrossRef]
- Galimberti, D.; Schoonenboom, N.; Scheltens, P.; Fenoglio, C.; Venturelli, E.; Pijnenburg, Y.A.L.; Bresolin, N.; Scarpini, E. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2006, 66, 146–147. [Google Scholar] [CrossRef]
- Huang, G.; Jian, J.; Liu, C.J. Progranulinopathy: A diverse realm of disorders linked to progranulin imbalances. Cytokine Growth Factor. Rev. 2024, 76, 142–159. [Google Scholar] [CrossRef]
- Boylan, M.A.; Pincetic, A.; Romano, G.; Tatton, N.; Kenkare-Mitra, S.; Rosenthal, A. Targeting Progranulin as an Immuno-Neurology Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 15946. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.D.; Abdul Hamid, H.; Md Hashim, N.F.; Cheema, M.S.; Chiroma, S.M.; Mustapha, M.; Mehat, M.Z. Could protein phosphatase 2A and glycogen synthase kinase-3 beta be targeted by natural compounds to ameliorate Alzheimer’s pathologies? Brain Res. 2024, 1829, 148793. [Google Scholar] [CrossRef]
- Fenoglio, C.; Serpente, M.; Visconte, C.; Arcaro, M.; Sorrentino, F.; D’Anca, M.; Arighi, A.; Rotondo, E.; Vimercati, R.; Rossi, G.; et al. Circulating Non-Coding RNA Levels Are Altered in Autosomal Dominant Frontotemporal Dementia. Int. J. Mol. Sci. 2022, 23, 14723. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, C.; Serpente, M.; Arcaro, M.; Carandini, T.; Sacchi, L.; Pintus, M.; Rotondo, E.; Borracci, V.; Ghezzi, L.; Bouzigues, A.; et al. Inflammatory plasma profile in genetic symptomatic and presymptomatic Frontotemporal Dementia—A GENFI study. Brain Behav. Immun. 2024, 122, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Barkhof, F.; Scheltens, P.; Schott, J.M.; Fox, N.C. An algorithmic approach to structural imaging in dementia. J. Neurol. Neurosurg. Psychiatry 2014, 85, 692–698. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Kipps, C.M.; Johnson, J.K.; Seeley, W.W.; Mendez, M.F.; Knopman, D.; Kertesz, A.; Mesulam, M.; Salmon, D.P.; et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer Dis. Assoc. Disord. 2007, 21, S14–S18. [Google Scholar] [CrossRef]
- Mueller, K.; Scherf, N.; Grimmer, T.; Diehl-Schmid, J.; Danek, A.; Levin, J.; Wiltfang, J.; Anderl-Straub, S.; Otto, M.; Schroeter, M.L. Criminal Behavior in Frontotemporal Dementia: A Multimodal MRI Study. Hum. Brain Mapp. 2025, 46, e70308. [Google Scholar] [CrossRef]
- Matyi, M.A.; Radhakrishnan, H.; Olm, C.A.; Phillips, J.S.; Cook, P.A.; Rhodes, E.; Gee, J.C.; Irwin, D.J.; McMillan, C.T.; Massimo, L. Executive dysfunction relates to salience network desegregation in behavioural variant frontotemporal dementia. Neuroimage Clin. 2025, 48, 103853. [Google Scholar] [CrossRef]
- Coupé, P.; Catheline, G.; Lanuza, E.; Manjón, J.V. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum. Brain Mapp. 2017, 38, 5501–5518. [Google Scholar] [CrossRef]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533, Erratum in Nature 2022, 610, E6. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Mansencal, B.; Manjon, J.V.; Tourdias, T.; Catheline, G.; Coupé, P. Anatomical MRI staging of frontotemporal dementia variants. Alzheimers Dement. 2023, 19, 3283–3294. [Google Scholar] [CrossRef]
- Fumagalli, G.G.; Basilico, P.; Arighi, A.; Bocchetta, M.; Dick, K.M.; Cash, D.M.; Harding, S.; Mercurio, M.; Fenoglio, C.; Pietroboni, A.M.; et al. Distinct patterns of brain atrophy in Genetic Frontotemporal Dementia Initiative (GENFI) cohort revealed by visual rating scales. Alzheimers Res. Ther. 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Todd, E.G.; Peakman, G.; Cash, D.M.; Convery, R.S.; Russell, L.L.; Thomas, D.L.; Eugenio Iglesias, J.; van Swieten, J.C.; Jiskoot, L.C.; et al. Differential early subcortical involvement in genetic FTD within the GENFI cohort. Neuroimage Clin. 2021, 30, 102646. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Millan, A.; Borrego-Écija, S.; van Swieten, J.C.; Jiskoot, L.; Moreno, F.; Laforce, R.; Graff, C.; Masellis, M.; Tartaglia, M.C.; Rowe, J.B.; et al. Loss of brainstem white matter predicts onset and motor neuron symptoms in C9orf72 expansion carriers: A GENFI study. J. Neurol. 2023, 270, 1573–1586. [Google Scholar] [CrossRef]
- Gazzina, S.; Grassi, M.; Premi, E.; Alberici, A.; Benussi, A.; Archetti, S.; Gasparotti, R.; Bocchetta, M.; Cash, D.M.; Todd, E.G.; et al. Structural brain splitting is a hallmark of Granulin-related frontotemporal dementia. Neurobiol. Aging 2022, 114, 94–104. [Google Scholar] [CrossRef]
- Bruffaerts, R.; Gors, D.; Bárcenas Gallardo, A.; Vandenbulcke, M.; Van Damme, P.; Suetens, P.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; et al. Hierarchical spectral clustering reveals brain size and shape changes in asymptomatic carriers of C9orf72. Brain Commun. 2022, 4, fcac182. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Costa, T.; Gazzina, S.; Benussi, A.; Cauda, F.; Gasparotti, R.; Archetti, S.; Alberici, A.; van Swieten, J.C.; Sanchez-Valle, R.; et al. An Automated Toolbox to Predict Single Subject Atrophy in Presymptomatic Granulin Mutation Carriers. J. Alzheimers Dis. 2022, 86, 205–218. [Google Scholar] [CrossRef]
- Pasternak, M.; Mirza, S.S.; Luciw, N.; Mutsaerts, H.J.M.M.; Petr, J.; Thomas, D.; Cash, D.; Bocchetta, M.; Tartaglia, M.C.; Mitchell, S.B.; et al. Longitudinal cerebral perfusion in presymptomatic genetic frontotemporal dementia: GENFI results. Alzheimers Dement. 2024, 20, 3525–3542. [Google Scholar] [CrossRef]
- Giunta, M.; Libri, I.; Premi, E.; Brattini, C.; Paghera, B.; Archetti, S.; Gasparotti, R.; Padovani, A.; Borroni, B.; Benussi, A. Clinical and radiological features of Posterior Cortical Atrophy (PCA) in a GRN mutation carrier: A case report. Eur. J. Neurol. 2021, 28, 344–348. [Google Scholar] [CrossRef]
- Bouzigues, A.; Du VLe Joulot, M.; Peysson, N.; Houot, M.; Béranger, B.; Russell, L.L.; Foster, P.H.; Ferry-Bolder, E.; van Swieten, J.C.; Jiskoot, L.; et al. Structural and functional connectivity in tau mutation carriers: From presymptomatic to symptomatic frontotemporal dementia. Alzheimers Dement. 2025, 21, e70367. [Google Scholar] [CrossRef]
- Borrego-Ecija, S.; Juncà-Parella, J.; Vandebergh, M.; Pérez Millan, A.; Balasa, M.; Llado, A.; Bouzigues, A.; Russell, L.L.; Foster, P.H.; Ferry-Bolder, E.; et al. Association of Initial Side of Brain Atrophy with Clinical Features and Disease Progression in Patients with GRN Frontotemporal Dementia. Neurology 2024, 103, e209944. [Google Scholar] [CrossRef]
- Harper, L.; Fumagalli, G.G.; Barkhof, F.; Scheltens, P.; O’Brien, J.T.; Bouwman, F.; Burton, E.J.; Rohrer, J.D.; Fox, N.C.; Ridgway, G.R.; et al. MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain 2016, 139, 1211. [Google Scholar] [CrossRef]
- Assogna, M.; Premi, E.; Gazzina, S.; Benussi, A.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Gasparotti, R.; Padovani, A.; Tadayon, E.; et al. Association of Choroid Plexus Volume with Serum Biomarkers, Clinical Features, and Disease Severity in Patients with Frontotemporal Lobar Degeneration Spectrum. Neurology 2023, 101, e1218–e1230. [Google Scholar] [CrossRef] [PubMed]
- Manera, A.L.; Dadar, M.; Van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R., Jr.; Graff, C.; Synofzik, M.; Galimberti, D.; et al. MRI data-driven algorithm for the diagnosis of behavioural variant frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 608–616. [Google Scholar] [CrossRef]
- Premi, E.; Giunta, M.; Iraji, A.; Rachakonda, S.; Calhoun, V.D.; Gazzina, S.; Benussi, A.; Gasparotti, R.; Archetti, S.; Bocchetta, M.; et al. Dissemination in time and space in presymptomatic granulin mutation carriers: A GENFI spatial chronnectome study. Neurobiol. Aging 2021, 108, 155–167. [Google Scholar] [CrossRef] [PubMed]
- McMillan, C.T.; Brun, C.; Siddiqui, S.; Churgin, M.; Libon, D.; Yushkevich, P.; Zhang, H.; Boller, A.; Gee, J.; Grossman, M. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology 2012, 78, 1761–1768. [Google Scholar] [CrossRef]
- McMillan, C.T.; Irwin, D.J.; Avants, B.B.; Powers, J.; Cook, P.A.; Toledo, J.B.; McCarty Wood, E.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q.; et al. White Matter Imaging Helps Dissociate Tau from TDP-43 in Frontotemporal Lobar Degeneration. J. Neurol. Neurosurg. Psychiatry 2013, 84, 949. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Mansencal, B.; Fonov, V.; Manjon, J.V.; Tourdias, T.; Bouzigues, A.; Russell, L.L.; Foster, P.H.; Ferry-Bolder, E.; van Swieten, J.C.; et al. Anatomical progression of genetic frontotemporal lobar degeneration across the lifespan. Brain 2025, 148, 3880–3892. [Google Scholar] [CrossRef]
- Higuchi, M.; Tagai, K.; Takahata, K.; Endo, H. Advances in PET imaging of protein aggregates associated with neurodegenerative disease. Nat. Rev. Neurol. 2025, 21, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Bevan-Jones, R.W.; Cope, T.E.; Jones, S.P.; Passamonti, L.; Hong, Y.T.; Fryer, T.; Arnold, R.; Coles, J.P.; Aigbirhio, F.A.; Patterson, K.; et al. [18F]AV-1451 binding is increased in frontotemporal dementia due to C9orf72 expansion. Ann. Clin. Transl. Neurol. 2018, 5, 1292–1296. [Google Scholar] [CrossRef]
- Jones, D.T.; Knopman, D.S.; Graff-Radford, J.; Syrjanen, J.A.; Senjem, M.L.; Schwarz, C.G.; Dheel, C.; Wszolek, Z.; Rademakers, R.; Kantarci, K.; et al. In vivo 18 F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology 2018, 90, e947–e954. [Google Scholar] [CrossRef] [PubMed]
- Gatto, R.G.; Carlos, A.F.; Reichard, R.R.; Lowe, V.J.; Whitwell, J.L.; Josephs, K.A. Comparative assessment of regional tau distribution by Tau-PET and Post-mortem neuropathology in a representative set of Alzheimer’s & frontotemporal lobar degeneration patients. PLoS ONE 2023, 18, e0284182. [Google Scholar] [CrossRef]
- Malarte, M.L.; Gillberg, P.G.; Kumar, A.; Bogdanovic, N.; Lemoine, L.; Nordberg, A. Discriminative binding of tau PET tracers PI2620, MK6240 and RO948 in Alzheimer’s disease, corticobasal degeneration and progressive supranuclear palsy brains. Mol. Psychiatry 2022, 28, 1272–1283, Erratum in Mol. Psychiatry 2023, 29, 38. [Google Scholar] [CrossRef]
- Kubota, M.; Endo, H.; Takahata, K.; Tagai, K.; Suzuki, H.; Onaya, M.; Sano, Y.; Yamamoto, Y.; Kurose, S.; Matsuoka, K.; et al. In vivo PET classification of tau pathologies in patients with frontotemporal dementia. Brain Commun. 2024, 6, fcae075. [Google Scholar] [CrossRef]
- Suzuki, H.; Kubota, M.; Kurose, S.; Tagai, K.; Endo, H.; Onaya, M.; Yamamoto, Y.; Sahara, N.; Ohgidani, M.; Haga, C.; et al. Neuropathological correlations of 18F-florzolotau PET in a case with pick’s disease. EJNMMI Res. 2025, 15, 96. [Google Scholar] [CrossRef]
- Vanderlinden, G.; Vandenberghe, R.; Vandenbulcke, M.; Van Laere, K. The Current Role of Tau PET Imaging in Neurodegeneration. Semin. Nucl. Med. 2025, 55, 548–564. [Google Scholar] [CrossRef]
- Nigro, S.; Tafuri, B.; Urso, D.; De Blasi, R.; Cedola, A.; Gigli, G.; Logroscino, G. Altered structural brain networks in linguistic variants of frontotemporal dementia. Brain Imaging Behav. 2022, 16, 1113–1122. [Google Scholar] [CrossRef]
- Neumann, M.; Kwong, L.K.; Sampathu, D.M.; Trojanowski, J.Q.; Lee, V.M.Y. TDP-43 Proteinopathy in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis: Protein Misfolding Diseases Without Amyloidosis. Arch. Neurol. 2007, 64, 1388–1394. [Google Scholar] [CrossRef]
- Tetzloff, K.A.; Duffy, J.R.; Clark, H.M.; Utianski, R.L.; Strand, E.A.; Machulda, M.M.; Botha, H.; Martin, P.R.; Schwarz, C.G.; Senjem, M.L.; et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain 2019, 142, 2466–2482. [Google Scholar] [CrossRef]
- Boxer, A.L.; Geschwind, M.D.; Belfor, N.; Gorno-Tempini, M.L.; Schauer, G.F.; Miller, B.L.; Weiner, M.W.; Rosen, H.J. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 2006, 63, 81–86. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Höglinger, G.U.; Antonini, A.; Bordelon, Y.; Boxer, A.L.; Colosimo, C.; Van Eimeren, T.; Golbe, L.I.; Kassubek, J.; Kurz, C.; et al. Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov. Disord. 2017, 32, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, H.; Xie, H.; Jia, Z.; Chen, Q. Molecular imaging biomarkers in familial frontotemporal lobar degeneration: Progress and prospects. Front. Neurol. 2022, 13, 933217. [Google Scholar] [CrossRef]
- Popuri, K.; Beg, M.F.; Lee, H.; Balachandar, R.; Wang, L.; Sossi, V.; Jacova, C.; Baker, M.; Shahinfard, E.; Rademakers, R.; et al. FDG-PET in presymptomatic C9orf72 mutation carriers. Neuroimage Clin. 2021, 31, 102687. [Google Scholar] [CrossRef]
- Malpetti, M.; Holland, N.; Jones, P.S.; Ye, R.; Cope, T.E.; Fryer, T.D.; Hong, Y.T.; Savulich, G.; Rittman, T.; Passamonti, L.; et al. Synaptic density in carriers of C9orf72 mutations: A [11C]UCB-J PET study. Ann. Clin. Transl. Neurol. 2021, 8, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Murley, A.G.; Rowe, J.B. Neurotransmitter deficits from fronto temporal lobar degeneration. Brain 2018, 141, 1263–1285. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Pengo, M.; Mattioli, I.; Cantoni, V.; Dukart, J.; Gasparotti, R.; Buratti, E.; Padovani, A.; Bocchetta, M.; Todd, E.G.; et al. Early neurotransmitters changes in prodromal frontotemporal dementia: A GENFI study. Neurobiol. Dis. 2023, 179, 106068. [Google Scholar] [CrossRef] [PubMed]
- Toller, G.; Cobigo, Y.; Callahan, P.; Appleby, B.S.; Brushaber, D.; Domoto-Reilly, K.; Forsberg, L.K.; Ghoshal, N.; Graff-Radford, J.; Graff-Radford, N.R. Multisite ALLFTD study modeling progressive empathy loss from the earliest stages of behavioral variant frontotemporal dementia. Alzheimers Dement. 2023, 19, 2842–2852. [Google Scholar] [CrossRef]
- Schuster, B.A.; Sowden, S.; Rybicki, A.J.; Fraser, D.S.; Press, C.; Holland, P.; Cook, J.L. Dopaminergic Modulation of Dynamic Emotion Perception. J. Neurosci. 2022, 42, 4394–4400. [Google Scholar] [CrossRef]
- Duerler, P.; Vollenweider, F.X.; Preller, K.H. A neurobiological perspective on social influence: Serotonin and social adaptation. J. Neurochem. 2022, 162, 60–79. [Google Scholar] [CrossRef]
- Kanen, J.W.; Arntz, F.E.; Yellowlees, R.; Cardinal, R.N.; Price, A.; Christmas, D.M.; Apergis-Schoute, A.M.; Sahakian, B.J.; Robbins, T.W. Serotonin depletion amplifies distinct human social emotions as a function of individual differences in personality. Transl. Psychiatry 2021, 11, 81. [Google Scholar] [CrossRef]
- Gérard, T.; Colmant, L.; Malotaux, V.; Salman, Y.; Huyghe, L.; Quenon, L.; Boyer, E.; Dricot, L.; Ivanoiu, A.; Lhommel, R.; et al. Tau PET Imaging with [18F]MK-6240: Limited Affinity for Primary Tauopathies and High Specificity for Alzheimer’s Disease. Eur. J. Neurol. 2025, 32, e70068. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Benussi, A.; Premi, E.; Alberici, A.; Marcello, E.; Gardoni, F.; Di Luca, M.; Padovani, A. Biological, Neuroimaging, and Neurophysiological Markers in Frontotemporal Dementia: Three Faces of the Same Coin. J. Alzheimers Dis. 2018, 62, 1113–1123. [Google Scholar] [CrossRef]
- Cosseddu, M.; Benussi, A.; Gazzina, S.; Turrone, R.; Archetti, S.; Bonomi, E.; Biasiotto, G.; Zanella, I.; Ferrari, R.; Cotelli, M.S.; et al. Mendelian forms of disease and age at onset affect survival in frontotemporal dementia. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Todd, E.G.; Bouzigues, A.; Cash, D.M.; Nicholas, J.M.; Convery, R.S.; Russell, L.L.; Thomas, D.L.; Malone, I.B.; Iglesias, J.E.; et al. Structural MRI predicts clinical progression in presymptomatic genetic frontotemporal dementia: Findings from the GENetic Frontotemporal dementia Initiative cohort. Brain Commun. 2023, 5, fcad061. [Google Scholar] [CrossRef]
- McCarthy, J.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; Synofzik, M.; Galimberti, D.; Rowe, J.B.; Masellis, M.; et al. Data-driven staging of genetic frontotemporal dementia using multi-modal MRI. Hum. Brain Mapp. 2022, 43, 1821–1835. [Google Scholar] [CrossRef]
- Staffaroni, A.M.; Quintana, M.; Wendelberger, B.; Heuer, H.W.; Russell, L.L.; Cobigo, Y.; Wolf, A.; Goh, S.M.; Petrucelli, L.; Gendron, T.F.; et al. Temporal order of clinical and biomarker changes in familial frontotemporal dementia. Nat. Med. 2022, 28, 2194–2206. [Google Scholar] [CrossRef]
- Guarnier, G.; Reinelt, J.; Molloy, E.N.; Mihai, P.G.; Einaliyan, P.; Valk, S.; Modestino, A.; Ugolini, M.; Mueller, K.; Wu, Q.; et al. Cascaded Multimodal Deep Learning in the Differential Diagnosis, Progression Prediction, and Staging of Alzheimer’s and Frontotemporal Dementia. MedRxiv 2025, in press. [Google Scholar] [CrossRef]
- Prinse, F.A.M.; Weerd Lvan der Swieten JCvan Ronen, I.; Seelaar, H.; Hirschler, L.; Najac, C.; Dopper, E.G.P. Investigating the role of neuroinflammation and brain clearance in frontotemporal lobar degeneration using 7T MRI and fluid biomarkers: Protocol for a cross-sectional study in a tertiary care setting. BMJ Open 2025, 15, e102668. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Seripa, D.; Daniele, A.; Watling, M.; Giannelli, G.; Imbimbo, B.P. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat. Rev. Neurol. 2020, 16, 213–228. [Google Scholar] [CrossRef]
- Giunta, M.; Solje, E.; Gardoni, F.; Borroni, B.; Benussi, A. Experimental Disease-Modifying Agents for Frontotemporal Lobar Degeneration. J. Exp. Pharmacol. 2021, 13, 359–376. [Google Scholar] [CrossRef]
- Carter, L.; Borroni, B.; Mummery, C.; Ber ILe Boeve, B.; Boxer, A.; Smithey, M.; Chow, T.; Huang, J.; Guizzetti, L.; Salvadore, G.; et al. Baseline Characteristics for INFRONT-3: A Phase 3 Double-Blind, Placebo-Controlled 96-Week Study Evaluating Latozinemab in FTD-GRN (P8-3.005). Neurology 2025, 104, 4085. [Google Scholar] [CrossRef]
- Chang Berger, A.; Cohen, I.; Jafarnejad, M.; Chiu, C.-L.; Bhalla, A.; Damo, L.; Shanbhag, N.M.; Simen, A.; Lu, H.; Zicha, S.; et al. Safety and pharmacokinetics of single ascending doses of TAK-594/DNL593, a brain-penetrant progranulin replacement therapy, in healthy volunteers: Interim results from Part A of a Phase 1/2 clinical trial. Alzheimers Dement. 2023, 19, e075068. [Google Scholar] [CrossRef]
- Study Details|A Study of PBFT02 in Participants with FTD and Mutations in the Granulin Precursor (GRN) or C9ORF72 Genes|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04747431 (accessed on 8 August 2025).
- AviadoBio Announces Completion of Second Cohort in Phase 1/2 ASPIRE-FTD Clinical Trial Studying AVB-101 for FTD-GRN. Available online: https://aviadobio.com/aviadobio-announces-completion-of-second-cohort-in-phase-1-2-aspire-ftd-clinical-trial/ (accessed on 8 August 2025).
- Tran, H.; Moazami, M.P.; Yang, H.; McKenna-Yasek, D.; Douthwright, C.L.; Pinto, C.; Metterville, J.; Shin, M.; Sanil, N.; Dooley, C.; et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide. Nat. Med. 2022, 28, 117–124. [Google Scholar] [CrossRef]
- Liu, Y.; Andreucci, A.; Iwamoto, N.; Yin, Y.; Yang, H.; Liu, F.; Bulychev, A.; Hu, X.S.; Lin, X.; Lamore, S.; et al. Preclinical evaluation of WVE-004, aninvestigational stereopure oligonucleotide forthe treatment of C9orf72-associated ALS or FTD. Mol. Ther. Nucleic Acids 2022, 28, 558–570. [Google Scholar] [CrossRef]
- van den Berg, L.H.; Rothstein, J.D.; Shaw, P.J.; Babu, S.; Benatar, M.; Bucelli, R.C.; Genge, A.; Glass, J.D.; Hardiman, O.; Libri, V.; et al. Safety, tolerability, and pharmacokinetics of antisense oligonucleotide BIIB078 in adults with C9orf72-associated amyotrophic lateral sclerosis: A phase 1, randomised, double blinded, placebo-controlled, multiple ascending dose study. Lancet Neurol. 2024, 23, 901–912. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Zuniga, G.; Ramirez, P.; Fernandez, R.; Wang, C.-P.; Li, J.; Davila, L.; Pelton, K.; Gomez, S.; Sohn, C.; et al. A Phase IIa clinical trial to evaluate the effects of anti-retroviral therapy in Alzheimer’s disease (ART-AD). NPJ Dement. 2025, 1, 2. [Google Scholar] [CrossRef]
- Babu, S.; Nicholson, K.A.; Rothstein, J.D.; Swenson, A.; Sampognaro, P.J.; Pant, P.; Macklin, E.A.; Spruill, S.; Paganoni, S.; Gendron, T.F.; et al. Apilimod dimesylate in C9orf72 amyotrophic lateral sclerosis: A randomized phase 2a clinical trial. Brain 2024, 147, 2998–3008. [Google Scholar] [CrossRef]
- McMillan, C.T.; Russ, J.; Wood, E.M.; Irwin, D.J.; Grossman, M.; McCluskey, L.; Elman, L.; Van Deerlin, V.; Lee, E.B. C9orf72 promoter hypermethylation is neuroprotective: Neuroimaging and neuropathologic evidence. Neurology 2015, 84, 1622. [Google Scholar] [CrossRef]
- Boros, B.D.; Schoch, K.M.; Kreple, C.J.; Miller, T.M. Antisense Oligonucleotides for the Study and Treatment of ALS. Neurotherapeutics 2022, 19, 1145. [Google Scholar] [CrossRef]
- Study Details|Ciprofloxacin/Celecoxib Combination in Patients with ALS|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04090684 (accessed on 8 August 2025).
- Writing Committee for the HEALEY ALS Platform Trial. Verdiperstat in Amyotrophic Lateral Sclerosis: Results from the Randomized HEALEY ALS Platform Trial. JAMA Neurol. 2025, 82, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Rayner, S.L.; Lee, A.; Chung, R.S. TDP-43 as a therapeutic target in neurodegenerative diseases: Focusing on motor neuron disease and frontotemporal dementia. Ageing Res. Rev. 2023, 92, 102085. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Litvan, I.; Mendonca, N.; Wang, D.; Zheng, H.; Rendenbach-Mueller, B.; Lon, H.K.; Jin, Z.; Fisseha, N.; Budur, K.; et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: A phase 2, randomised, placebo-controlled trial. Lancet Neurol. 2021, 20, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.; Kong, J.; O’Gorman, J.; Ratti, E.; Rajagovindan, R.; Viollet, L.; Huang, E.; Sharma, S.; Racine, A.M.; Czerkowicz, J.; et al. TANGO: A placebo-controlled randomized phase 2 study of efficacy and safety of the anti-tau monoclonal antibody gosuranemab in early Alzheimer’s disease. Nat. Aging 2023, 3, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Anti-tau antibody stumbles in phase II Alzheimer trial. Nat. Rev. Drug Discov. 2024, 23, 883. [Google Scholar] [CrossRef]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- Study of Safety, Tolerability, Pharmacodynamics and Pharmacokinetics of NIO752 in Early Alzheimer’s Disease Participants|Novartis. Available online: https://www.novartis.com/clinicaltrials/study/nct05469360 (accessed on 8 August 2025).
- Vivash, L.; Malpas, C.B.; Meletis, C.; Gollant, M.; Eratne, D.; Li, Q.X.; McDonald, S.; O’Brien, W.T.; Brodtmann, A.; Darby, D.; et al. A phase 1b open-label study of sodium selenate as a disease-modifying treatment for possible behavioral variant frontotemporal dementia. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12299. [Google Scholar] [CrossRef]
- Codron, P.; Cassereau, J.; Vourc’h, P. InFUSing antisense oligonucleotides for treating ALS. Trends Mol. Med. 2022, 28, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Altomare, D.; Benussi, A.; Bréchet, L.; Casula, E.P.; Dodich, A.; Pievani, M.; Santarnecchi, E.; Frisoni, G.B. The emerging field of non-invasive brain stimulation in Alzheimer’s disease. Brain 2024, 147, 4003–4016. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’era, V.; Cosseddu, M.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Manenti, R.; Benussi, L.; Brattini, C.; Alberici, A.; et al. Transcranial stimulation in frontotemporal dementia: A randomized, double-blind, sham-controlled trial. Alzheimers Dement. Transl. Res. Clin. Interv. 2020, 6, e12033. [Google Scholar] [CrossRef]
- Teichmann, M.; Lesoil, C.; Godard, J.; Vernet, M.; Bertrand, A.; Levy, R.; Dubois, B.; Lemoine, L.; Truong, D.Q.; Bikson, M.; et al. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann. Neurol. 2016, 80, 693–707. [Google Scholar] [CrossRef]
- Gervits, F.; Ash, S.; Coslett, H.B.; Rascovsky, K.; Grossman, M.; Hamilton, R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang. 2016, 162, 35–41. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Petesi, M.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miniussi, C.; Padovani, A.; Borroni, B. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J. Alzheimers Dis. 2014, 39, 799–808. [Google Scholar] [CrossRef]
- Godoi, D.C.; Pandiá, E.; Rodrigues, M.E.; Araújo, L.; Barbosa, M.; Alhwaishel, K.; Godoi, A.; McGonigle, D.J. Linguistic effects of transcranial Direct Current Stimulation (tDCS) in patients with primary progressive aphasia: A systematic review and meta-analysis of randomised controlled trials. Neurosci. Biobehav. Rev. 2025, 176, 106264. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Adenzato, M.; Cantoni, V.; Manenti, R.; Alberici, A.; Enrici, I.; Benussi, A.; Dell’Era, V.; Bonetta, E.; Padovani, A.; et al. Enhancing theory of mind in behavioural variant frontotemporal dementia with transcranial direct current stimulation. Cogn. Affect. Behav. Neurosci. 2018, 18, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Antczak, J.; Kowalska, K.; Klimkowicz-Mrowiec, A.; Wach, B.; Kasprzyk, K.; Banach, M.; Rzeźnicka-Brzegowy, K.; Kubica, J.; Słowik, A. Repetitive transcranial magnetic stimulation for the treatment of cognitive impairment in frontotemporal dementia: An open-label pilot study. Neuropsychiatr. Dis. Treat. 2018, 14, 749. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Alberici, A.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miozzo, A.; Padovani, A.; Miniussi, C.; Borroni, B. Prefrontal cortex rTMS enhances action naming in progressive non-fluent aphasia. Eur. J. Neurol. 2012, 19, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Trebbastoni, A.; Raccah, R.; de Lena, C.; Zangen, A.; Inghilleri, M. Repetitive Deep Transcranial Magnetic Stimulation Improves Verbal Fluency and Written Language in a Patient with Primary Progressive Aphasia-Logopenic Variant (LPPA). Brain Stimul. 2013, 6, 545–553. [Google Scholar] [CrossRef]
- Benussi, A.; Di Lorenzo, F.; Dell’Era, V.; Cosseddu, M.; Alberici, A.; Caratozzolo, S.; Cotelli, M.S.; Micheli, A.; Rozzini, L.; Depari, A.; et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology 2017, 89, 665–672. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Cosseddu, M.; Spallazzi, M.; Micheli, A.; Turrone, R.; Alberici, A.; Borroni, B. TMS for staging and predicting functional decline in frontotemporal dementia. Brain Stimul. 2020, 13, 386–392. [Google Scholar] [CrossRef]
- Vucic, S.; Stanley Chen, K.H.; Kiernan, M.C.; Hallett, M.; Benninger, D.H.; Di Lazzaro, V.; Rossini, P.M.; Benussi, A.; Berardelli, A.; Currà, A.; et al. Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee. Clin. Neurophysiol. 2023, 150, 131–175. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.V.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. 2021, 132, 2568–2607. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Borroni, B. The role of transcranial magnetic stimulation in the diagnosis of dementia. In Horizons in Neuroscience Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; Volume 44, pp. 55–104. [Google Scholar]
- Benussi, A.; Premi, E.; Gazzina, S.; Cantoni, V.; Cotelli, M.S.; Giunta, M.; Gasparotti, R.; Calhoun, V.D.; Borroni, B. Neurotransmitter imbalance dysregulates brain dynamic fluidity in frontotemporal degeneration. Neurobiol. Aging 2020, 94, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Benussi, A. Transcranial Magnetic Stimulation Across the Lifespan: Impact of Developmental and Degenerative Processes. Biol. Psychiatry 2023, 95, 581–591. [Google Scholar] [CrossRef]
- Padovani, A.; Benussi, A.; Cotelli, M.S.; Ferrari, C.; Cantoni, V.; Dell’Era, V.; Turrone, R.; Paghera, B.; Borroni, B. Transcranial magnetic stimulation and amyloid markers in mild cognitive impairment: Impact on diagnostic confidence and diagnostic accuracy. Alzheimers Res. Ther. 2019, 11, 95. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Cosseddu, M.; Spallazzi, M.; Alberici, A.; Padovani, A.; Borroni, B. Neurophysiological Correlates of Positive and Negative Symptoms in Frontotemporal Dementia. J. Alzheimers Dis. 2020, 73, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Gazzina, S.; Benussi, A.; Premi, E.; Paternicò, D.; Cristillo, V.; Dell’Era, V.; Cosseddu, M.V.; Archetti, S.; Alberici, A.; Gasparotti, R.; et al. Neuroanatomical Correlates of Transcranial Magnetic Stimulation in Presymptomatic Granulin Mutation Carriers. Brain Topogr. 2018, 31, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Palese, F.; Bonomi, E.; Nuzzo, T.; Benussi, A.; Mellone, M.; Zianni, E.; Cisani, F.; Casamassa, A.; Alberici, A.; Scheggia, D.; et al. Anti-GluA3 antibodies in frontotemporal dementia: Effects on glutamatergic neurotransmission and synaptic failure. Neurobiol. Aging 2020, 86, 143–155. [Google Scholar] [CrossRef]
- Burrell, J.R.; Kiernan, M.C.; Vucic, S.; Hodges, J.R. Motor Neuron dysfunction in frontotemporal dementia. Brain 2011, 134, 2582–2594. [Google Scholar] [CrossRef]
- Benussi, A.; Grassi, M.; Palluzzi, F.; Cantoni, V.; Cotelli, M.S.; Premi, E.; Di Lorenzo, F.; Pellicciari, M.C.; Ranieri, F.; Musumeci, G.; et al. Classification accuracy of TMS for the diagnosis of mild cognitive impairment. Brain Stimul. 2021, 14, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Gazzina, S.; Premi, E.; Cosseddu, M.; Archetti, S.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Alberici, A.; Micheli, A.; et al. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol. Aging 2019, 76, 133–140. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Ferrari, C.; Caratozzolo, S.; Rozzini, L.; Alberici, A.; Padovani, A.; Borroni, B. Discrimination of atypical parkinsonisms with transcranial magnetic stimulation. Brain Stimul. 2018, 11, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Alberici, A.; Buratti, E.; Ghidoni, R.; Gardoni, F.; Di Luca, M.; Padovani, A.; Borroni, B. Toward a Glutamate Hypothesis of Frontotemporal Dementia. Front. Neurosci. 2019, 13, 304. [Google Scholar] [CrossRef]
- Benussi, A.; Grassi, M.; Palluzzi, F.; Koch, G.; Di Lazzaro, V.; Nardone, R.; Cantoni, V.; Dell’Era, V.; Premi, E.; Martorana, A.; et al. Classification Accuracy of Transcranial Magnetic Stimulation for the Diagnosis of Neurodegenerative Dementias. Ann. Neurol. 2020, 87, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Assogna, M.; Sprugnoli, G.; Press, D.; Dickerson, B.; Macone, J.; Bonnì, S.; Borghi, I.; Connor, A.; Hoffman, M.; Grover, N.; et al. Gamma-induction in frontotemporal dementia (GIFTeD) randomized placebo-controlled trial: Rationale, noninvasive brain stimulation protocol, and study design. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12219. [Google Scholar] [CrossRef]
- Pagnoni, I.; Gobbi, E.; Premi, E.; Borroni, B.; Binetti, G.; Cotelli, M.; Manenti, R. Language training for oral and written naming impairment in primary progressive aphasia: A review. Transl. Neurodegener. 2021, 10, 24. [Google Scholar] [CrossRef]
- Massimo, L.; Hirschman, K.B.; Aryal, S.; Quinn, R.; Fisher, L.; Sharkey, M.; Thomas, G.; Bowles, K.H.; Riegel, B. iCare4Me for FTD: A pilot randomized study to improve self-care in caregivers of persons with frontotemporal degeneration. Alzheimers Dement. Transl. Res. Clin. Interv. 2023, 9, e12381. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.M.; Boxer, A.L. Treatment of frontotemporal dementia. Curr. Treat. Options Neurol. 2014, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
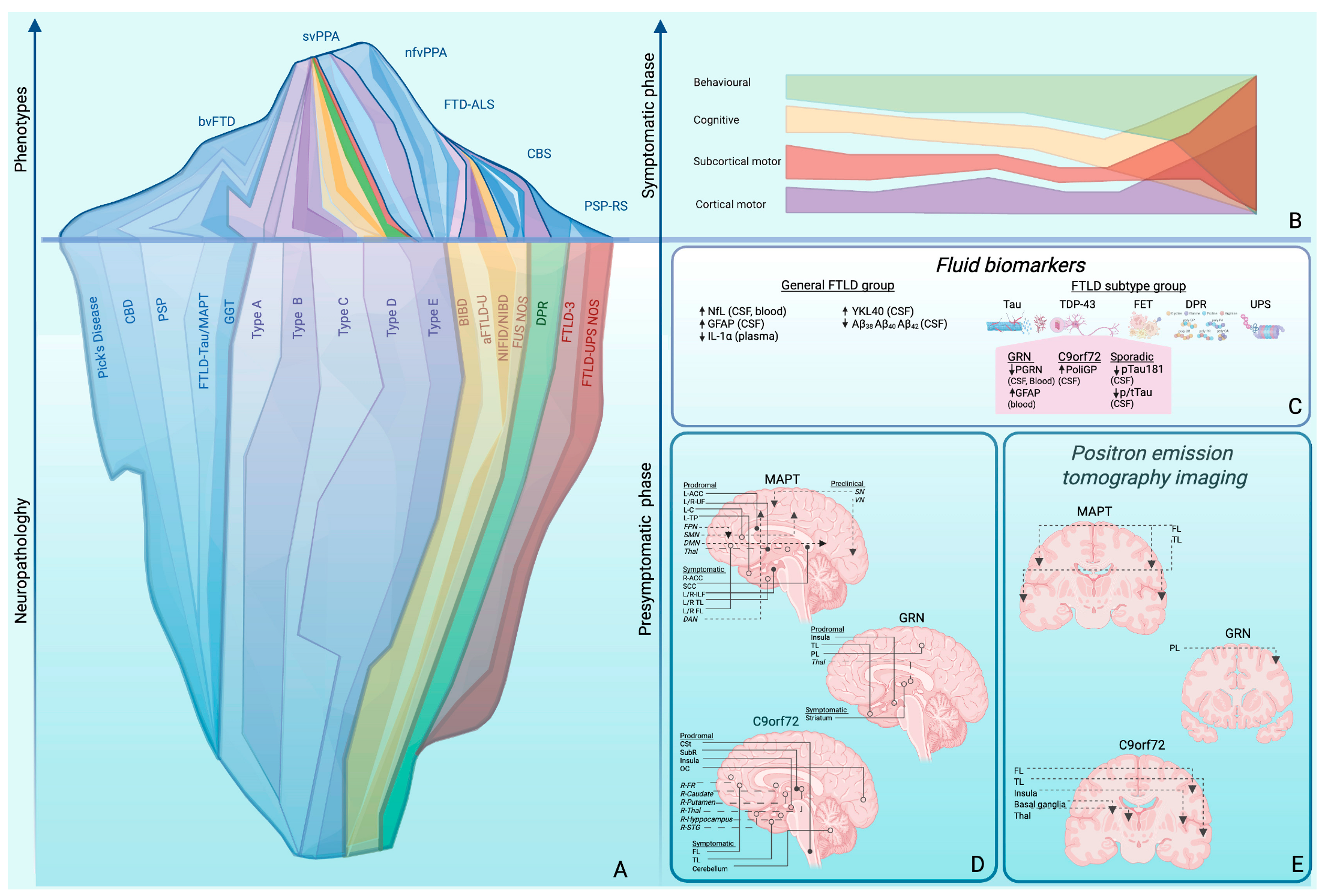

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacino, F.; Manganotti, P.; Benussi, A. Preclinical and Prodromal Frontotemporal Dementia: Challenges and Opportunities. Int. J. Transl. Med. 2025, 5, 52. https://doi.org/10.3390/ijtm5040052
Palacino F, Manganotti P, Benussi A. Preclinical and Prodromal Frontotemporal Dementia: Challenges and Opportunities. International Journal of Translational Medicine. 2025; 5(4):52. https://doi.org/10.3390/ijtm5040052
Chicago/Turabian StylePalacino, Federica, Paolo Manganotti, and Alberto Benussi. 2025. "Preclinical and Prodromal Frontotemporal Dementia: Challenges and Opportunities" International Journal of Translational Medicine 5, no. 4: 52. https://doi.org/10.3390/ijtm5040052
APA StylePalacino, F., Manganotti, P., & Benussi, A. (2025). Preclinical and Prodromal Frontotemporal Dementia: Challenges and Opportunities. International Journal of Translational Medicine, 5(4), 52. https://doi.org/10.3390/ijtm5040052





