Concentration-Dependent Pleiotropic Effects of Thymosin Beta4 and Cofilin on the Migratory Activity of Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines, Cell Culture, and Transfection Vectors
2.3. Cell Migration Assays and Immunofluorescence Microscopy
2.4. Protein Preparations and Immunoblotting
2.5. Determination of the Intracellular Beta-Thymosin Content by Reverse-Phase High-Performance Liquid Chromatography (HPLC) After Equilibrium Centrifugation
3. Results
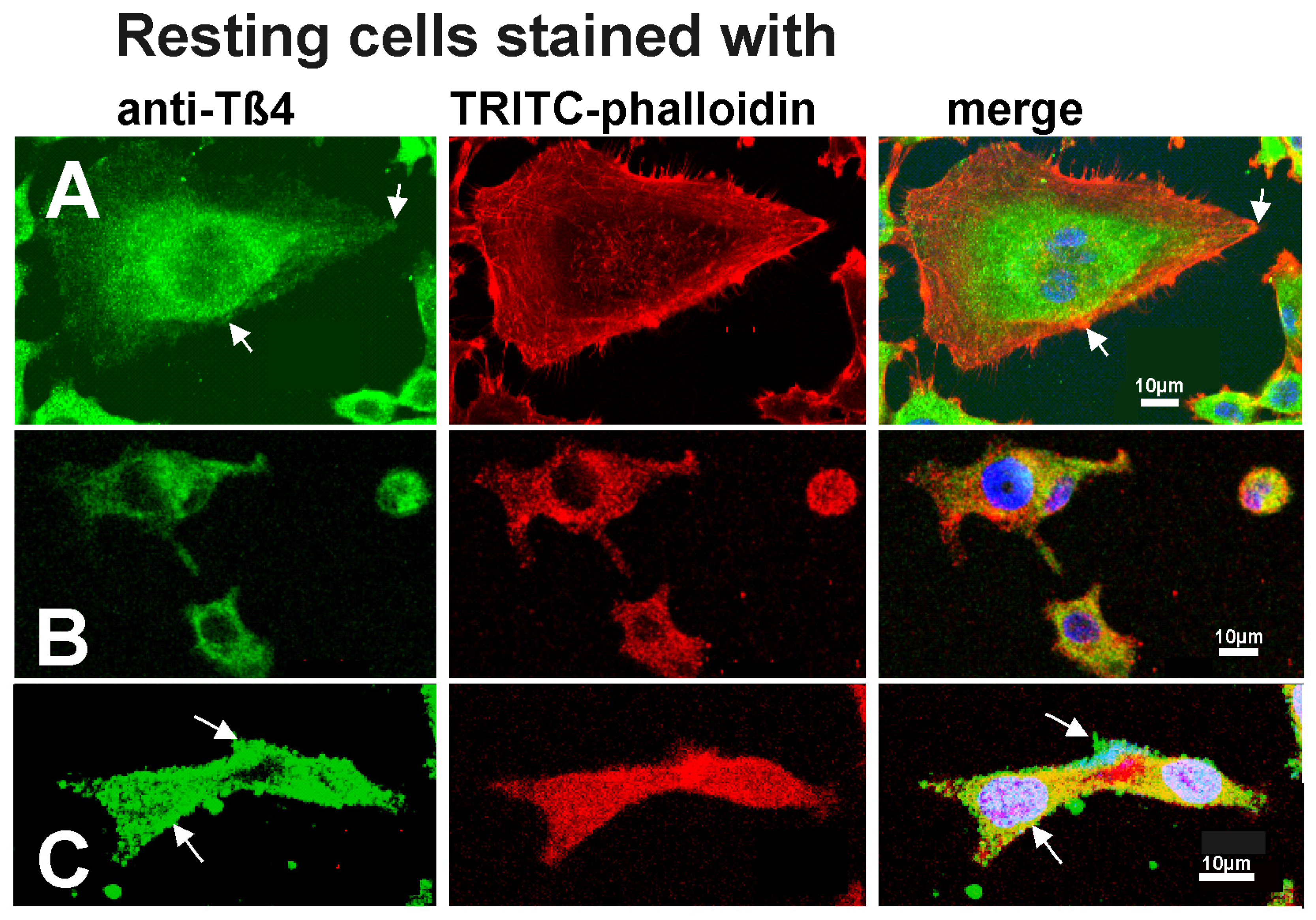
3.1. The Distribution of Tß4 and Organization of the Actin Cytoskeleton in Tumor Cell Lines
3.2. Effect of Cofilin Overexpression on Tumor Cell Migration
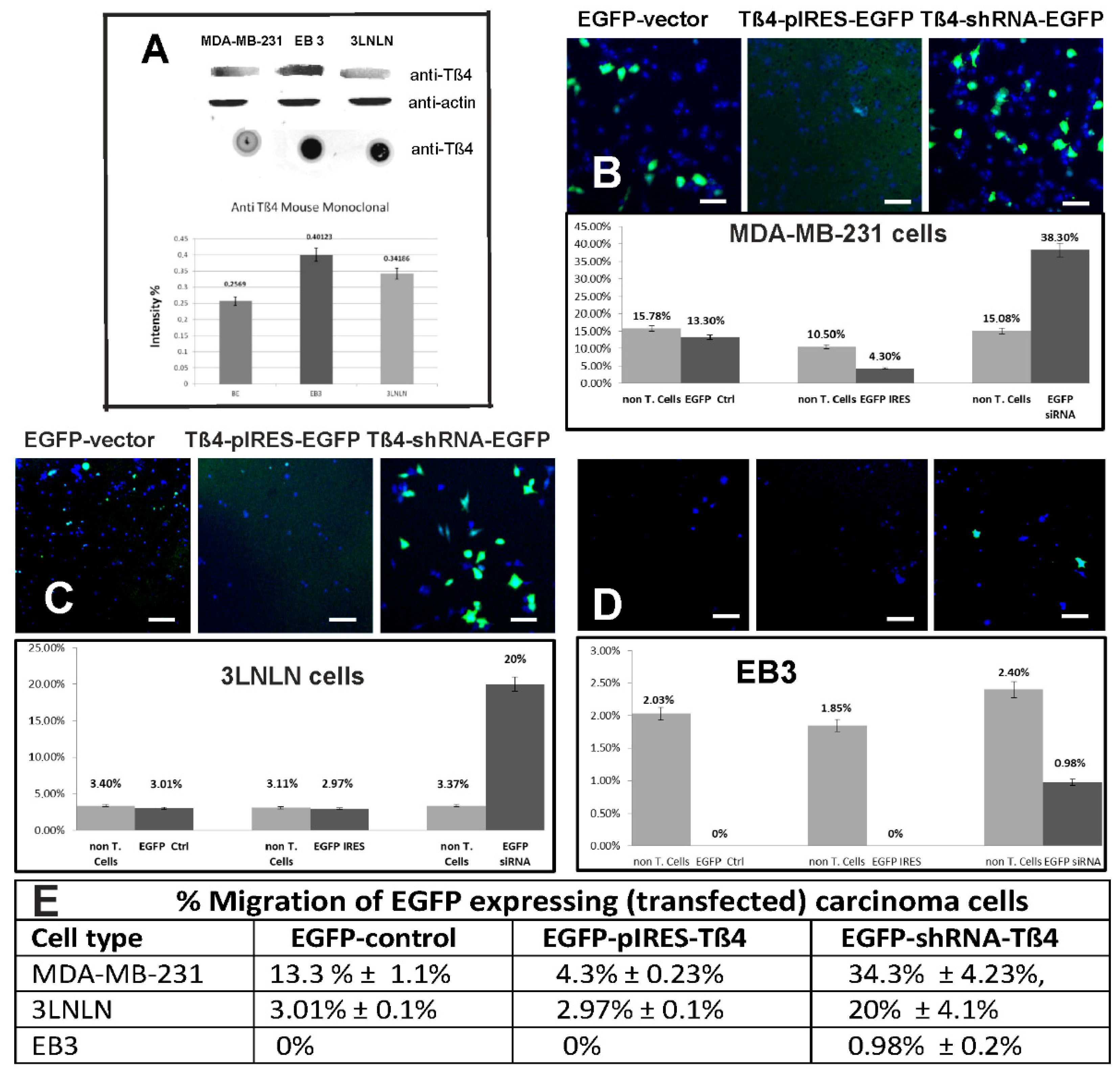
3.3. Effect of Modulating the Intracellular Tß4 Concentration on Tumor Cell Migration Analyzed by the Transwell Assay
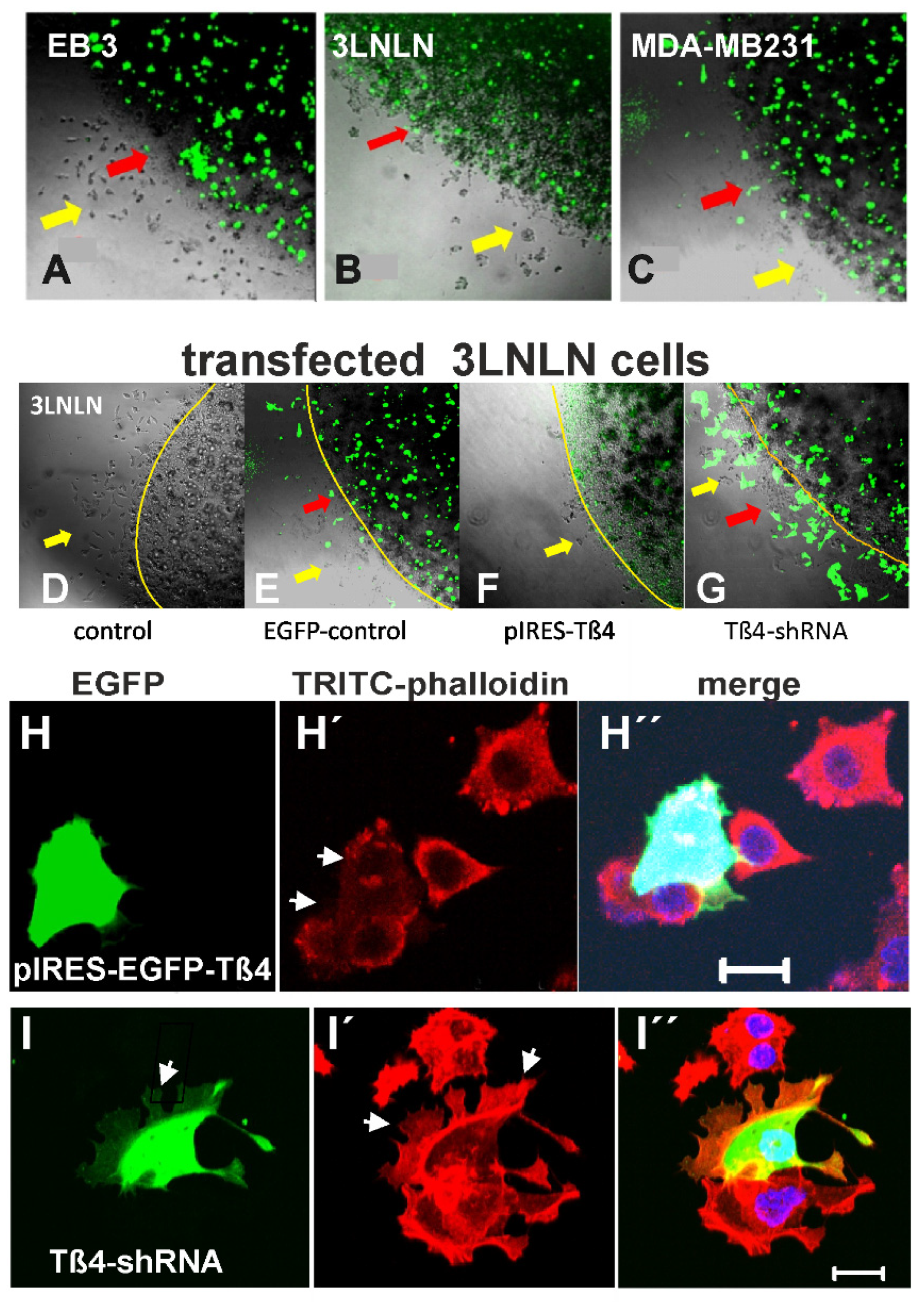
3.4. Cell Migration Analyzed by the Agarose Drop Assay
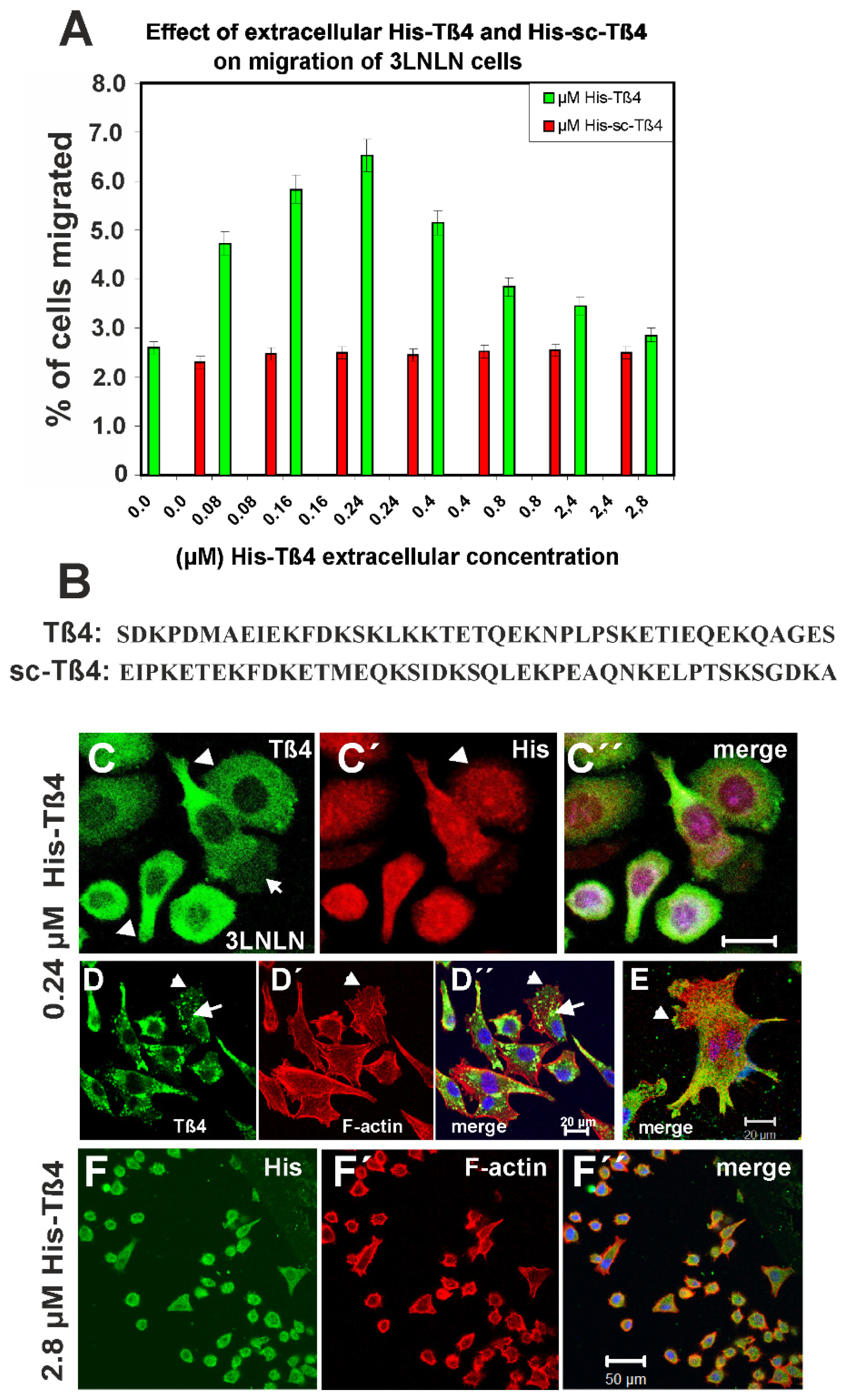
3.5. Influence of Extracellular Tß4 on Tumor Cell Migration
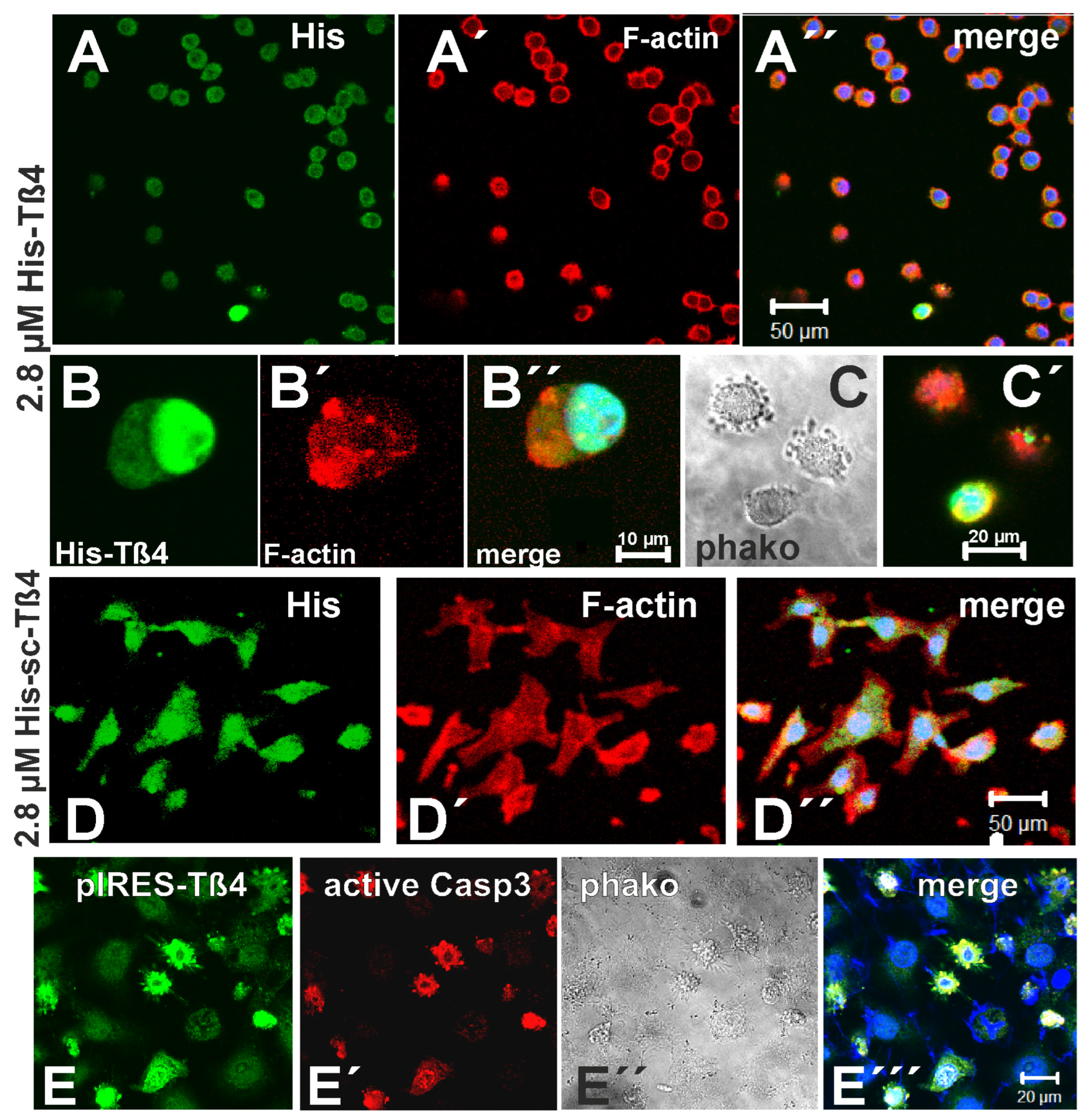
3.6. Effect of Exposure to 2.8 µM Tß4 and of Overexpressing EGFP-Tß4 on 3LNLN Cells
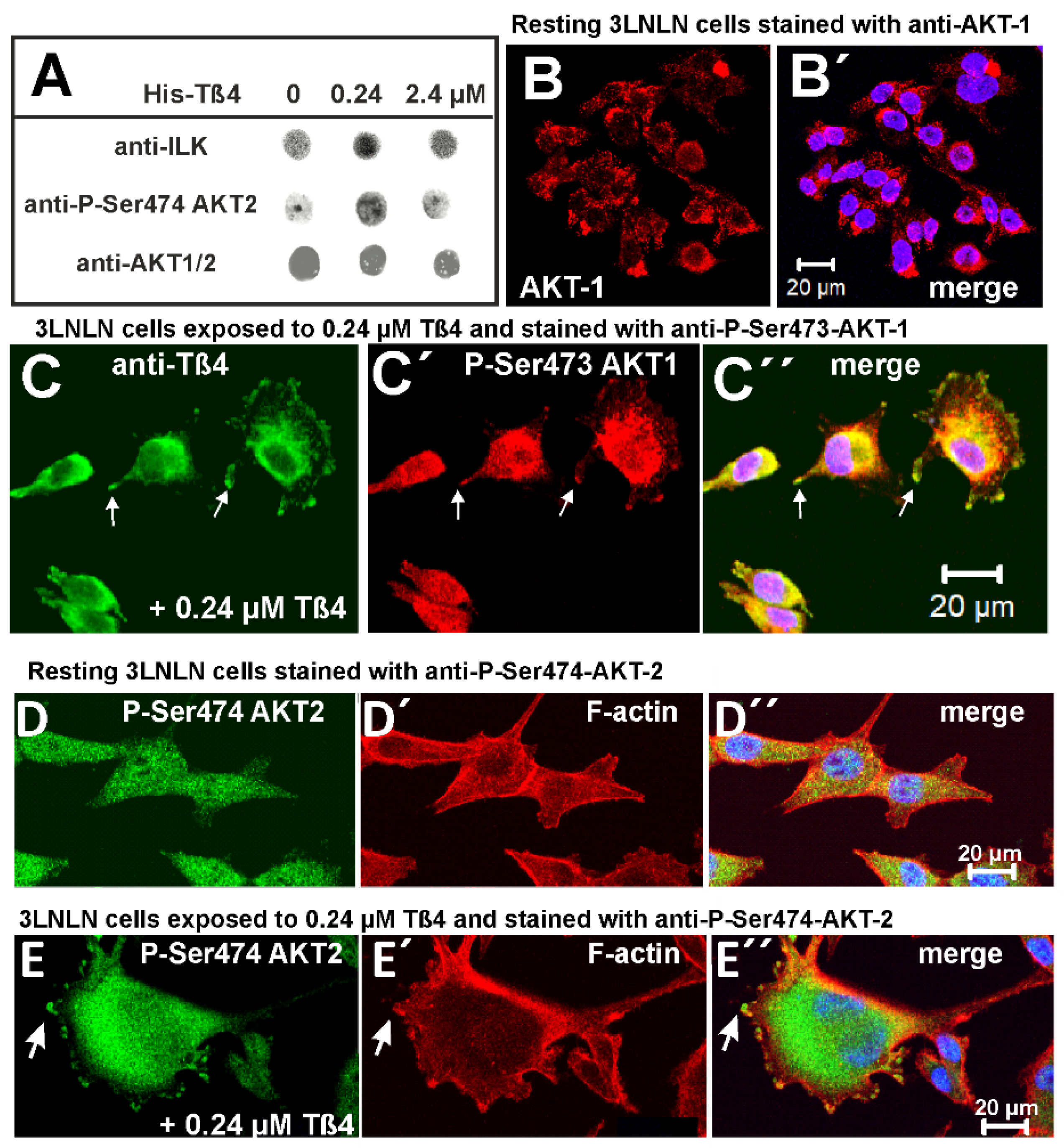
3.7. Co-Localization of Tß4 with ILK in the Presence of Extracellular Tß4
3.8. Extracellular Tß4 Increases the Phosphorylation/Activation of AKT/PKB Proteins
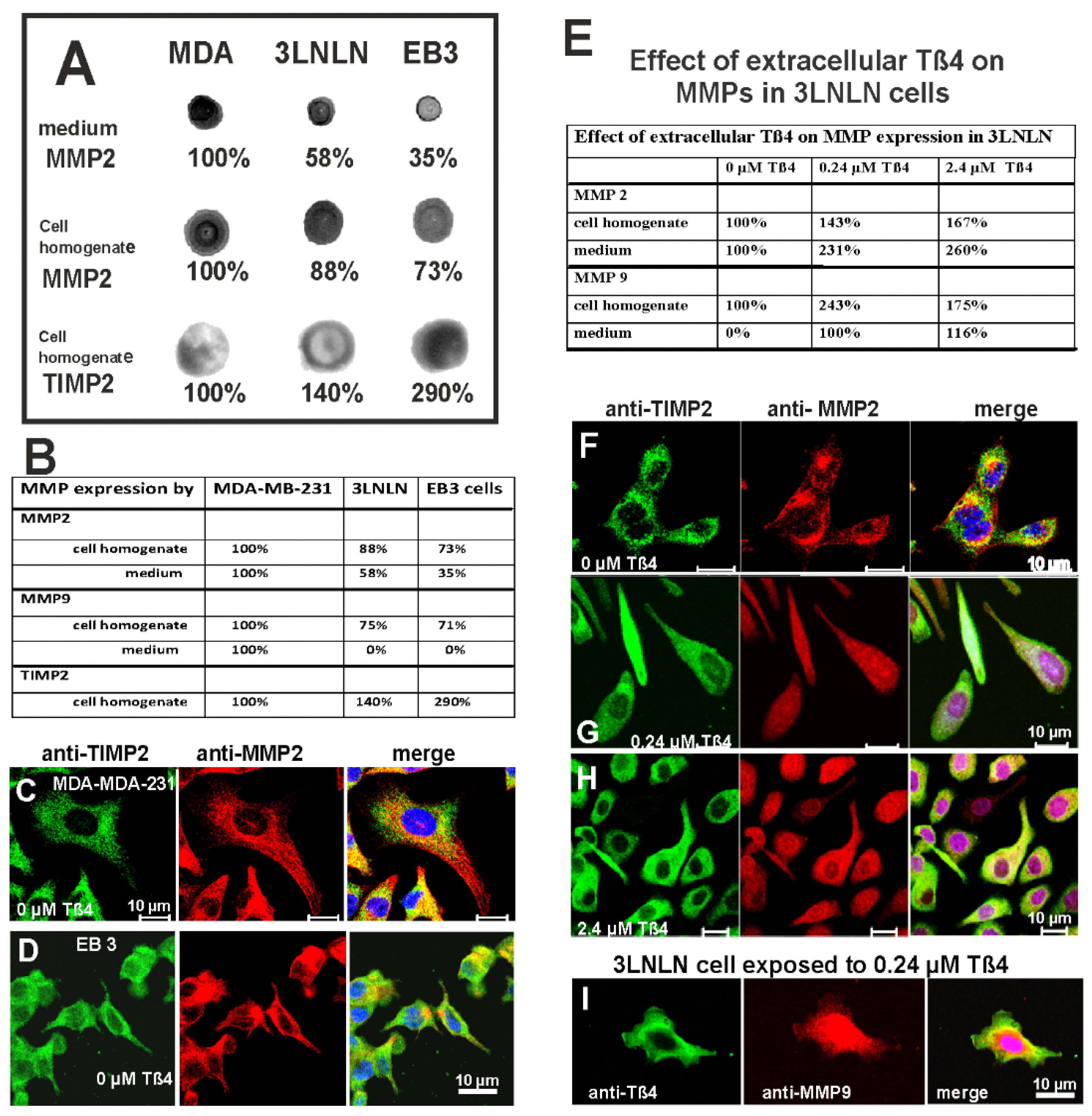
3.9. Expression of Matrix-Metalloproteinases in Non-Stimulated Carcinoma Cells
3.10. Exposure to Extracellular Tß4 Increases MMP Expression by the Carcinoma Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | actin-binding protein |
| ADF | actin-depolymerizing factor |
| AKT | protein kinase B |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| F-actin | filamentous actin |
| FCS | fetal calf serum |
| FITC | fluorescein isothiocyanate |
| G-actin | monomeric/globular actin |
| HEPES | 4-(hydroxyethyl)-1-piperazineethanesulfonic acid |
| ILK | integrin-linked kinase |
| shRNA | small hairpin RNA |
| Tß4 | thymosin beta4 |
References
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fife, C.M.; McCarroll, J.A.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2013, 171, 5507–5523. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoenenberger, C.A.; Bischler, N.; Fahrenkrog, B.; Aebi, U. Actin’s propensity for dynamic filament patterning. FEBS Lett. 2002, 529, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Safer, D.; Elzinga, M.; Nachmias, V.T. Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J. Biol. Chem. 1991, 266, 4029–4032. [Google Scholar] [CrossRef] [PubMed]
- Cassimeris, L.; Safer, D.; Nachmias, V.T.; Zigmond, S.H. Thymosin beta 4 sequesters the majority of G-actin in resting human polymorphonuclear leukocytes. J. Cell Biol. 1992, 119, 1261–1270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mannherz, H.G.; Hannappel, E. The ß-thymosins: Intracellular and extracellular activities of a versatile actin binding protein family. Cell Motil. Cytoskelet. 2009, 66, 839–851. [Google Scholar] [CrossRef]
- Bamburg, J.R. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999, 15, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Robinson, R.C. Guardians of the actin monomer. Eur. J. Cell Biol. 2013, 92, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Bravo-Cordero, J.J.; Magalhaes, M.A.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef]
- Lai, F.P.L.; Szczodrak, M.; Block, J.; Faix, J.; Breitsprecher, D.; Mannherz, H.G.; Stradal, T.E.B.; Dunn, G.A.; Small, J.V.; Rottner, K. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008, 27, 982–992. [Google Scholar] [CrossRef]
- Linder, S.; Cervero, P.; Eddy, R.; Condeelis, J. Mechanisms and roles of podosomes and invadopodia. Nat. Rev. Mol. Cell Biol. 2023, 24, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Simiczyjew, A.; Mazur, A.J.; Ampe, C.; Malicka-Błaszkiewicz, M.; van Troys, M.; Nowak, D. Active invadopodia of mesenchymally migrating cancer cells contain both β and γ cytoplasmic actin isoforms. Exp. Cell Res. 2016, 339, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Bröcker, E.B. T cell migration in three-dimensional extracellular matrix: Guidance by polarity and sensations. Dev. Immunol. 2000, 7, 249–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wegner, A. Head to tail polymerization of actin. J. Mol. Biol. 1976, 108, 139–150. [Google Scholar] [CrossRef]
- Carlier, M.F.; Pernier, J.; Montaville, P.; Shekhar, S.; Kühn, S. Control of polarized assembly of actin filaments in cell motility. Cell. Mol. Life Sci. 2015, 72, 3051–3067. [Google Scholar] [CrossRef]
- dos Remedios, C.G.; Chhabra, D.; Kekic, M.; Dedova, I.V.; Tsubakihara, M.; Berry, D.A.; Nosworthy, N.J. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol. Rev. 2003, 83, 433–473. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.F.; Laurent, V.; Santolini, J.; Melki, R.; Didry, D.; Xia, G.-X.; Hong, Y.; Chua, N.-H.; Pantaloni, D. Actin filament depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: Implication in actin-based motility. J. Cell Biol. 1997, 136, 1307–1323. [Google Scholar] [CrossRef]
- Andrianantoandro, E.; Pollard, T.D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 2006, 24, 13–23. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, J.; Condeelis, J.; Glogauer, M. A common cofilin activity cycle in invasive tumor cells and inflammatory cells. J. Cell Sci. 2009, 122, 305–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tania, N.; Prosk, E.; Condeelis, J.; Edelstein-Keshet, L. A temporal model of cofilin regulation and the early peak of actin barbed ends in invasive tumor cells. Biophys. J. 2011, 100, 1883–1892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarbock, J.; Oschkinat, H.; Hannappel, E.; Kalbacher, H.; Voelter, W.; Holak, T.A. Solution conformation of thymosin beta 4: A nuclear magnetic resonance and simulated annealing study. Biochemistry 1990, 29, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Irobi, E.; Aguda, A.H.; Larsson, M.; Guerin, C.; Yin, H.L.; Burtnick, L.D.; Blanchoin, L.; Robinson, R.C. Structural basis of actin sequestration by thymosin-beta4: Implications for WH2 proteins. EMBO J. 2004, 23, 3599–3608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mannherz, H.G.; Mazur, A.; Jockusch, B.M. Repolymerization of Actin from Actin:Thymosin ß4 complex induced by Diaphanous related formins and Gelsolin. Ann. N. Y. Acad. Sci. 2010, 1194, 36–43. [Google Scholar] [CrossRef]
- Schönichen, A.; Geyer, M. Fifteen formins for an actin filament: A molecular view on the regulation of human formins. Biochim. Biophys. Acta. 2010, 1803, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Al Haj, A.; Mazur, A.J.; Buchmeier, S.; App, C.; Theiss, C.; Silvan, U.; Schoenenberger, C.A.; Jockusch, B.M.; Hannappel, E.; Weeds, A.G.; et al. Thymosin beta4 inhibits ADF/cofilin stimulated F-actin cycling and HeLa cell migration: Reversal by active Arp2/3 complex. Cytoskeleton 2014, 71, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Jeong, M.J.; Kleinman, H.K. Role of thymosin beta4 in tumor metastasis andangiogenesis. J. Natl. Cancer Inst. 2003, 95, 1674–1680. [Google Scholar] [CrossRef]
- Yamamoto, T.; Gotoh, M.; Kitajima, M.; Hirohashi, S. Thymosin beta-4 expression is correlated with metastatic capacity of colorectal carcinomas. Biochem. Biophys. Res. Commun. 1993, 193, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Mollinari, C.; di Martino, S.; Biffoni, M.; Pilozzi, E.; Pagliuca, A.; de Stefano, M.C.; Circo, R.; Merlo, D.; De Maria, R.; et al. Thymosin beta4 targeting impairs activity of colon cancer stem cells. FASEB J. 2010, 24, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Popow-Wozniak, A.; Mazur, A.J.; Mannherz, H.G.; Malicka-Blaszkiewicz, M.; Nowak, D. Cofilin overexpression affects actin cytoskeleton organization and migration of human colon adenocarcinoma cells. Histochem. Cell Biol. 2012, 138, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Califano, D.; Monaco, C.; Santelli, G.; Giuliano, A.; Veronese, M.L.; Berlingieri, M.T.; de Franciscis, V.; Berger, N.; Trapasso, F.; Santoro, M.; et al. Thymosin beta-10 gene overexpression correlated with the highly malignant neoplastic phenotype of transformed thyroid cells in vivo and in vitro. Cancer Res. 1998, 58, 823–828. [Google Scholar] [PubMed]
- Wang, B.; Wang, Z.; Zhang, T.; Yang, G. Overexpression of thymosin β10 correlates with disease progression and poor prognosis in bladder cancer. Exp. Ther. Med. 2019, 18, 3759–3766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, F.X.; Lin, S.C.; Morrison-Bogorad, M.; Atkinson, M.A.; Yin, H.L. Thymosin beta 10 and thymosin beta 4 are both actin monomer sequestering proteins. J. Biol. Chem. 1993, 268, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Lin, S.C.; Morrison-Bogorad, M.; Yin, H.L. Effects of thymosin beta 4 and thymosin beta 10 on actin structures in living cells. Cell Motil. Cytoskelet. 1994, 27, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bock-Marquette, I.; Saxena, A.; White, M.D.; Dimaio, J.M.; Srivastava, D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004, 432, 466–472. [Google Scholar] [CrossRef]
- Fan, Y.; Gong, Y.; Ghosh, P.K.; Graham, L.M.; Fox, P.L. Spatial coordination of actin polymerization and ILK-Akt2 activity during endothelial cell migration. Dev. Cell. 2009, 16, 661–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix metalloproteinases shape the tumor microenviron-ment in cancer progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mannherz, H.G.; Gonsior, S.M.; Gremm, D.; Wu, X.; Pope, B.J.; Weeds, A.G. Activated cofilin colocalizes with Arp2/3 complex in apoptotic blebs during programmed cell death. Eur. J. Cell Biol. 2005, 84, 503–515. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Kretz, O.; Morosan-Puopolo, G.; Chernogorova, P.; Theiss, C.; Brand-Saberi, B. Thymosin ß4 induces folding of the developing optic tectum in the chicken (Gallusdomesticus). J. Comp. Neurol. 2011, 520, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Yusuf, F.; Farjah, G.H.; Brand-Saberi, B. RNAi-induced targeted silencing of developmental control genes during chicken embryogenesis. Dev. Biol. 2005, 285, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Krawczenko, A.; Duoe, D.; Malicka-Blaszkiewicz, M. Actin in human colonadenocarcinoma cells with different metastatic potential. Acta Biochim Pol. 2002, 49, 823–828. [Google Scholar] [CrossRef]
- Harrington, J.T., Jr.; Stastny, P. Macrophage migration from an agarose droplet: Development of a micromethod for assay of delayed hypersensitivity. J. Immunol. 1973, 110, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Low, T.L.; Lin, C.Y.; Pan, T.L.; Chiou, A.J.; Tsugita, A. Structure and immunological properties of thymosin beta 9 Met, a new analog of thymosin beta 4 isolated from porcine thymus. Int. J. Pept. Protein Res. 1990, 36, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Röder, B. Biochemische Und Zellbiologische Untersuchungen Des Intrinsisch Unstrukturierten Proteins Thymosin Beta 4. Ph.D. Thesis, Ruhr-University, Bochum, Germany, 2012. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hannappel, E.; van Kampen, M. Determination of thymosin beta 4 in human blood cells and serum. J. Chromatogr. 1987, 397, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, M.; Olivero, P.; Torres, R.; Riveros, A.; Quest, A.F.; Stutzin, A. Cell volume regulation in response to hypotonicity is impaired in HeLa cells expressing a protein kinase-C alpha mutant lacking kinase activity. J. Biol. Chem. 2004, 279, 17681–17689. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol. Rep. 2010, 2, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Low, T.L.; Goldstein, A.L. Thymosins: Structure, function and therapeutic applications. Thymus 1984, 6, 27–42. [Google Scholar] [PubMed]
- Aoki, K.; Satoi, S.; Harada, S.; Uchida, S.; Iwasa, Y.; Ikenouchi, J. Coordinated changes in cell membrane and cytoplasm during maturation of apoptotic bleb. Mol. Biol. Cell. 2020, 31, 833–844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piao, Z.; Hong, C.S.; Jung, M.R.; Choi, C.; Park, Y.K. Thymosin β4 induces invasion and migration of human colorectal cancer cells through the ILK/AKT/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2014, 452, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NF-kappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarmola, E.G.; Parikh, S.; Bubb, M.R. Formation and implications of a ternary complex of profilin, thymosin beta 4, and actin. J. Biol. Chem. 2001, 276, 45555–45563. [Google Scholar] [CrossRef] [PubMed]
- Ebner, M.; Lučić, I.; Leonard, T.A.; Yudushkin, I. PI(3,4,5)P3 Engagement Restricts Akt Activity to Cellular Membranes. Mol Cell. 2017, 65, 416–431.e6. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.; Koh, H.; Yoon, S.O.; Chung, A.S.; Cho, K.S.; Chung, J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001, 11, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Hey, S.; Linder, S. Matrix metalloproteinases at a glance. J. Cell Sci. 2024, 137, jcs261898. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambert, A.W.; Zhang, Y.; Weinberg, R.A. Cell-intrinsic and microenvironmental determinants of metastatic colonization. Nat. Cell Biol. 2024, 26, 687–697. [Google Scholar] [CrossRef]
- Hall, A.K. Thymosin beta-10 accelerates apoptosis. Cell Mol. Biol. Res. 1995, 41, 167–180. [Google Scholar] [PubMed]
- Huff, T.; Rosorius, O.; Otto, A.M.; Müller, C.S.; Ballweber, E.; Hannappel, E.; Mannherz, H.G. Nuclear localization of the G-actin sequestering peptide thymosin beta4. J. Cell Sci. 2004, 117, 5333–5341. [Google Scholar] [CrossRef] [PubMed]
- Renault, L. Intrinsic, Functional, and Structural Properties of β-Thymosins and β-Thymosin/WH2 Domains in the Regulation and Coordination of Actin Self-Assembly Dynamics and Cytoskeleton Remodeling. Vitam. Horm. 2016, 102, 25–54. [Google Scholar] [PubMed]
- Mannherz, H.G.; Budde, H.; Jarkas, M.; Hassoun, R.; Malek-Chudzik, N.; Mazur, A.J.; Skuljec, J.; Pul, R.; Napirei, M.; Hamdani, N. Reorganization of the actin cytoskeleton during the formation of neutrophil extracellular traps (NETs). Eur. J. Cell Biol. 2024, 103, 151407. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Chowdhury, S.; Billah, M.M.; Islam, K.M.D.; Thorlacius, H.; Rahman, M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int. J. Mol. Sci. 2021, 22, 7260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mousset, A.; Bellone, L.; Gaggioli, C.; Albrengues, J. NETscape or NEThance: Tailoring anti-cancer therapy. Trends Cancer 2024, 10, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.; Müller, C.S.; Otto, A.M.; Netzker, R.; Hannappel, E. β-Thymosins, small acidic peptides with multiple functions. Int. J. Biochem. Cell Biol. 2001, 33, 205–220. [Google Scholar] [CrossRef]
- Pope, B.J.; Gonsior, S.M.; Yeoh, S.; McGough, A.; Weeds, A.G. Uncoupling actin filament fragmentation by cofilin from increased subunit turnover. J. Mol. Biol. 2000, 298, 649–661. [Google Scholar] [CrossRef]

| EB3 | 3LNLN | MDA-MB-231 | Cell Type |
|---|---|---|---|
| 0.466 µM | 0.33 µM | 0.306 µM | Intracellular Tß4 |
| 0.66 µM | 0.62 µM | 0.353 µM | Intracellular Tß10 |
| 1.126 µM | 0.95 µM | 0.659 µM | Total ß-thymosins |
| 2.4 µM His-Tß4 | 0.24 µM His-Tß4 | Control | |
|---|---|---|---|
| 107% | 141% | 100% | anti-ILK |
| 121% | 208% | 100% | anti-P-Ser474-AKT2 |
| 72% | 85% | 100% | anti-AKT1,2 |
| 99% | 97% | 100% | anti-actin |
| 98% | 98% | 100% | anti-tubulin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Haj, A.; Ćwikłowska, K.; Mazur, A.J.; Brand-Saberi, B.; Hannappel, E.; Mannherz, H.G. Concentration-Dependent Pleiotropic Effects of Thymosin Beta4 and Cofilin on the Migratory Activity of Carcinoma Cells. Int. J. Transl. Med. 2025, 5, 16. https://doi.org/10.3390/ijtm5020016
Al Haj A, Ćwikłowska K, Mazur AJ, Brand-Saberi B, Hannappel E, Mannherz HG. Concentration-Dependent Pleiotropic Effects of Thymosin Beta4 and Cofilin on the Migratory Activity of Carcinoma Cells. International Journal of Translational Medicine. 2025; 5(2):16. https://doi.org/10.3390/ijtm5020016
Chicago/Turabian StyleAl Haj, Abdulatif, Kamila Ćwikłowska, Antonina Joanna Mazur, Beate Brand-Saberi, Ewald Hannappel, and Hans Georg Mannherz. 2025. "Concentration-Dependent Pleiotropic Effects of Thymosin Beta4 and Cofilin on the Migratory Activity of Carcinoma Cells" International Journal of Translational Medicine 5, no. 2: 16. https://doi.org/10.3390/ijtm5020016
APA StyleAl Haj, A., Ćwikłowska, K., Mazur, A. J., Brand-Saberi, B., Hannappel, E., & Mannherz, H. G. (2025). Concentration-Dependent Pleiotropic Effects of Thymosin Beta4 and Cofilin on the Migratory Activity of Carcinoma Cells. International Journal of Translational Medicine, 5(2), 16. https://doi.org/10.3390/ijtm5020016







