Clinical Course of Parotid Carcinoma with Hepatic and Nodal Metastases: A Case Report
Abstract
1. Introduction
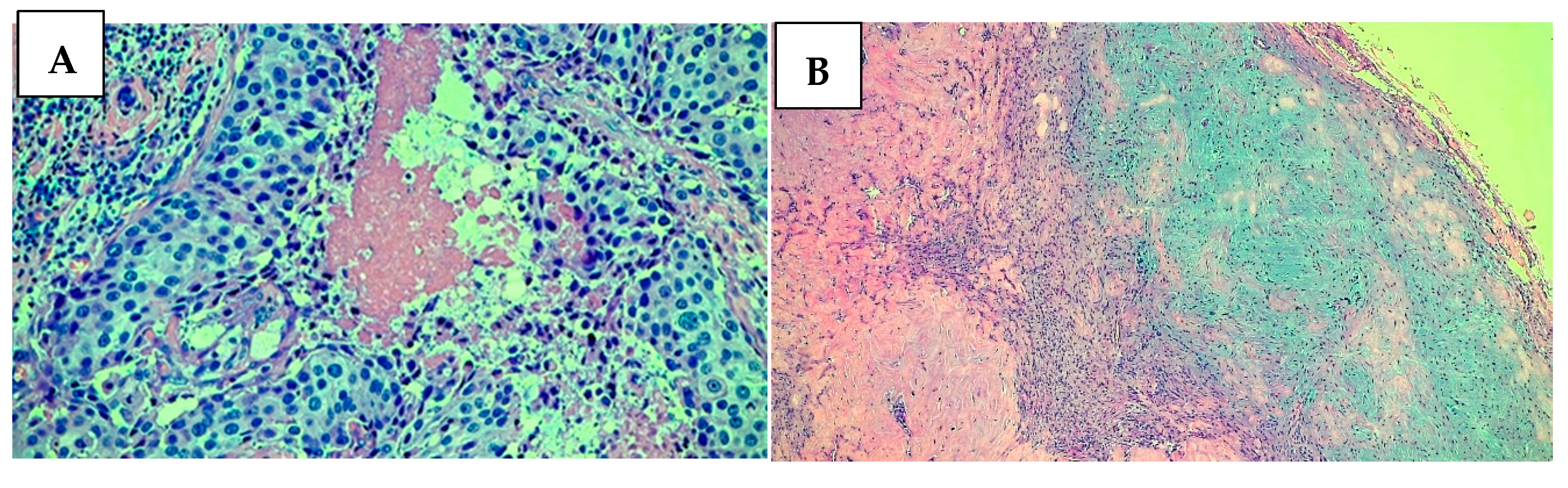
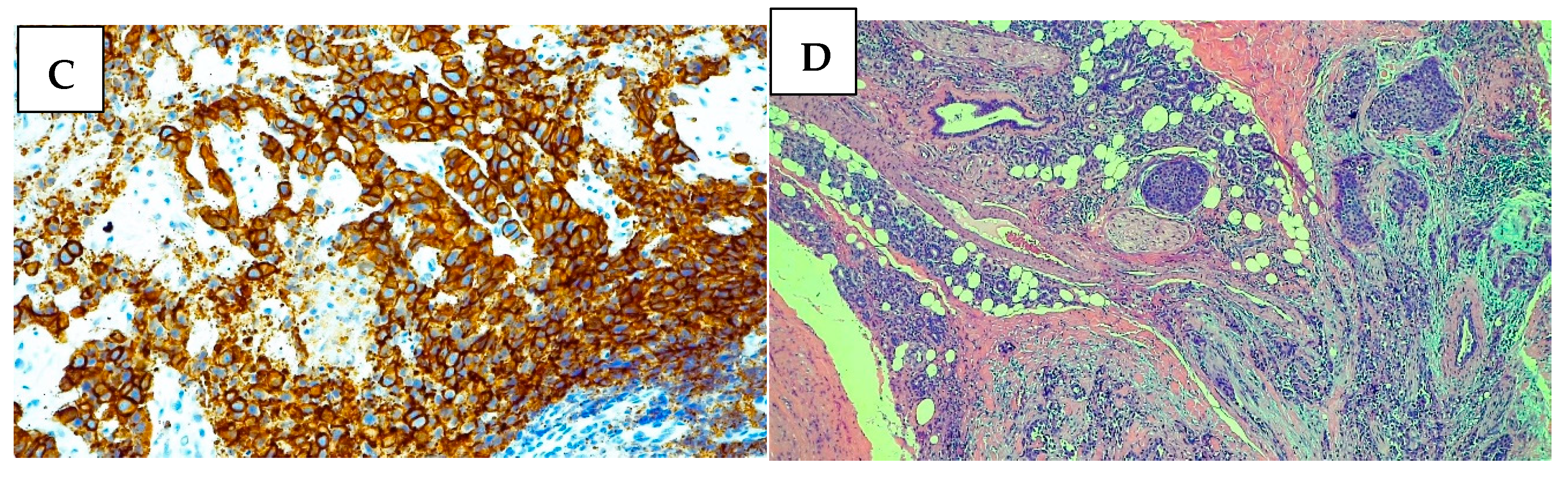
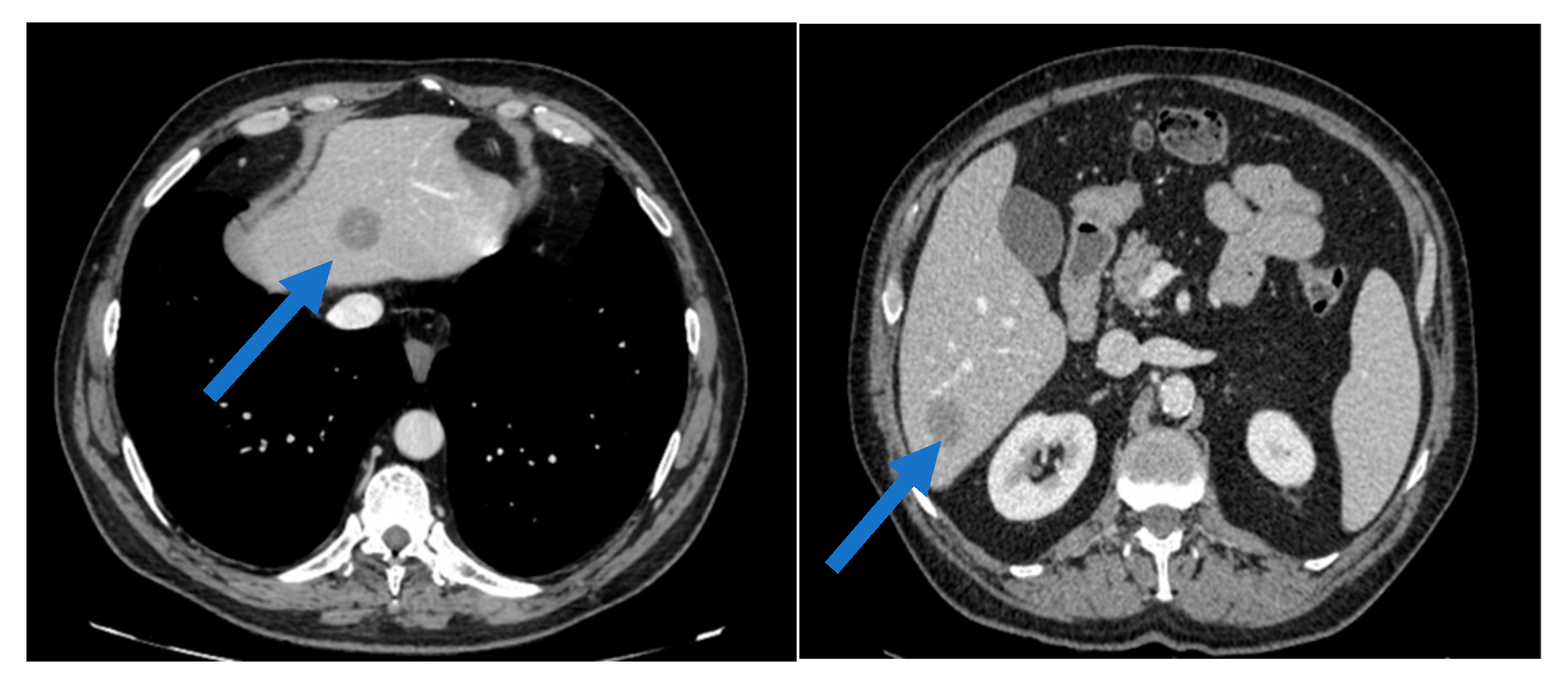
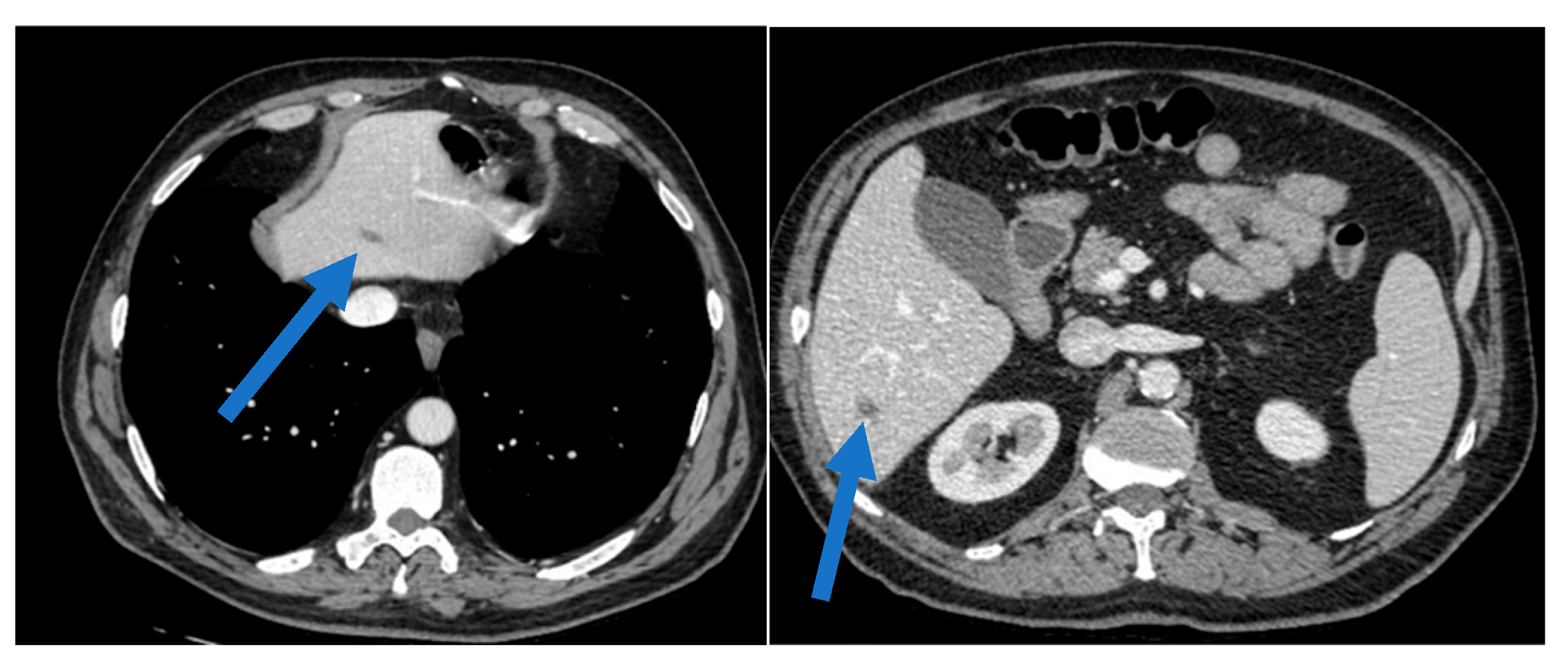
2. Case Presentation
- -
- Docetaxel: 75 mg/m2, administered intravenously every 3 weeks.
- -
- Trastuzumab: 8 mg/kg loading dose, followed by 6 mg/kg every 3 weeks, administered intravenously.
- -
- Pertuzumab: 840 mg loading dose, followed by 420 mg every 3 weeks, administered intravenously.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Herpen, C.; Vander Poorten, V.; Skalova, A.; Terhaard, C.; Maroldi, R.; van Engen, A.; Baujat, B.; Locati, L.D.; Jensen, A.D.; Smeele, L.; et al. Salivary gland cancer: ESMO–European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up†. ESMO Open 2022, 7, 100602. [Google Scholar] [CrossRef]
- Steuer, C.E.; Hanna, G.J.; Viswanathan, K.; Bates, J.E.; Kaka, A.S.; Schmitt, N.C.; Ho, A.L.; Saba, N.F. The evolving landscape of salivary gland tumors. CA Cancer J. Clin. 2023, 73, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Dalin, M.; Watson, P.; Ho, A.; Morris, L. Androgen Receptor Signaling in Salivary Gland Cancer. Cancers 2017, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II Trial of Trastuzumab and Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2–Positive Salivary Duct Carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Voora, R.S.; Panuganti, B.; Califano, J.; Coffey, C.; Guo, T. Patterns of Lymph Node Metastasis in Parotid Cancer and Implications for Extent of Neck Dissection. Otolaryngol. Neck Surg. 2023, 168, 1067–1078. [Google Scholar] [CrossRef]
- Nobis, C.P.; Rohleder, N.H.; Wolff, K.D.; Wagenpfeil, S.; Scherer, E.Q.; Kesting, M.R. Head and Neck Salivary Gland Carcinomas—Elective Neck Dissection, Yes or No? J. Oral Maxillofac. Surg. 2014, 72, 205–210. [Google Scholar] [CrossRef]
- da Silva, L.P.; Serpa, M.S.; Viveiros, S.K.; Sena, D.A.C.; Pinho, R.F.d.C.; Guimarães, L.D.d.A.; Andrade, E.S.d.S.; Pereira, J.R.D.; da Silveira, M.M.F.; Sobral, A.P.V.; et al. Salivary gland tumors in a Brazilian population: A 20-year retrospective and multicentric study of 2292 cases. J. Cranio-Maxillofac. Surg. 2018, 46, 2227–2233. [Google Scholar] [CrossRef]
- Nair, V.J.; Pantarotto, J.R. Treatment of metastatic liver tumors using stereotactic ablative radiotherapy. World J. Radiol. 2014, 6, 18–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodman, K.A.; Kavanagh, B.D. Stereotactic Body Radiotherapy for Liver Metastases. Semin. Radiat. Oncol. 2017, 27, 240–246. [Google Scholar] [CrossRef]
- Mahadevan, A.; Blanck, O.; Lanciano, R.; Peddada, A.; Sundararaman, S.; D’ambrosio, D.; Sharma, S.; Perry, D.; Kolker, J.; Davis, J. Stereotactic Body Radiotherapy (SBRT) for liver metastasis—Clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat. Oncol. 2018, 13, 26. [Google Scholar] [CrossRef]
- Kurzrock, R.; Bowles, D.W.; Kang, H.; Meric-Bernstam, F.; Hainsworth, J.; Spigel, D.R.; Bose, R.; Burris, H.; Sweeney, C.J.; Beattie, M.S.; et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: Results from MyPathway, a phase IIa multiple basket study. Ann. Oncol. 2020, 31, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef] [PubMed]
- Mounsey, L.; Corrêa, T.S.; Matos, G.D.R.; Segura, M.; dos Anjos, C.H. Second-Line Treatment of HER2-Positive Salivary Gland Tumor: Ado-Trastuzumab Emtansine (T-DM1) after Progression on Trastuzumab. Case Rep. Oncol. 2018, 11, 252–257. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Offin, M.; Buonocore, D.J.; Myers, M.L.; Venkatesh, A.; Razavi, P.; Ginsberg, M.S.; Ulaner, G.A.; Solit, D.B.; et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): Results from a phase II basket trial. JCO 2019, 37, 6001. [Google Scholar] [CrossRef]
- Kinoshita, I.; Kano, S.; Honma, Y.; Kiyota, N.; Tahara, M.; Takahashi, S.; Ito, Y.; Hatanaka, Y.; Matsuno, Y.; Dosaka-Akita, H. 849O Phase II study of trastuzumab deruxtecan in patients with HER2-positive recurrent/metastatic salivary gland cancer: Results from the MYTHOS trial. Ann. Oncol. 2024, 35, S613–S614. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, S.J.; Park, S.; Jung, H.A.; Lee, S.H.; Jeong, H.S.; Chung, M.K.; Ahn, M.J. A Single-Arm, Prospective, Phase II Study of Cisplatin Plus Weekly Docetaxel as First-Line Therapy in Patients with Metastatic or Recurrent Salivary Gland Cancer. Cancer Res. Treat. 2022, 54, 719–727. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.-Q.; Chen, F.-P.; Yan, J.-Y.; Huang, X.-D.; Li, F.; Sun, Y.; Zhou, G.-Q. Role of postoperative radiotherapy in nonmetastatic head and neck adenoid cystic carcinoma. J. Natl. Compr. Cancer Netw. 2020, 18, 1476–1484. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Jung, H.A.; Lee, S.; Seo, S.; Kim, S.; Kim, J.; Lee, K.; Kang, E.J.; Kim, J.W.; et al. A phase 2 multicenter study of docetaxel-PM and trastuzumab-pkrb combination therapy in recurrent or metastatic salivary gland carcinomas. Cancer 2023, 129, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, S.; Ntokou, A.; Drizou, M.; Perdikari, K.; Makaronis, P.; Katsarou, E.; Koufopoulos, N.; Tzovaras, A.; Ardavanis, A. Nivolumab in patients with rare head and neck carcinomas: A single center’s experience. Oral. Oncol. 2020, 101, 104359. [Google Scholar] [CrossRef]
- Honma, Y.; Monden, N.; Yamazaki, K.; Kano, S.; Satake, H.; Kadowaki, S.; Utsumi, Y.; Nakatogawa, T.; Takano, R.; Fujii, K.; et al. Apalutamide and Goserelin for Androgen Receptor–Positive Salivary Gland Carcinoma: A Phase II Nonrandomized Clinical Trial, YATAGARASU. Clin. Cancer Res. 2024, 30, 3416–3427. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; Acosta, A.D.J.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
| Study/Authors | Drug | Year | Patients | Outcome | PFS | Purpose |
|---|---|---|---|---|---|---|
| Li et al. [14] | Ado-trastuzumab emtansine | 2021 | 10 | ORR 90%, SD IN 4/9 pts | 9 months | Metastatic |
| Takahashi et al. [4] | Docetaxel+perutuzmab+trastuzumab | 2020 | 5 | Tumor reduction in all patients | 12 months | neoadjuvant |
| Ahn et al. [16] | Cisplatin+weekly docetaxel | 2022 | 8 | 50% ORR, with 3 achieving CR | 10 months | Metastatic |
| Chen et al. [17] | Radiotherapy | 2023 | 6 | Improved symptoms and tumor size reduction | 8 months | Adjuvant |
| Lee et al. [18] | Docetaxel-PM+trastuzumab-pkrb | 2021 | 12 | ORR of 75%, 6 patients with PR | 14 months | Metastatic |
| Kakkali et al. [19] | NIvolumab | 2020 | 6 | Prolonged disease control | 11 months | metastatic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doronzo, A.; Musci, G.; Gadaleta-Caldarola, G.; Sergi, M.C. Clinical Course of Parotid Carcinoma with Hepatic and Nodal Metastases: A Case Report. Int. J. Transl. Med. 2025, 5, 3. https://doi.org/10.3390/ijtm5010003
Doronzo A, Musci G, Gadaleta-Caldarola G, Sergi MC. Clinical Course of Parotid Carcinoma with Hepatic and Nodal Metastases: A Case Report. International Journal of Translational Medicine. 2025; 5(1):3. https://doi.org/10.3390/ijtm5010003
Chicago/Turabian StyleDoronzo, Antonio, Giovanni Musci, Gennaro Gadaleta-Caldarola, and Maria Chiara Sergi. 2025. "Clinical Course of Parotid Carcinoma with Hepatic and Nodal Metastases: A Case Report" International Journal of Translational Medicine 5, no. 1: 3. https://doi.org/10.3390/ijtm5010003
APA StyleDoronzo, A., Musci, G., Gadaleta-Caldarola, G., & Sergi, M. C. (2025). Clinical Course of Parotid Carcinoma with Hepatic and Nodal Metastases: A Case Report. International Journal of Translational Medicine, 5(1), 3. https://doi.org/10.3390/ijtm5010003






