The Early Response After Radiation Therapy on Three-Dimensional Oral Cancer Model Using Patient-Derived Cancer-Associated Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Fabrication of a Human 3D Oral Cancer Model Using Patient-Derived Fibroblasts
2.3. X-Ray Irradiation for 3D Oral Cancer Model
2.4. BNCT for 3D Oral Cancer Model
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
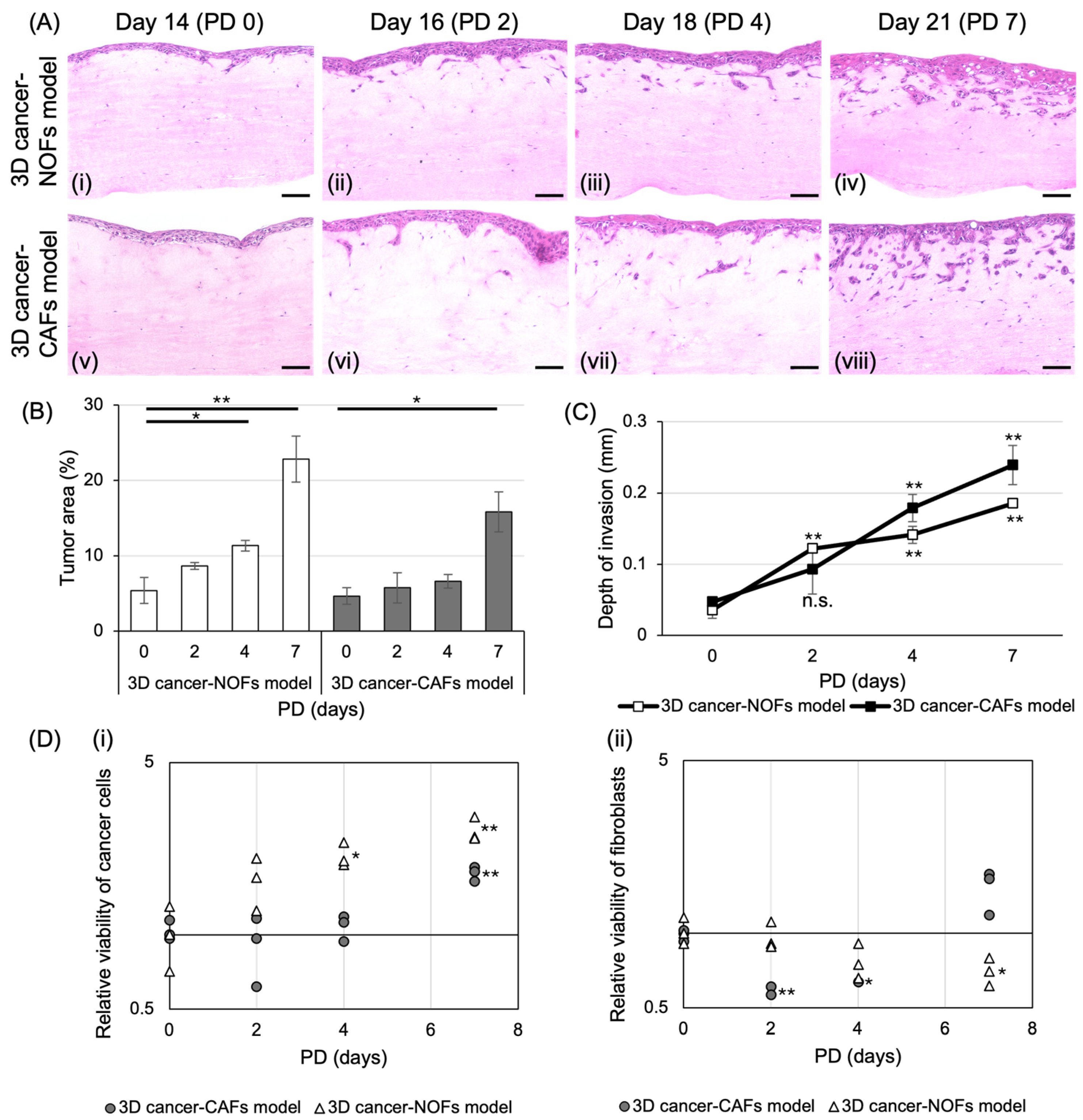
3.1. Fabrication of 3D Oral Cancer Models and Histological Evaluation
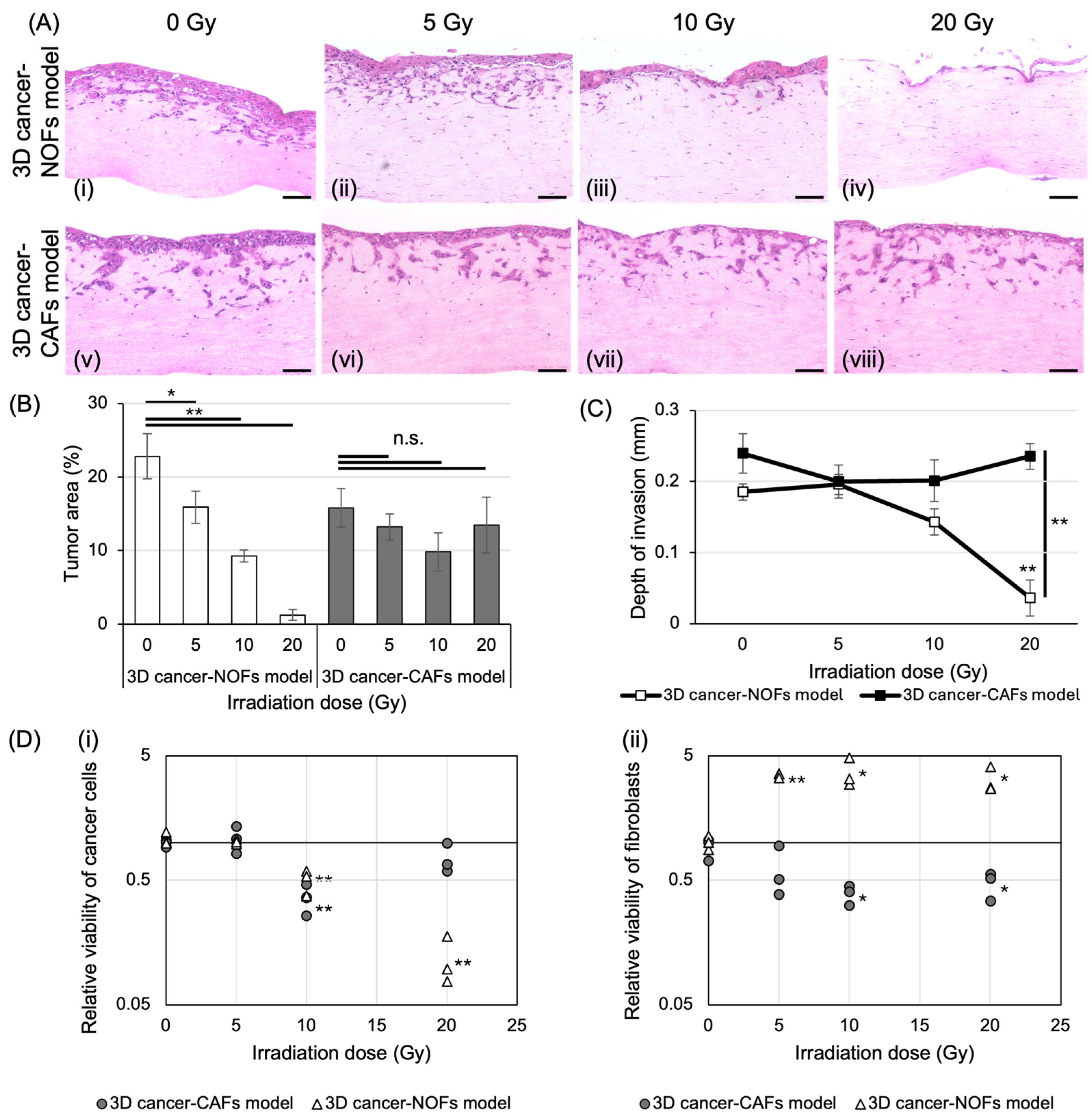
3.2. The Effect of X-Ray Irradiation on 3D Oral Cancer Models
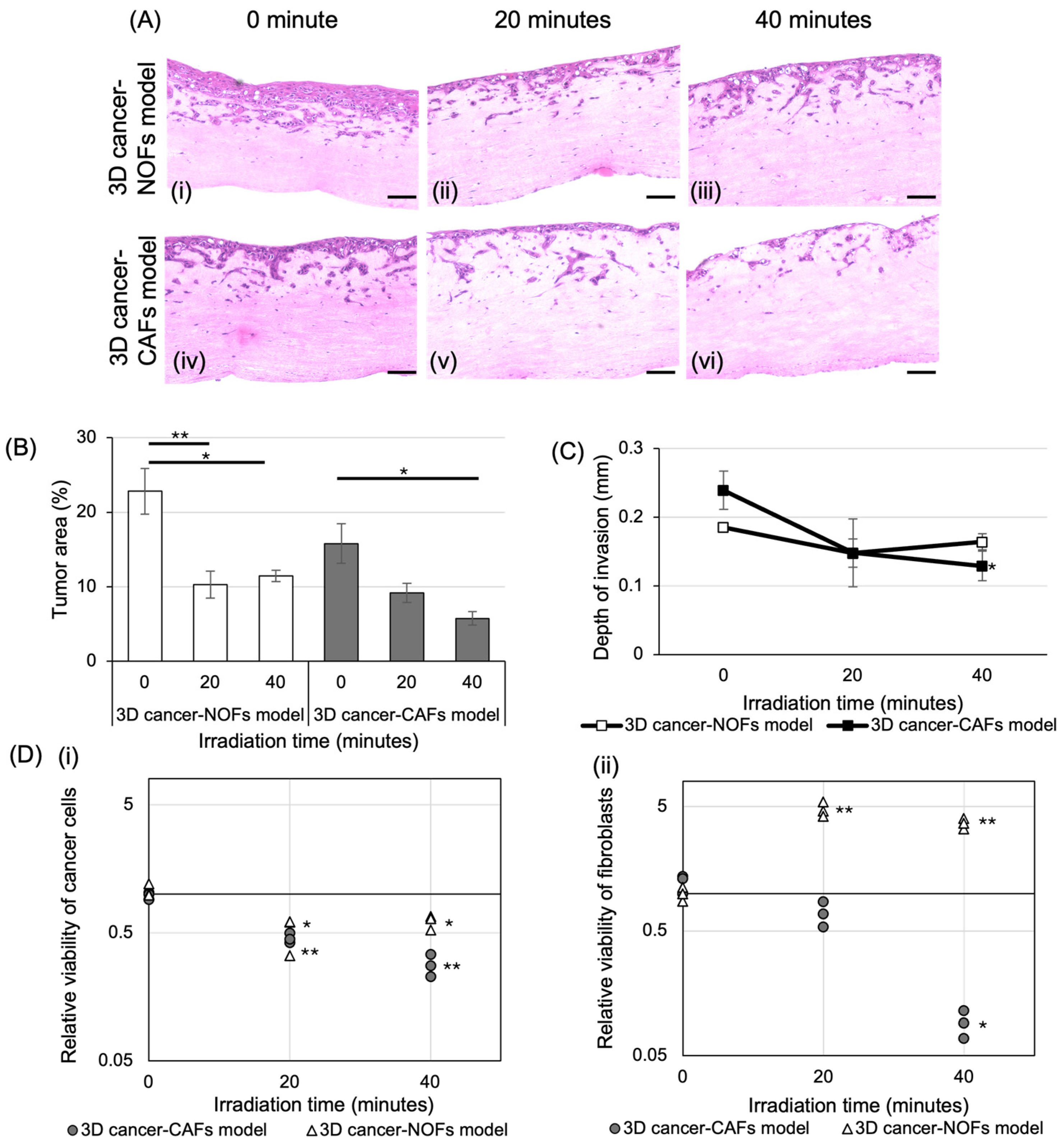
3.3. The Effect of BNCT on 3D Oral Cancer Models
3.4. Comparison of the Effect of X-Ray and BNCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Lv, N.; Li, M.; Liu, M.; Wu, C. Cancer-associated fibroblasts: Tumor defenders in radiation therapy. Cell Death Dis. 2023, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Bienkowska, K.J.; Hanley, C.J.; Thomas, G.J. Cancer-Associated Fibroblasts in Oral Cancer: A Current Perspective on Function and Potential for Therapeutic Targeting. Front. Oral Health 2021, 2, 686337. [Google Scholar] [CrossRef] [PubMed]
- Arebro, J.; Lee, C.M.; Bennewith, K.L.; Garnis, C. Cancer-Associated Fibroblast Heterogeneity in Malignancy with Focus on Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2024, 25, 1300. [Google Scholar] [CrossRef] [PubMed]
- El Herch, I.; Tornaas, S.; Dongre, H.N.; Costea, D.E. Heterogeneity of cancer-associated fibroblasts and tumor-promoting roles in head and neck squamous cell carcinoma. Front. Mol. Biosci. 2024, 11, 1340024. [Google Scholar] [CrossRef]
- Piper, M.; Mueller, A.C.; Karam, S.D. The interplay between cancer associated fibroblasts and immune cells in the context of radiation therapy. Mol. Carcinog. 2020, 59, 754–765. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Monti Hughes, A.; Hu, N. Optimizing Boron Neutron Capture Therapy (BNCT) to Treat Cancer: An Updated Review on the Latest Developments on Boron Compounds and Strategies. Cancers 2023, 15, 4091. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Miao, L.; Li, Y. Boron Neutron Capture Therapy: Current Status and Challenges. Front. Oncol. 2022, 12, 788770. [Google Scholar] [CrossRef]
- Takeno, S.; Yoshino, Y.; Aihara, T.; Higashino, M.; Kanai, Y.; Hu, N.; Kakino, R.; Kawata, R.; Nihei, K.; Ono, K. Preliminary outcomes of boron neutron capture therapy for head and neck cancers as a treatment covered by public health insurance system in Japan: Real-world experiences over a 2-year period. Cancer Med. 2024, 13, e7250. [Google Scholar] [CrossRef]
- Fontana, F.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. In Vitro 3D Cultures to Model the Tumor Microenvironment. Cancers 2021, 13, 2970. [Google Scholar] [CrossRef]
- Wishart, G.; Gupta, P.; Schettino, G.; Nisbet, A.; Velliou, E. 3D tissue models as tools for radiotherapy screening for pancreatic cancer. Br. J. Radiol. 2021, 94, 20201397. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Haga, K.; Yoshiba, N.; Yortchan, W.; Takada, S.; Tanaka, R.; Naito, E.; Abé, T.; Maruyama, S.; Yamazaki, M.; et al. Development and Characterization of a Three-Dimensional Organotypic In Vitro Oral Cancer Model with Four Co-Cultured Cell Types, Including Patient-Derived Cancer-Associated Fibroblasts. Biomedicines 2024, 12, 2373. [Google Scholar] [CrossRef]
- Naito, E.; Igawa, K.; Takada, S.; Haga, K.; Yortchan, W.; Suebsamarn, O.; Kobayashi, R.; Yamazaki, M.; Tanuma, J.I.; Hamano, T.; et al. The effects of carbon-ion beam irradiation on three-dimensional in vitro models of normal oral mucosa and oral cancer: Development of a novel tool to evaluate cancer therapy. In Vitro Cell. Dev. Biol. Anim. 2024, 60, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Igawa, K.; Izumi, K.; Sakurai, Y. Development of the Follow-Up Human 3D Oral Cancer Model in Cancer Treatment. BioTech 2023, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kobayash, T. Spectrum evaluation at the filter-modified neutron irradiation field for neutron capture therapy in Kyoto University Research Reactor. Nucl. Instrum. Methods Phys. Res. A 2004, 531, 585–595. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kobayashi, T. Characteristics of the KUR Heavy Water Neutron Irradiation Facility as a neutron irradiation field with variable energy spectra. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2000, 453, 569–596. [Google Scholar] [CrossRef]
- Sercombe, L.; Igawa, K.; Izumi, K. Radiation evaluation assay using a human three-dimensional oral cancer model for clinical radiation therapy. Talanta Open 2024, 9, 100297. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Wu, B.; Li, Y.; Liu, Z.; Gao, Y.; Gao, L.; Song, Q.; Zheng, Z.; Yao, Y. Irradiation enhances the malignancy-promoting behaviors of cancer-associated fibroblasts. Front. Oncol. 2022, 12, 965660. [Google Scholar] [CrossRef]
- Sato, M.; Hirose, K.; Takeno, S.; Aihara, T.; Nihei, K.; Takai, Y.; Hayashi, T.; Bando, K.; Kimura, H.; Tsurumi, K.; et al. Safety of Boron Neutron Capture Therapy with Borofalan(10B) and Its Efficacy on Recurrent Head and Neck Cancer: Real-World Outcomes from Nationwide Post-Marketing Surveillance. Cancers 2024, 16, 869. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-Y.; Hsu, C.-H.; Li, C.-Y.; Hong, S.-Y.; Chen, C.-R.; Chen, C.-S. Evaluating the biological effectiveness of boron neutron capture therapy by using microfluidics-based pancreatic tumor spheroids. Analyst 2023, 148, 3045–3056. [Google Scholar] [CrossRef]
- Yu, L.S.; Jhunjhunwala, M.; Hong, S.Y.; Yu, L.Y.; Lin, W.R.; Chen, C.S. Tissue Architecture Influences the Biological Effectiveness of Boron Neutron Capture Therapy in In Vitro/In Silico Three-Dimensional Self-Assembly Cell Models of Pancreatic Cancers. Cancers 2021, 13, 4058. [Google Scholar] [CrossRef] [PubMed]
- Yura, Y.; Fujita, Y. Boron neutron capture therapy as a novel modality of radiotherapy for oral cancer: Principle and antitumor effect. Oral Sci. Int. 2013, 10, 9–14. [Google Scholar]
- Martinez-Zubiaurre, I.; Hellevik, T. Cancer-associated fibroblasts in radiotherapy: Bystanders or protagonists? Cell Commun. Signal. 2023, 21, 108. [Google Scholar] [CrossRef]
- Berzaghi, R.; Gundersen, K.; Dille Pedersen, B.; Utne, A.; Yang, N.; Hellevik, T.; Martinez-Zubiaurre, I. Immunological signatures from irradiated cancer-associated fibroblasts. Front. Immunol. 2024, 15, 1433237. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyata, H.; Sugimura, K.; Fukuda, S.; Kanemura, T.; Yamashita, K.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; et al. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis 2015, 36, 894–903. [Google Scholar] [CrossRef]
- Engrácia, D.M.; Pinto, C.I.G.; Mendes, F. Cancer 3D Models for Metallodrug Preclinical Testing. Int. J. Mol. Sci. 2023, 24, 11915. [Google Scholar] [CrossRef]
- De Felice, F.; Musio, D.; Tombolini, V. Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment. J. Pers. Med. 2021, 11, 393. [Google Scholar] [CrossRef]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D cancer models: One step closer to in vitro human studies. Front. Immunol. 2023, 14, 1175503. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Bessho, T.; Takagi, T.; Igawa, K.; Sato, K. Gelatin-based cell culture device for construction and X-ray irradiation of a three-dimensional oral cancer model. Anal. Sci. 2023, 39, 771–778. [Google Scholar] [CrossRef] [PubMed]

| Irradiation Time | Type of Model | Boron Concentration (ppm) | Thermal Neutron Fluence (cm−2) | Total Dose (Gy-eq) |
|---|---|---|---|---|
| 0 min | 3D cancer-NOFs model | 53.91 ± 4.14 | 0 | 0 |
| 3D cancer-CAFs model | 56.14 ± 6.24 | 0 | 0 | |
| 20 min | 3D cancer-NOFs model | 53.91 ± 4.14 | 2.21 × 1012 | 8.71 ± 0.67 |
| 3D cancer-CAFs model | 56.14 ± 6.24 | 2.21 × 1012 | 9.07 ± 1.01 | |
| 40 min | 3D cancer-NOFs model | 53.91 ± 4.14 | 4.48 × 1012 | 17.66 ± 1.36 |
| 3D cancer-CAFs model | 56.14 ± 6.24 | 4.48 × 1012 | 18.38 ± 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, I.; Igawa, K.; Kondo, N.; Sakurai, Y.; Fujimura, A.; Takabatake, K.; Huang, P.; Michiue, H.; Ibaragi, S.; Izumi, K. The Early Response After Radiation Therapy on Three-Dimensional Oral Cancer Model Using Patient-Derived Cancer-Associated Fibroblasts. Int. J. Transl. Med. 2025, 5, 12. https://doi.org/10.3390/ijtm5010012
Yamamoto I, Igawa K, Kondo N, Sakurai Y, Fujimura A, Takabatake K, Huang P, Michiue H, Ibaragi S, Izumi K. The Early Response After Radiation Therapy on Three-Dimensional Oral Cancer Model Using Patient-Derived Cancer-Associated Fibroblasts. International Journal of Translational Medicine. 2025; 5(1):12. https://doi.org/10.3390/ijtm5010012
Chicago/Turabian StyleYamamoto, Izumi, Kazuyo Igawa, Natsuko Kondo, Yoshinori Sakurai, Atsushi Fujimura, Kiyofumi Takabatake, Peng Huang, Hiroyuki Michiue, Soichiro Ibaragi, and Kenji Izumi. 2025. "The Early Response After Radiation Therapy on Three-Dimensional Oral Cancer Model Using Patient-Derived Cancer-Associated Fibroblasts" International Journal of Translational Medicine 5, no. 1: 12. https://doi.org/10.3390/ijtm5010012
APA StyleYamamoto, I., Igawa, K., Kondo, N., Sakurai, Y., Fujimura, A., Takabatake, K., Huang, P., Michiue, H., Ibaragi, S., & Izumi, K. (2025). The Early Response After Radiation Therapy on Three-Dimensional Oral Cancer Model Using Patient-Derived Cancer-Associated Fibroblasts. International Journal of Translational Medicine, 5(1), 12. https://doi.org/10.3390/ijtm5010012






