1. Introduction
Anxiety disorders are among the most prevalent mental health conditions, affecting approximately 19% of individuals in the United States over their lifetime. According to the 2019 National Center for Health Statistics’ National Health Interview Survey, 15% of adults reported experiencing anxiety symptoms in the two weeks preceding the survey [
1]. These disorders are often characterized by heightened arousal and fear, accompanied by physical and cognitive manifestations such as insomnia, restlessness, and difficulty concentrating. Despite the high prevalence of clinical anxiety disorders worldwide, there is a comparatively limited understanding of how symptoms of anxiety that do not meet diagnostic criteria impact individuals, leaving those affected with fewer available resources [
2].
Those who suffer from symptoms of anxiety report pronounced stress responses in challenging situations, with these heightened feelings often persisting well beyond the resolution of the initial stressor. This form of anxiety may present as persistent dread or apprehension regarding upcoming events. Stress and anxiety are closely tied to the human fear response, commonly referred to as the fight-or-flight mechanism. This mechanism is important to respond adaptively to short-term challenges. However, when such responses persist over extended periods, they can significantly impair quality of life, contributing to adverse health outcomes such as disrupted sleep, elevated stress levels, and impaired cognitive functioning [
3].
Stress and anxiety are often used interchangeably, and while they are similar, they refer to different experiences. Anxiety involves excessive worry and anticipation of future threats, while stress typically arises from external demands. Anxiety can occur without a clear trigger and enhances the perception of events as stressful. Thus, interventions to lower anxiety would likely lead to the modulation of stress [
4].
Perceived stress refers to an individual’s subjective appraisal of any situation or stimulus as stressful. Many factors, including anxiety, a lack of sleep, impaired cognitive function, poor health, and other psychological or physiological factors can influence one’s perception of stress. Prolonged perceived stress negatively influences overall health and quality of life and has been associated with increased mortality at any age, particularly in midlife [
5].
Stress, anxiety, and mood are closely linked. Not only do these conditions often manifest in parallel, but perceived stress has also been suggested to play a role in the pathophysiology of mood and anxiety disorders [
6,
7]. Additionally, those who report poor mood or feelings of anxiety feel more stressed during psychologically stressful situations than those who do not feel anxious.
It is well known that anxiety and stress are associated with other conditions that negatively impact health. For example, anxiety is associated with poor sleep quality [
8]. Poor sleep quality has been associated with increased obesity, systemic inflammation, compromised immune function, and reduced antioxidant defenses. Moreover, poor sleep negatively impacts mood, emotional regulation, attention, memory, and cognitive performance [
9,
10,
11,
12]. All of the above-mentioned symptoms negatively impact overall health. Pharmaceutical interventions are commonly prescribed for many of these conditions. Still, they often are associated with side effects, leading many individuals to seek alternate solutions such as dietary supplements and herbal ingredients [
13,
14,
15].
Apocynum venetum L. (
A. venetum), commonly known as “Luobuma” in Chinese and “Rafuma” in Japanese, is a perennial herbaceous shrub commonly used to produce herbal remedies and tea. Numerous bioactives have been identified in
A. venetum such as flavonoids, terpenes, steroids, phenylpropanoids, polysaccharides, and other glycosides found in root, stem, and leaf extracts. It is perhaps best known for its flavonoids including hyperoside, isoquercetrin, kaempferol, quercetin, and astragalin [
16].
A. venetum has demonstrated antioxidant, hepatoprotective, anti-inflammatory, and cardioprotective effects [
4]. Acute supplementation demonstrated anti-anxiety effects in mice undergoing a stressful maze test [
17]. The leaf extract has been shown to decrease sleep latency and increase non-REM sleep [
18]. Safety has been demonstrated in animal and human studies [
19].
While several studies have assessed the safety of A. venetum and the efficacy for various outcomes, to our knowledge, none have evaluated the potential interconnected effects related to stress and anxiety, nor have they included real-world data from large, diverse populations. Therefore, in this study, we aimed to assess the effect of Venetron®, which is a trademarked (A. venetum) leaf extract, on feelings of anxiety, stress, sleep, and overall health outcomes relative to placebo control over 6 weeks in a large group of healthy individuals from across the United States.
2. Materials and Methods
2.1. Study Design and Participants
This study investigated the effects of 50 mg Venetron® manufactured by Tokiwa Phytochemical Co., Ltd. (TM01) (Chiba, Japan), on feelings of anxiety, perceived stress, cognitive function, and sleep disturbance using a randomized, placebo-controlled, double-blind protocol, referred to as Radicle™ Calm 2. Each TM01 capsule contained 50 mg of Venetron®. Venetron® is specified to contain no less than 2% of each hyperoside and isoquercitrin, resulting in no less than 1 mg of each compound per capsule.
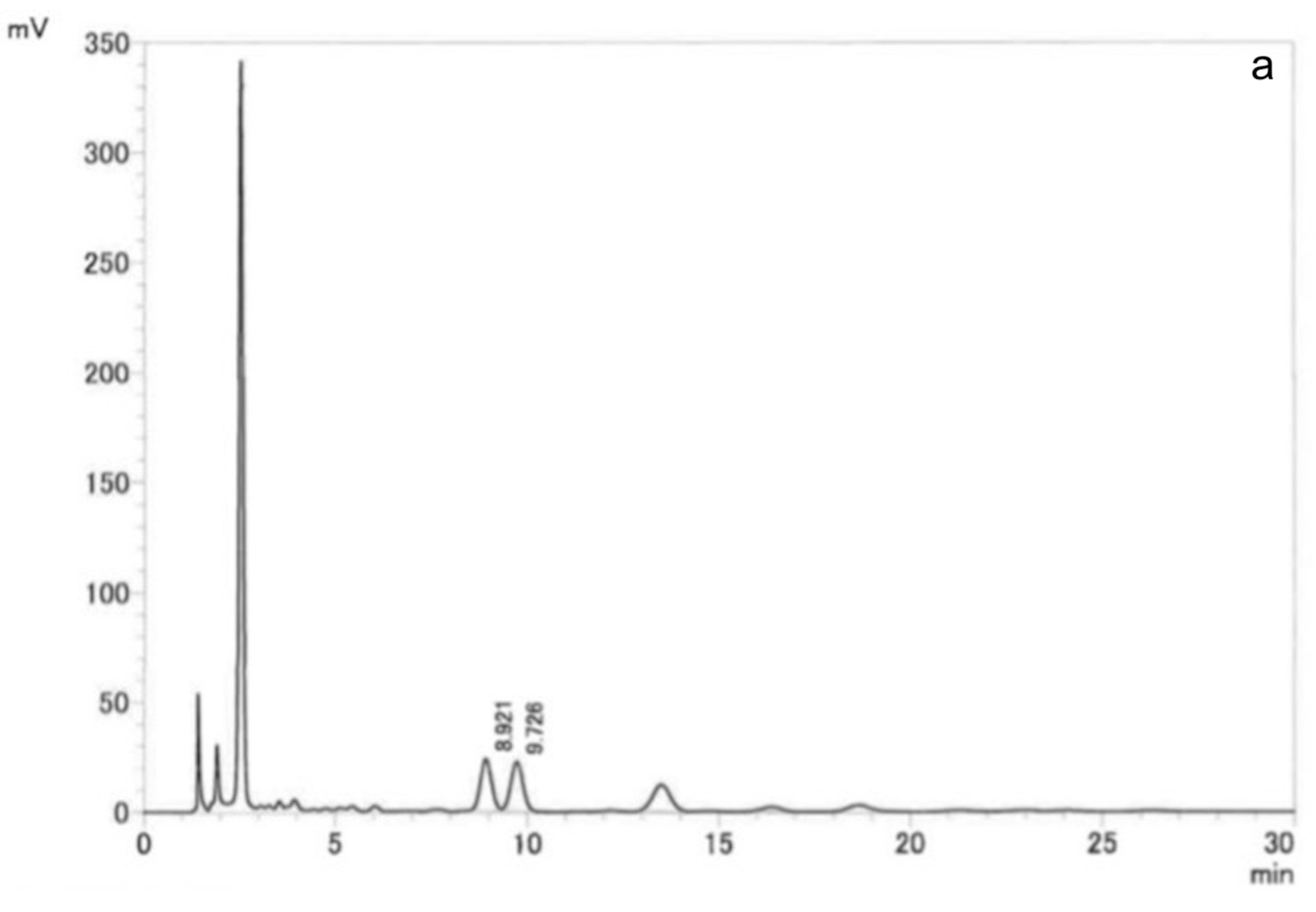
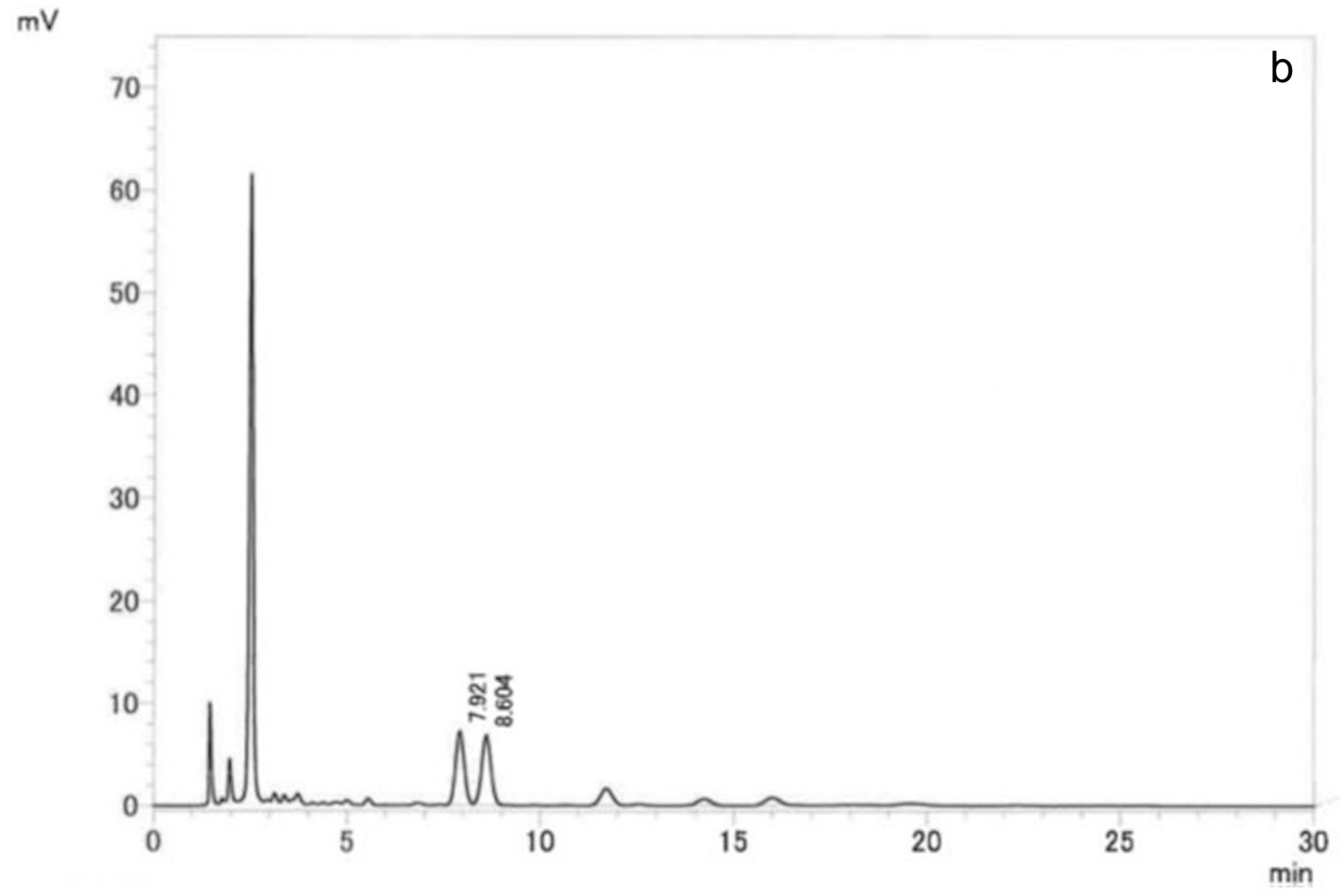
Venetron
® and TM01 capsules were analyzed by the following HPLC condition: a UV-visible spectrophotometer at a wavelength of 330 nm; a 4.6 mm × 15 cm, 5 μm ODS column; a column temperature of 35 °C; and a mobile phase of water/acetonitrile/trifluoroacetic acid (850:150:1). The HPLC chromatograms of Venetron
® and TM01 capsules are shown in
Figure 1. The standard compounds, hyperoside and isoquercitrin, were detected in both Venetron
® and TM01 capsules.
Recruitment, consent, and participation were entirely virtual. Participants were recruited using social media, a third-party consumer network, and Radicle Science’s electronic mailing list. The capsules were tested by a third-party laboratory for contaminants and composition. The active component, Rafuma (
Apocynum ventum) leaf extract, was present in the capsules. Those recruited completed a screener assessment to determine eligibility, provided informed consent, and completed all assessments using their computer or mobile device. All communication with participants occurred via SMS text messaging or email, and all study products were delivered through the mail to participants’ homes. For the flow of participant activity, please see
Figure 2.
Participants were eligible to participate if they were at least 21 years old, residing in the USA, indicated a desire to reduce feelings of anxiety, and could improve by at least 20% in feelings of anxiety, which was indicated during enrollment. Those who reported being pregnant, trying to become pregnant or breastfeeding, being a heavy drinker, having liver or kidney disease, moderate-to-severe heart disease, atrial fibrillation, or an irregular heartbeat even when taking medication for it, undergoing current or recent chemotherapy or immunotherapy treatments, or taking medications with known moderate or severe interactions with any of the active ingredients in the study products were ineligible to participate. Enrolment began on 4 October 2023 and ended on 12 October 2023. Eligible participants were provided with an electronic informed consent form and a digital copy of their completed consent form. Next, participants completed a baseline assessment regarding their demographics, health behaviors, feelings of anxiety, perceived stress, cognitive function, and sleep disturbance.
After completing the baseline assessment, participants were stratified by their sex at birth and their self-reported feelings of anxiety score collected during enrollment. Next, participants were randomly assigned to receive either the active product (TM01) or a placebo control (TM02). The placebo was created to match the active product in appearance, color, and smell, and dosage instructions were exactly the same between the groups. Participants were provided instructions for product consumption and a 6-week supply of their assigned study product (see
Table 1). Product assignments were masked to researchers and participants until after the study’s conclusion. Each week for 6 weeks after receiving their assigned product, participants received online assessments containing questions regarding their product consumption and any observed side effects, as well as validated health measures assessing their feelings of anxiety, perceived stress, cognitive function, and sleep quality.
The Sterling Review Board (IRB) [Identification number: 10678-EKPauli; approved on: 9 August 2023] approved this study. The registration of this protocol on
ClinicalTrials.gov [NCT05837910] was completed on 1 May 2023.
2.2. Outcomes
The primary health outcome for this study was feelings of anxiety, measured using the Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety 8a instrument. Perceived stress, cognitive function, and sleep quality were secondary health outcomes for this study. They were assessed using the NIH Toolbox Perceived Stress Survey, PROMIS Cognitive Function 4a, and PROMIS Sleep Disturbance 4a, respectively. The odds of achieving a minimal clinically important difference (MCID) were assessed for each health outcome. An MCID was operationalized as an improvement in a health outcome score that is greater than or equal to one-half of the standard deviation of participants’ scores collected at baseline. The standard deviation criterion for an MCID was calculated independently for both study arms.
Instances of spontaneously reported side effects were examined to assess the safety of the study products. Side effects severity was assessed using participants’ reported use of medical services due to observed side effects, based on the Common Terminology Criteria for Adverse Events (CTCAE; v5.0 USDHHS), a standardized classification and grading system for adverse effects. A mild side effect grade denotes that no intervention (medication or medical advice) was needed; a moderate grade denotes that a medication was taken or care from a healthcare provider was requested or obtained. A severe grade denotes that the participant was hospitalized or required significant medical intervention due to the side effects.
2.2.1. PROMIS Anxiety 8a
The Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety 8a is an 8-item self-report measure of anxiety symptoms experienced within the previous 7 days. Each item is evaluated on a 5-point Likert scale. The PROMIS Anxiety 8a scores range from 8 to 40, with higher scores indicating greater feelings of anxiety [
20,
21].
2.2.2. NIH Toolbox Perceived Stress Survey
The NIH Toolbox Perceived Stress Survey is a 10-item self-report measure of perceived stress experienced within the previous month. Each item is evaluated on a 5-point Likert scale. The NIH Toolbox Perceived Stress Survey scores range from 10 to 50, with higher scores indicating more perceived stress [
22].
2.2.3. PROMIS Cognitive Function 4a
PROMIS Cognitive Function 4a is a 4-item self-report measure of cognitive ability within the previous 7 days. Each item is evaluated on a 5-point Likert scale. The PROMIS Cognitive Function 4a scores range from 4 to 20, with higher scores indicating better cognitive function ability [
23].
2.2.4. PROMIS Sleep Disturbance 4a
PROMIS Sleep Disturbance 4a is a 4-item self-report measure of sleep disturbances within the previous 7 days. Each item is evaluated on a 5-point Likert scale. The PROMIS Sleep Disturbance 4a scores range from 4 to 20, with higher scores indicating greater sleep disturbances [
24].
2.3. Power Analysis
A Monte Carlo power analysis was conducted to determine the sample size required to detect a small effect size equal to a generalized Cohen’s D = 0.3 (approximately equals Cohen’s D = 0.3) between the slopes of the groups at a two-sided p-value of 0.05 with a Bonferroni multiplicity adjustment correcting for the planned contrasts and number of outcomes. The Monte Carlo power analysis used 1000 simulations, fitting a linear mixed-effect model with an interaction between groups and time, a random intercept at the participant level, a random slope for the study week, and an unstructured covariance matrix, and accounted for 5 covariates. The design parameters specified a slope difference of −0.19, a random intercept standard deviation of 2.83, a random slope standard deviation of 0.39, and an error standard deviation of 2.48. For a power of 80% and accounting for up to 40% attrition, the appropriate sample size is 250 participants per study arm.
2.4. Statistical Analysis
The primary statistical model was a mixed-effect regression to test for a significant difference in the change in feelings of anxiety, perceived stress, cognitive function, and sleep quality between the active arm and placebo. The statistical model included the study arm, time, and the interaction between the study arm and time with sex, age, body mass index (BMI), race, and ethnicity as covariates, with a random intercept at the participant level and a random slope for the study week using an unstructured covariance matrix. Time, age, and BMI were fit as continuous variables, while the study arm, sex at birth, race, and ethnicity were fit as categorical variables. To ensure that the mixed-effects regression captured the relationship between the study arm and time, an initial reduced model was fit to test the linear hypothesis and identify the appropriate model (linear, growth, etc.) for the interaction between them. The full mixed-effect regression model was then conducted using the optimal relationship. The reduced model included the study arm, time, and the interaction between study arm and time with a random intercept at the participant level and a random slope for the study week using an unstructured covariance matrix.
A secondary statistical model was fit to test for a statistical difference in the relative risk of achieving an MCID in feelings of anxiety, perceived stress, cognitive function, and sleep quality between the active and placebo study arms. The secondary model fit a general linear model using a Poisson distribution with a log link function and robust standard errors that included sex, age, ethnicity, and BMI as covariates to test for differences between study arms. Age and BMI were included as continuous variables, while sex, ethnicity, and study arm were included as categorical.
All analyses were performed on an intent-to-treat cohort, meaning that all participants who were assigned to a study product and completed the baseline were included in the cohort regardless of adherence to protocol. Stata 18 was used to perform the analysis.
3. Results
Four hundred seventy-six volunteers were eligible, consented to participate, and completed the baseline assessment. Of those who completed the baseline assessment, 370 participants completed at least one additional assessment and were included in the analysis: 179 in the active arm and 191 in the placebo arm. In total, 106 participants were excluded from the analysis for not completing any assessments after the baseline assessment.
3.1. Sample Demographics and Characteristics
Of the participants included in the analysis, the majority were female (60%) and most identified as white (88%). The mean age of participants at baseline was 43.77 (SD = 10.97, range = 21–75), and the mean body mass index (BMI) was 28.66 (SD = 6.93, range = 16–59). Participants were distributed across BMI categories as follows: underweight (2%), normal weight (31%), overweight (32%), and obese (35%). The majority of participants had a bachelor’s or associate degree (43%), had completed some college but did not have a degree (26%), or had a master’s or professional degree (23%). Approximately 96% reported that they were experiencing chronic (i.e., lasting 3 months or longer) feelings of anxiety, 97% reported experiencing chronic stress, 90% reported experiencing chronic poor attention or focus, and 91% reported experiencing chronic sleep disturbances. There were no significant differences between the active arm and placebo arm in the previously mentioned characteristics or demographics.
3.2. Product Usage
Throughout the duration of the study, the average number of days participants took the product each week ranged from 5.50 to 6.39 in the active arm and 6.02–6.43 in the placebo arm. The average daily capsule intake ranged from 1.13 to 1.37 in the active arm and 1.09–1.30 in the placebo arm.
3.3. Change in Health Outcomes
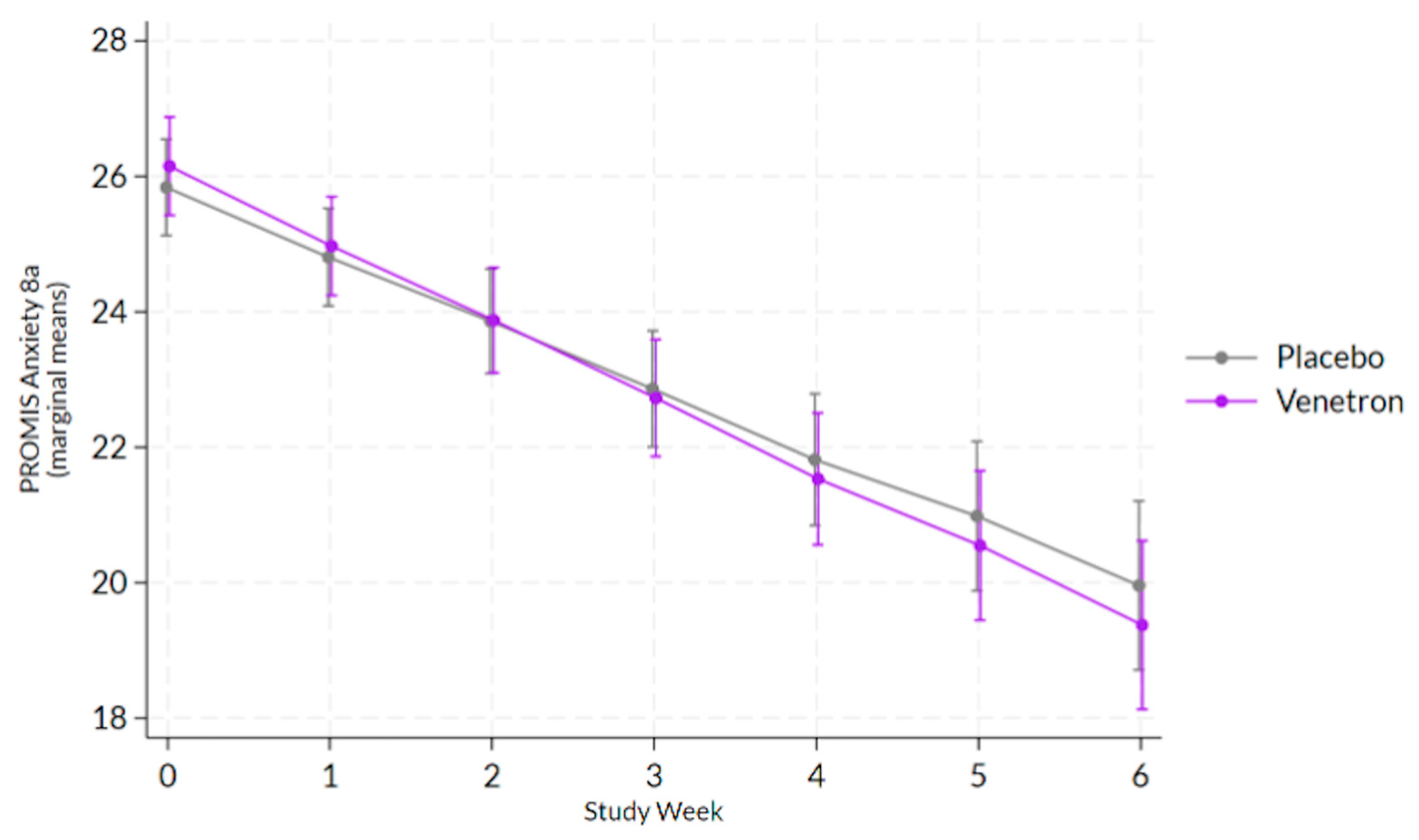
3.3.1. Feelings of Anxiety
The overall model for feelings of anxiety was statistically significant, Wald χ
2(14) = 283.16,
p < 0.0001; however, the interaction between study arms and time was non-significant (see
Figure 3), χ
2(1) = 1.34,
p = 0.2470.
3.3.2. MCID in Feelings of Anxiety
Approximately 75% of those in the active arm experienced an MCID compared to 65% of those in the placebo arm. A marginally significant difference was observed between arms in the odds of experiencing an MCID in participants’ PROMIS Anxiety 8a score, RR = 1.20, χ2(1) = 3.75, p = 0.0528, indicating those in the active arm had a greater likelihood of experiencing an MCID.
3.3.3. Perceived Stress
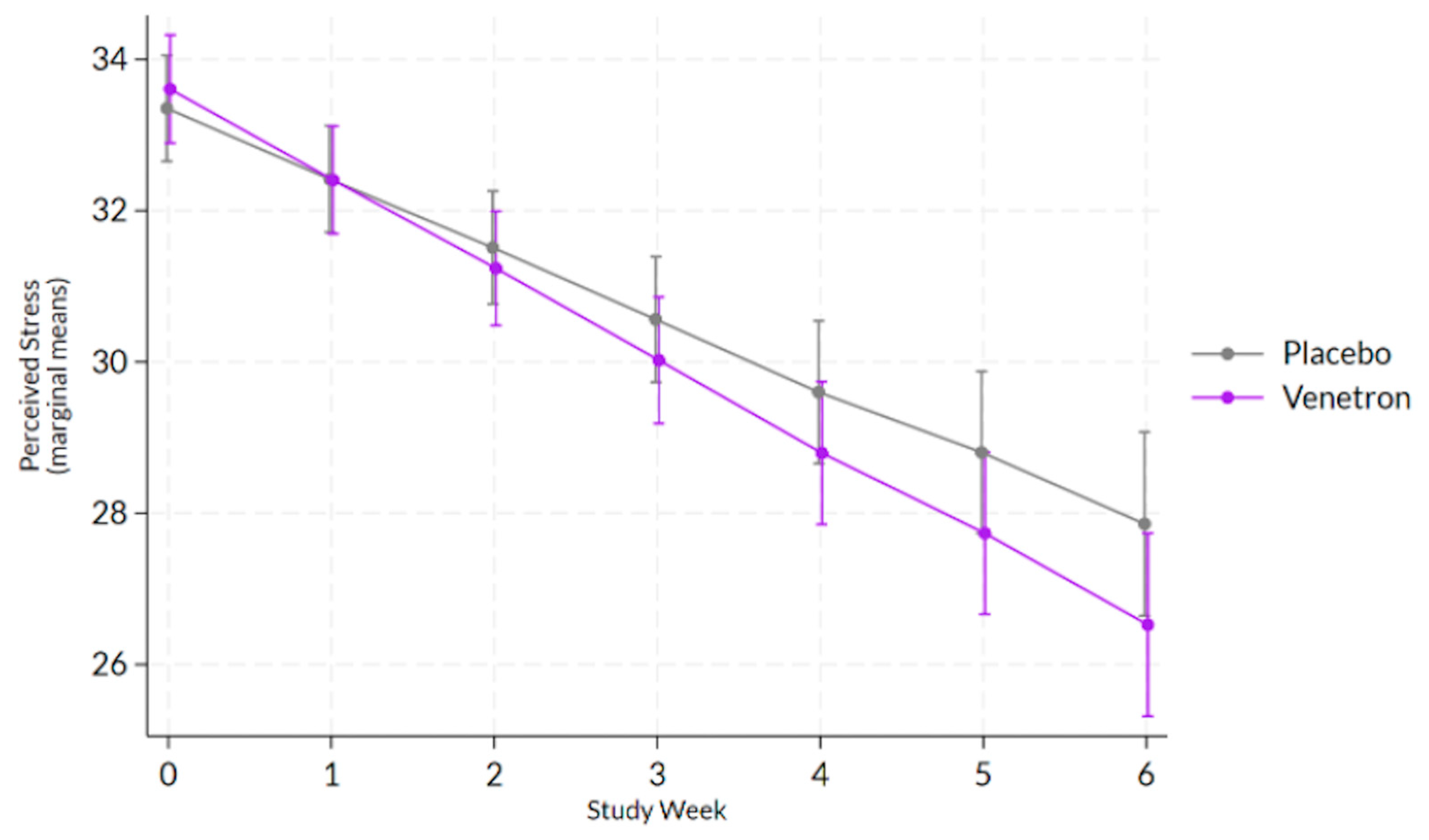
The overall model for perceived stress was statistically significant, Wald χ
2(14) = 286.84,
p < 0.0001, as well as the interaction between study arms and time (see
Figure 4), indicating that those in the active arm reported a greater decrease in perceived stress than those in the placebo arm, coefficient = −0.26, χ
2(1) = 4.19,
p = 0.0407.
3.3.4. MCID in Perceived Stress
Approximately 78% of those in the active arm experienced an MCID compared to 62% of those in the placebo arm. A significant difference was observed between arms in the odds of experiencing an MCID in participants’ NIH Toolbox Perceived Stress Scale score, χ2(1) = 6.80, p = 0.0091, indicating that those in the active arm had a greater likelihood of experiencing an MCID.
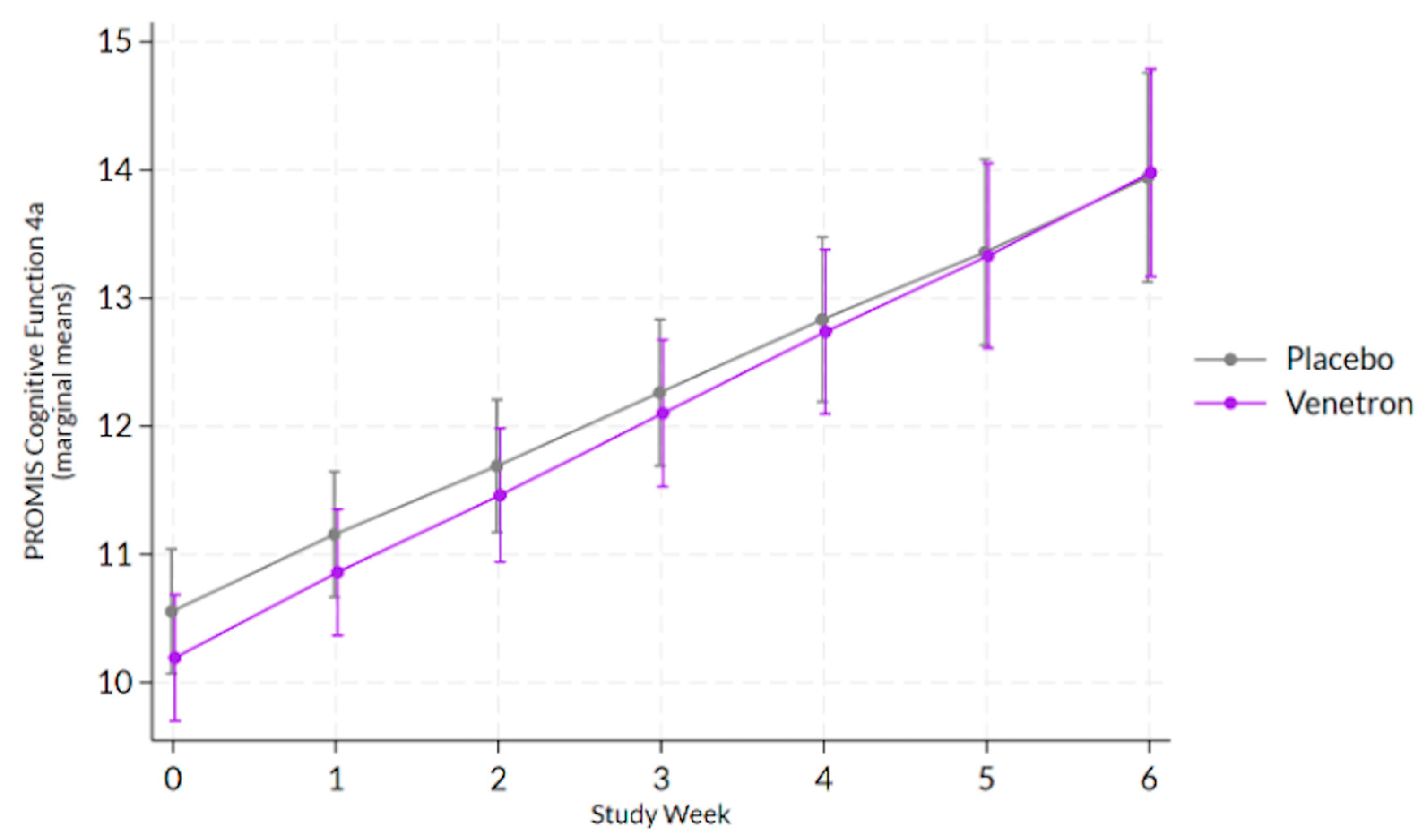
3.3.5. Cognitive Function
The overall model for cognitive function was statistically significant, Wald χ
2(13) = 201.49,
p < 0.0001; however, the interaction between study arms and time was non-significant (see
Figure 5), χ
2(1) = 0.63,
p = 0.4275.
3.3.6. Achievement of MCID in Cognitive Function
Approximately 74% of those in the active arm experienced an MCID compared to 71% of those in the placebo arm. No significant difference was observed between arms in the odds of experiencing an MCID in the participant’s PROMIS Cognitive Function 4a score, χ2(1) = 0.44, p = 0.5087.
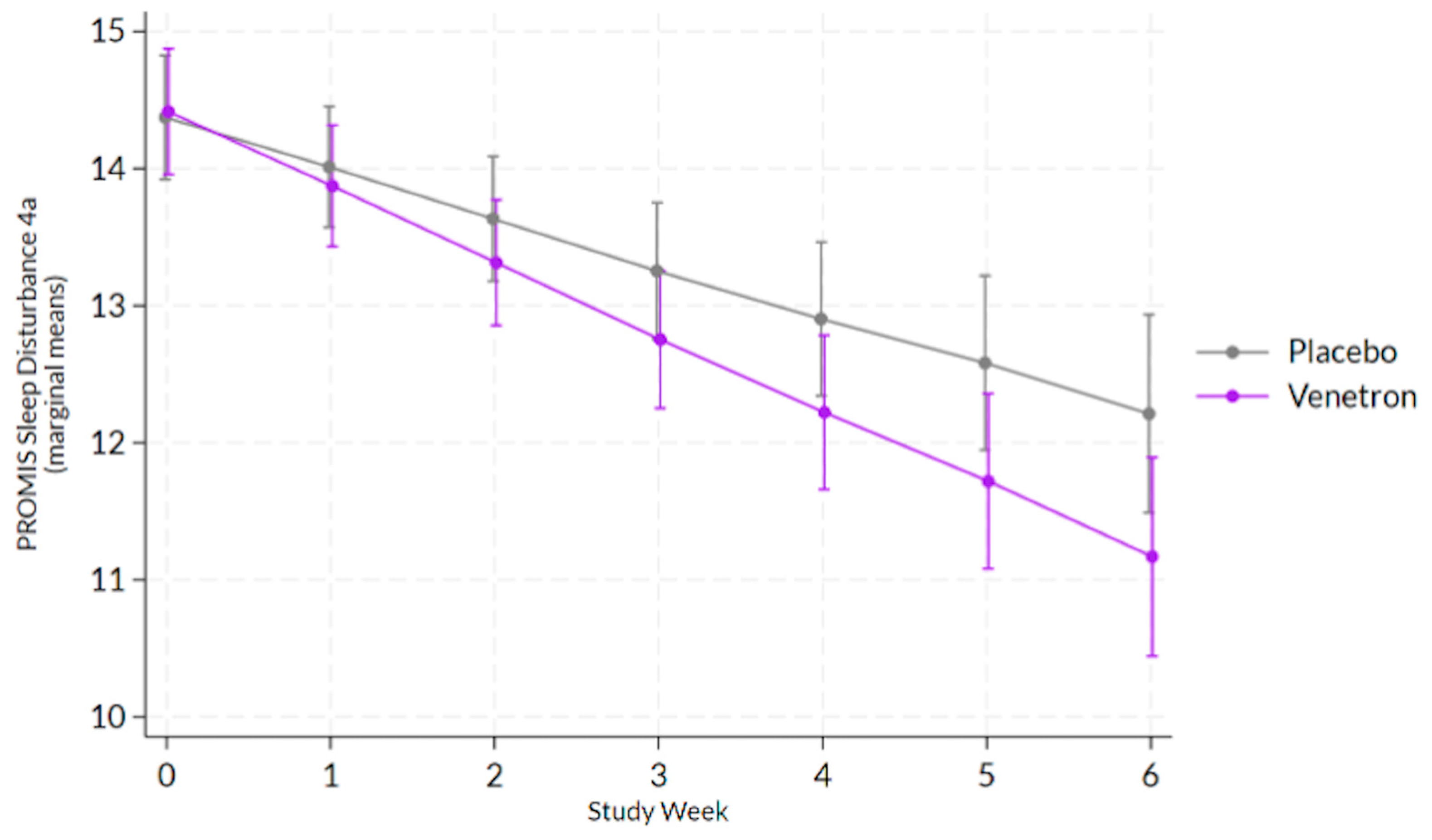
3.3.7. Sleep Disturbance
The overall model for sleep disturbance was statistically significant, Wald χ
2(13) = 135.93,
p < 0.0001, as well as the interaction between study arms and time (see
Figure 6), indicating that those in the active arm reported a greater decrease in sleep disturbance than those in the placebo arm, coefficient = −0,18, χ
2(1) = 4.91,
p = 0.0267.
3.3.8. Achievement of MCID in Sleep Disturbance
Approximately 65% of those in the active arm experience an MCID compared to 52% of those in the placebo arm. No significant difference was observed between arms in the odds of experiencing an MCID in participants’ PROMIS Sleep Disturbance 4a score, χ2(1) = 2.91, p = 0.0879.
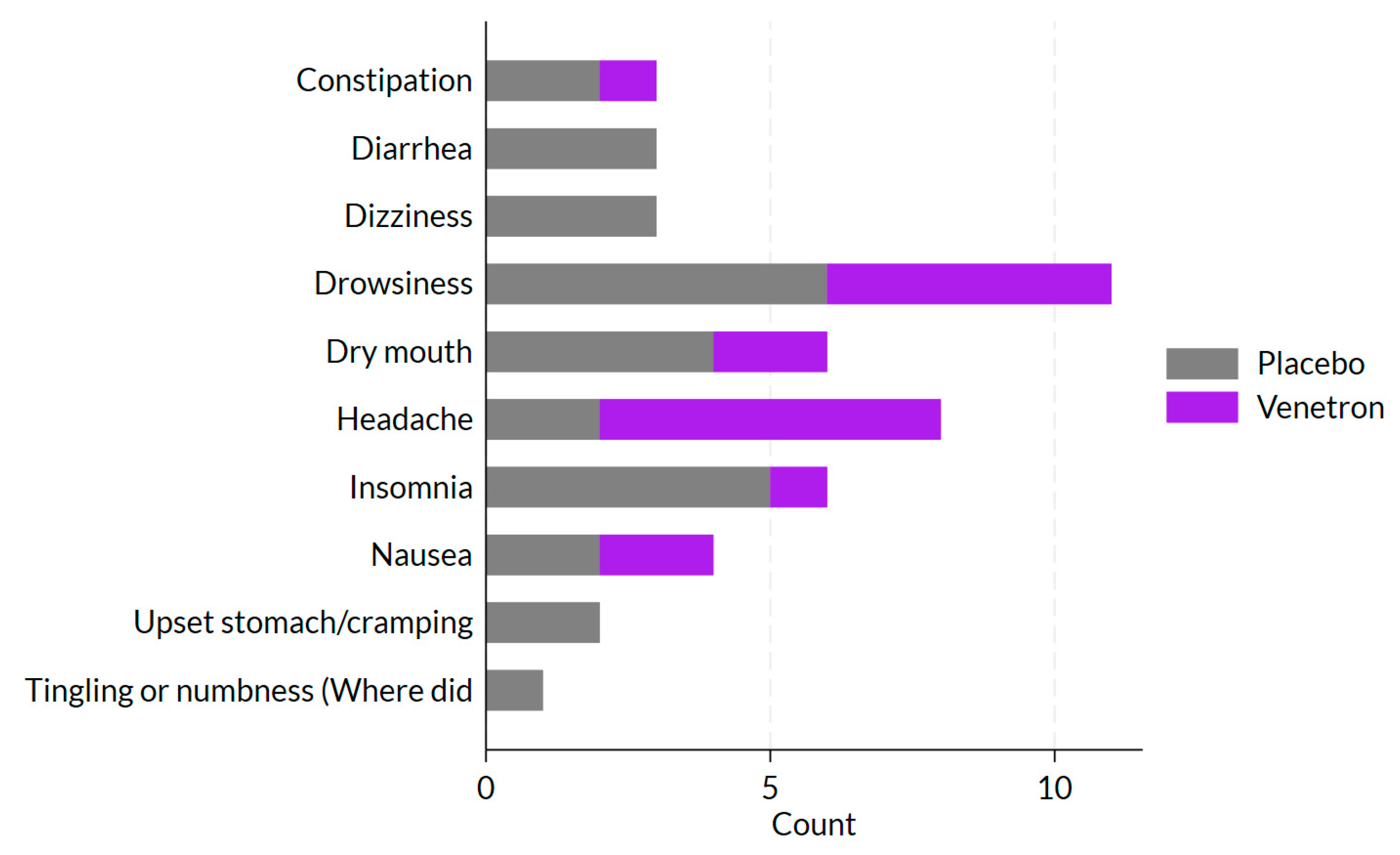
3.4. Safety
Throughout the study, approximately 10% (
n = 17) of participants in the active arm reported at least one side effect compared to 12% (
n = 22) of participants in the placebo arm (see
Figure 7). The most commonly reported side effects included drowsiness (17%;
n = 11), headache (12%;
n = 8), dry mouth (9%;
n = 6), and insomnia (9%;
n = 6). There was no significant difference between study arms in the percentage of participants who reported experiencing a side effect (
p = 0.527).
4. Discussion
In this randomized, double-blind, placebo-controlled study assessing health outcomes, participants taking A. venetum showed significant improvements in stress levels and sleep disturbances compared to the placebo group. Those taking Venetron® were also significantly more likely to experience clinically meaningful improvements in stress and showed a marginally significantly greater likelihood of clinically meaningful improvements in anxiety. However, no evidence was found to suggest that Venetron® had a beneficial effect beyond the placebo for cognitive function or depression. Venetron® was well tolerated, with no severe side effects reported.
A. venetum has a long history of use in traditional Chinese medicine for various purposes, including its antidepressant and anxiolytic effects [
4]. However, human research remains limited, and the mechanisms underlying these effects are not fully understood. Preclinical studies using rodent models suggest that
A. venetum’s anxiolytic effects may be mediated via the GABAergic system and can be antagonized by a benzodiazepine antagonist [
17]. Additionally, disruptions in sleep patterns are often linked to anxiety [
25], and it is plausible that reductions in anxiety contributed to improvements in stress and sleep, even in the absence of significant anxiety reduction. Preclinical research also indicates that
A. venetum extracts may regulate brain monoamine levels [
26], offering another potential mechanism for its effects on stress and sleep [
27].
This study aimed to assess the “real-world” effectiveness of the study product by administering it to a broad population in a manner and setting that is reflective of actual consumer use. Traditional clinical trials often employ restrictive eligibility criteria and rigorous in-person monitoring, creating a tightly controlled environment and method of use that is not reflective of actual usage by consumers. Traditional trials also frequently suffer from limited sample sizes and reduced external validity, as participants’ characteristics and behaviors may not accurately reflect those of real-world users. Our approach does not employ such a restrictive environment and inherently involves higher levels of missing data and greater heterogeneity. However, our intent-to-treat analysis utilizes linear-mixed regression models that account for missing data. Our approach is intended to better capture the real-world effects of such products and offer unique value in informing regulatory and clinical decision-making and guiding the design of future clinical trials [
26,
28].
This study has several notable limitations. First, approximately 22% of participants did not complete follow-up assessments. Nevertheless, the overall attrition rate was below the anticipated threshold of 50%, and the study maintained adequate statistical power to detect significant changes in anxiety outcomes. Conducted entirely in the USA and aligning with the body mass index (BMI) of the US adult population [
29], the sample population was predominantly overweight or obese, which aligns with findings from previous meta-analyses indicating a higher prevalence of anxiety in these groups, thereby rendering the sample composition unsurprising [
30]. Lastly, while participants self-reported being in good health, no formal screening for diagnosed medical conditions was conducted. This approach was designed to represent a real-world consumer demographic likely to seek anxiety-related interventions, enhancing the study’s external validity.