Contribution of Sex Differences to Development of Cardiovascular Disease in Metabolic-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Introduction
2. Prevalence of Sex Differences in the Development of MASLD and CVD
3. Factors Contributing to Sex Differences in MASLD-Induced CVD
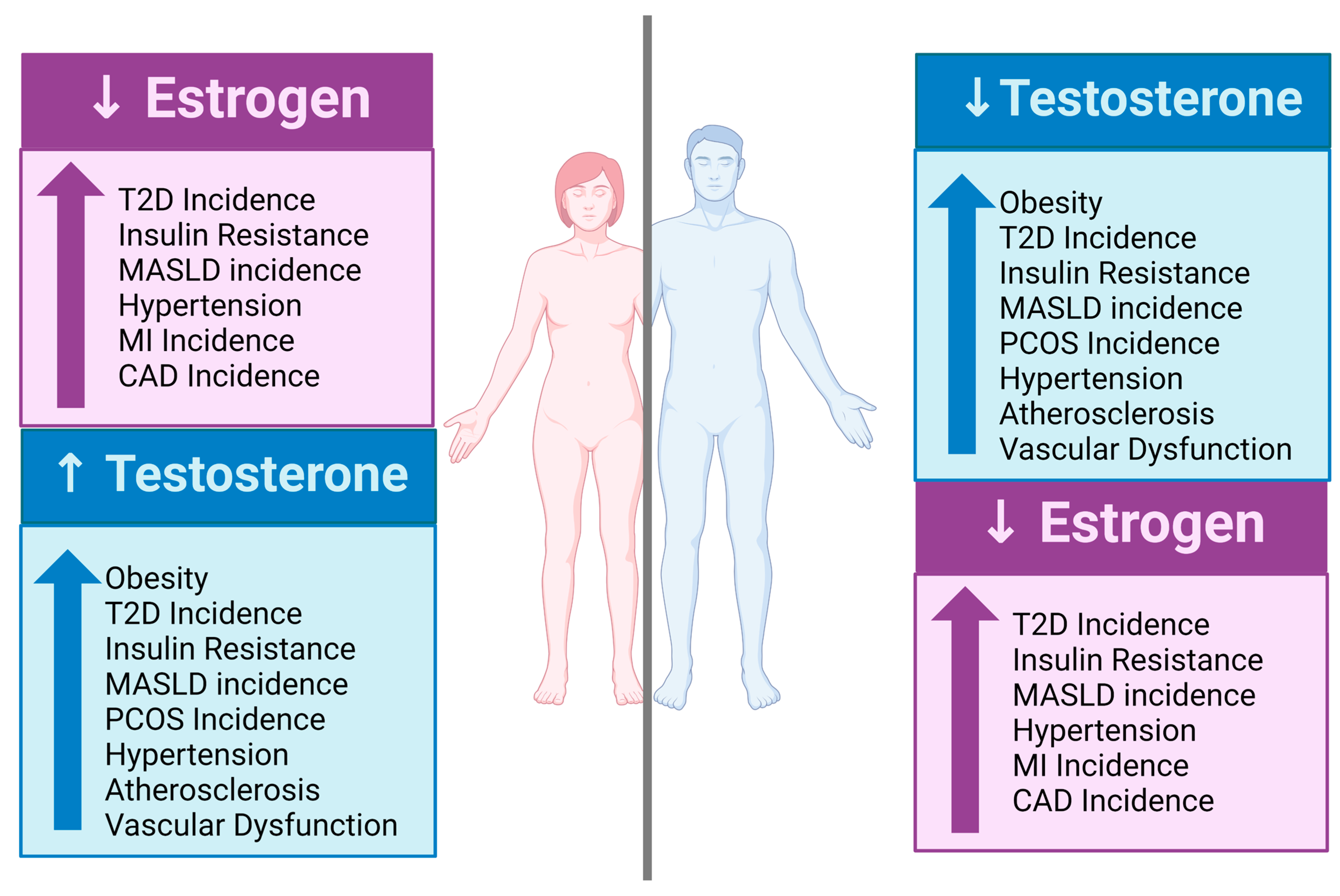
3.1. Estrogen and the Estrogen Receptor
3.2. Progesterone
3.3. Androgens
3.4. Insulin-like Growth Factor-1 (IGF-1)/Growth Hormone (GH)–Axis
3.5. Adipokines
3.6. Plasma Bilirubin Levels
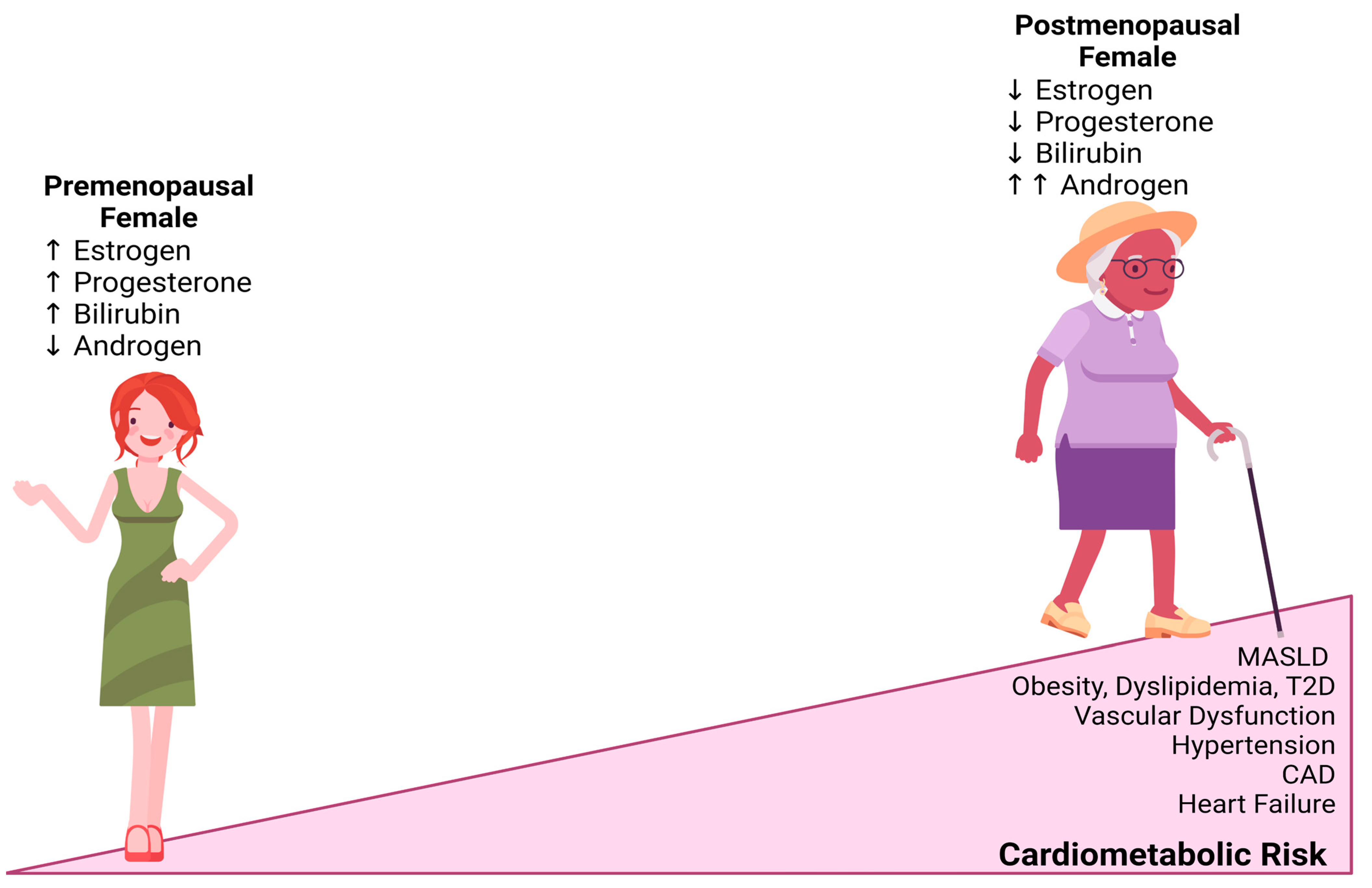
4. Reproductive Status
5. Age
6. Race and Ethnicity
7. Genetic Predispositions
8. Lifestyle
9. Mechanistic Factors Potentially Responsible for Sex Differences in MASLD-Induced CVD
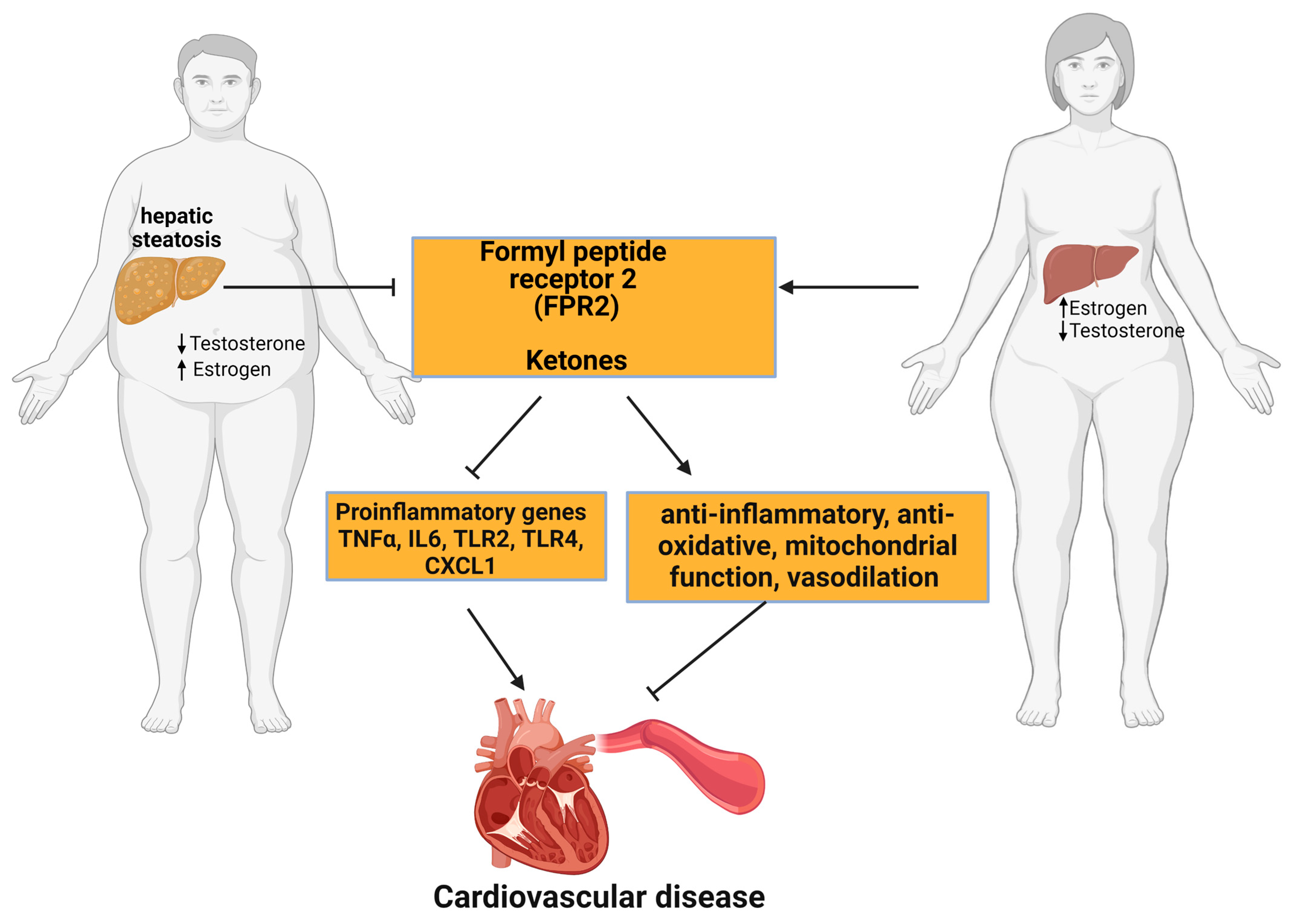
9.1. Formyl Peptide Receptor 2
9.2. Adipose Tissue Distribution
9.3. Liver Pyruvate Kinase
9.4. Ketone Bodies
9.5. Mitochondrial Bioenergetics
9.6. Insulin Resistance
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Junior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hinds, T.D., Jr.; Stec, D.E. Mechanisms Linking Metabolic-Associated Fatty Liver Disease (MAFLD) to Cardiovascular Disease. Curr. Hypertens. Rep. 2023, 25, 151–162. [Google Scholar] [CrossRef]
- Platek, A.E.; Szymanska, A. Metabolic dysfunction-associated steatotic liver disease as a cardiovascular risk factor. Clin. Exp. Hepatol. 2023, 9, 187–192. [Google Scholar] [CrossRef]
- Driessen, S.; Francque, S.M.; Anker, S.D.; Castro Cabezas, M.; Grobbee, D.E.; Tushuizen, M.E.; Holleboom, A.G. Metabolic dysfunction-associated steatotic liver disease and the heart. Hepatology 2023. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Athyros, V.G.; Alexandrides, T.K.; Bilianou, H.; Cholongitas, E.; Doumas, M.; Ganotakis, E.S.; Goudevenos, J.; Elisaf, M.S.; Germanidis, G.; Giouleme, O.; et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism 2017, 71, 17–32. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kutting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef]
- Niederseer, D.; Wernly, B.; Aigner, E.; Stickel, F.; Datz, C. NAFLD and Cardiovascular Diseases: Epidemiological, Mechanistic and Therapeutic Considerations. J. Clin. Med. 2021, 10, 467. [Google Scholar] [CrossRef]
- Goliopoulou, A.; Theofilis, P.; Oikonomou, E.; Anastasiou, A.; Pantelidis, P.; Gounaridi, M.I.; Zakynthinos, G.E.; Katsarou, O.; Kassi, E.; Lambadiari, V.; et al. Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 14292. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P.; et al. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement from the American Heart Association. Arter. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Wilcox, J.E.; Ning, H.; Lewis, C.E.; Carr, J.J.; Rinella, M.E.; Shah, S.J.; Lima, J.A.C.; Lloyd-Jones, D.M. Longitudinal Association of Non-Alcoholic Fatty Liver Disease with Changes in Myocardial Structure and Function: The CARDIA Study. J. Am. Heart Assoc. 2020, 9, e014279. [Google Scholar] [CrossRef]
- Nielsen, R.; Moller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frokiaer, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment with the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef] [PubMed]
- Nagral, A.; Bangar, M.; Menezes, S.; Bhatia, S.; Butt, N.; Ghosh, J.; Manchanayake, J.H.; Mahtab, M.A.; Singh, S.P. Gender Differences in Nonalcoholic Fatty Liver Disease. Euroasian J. Hepatogastroenterol. 2022, 12, S19–S25. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71 e15. [Google Scholar] [CrossRef] [PubMed]
- Couchepin, C.; Le, K.A.; Bortolotti, M.; da Encarnacao, J.A.; Oboni, J.B.; Tran, C.; Schneiter, P.; Tappy, L. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 2008, 31, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef]
- Long, M.T.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Ma, J.; Loomba, R.; Chung, R.T.; Benjamin, E.J. A simple clinical model predicts incident hepatic steatosis in a community-based cohort: The Framingham Heart Study. Liver Int. 2018, 38, 1495–1503. [Google Scholar] [CrossRef]
- Lee, N.H.; Jeong, S.J.; Wang, J.H.; Choi, Y.J.; Oh, H.M.; Cho, J.H.; Ahn, Y.C.; Son, C.G. The Clinical Diagnosis-Based Nationwide Epidemiology of Metabolic Dysfunction-Associated Liver Disease in Korea. J. Clin. Med. 2023, 12, 7634. [Google Scholar] [CrossRef]
- Norheim, F.; Hui, S.T.; Kulahcioglu, E.; Mehrabian, M.; Cantor, R.M.; Pan, C.; Parks, B.W.; Lusis, A.J. Genetic and hormonal control of hepatic steatosis in female and male mice. J. Lipid Res. 2017, 58, 178–187. [Google Scholar] [CrossRef]
- Badmus, O.O.; Kipp, Z.A.; Bates, E.A.; da Silva, A.A.; Taylor, L.C.; Martinez, G.J.; Lee, W.H.; Creeden, J.F.; Hinds, T.D., Jr.; Stec, D.E. Loss of hepatic PPARalpha in mice causes hypertension and cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R81–R95. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Sheng, L.; Liu, H.X.; Kalanetra, K.M.; Mirsoian, A.; Murphy, W.J.; French, S.W.; Krishnan, V.V.; Mills, D.A.; Wan, Y.Y. Western Diet-Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment. Am. J. Pathol. 2017, 187, 1800–1813. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.; Clarkson, V.; Shephard, E.G.; Marais, D.A.; Jaffer, M.A.; Woodburne, V.E.; Kirsch, R.E.; Hall Pde, L. Rodent nutritional model of non-alcoholic steatohepatitis: Species, strain and sex difference studies. J. Gastroenterol. Hepatol. 2003, 18, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; Delbare, S.; Wilson, R.A.; Manigrasso, M.B.; Zhou, B.; Ruiz, H.H.; Mangar, K.; Higa, R.; Brown, E.; Li, H.; et al. Sex differences in murine MASH evoked by a fructose-pamitate-cholesterol-enriched diet. JHEP Rep. 2024, 101222. [Google Scholar] [CrossRef]
- Solomon, A.; Negrea, M.O.; Cipaian, C.R.; Boicean, A.; Mihaila, R.; Rezi, C.; Cristinescu, B.A.; Berghea-Neamtu, C.S.; Popa, M.L.; Teodoru, M.; et al. Interactions between Metabolic Syndrome, MASLD, and Arterial Stiffening: A Single-Center Cross-Sectional Study. Healthcare 2023, 11, 2696. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, C.; Huang, Y.Z.; Zhang, L.L. Nonalcoholic fatty liver disease shows significant sex dimorphism. World J. Clin. Cases 2022, 10, 1457–1472. [Google Scholar] [CrossRef]
- Allen, A.M.; Therneau, T.M.; Mara, K.C.; Larson, J.J.; Watt, K.D.; Hayes, S.N.; Kamath, P.S. Women with Nonalcoholic Fatty Liver Disease Lose Protection Against Cardiovascular Disease: A Longitudinal Cohort Study. Am. J. Gastroenterol. 2019, 114, 1764–1771. [Google Scholar] [CrossRef]
- Burra, P.; Bizzaro, D.; Gonta, A.; Shalaby, S.; Gambato, M.; Morelli, M.C.; Trapani, S.; Floreani, A.; Marra, F.; Brunetto, M.R.; et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021, 41, 1713–1733. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Chang, B.; Kim, D.S.; Kim, S. Improvement in Menopause-Associated Hepatic Lipid Metabolic Disorders by Herbal Formula HPC03 on Ovariectomized Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 1409376. [Google Scholar] [CrossRef]
- Ruggiero, R.J.; Likis, F.E. Estrogen: Physiology, pharmacology, and formulations for replacement therapy. J. Midwifery Womens Health 2002, 47, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Grobe, Y.; Ponciano-Rodriguez, G.; Ramos, M.H.; Uribe, M.; Mendez-Sanchez, N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann. Hepatol. 2010, 9, 402–409. [Google Scholar] [CrossRef]
- Jones, M.E.; Thorburn, A.W.; Britt, K.L.; Hewitt, K.N.; Wreford, N.G.; Proietto, J.; Oz, O.K.; Leury, B.J.; Robertson, K.M.; Yao, S.; et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA 2000, 97, 12735–12740. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Toda, K.; Ono, M.; Fujikawa-Adachi, K.; Saibara, T.; Onishi, S.; Enzan, H.; Okada, T.; Shizuta, Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J. Clin. Invest. 2000, 105, 1819–1825. [Google Scholar] [CrossRef]
- Tian, G.X.; Sun, Y.; Pang, C.J.; Tan, A.H.; Gao, Y.; Zhang, H.Y.; Yang, X.B.; Li, Z.X.; Mo, Z.N. Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes. Rev. 2012, 13, 381–387. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Craig, T.; Zheng, W.; Ji, H.; Haywood, J.R.; Sandberg, K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 2004, 44, 405–409. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Jung, Y. Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Cells 2019, 8, 1259. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Min, J.S.; Lee, S.; Lee, D.Y.; Choi, D. Different effects of menopausal hormone therapy on non-alcoholic fatty liver disease based on the route of estrogen administration. Sci. Rep. 2023, 13, 15461. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Moolman, J.A. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc. Res. 2006, 69, 777–780. [Google Scholar] [CrossRef]
- Arias-Loza, P.A.; Muehlfelder, M.; Pelzer, T. Estrogen and estrogen receptors in cardiovascular oxidative stress. Pflugers Arch. 2013, 465, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, J.; Shetty, N.; Menon, M.B.; Ramaswamy, A.; Gupta, S. Tamoxifen and fulvestrant induced steatohepatitis with cirrhosis: A rare case report. South Asian J. Cancer 2019, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Djouadi, F.; Weinheimer, C.J.; Saffitz, J.E.; Pitchford, C.; Bastin, J.; Gonzalez, F.J.; Kelly, D.P. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. J. Clin. Invest. 1998, 102, 1083–1091. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Lanari, A.; Garegnani, T.C.; Heinrichs, G.; Castresana, M.P. Hepatic fibrosis and progesterone. Acta Gastroenterol. Latinoam. 1988, 18, 161–171. [Google Scholar]
- Dhote, V.V.; Balaraman, R. Gender specific effect of progesterone on myocardial ischemia/reperfusion injury in rats. Life Sci. 2007, 81, 188–197. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Licata, G.; Shan, J.; Bing, L.; Karpinski, E.; Pang, P.K.; Resnick, L.M. Vascular Effects of Progesterone: Role of Cellular Calcium Regulation. Hypertension 2001, 37, 142–147. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Protective actions of progesterone in the cardiovascular system: Potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids 2013, 78, 583–588. [Google Scholar] [CrossRef]
- Cui, P.; Hu, W.; Ma, T.; Hu, M.; Tong, X.; Zhang, F.; Shi, J.; Xu, X.; Li, X.; Shao, L.R.; et al. Long-term androgen excess induces insulin resistance and non-alcoholic fatty liver disease in PCOS-like rats. J. Steroid Biochem. Mol. Biol. 2021, 208, 105829. [Google Scholar] [CrossRef]
- Song, M.J.; Choi, J.Y. Androgen dysfunction in non-alcoholic fatty liver disease: Role of sex hormone binding globulin. Front. Endocrinol. 2022, 13, 1053709. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chen, P.; Li, Y.; Song, Q.; Han, J.; Fang, L.; Guan, Q.; Yu, C. The Fatty Liver Index, the Strongest Risk Factor for Low Testosterone Level. Obes. Facts 2023, 16, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B.; Jing, T.Y.; Resnick, L.M.; Barbagallo, M.; Laragh, J.H.; Sealey, J.E. Sex hormones and hemostatic risk factors for coronary heart disease in men with hypertension. J Hypertens. 1993, 11, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B.; Pinkernell, B.H.; Jing, T.Y. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler. Thromb. 1994, 14, 701–706. [Google Scholar] [CrossRef]
- Mody, A.; White, D.; Kanwal, F.; Garcia, J.M. Relevance of low testosterone to non-alcoholic fatty liver disease. Cardiovasc. Endocrinol. 2015, 4, 83–89. [Google Scholar] [CrossRef]
- Scicchitano, P.; Dentamaro, I.; Carbonara, R.; Bulzis, G.; Dachille, A.; Caputo, P.; Riccardi, R.; Locorotondo, M.; Mandurino, C.; Matteo Ciccone, M. Cardiovascular Risk in Women With PCOS. Int. J. Endocrinol. Metab. 2012, 10, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Sung, Y.A.; Hong, Y.S.; Song, D.K.; Jung, H.; Jeong, K.; Chung, H.; Lee, H. Non-alcoholic fatty liver disease is associated with hyperandrogenism in women with polycystic ovary syndrome. Sci. Rep. 2023, 13, 13397. [Google Scholar] [CrossRef]
- Talbott, E.O.; Zborowski, J.V.; Rager, J.R.; Boudreaux, M.Y.; Edmundowicz, D.A.; Guzick, D.S. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 5454–5461. [Google Scholar] [CrossRef]
- Cascella, T.; Palomba, S.; Tauchmanova, L.; Manguso, F.; Di Biase, S.; Labella, D.; Giallauria, F.; Vigorito, C.; Colao, A.; Lombardi, G.; et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 4395–4400. [Google Scholar] [CrossRef] [PubMed]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef]
- Blum, W.F.; Alherbish, A.; Alsagheir, A.; El Awwa, A.; Kaplan, W.; Koledova, E.; Savage, M.O. The growth hormone-insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr. Connect 2018, 7, R212–R222. [Google Scholar] [CrossRef]
- Hutchison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Della Volpe, L.; Alisi, A.; Panera, N.; Maggiore, G.; Vania, A. The Role of the GH/IGF1 Axis on the Development of MAFLD in Pediatric Patients with Obesity. Metabolites 2022, 12, 1221. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska-Gladysz, J.; Gorecka, A.E.; Stepien, J.; Rysz, I.; Ben-Skowronek, I. IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3966. [Google Scholar] [CrossRef]
- Span, J.P.; Pieters, G.F.; Sweep, C.G.; Hermus, A.R.; Smals, A.G. Gender difference in insulin-like growth factor I response to growth hormone (GH) treatment in GH-deficient adults: Role of sex hormone replacement. J. Clin. Endocrinol. Metab. 2000, 85, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.; Johannsson, G.; Leong, G.M.; Ho, K.K. Estrogen regulation of growth hormone action. Endocr. Rev. 2004, 25, 693–721. [Google Scholar] [CrossRef]
- Clemente-Suarez, V.J.; Redondo-Florez, L.; Beltran-Velasco, A.I.; Martin-Rodriguez, A.; Martinez-Guardado, I.; Navarro-Jimenez, E.; Laborde-Cardenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Shklyaev, S.S.; Melnichenko, G.A.; Volevodz, N.N.; Falaleeva, N.A.; Ivanov, S.A.; Kaprin, A.D.; Mokrysheva, N.G. Adiponectin: A pleiotropic hormone with multifaceted roles. Probl. Endokrinol. 2021, 67, 98–112. [Google Scholar] [CrossRef]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and metabolic cardiovascular diseases: Therapeutic opportunities and challenges. Genes Dis. 2023, 10, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 2012, 165, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Bottner, A.; Kratzsch, J.; Muller, G.; Kapellen, T.M.; Bluher, S.; Keller, E.; Bluher, M.; Kiess, W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J. Clin. Endocrinol. Metab. 2004, 89, 4053–4061. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yanase, T.; Nomura, M.; Okabe, T.; Goto, K.; Sato, T.; Kawano, H.; Kato, S.; Nawata, H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 2005, 54, 1000–1008. [Google Scholar] [CrossRef]
- Capllonch-Amer, G.; Sbert-Roig, M.; Galmes-Pascual, B.M.; Proenza, A.M.; Llado, I.; Gianotti, M.; Garcia-Palmer, F.J. Estradiol stimulates mitochondrial biogenesis and adiponectin expression in skeletal muscle. J. Endocrinol. 2014, 221, 391–403. [Google Scholar] [CrossRef]
- Sieminska, L.; Cichon-Lenart, A.; Kajdaniuk, D.; Kos-Kudla, B.; Marek, B.; Lenart, J.; Nowak, M. Sex hormones and adipocytokines in postmenopausal women. Pol. Merkur. Lekarski. 2006, 20, 727–730. [Google Scholar]
- Valenzuela-Vallejo, L.; Chrysafi, P.; Kouvari, M.; Guatibonza-Garcia, V.; Mylonakis, S.C.; Katsarou, A.; Verrastro, O.; Markakis, G.; Eslam, M.; Papatheodoridis, G.; et al. Circulating hormones in biopsy-proven steatotic liver disease and steatohepatitis: A Multicenter Observational Study. Metabolism 2023, 148, 155694. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Hall, J.E.; da Silva, A.A.; do Carmo, J.M.; Dubinion, J.; Hamza, S.; Munusamy, S.; Smith, G.; Stec, D.E. Obesity-induced hypertension: Role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 2010, 285, 17271–17276. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Color, L.; Dominguez-Rosales, J.A.; Maldonado-Gonzalez, M.; Ruiz-Madrigal, B.; Sanchez Munoz, M.P.; Zaragoza-Guerra, V.A.; Espinoza-Padilla, V.H.; Ruelas-Cinco, E.D.C.; Ramirez-Meza, S.M.; Torres Baranda, J.R.; et al. Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans. Int. J. Mol. Sci. 2024, 25, 6420. [Google Scholar] [CrossRef]
- de Castro, M.A.; Baltar, V.T.; Marchioni, D.M.; Fisberg, R.M. Sex differences in serum leptin and its relation to markers of cardiometabolic risk in middle-aged adults: Evidence from a population-based study. Nutrition 2015, 31, 491–497. [Google Scholar] [CrossRef]
- Corsonello, A.; Perticone, F.; Malara, A.; De Domenico, D.; Loddo, S.; Buemi, M.; Ientile, R.; Corica, F. Leptin-dependent platelet aggregation in healthy, overweight and obese subjects. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Galletti, F.; D’Elia, L.; De Palma, D.; Russo, O.; Barba, G.; Siani, A.; Miller, M.A.; Cappuccio, F.P.; Rossi, G.; Zampa, G.; et al. Hyperleptinemia is associated with hypertension, systemic inflammation and insulin resistance in overweight but not in normal weight men. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Wauters, M.; Van Gaal, L. Gender differences in leptin levels and physiology: A role for leptin in human reproduction. J. Gend. Specif. Med. 1999, 2, 46–51. [Google Scholar]
- Hellstrom, L.; Wahrenberg, H.; Hruska, K.; Reynisdottir, S.; Arner, P. Mechanisms behind gender differences in circulating leptin levels. J. Intern. Med. 2000, 247, 457–462. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Guseh, J.S.; Bhambhani, V.; Zanni, M.V.; Courchesne, P.; Lyass, A.; Larson, M.G.; Levy, D.; Ho, J.E. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Feitosa, M.F.; Wilk, J.B.; Laramie, J.M.; Yu, K.; Leiendecker-Foster, C.; Myers, R.H.; Province, M.A.; Borecki, I.B. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 2009, 53, 473–479. [Google Scholar] [CrossRef]
- Manrique-Acevedo, C.; Chinnakotla, B.; Padilla, J.; Martinez-Lemus, L.A.; Gozal, D. Obesity and cardiovascular disease in women. Int. J. Obes. 2020, 44, 1210–1226. [Google Scholar] [CrossRef]
- Becerril, S.; Rodriguez, A.; Catalan, V.; Ramirez, B.; Mentxaka, A.; Neira, G.; Gomez-Ambrosi, J.; Fruhbeck, G. Sex- and Age-Dependent Changes in the Adiponectin/Leptin Ratio in Experimental Diet-Induced Obesity in Mice. Nutrients 2022, 15, 73. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Creeden, J.F.; Gordon, D.M.; Stec, D.F.; Donald, M.C.; Stec, D.E. Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma beta-Hydroxybutyrate. Front. Pharmacol. 2020, 11, 594574. [Google Scholar] [CrossRef]
- Liang, C.; Yu, Z.; Bai, L.; Hou, W.; Tang, S.; Zhang, W.; Chen, X.; Hu, Z.; Duan, Z.; Zheng, S. Association of Serum Bilirubin with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 869579. [Google Scholar] [CrossRef] [PubMed]
- Kipp, Z.A.; Martinez, G.J.; Bates, E.A.; Maharramov, A.B.; Flight, R.M.; Moseley, H.N.B.; Morris, A.J.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin Nanoparticle Treatment in Obese Mice Inhibits Hepatic Ceramide Production and Remodels Liver Fat Content. Metabolites 2023, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D., Jr.; Stec, D.E. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 2018, 72, 788–795. [Google Scholar] [CrossRef]
- McArdle, P.F.; Whitcomb, B.W.; Tanner, K.; Mitchell, B.D.; Shuldiner, A.R.; Parsa, A. Association between bilirubin and cardiovascular disease risk factors: Using Mendelian randomization to assess causal inference. BMC Cardiovasc. Disord. 2012, 12, 16. [Google Scholar] [CrossRef]
- Wen, G.; Yao, L.; Hao, Y.; Wang, J.; Liu, J. Bilirubin ameliorates murine atherosclerosis through inhibiting cholesterol synthesis and reshaping the immune system. J. Transl. Med. 2022, 20, 1. [Google Scholar] [CrossRef]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D., Jr. Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Kim, D.; Chung, G.E.; Kang, S.J.; Park, M.J.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012, 18, 383–390. [Google Scholar] [CrossRef]
- Stojanov, M.; Stefanovic, A.; Dzingalasevic, G.; Ivanisevic, J.; Miljkovic, M.; Mandic-Radic, S.; Prostran, M. Total bilirubin in young men and women: Association with risk markers for cardiovascular diseases. Clin. Biochem. 2013, 46, 1516–1519. [Google Scholar] [CrossRef]
- Kim, A.H.; Son, D.H.; Moon, M.E.; Jeon, S.; Lee, H.S.; Lee, Y.J. Sex differences in the relationship between serum total bilirubin and risk of incident metabolic syndrome in community-dwelling adults: Propensity score analysis using longitudinal cohort data over 16 years. Cardiovasc. Diabetol. 2024, 23, 92. [Google Scholar] [CrossRef]
- Kao, T.L.; Chen, Y.L.; Kuan, Y.P.; Chang, W.C.; Ho, Y.C.; Yeh, S.; Jeng, L.B.; Ma, W.L. Estrogen-Estrogen Receptor alpha Signaling Facilitates Bilirubin Metabolism in Regenerating Liver Through Regulating Cytochrome P450 2A6 Expression. Cell Transpl. 2017, 26, 1822–1829. [Google Scholar] [CrossRef]
- Toth, B.; Yokoyama, Y.; Kuebler, J.F.; Schwacha, M.G.; Rue, L.W., 3rd; Bland, K.I.; Chaudry, I.H. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch. Surg. 2003, 138, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bai, Y.; Wang, R.; Bai, Z.; Yang, J.; Chen, Y.; Li, J.; Xu, L.; Li, S.; Hu, Y.; et al. Elevated serum bilirubin may significantly reduce coronary heart disease risk in females: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Coltell, O.; Asensio, E.M.; Sorli, J.V.; Barragan, R.; Fernandez-Carrion, R.; Portoles, O.; Ortega-Azorin, C.; Martinez-LaCruz, R.; Gonzalez, J.I.; Zanon-Moreno, V.; et al. Genome-Wide Association Study (GWAS) on Bilirubin Concentrations in Subjects with Metabolic Syndrome: Sex-Specific GWAS Analysis and Gene-Diet Interactions in a Mediterranean Population. Nutrients 2019, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Yim, J.Y.; Kim, D.; Lim, S.H.; Yang, J.I.; Kim, Y.S.; Yang, S.Y.; Kwak, M.S.; Kim, J.S.; Cho, S.H. The influence of metabolic factors for nonalcoholic Fatty liver disease in women. Biomed. Res. Int. 2015, 2015, 131528. [Google Scholar] [CrossRef]
- Booijink, R.; Ramachandran, P.; Bansal, R. Implications of innate immune sexual dimorphism for MASLD pathogenesis and treatment. Trends Pharmacol. Sci. 2024, 45, 614–627. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.L.; Golshan, S.; Harlow, K.E.; Angeles, J.E.; Durelle, J.; Goyal, N.P.; Newton, K.P.; Sawh, M.C.; Hooker, J.; Sy, E.Z.; et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. J. Pediatr. 2019, 207, 64–70. [Google Scholar] [CrossRef]
- Yu, E.L.; Schwimmer, J.B. Epidemiology of Pediatric Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2021, 17, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wu, Z.; Wang, S.; Yu, Z.; Ullah, R.; Liang, X.; Wu, W.; Huang, K.; Ni, Y.; Wang, J.; et al. Gender differences in non-alcoholic fatty liver disease in obese children and adolescents: A large cross-sectional study. Hepatol. Int. 2024, 18, 179–187. [Google Scholar] [CrossRef]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, G.J.; Wi, W.Y.; Norwitz, E.R.; Koo, B.K.; Lee, J.; Jung, Y.M.; Kwak, S.H.; Park, C.W.; Jun, J.K.; et al. Metabolic dysfunction-associated fatty liver disease as a risk factor for adverse outcomes in subsequent pregnancy: A nationwide cohort study. Hepatol. Int. 2023, 17, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Grab, J.; Dodge, J.L.; Gunderson, E.P.; Rubin, J.; Irani, R.A.; Cedars, M.; Terrault, N. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J. Hepatol. 2020, 73, 516–522. [Google Scholar] [CrossRef]
- Hershman, M.; Mei, R.; Kushner, T. Implications of Nonalcoholic Fatty Liver Disease on Pregnancy and Maternal and Child Outcomes. Gastroenterol. Hepatol. 2019, 15, 221–228. [Google Scholar]
- Karachaliou, G.S.; Suzuki, A. Metabolic dysfunction-associated steatotic liver disease: Emerging risk factors for adverse pregnancy outcomes. Clin. Liver Dis. 2024, 23, e0121. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, J.; Khalid, N.; Zhu, K.; Syed, T.; Liu, H.; Okolo, P.I., 3rd. Cardiovascular complications during delivery hospitalizations in patients with nonalcoholic fatty liver disease in pregnancy. Eur. J. Gastroenterol. Hepatol. 2024, 36, 1141–1148. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Kaplan, J.R.; Manuck, S.B. Premenopausal Reproductive Health Modulates Future Cardiovascular Risk-Comparative Evidence from Monkeys and Women. Yale J. Biol. Med. 2017, 90, 499–507. [Google Scholar] [PubMed]
- Karoli, R.; Fatima, J.; Chandra, A.; Gupta, U.; Islam, F.U.; Singh, G. Prevalence of hepatic steatosis in women with polycystic ovary syndrome. J. Hum. Reprod. Sci. 2013, 6, 9–14. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Lavine, J.E.; Yates, K.; Klair, J.; Terrault, N.A.; Clark, J.M.; Unalp-Arida, A.; et al. Patient Sex, Reproductive Status, and Synthetic Hormone Use Associate with Histologic Severity of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 127–131. [Google Scholar] [CrossRef]
- Gorenoi, V.; Schonermark, M.P.; Hagen, A. Benefits and risks of hormonal contraception for women. GMS Health Technol. Assess. 2007, 3, Doc06. [Google Scholar]
- Dou, W.; Huang, Y.; Liu, X.; Huang, C.; Huang, J.; Xu, B.; Yang, L.; Liu, Y.; Lei, X.; Li, X.; et al. Associations of Oral Contraceptive Use with Cardiovascular Disease and All-Cause Death: Evidence From the UK Biobank Cohort Study. J. Am. Heart Assoc. 2023, 12, e030105. [Google Scholar] [CrossRef]
- Roach, R.E.; Helmerhorst, F.M.; Lijfering, W.M.; Stijnen, T.; Algra, A.; Dekkers, O.M. Combined oral contraceptives: The risk of myocardial infarction and ischemic stroke. Cochrane Database Syst. Rev. 2015, 2015, CD011054. [Google Scholar] [CrossRef]
- Fabunmi, O.A.; Dludla, P.V.; Nkambule, B.B. Investigating cardiovascular risk in premenopausal women on oral contraceptives: Systematic review with meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1127104. [Google Scholar] [CrossRef]
- Kaminski, P.; Szpotanska-Sikorska, M.; Wielgos, M. Cardiovascular risk and the use of oral contraceptives. Neuro Endocrinol. Lett. 2013, 34, 587–589. [Google Scholar]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Mehta, J.; Kling, J.M.; Manson, J.E. Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front. Endocrinol. 2021, 12, 564781. [Google Scholar] [CrossRef]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]
- Yang, X.P.; Reckelhoff, J.F. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 133–138. [Google Scholar] [CrossRef]
- Boardman, H.M.; Hartley, L.; Eisinga, A.; Main, C.; Roque i Figuls, M.; Bonfill Cosp, X.; Gabriel Sanchez, R.; Knight, B. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst. Rev. 2015, 2015, CD002229. [Google Scholar] [CrossRef]
- Hulley, S.; Furberg, C.; Barrett-Connor, E.; Cauley, J.; Grady, D.; Haskell, W.; Knopp, R.; Lowery, M.; Satterfield, S.; Schrott, H.; et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 58–66. [Google Scholar] [CrossRef]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef]
- Beral, V.; Bull, D.; Reeves, G.; Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar] [CrossRef]
- Eskridge, W.; Cryer, D.R.; Schattenberg, J.M.; Gastaldelli, A.; Malhi, H.; Allen, A.M.; Noureddin, M.; Sanyal, A.J. Metabolic Dysfunction-Associated Steatotic Liver Disease and Metabolic Dysfunction-Associated Steatohepatitis: The Patient and Physician Perspective. J. Clin. Med. 2023, 12, 6216. [Google Scholar] [CrossRef]
- Moon, J.H.; Jeong, S.; Jang, H.; Koo, B.K.; Kim, W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: A nationwide cohort study. EClinicalMedicine 2023, 65, 102292. [Google Scholar] [CrossRef]
- Ren, R.; Zheng, Y. Sex differences in cardiovascular and all-cause mortality in nonalcoholic fatty liver disease in the US population. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1349–1357. [Google Scholar] [CrossRef]
- Dunn, W.; Xu, R.; Wingard, D.L.; Rogers, C.; Angulo, P.; Younossi, Z.M.; Schwimmer, J.B. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am. J. Gastroenterol. 2008, 103, 2263–2271. [Google Scholar] [CrossRef]
- Kagansky, N.; Levy, S.; Keter, D.; Rimon, E.; Taiba, Z.; Fridman, Z.; Berger, D.; Knobler, H.; Malnick, S. Non-alcoholic fatty liver disease—A common and benign finding in octogenarian patients. Liver Int. 2004, 24, 588–594. [Google Scholar] [CrossRef]
- Fu, C.E.; Teng, M.; Tung, D.; Ramadoss, V.; Ong, C.; Koh, B.; Lim, W.H.; Tan, D.J.H.; Koh, J.H.; Nah, B.; et al. Sex and Race-Ethnic Disparities in Metabolic Dysfunction-Associated Steatotic Liver Disease: An Analysis of 40,166 Individuals. Dig. Dis. Sci. 2024, 69, 3195–3205. [Google Scholar] [CrossRef]
- Gulati, R.; Moylan, C.A.; Wilder, J.; Wegermann, K. Racial and ethnic disparities in metabolic dysfunction-associated steatotic liver disease. Metab. Target Organ Damage 2024, 4, 9. [Google Scholar] [CrossRef]
- Gosnell, J.M.; Golovko, G.; Arroyave, E.; Moghe, A.; Kueht, M.L.; Saldarriaga, O.A.; McKinney, K.H.; Stevenson, H.L.; Ferguson, M.R. Disparate outcomes in Hispanic patients with metabolic dysfunction-associated steatotic liver disease/steatohepatitis and type 2 diabetes: Large cohort study. World J Diabetes. 2024, 15, 886–897. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Aboona, M.B.; Faulkner, C.; Rangan, P.; Ng, C.H.; Huang, D.Q.; Muthiah, M.; Nevah Rubin, M.I.; Han, M.A.T.; Fallon, M.B.; Kim, D.; et al. Disparities among ethnic groups in mortality and outcomes among adults with MASLD: A multicenter study. Liver Int. 2024, 44, 1316–1328. [Google Scholar] [CrossRef]
- Elsaid, M.I.; Bridges, J.F.P.; Mumtaz, K.; Li, N.; Sobotka, L.; Rustgi, V.K.; Paskett, E.D. The impact of metabolic syndrome severity on racial and ethnic disparities in Metabolic Dysfunction-Associated Steatotic Liver Disease. PLoS ONE 2024, 19, e0299836. [Google Scholar] [CrossRef]
- Chen, Y.; Du, X.; Kuppa, A.; Feitosa, M.F.; Bielak, L.F.; O’Connell, J.R.; Musani, S.K.; Guo, X.; Kahali, B.; Chen, V.L.; et al. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat. Genet. 2023, 55, 1640–1650. [Google Scholar] [CrossRef]
- Marigorta, U.M.; Millet, O.; Lu, S.C.; Mato, J.M. Dysfunctional VLDL metabolism in MASLD. NPJ Metab. Health Dis. 2024, 2, 16. [Google Scholar] [CrossRef]
- Petta, S.; Armandi, A.; Bugianesi, E. Impact of PNPLA3 I148M on Clinical Outcomes in Patients With MASLD. Liver Int. 2024. [Google Scholar] [CrossRef]
- Kozlitina, J.; Sookoian, S. Global Epidemiological Impact of PNPLA3 I148M on Liver Disease. Liver Int. 2024. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Nick, A.; Holtta-Vuori, M.; Thiele, C.; Isokuortti, E.; Lallukka-Bruck, S.; Zhou, Y.; Hakkarainen, A.; Lundbom, N.; Peltonen, M.; et al. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight 2019, 4, e127902. [Google Scholar] [CrossRef]
- Cherubini, A.; Ostadreza, M.; Jamialahmadi, O.; Pelusi, S.; Rrapaj, E.; Casirati, E.; Passignani, G.; Norouziesfahani, M.; Sinopoli, E.; Baselli, G.; et al. Interaction between estrogen receptor-alpha and PNPLA3 p.I148M variant drives fatty liver disease susceptibility in women. Nat. Med. 2023, 29, 2643–2655. [Google Scholar] [CrossRef]
- Chinwong, D.; Mookmanee, N.; Chongpornchai, J.; Chinwong, S. A Comparison of Gender Differences in Smoking Behaviors, Intention to Quit, and Nicotine Dependence among Thai University Students. J. Addict. 2018, 2018, 8081670. [Google Scholar] [CrossRef]
- Zein, C.O.; Unalp, A.; Colvin, R.; Liu, Y.C.; McCullough, A.J.; Nonalcoholic Steatohepatitis Clinical Research, N. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J. Hepatol. 2011, 54, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Fu, Y.; Liao, W.; Zheng, C.; Wu, X. Association between Smoking and Liver Fibrosis among Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6028952. [Google Scholar] [CrossRef]
- Balogun, O.; Wang, J.Y.; Shaikh, E.S.; Liu, K.; Stoyanova, S.; Memel, Z.N.; Schultz, H.; Mun, L.; Bertman, J.; Rogen, C.A.; et al. Effect of combined tobacco use and type 2 diabetes mellitus on prevalent fibrosis in patients with MASLD. Hepatol. Commun. 2023, 7, e0300. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Eto, K.; Furuno, K.; Mori, T.; Kawasaki, H.; Gomita, Y. Effect of cigarette smoke on lipid peroxidation and liver function tests in rats. Acta Med. Okayama 1995, 49, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Soeda, J.; Morgan, M.; McKee, C.; Mouralidarane, A.; Lin, C.; Roskams, T.; Oben, J.A. Nicotine induces fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on hepatic stellate cells. Biochem. Biophys. Res. Commun. 2012, 417, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, S.M.; Asgharpour, A. Smoking and Liver Disease. Gastroenterol. Hepatol. 2020, 16, 617–625. [Google Scholar]
- Lee, J.; Cooke, J.P. The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis 2011, 215, 281–283. [Google Scholar] [CrossRef]
- Jensen, J.A.; Goodson, W.H.; Hopf, H.W.; Hunt, T.K. Cigarette smoking decreases tissue oxygen. Arch. Surg. 1991, 126, 1131–1134. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Pujani, M.; Chauhan, V.; Singh, K.; Rastogi, S.; Agarwal, C.; Gera, K. The effect and correlation of smoking with platelet indices, neutrophil lymphocyte ratio and platelet lymphocyte ratio. Hematol. Transfus. Cell Ther. 2021, 43, 424–429. [Google Scholar] [CrossRef]
- Syamlal, G.; Mazurek, J.M.; Dube, S.R. Gender differences in smoking among U.S. working adults. Am. J. Prev. Med. 2014, 47, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, E.M.; Kock, L.; Feinstein, M.J.P.; Coleman, S.R.M.; Gaalema, D.E.; Higgins, S.T. Sex differences in tobacco use, attempts to quit smoking, and cessation among dual users of cigarettes and e-cigarettes: Longitudinal findings from the US Population Assessment of Tobacco and Health Study. Prev. Med. 2024, 108112. [Google Scholar] [CrossRef] [PubMed]
- Hagen, E.H.; Garfield, M.J.; Sullivan, R.J. The low prevalence of female smoking in the developing world: Gender inequality or maternal adaptations for fetal protection? Evol. Med. Public Health 2016, 2016, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms-A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Szudzik, M.; Hutsch, T.; Chabowski, D.; Zajdel, M.; Ufnal, M. Normal caloric intake with high-fat diet induces metabolic dysfunction-associated steatotic liver disease and dyslipidemia without obesity in rats. Sci. Rep. 2024, 14, 22796. [Google Scholar] [CrossRef]
- Wu, B.N.; O’Sullivan, A.J. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, M.; Paye, S.; Benayoun, B.A. Sex as a Biological Variable in Nutrition Research: From Human Studies to Animal Models. Annu. Rev. Nutr. 2022, 42, 227–250. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef]
- Butera, P.C. Estradiol and the control of food intake. Physiol. Behav. 2010, 99, 175–180. [Google Scholar] [CrossRef]
- Chai, J.K.; Blaha, V.; Meguid, M.M.; Laviano, A.; Yang, Z.J.; Varma, M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am. J. Physiol. 1999, 276, R1366–R1373. [Google Scholar] [CrossRef]
- Prince, S.A.; Roberts, K.C.; Melvin, A.; Butler, G.P.; Thompson, W. Gender and education differences in sedentary behaviour in Canada: An analysis of national cross-sectional surveys. BMC Public Health 2020, 20, 1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, P.; Liu, F.; Chen, Y.; Xie, J.; Bai, B.; Liu, Q.; Ma, H.; Geng, Q. Gender Differences in Unhealthy Lifestyle Behaviors among Adults with Diabetes in the United States between 1999 and 2018. Int. J. Environ. Res. Public Health 2022, 19, 16412. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Gulati, M.; Huang, T.Y.; Kwan, A.C.; Ouyang, D.; Ebinger, J.E.; Casaletto, K.; Moreau, K.L.; Skali, H.; Cheng, S. Sex Differences in Association of Physical Activity with All-Cause and Cardiovascular Mortality. J. Am. Coll. Cardiol. 2024, 83, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chen, K.; Gong, W.; Yoshimura, T.; Huang, J.; Wang, J.M. The G-Protein Coupled Formyl Peptide Receptors and Their Role in the Progression of Digestive Tract Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820973280. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef]
- Lee, C.; Han, J.; Jung, Y. Formyl peptide receptor 2 is an emerging modulator of inflammation in the liver. Exp. Mol. Med. 2023, 55, 325–332. [Google Scholar] [CrossRef]
- Tylek, K.; Trojan, E.; Regulska, M.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Formyl peptide receptor 2, as an important target for ligands triggering the inflammatory response regulation: A link to brain pathology. Pharmacol. Rep. 2021, 73, 1004–1019. [Google Scholar] [CrossRef]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef]
- Liu, M.; Chen, K.; Yoshimura, T.; Liu, Y.; Gong, W.; Wang, A.; Gao, J.L.; Murphy, P.M.; Wang, J.M. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2012, 2, 786. [Google Scholar] [CrossRef]
- Kang, J.W.; Choi, H.S.; Lee, S.M. Resolvin D1 attenuates liver ischaemia/reperfusion injury through modulating thioredoxin 2-mediated mitochondrial quality control. Br. J. Pharmacol. 2018, 175, 2441–2453. [Google Scholar] [CrossRef]
- Xu, F.; Zhou, X.; Hao, J.; Dai, H.; Zhang, J.; He, Y.; Hao, H. Lipoxin A(4) and its analog suppress hepatocarcinoma cell epithelial-mesenchymal transition, migration and metastasis via regulating integrin-linked kinase axis. Prostaglandins Other Lipid Mediat. 2018, 137, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Laguna-Fernandez, A.; Gonzalez-Diez, M.; Paulsson-Berne, G.; Hansson, G.K.; Back, M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 2015, 105, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xue, S.; Chen, K.; Le, Y.; Zhu, R.; Wang, S.; Liu, S.; Cheng, X.; Guan, H.; Wang, J.M.; et al. The G-protein-coupled chemoattractant receptor Fpr2 exacerbates neuroglial dysfunction and angiogenesis in diabetic retinopathy. FASEB Bioadv. 2020, 2, 613–623. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Han, J.; Oh, D.; Kim, M.; Jeong, H.; Kim, T.J.; Kim, S.W.; Kim, J.N.; Seo, Y.S.; et al. Formyl peptide receptor 2 determines sex-specific differences in the progression of nonalcoholic fatty liver disease and steatohepatitis. Nat. Commun. 2022, 13, 578. [Google Scholar] [CrossRef]
- Giebeler, A.; Streetz, K.L.; Soehnlein, O.; Neumann, U.; Wang, J.M.; Brandenburg, L.O. Deficiency of formyl peptide receptor 1 and 2 is associated with increased inflammation and enhanced liver injury after LPS-stimulation. PLoS ONE 2014, 9, e100522. [Google Scholar] [CrossRef]
- Chen, X.; Zhuo, S.; Zhu, T.; Yao, P.; Yang, M.; Mei, H.; Li, N.; Ma, F.; Wang, J.M.; Chen, S.; et al. Fpr2 Deficiency Alleviates Diet-Induced Insulin Resistance Through Reducing Body Weight Gain and Inhibiting Inflammation Mediated by Macrophage Chemotaxis and M1 Polarization. Diabetes 2019, 68, 1130–1142. [Google Scholar] [CrossRef]
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol. 2011, 46 (Suppl. 1), 63–69. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Choi, H.; Kim, H.J.; Lee, M.O. Chemotactic cytokines secreted from Kupffer cells contribute to the sex-dependent susceptibility to non-alcoholic fatty liver diseases in mice. Life Sci. 2022, 306, 120846. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, H.; Guo, Y.; Peng, F.; Meng, L. Immune-Related Genes in the Pathogenesis of Atherosclerosis: Based on Sex Differences. J. Inflamm. Res. 2023, 16, 4713–4724. [Google Scholar] [CrossRef]
- Petri, M.H.; Laguna-Fernandez, A.; Arnardottir, H.; Wheelock, C.E.; Perretti, M.; Hansson, G.K.; Back, M. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E(−/−) mice. Br. J. Pharmacol. 2017, 174, 4043–4054. [Google Scholar] [CrossRef]
- Vital, S.A.; Becker, F.; Holloway, P.M.; Russell, J.; Perretti, M.; Granger, D.N.; Gavins, F.N. Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation 2016, 133, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002, 45, 1201–1210. [Google Scholar] [CrossRef]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e716. [Google Scholar] [CrossRef]
- Frank, A.P.; de Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef]
- Rabadan-Chavez, G.; Diaz de la Garza, R.I.; Jacobo-Velazquez, D.A. White adipose tissue: Distribution, molecular insights of impaired expandability, and its implication in fatty liver disease. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166853. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Wells, J.C. Sexual dimorphism of body composition. Best Pr. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Blouin, K.; Boivin, A.; Tchernof, A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2008, 108, 272–280. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Joseph, F. Adipose tissue and adipokines: The association with and application of adipokines in obesity. Scientifica 2014, 2014, 328592. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.; Pinkerton, M.A.; Spradley, F.T.; Palei, A.C.; Hall, J.E.; do Carmo, J.M. Chronic CNS-mediated cardiometabolic actions of leptin: Potential role of sex differences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R173–R181. [Google Scholar] [CrossRef]
- Song, H.J.; Oh, S.; Quan, S.; Ryu, O.H.; Jeong, J.Y.; Hong, K.S.; Kim, D.H. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatr. 2014, 14, 8. [Google Scholar] [CrossRef]
- Poret, J.M.; Gaudet, D.A.; Braymer, H.D.; Primeaux, S.D. Sex differences in markers of metabolic syndrome and adipose tissue inflammation in obesity-prone, Osborne-Mendel and obesity-resistant, S5B/Pl rats. Life Sci. 2021, 273, 119290. [Google Scholar] [CrossRef]
- Ekdahl, K.N. Studies on the regulation of rat liver pyruvate kinase and fructose-1,6-bisphosphatase. Ups J. Med. Sci. 1987, 92, 217–232. [Google Scholar] [CrossRef][Green Version]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. 2019, 28, 1771–1784. [Google Scholar] [CrossRef]
- Imamura, K.; Tanaka, T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. J. Biochem. 1972, 71, 1043–1051. [Google Scholar] [CrossRef]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef]
- Noguchi, T.; Inoue, H.; Tanaka, T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J. Biol. Chem. 1986, 261, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhang, C.; Liu, Z.; Klevstig, M.; Mukhopadhyay, B.; Bergentall, M.; Cinar, R.; Stahlman, M.; Sikanic, N.; Park, J.K.; et al. Network analyses identify liver-specific targets for treating liver diseases. Mol. Syst. Biol. 2017, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Chella Krishnan, K.; Kurt, Z.; Barrere-Cain, R.; Sabir, S.; Das, A.; Floyd, R.; Vergnes, L.; Zhao, Y.; Che, N.; Charugundla, S.; et al. Integration of Multi-omics Data from Mouse Diversity Panel Highlights Mitochondrial Dysfunction in Non-alcoholic Fatty Liver Disease. Cell Syst. 2018, 6, 103–115.e107. [Google Scholar] [CrossRef]
- Chella Krishnan, K.; Floyd, R.R.; Sabir, S.; Jayasekera, D.W.; Leon-Mimila, P.V.; Jones, A.E.; Cortez, A.A.; Shravah, V.; Peterfy, M.; Stiles, L.; et al. Liver Pyruvate Kinase Promotes NAFLD/NASH in Both Mice and Humans in a Sex-Specific Manner. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 389–406. [Google Scholar] [CrossRef]
- Norheim, F.; Hasin-Brumshtein, Y.; Vergnes, L.; Chella Krishnan, K.; Pan, C.; Seldin, M.M.; Hui, S.T.; Mehrabian, M.; Zhou, Z.; Gupta, S.; et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab. 2019, 29, 932–949 e934. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Yin, Y.; Zheng, J.T.; Jiang, C.F.; Wang, J.; Shen, H.; Li, C.Y.; Wang, M.; Liu, L.Z.; et al. Insulin regulates glucose consumption and lactate production through reactive oxygen species and pyruvate kinase M2. Oxid. Med. Cell Longev. 2014, 2014, 504953. [Google Scholar] [CrossRef]
- Meoli, L.; Gupta, N.K.; Saeidi, N.; Panciotti, C.A.; Biddinger, S.B.; Corey, K.E.; Stylopoulos, N. Nonalcoholic fatty liver disease and gastric bypass surgery regulate serum and hepatic levels of pyruvate kinase isoenzyme M2. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E613–E621. [Google Scholar] [CrossRef]
- Fan, N.; Zhang, X.; Zhao, W.; Zhao, J.; Luo, D.; Sun, Y.; Li, D.; Zhao, C.; Wang, Y.; Zhang, H.; et al. Covalent Inhibition of Pyruvate Kinase M2 Reprograms Metabolic and Inflammatory Pathways in Hepatic Macrophages against Non-alcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2022, 18, 5260–5275. [Google Scholar] [CrossRef] [PubMed]
- Rihan, M.; Sharma, S.S. Role of Pyruvate Kinase M2 (PKM2) in Cardiovascular Diseases. J. Cardiovasc. Transl. Res. 2023, 16, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Han, S.N.; Zhang, J.Y.; Dioletis, E.; Nemeth, B.T.; Pacher, P.; Feng, D.; Bataller, R.; Cabezas, J.; Starkel, P.; et al. Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1alpha Transactivation in Steatohepatitis. Cell Metab. 2018, 27, 339–350 e333. [Google Scholar] [CrossRef] [PubMed]
- Doddapattar, P.; Dev, R.; Ghatge, M.; Patel, R.B.; Jain, M.; Dhanesha, N.; Lentz, S.R.; Chauhan, A.K. Myeloid Cell PKM2 Deletion Enhances Efferocytosis and Reduces Atherosclerosis. Circ. Res. 2022, 130, 1289–1305. [Google Scholar] [CrossRef]
- Rees, M.L.; Subramaniam, J.; Li, Y.; Hamilton, D.J.; Frazier, O.H.; Taegtmeyer, H. A PKM2 signature in the failing heart. Biochem. Biophys. Res. Commun. 2015, 459, 430–436. [Google Scholar] [CrossRef]
- Ni, L.; Lin, B.; Hu, L.; Zhang, R.; Fu, F.; Shen, M.; Yang, J.; Shi, D. Pyruvate Kinase M2 Protects Heart from Pressure Overload-Induced Heart Failure by Phosphorylating RAC1. J. Am. Heart Assoc. 2022, 11, e024854. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, T.; Wu, X.; Yi Qiu, J. Ketone production by ketogenic diet and by intermittent fasting has different effects on the gut microbiota and disease progression in an Alzheimer’s disease rat model. J. Clin. Biochem. Nutr. 2020, 67, 188–198. [Google Scholar] [CrossRef]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, W.H.; Wu, J.B.; Xiao, W.H. beta-hydroxybutyrate: A crucial therapeutic target for diverse liver diseases. Biomed. Pharmacother. 2023, 165, 115191. [Google Scholar] [CrossRef]
- Soni, S.; Tabatabaei Dakhili, S.A.; Ussher, J.R.; Dyck, J.R.B. The therapeutic potential of ketones in cardiometabolic disease: Impact on heart and skeletal muscle. Am. J. Physiol. Cell Physiol. 2024, 326, C551–C566. [Google Scholar] [CrossRef]
- Mooli, R.G.R.; Ramakrishnan, S.K. Emerging Role of Hepatic Ketogenesis in Fatty Liver Disease. Front. Physiol. 2022, 13, 946474. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Horowitz, J.F.; Klein, S. Gender differences in lipid and glucose kinetics during short-term fasting. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1333–E1339. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Abildgaard, J.; Lindegaard, B.; Febbraio, M.A. Metabolic control and sex: A focus on inflammatory-linked mediators. Br. J. Pharmacol. 2019, 176, 4193–4207. [Google Scholar] [CrossRef]
- Stec, D.E.; Gordon, D.M.; Hipp, J.A.; Hong, S.; Mitchell, Z.L.; Franco, N.R.; Robison, J.W.; Anderson, C.D.; Stec, D.F.; Hinds, T.D., Jr. Loss of hepatic PPARalpha promotes inflammation and serum hyperlipidemia in diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R733–R745. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Kimura, T.; Ago, Y.; Nakama, M.; Aoyama, Y.; Abdelkreem, E.; Matsumoto, H.; Ohnishi, H.; Sasai, H.; Osawa, M.; et al. Deficiency of 3-hydroxybutyrate dehydrogenase (BDH1) in mice causes low ketone body levels and fatty liver during fasting. J. Inherit. Metab. Dis. 2020, 43, 960–968. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef]
- Scott, S.R.; Singh, K.; Yu, Q.; Sen, C.K.; Wang, M. Sex as Biological Variable in Cardiac Mitochondrial Bioenergetic Responses to Acute Stress. Int. J. Mol. Sci. 2022, 23, 9312. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Piquereau, J.; Garnier, A.; Mericskay, M.; Lemaire, C.; Crozatier, B. Gender issues in cardiovascular diseases. Focus on energy metabolism. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165722. [Google Scholar] [CrossRef]
- Sultanova, R.F.; Schibalski, R.; Yankelevich, I.A.; Stadler, K.; Ilatovskaya, D.V. Sex differences in renal mitochondrial function: A hormone-gous opportunity for research. Am. J. Physiol. Renal. Physiol. 2020, 319, F1117–F1124. [Google Scholar] [CrossRef]
- Justo, R.; Boada, J.; Frontera, M.; Oliver, J.; Bermudez, J.; Gianotti, M. Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am. J. Physiol. Cell Physiol. 2005, 289, C372–C378. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, Q.; Feng, X.; Liu, Y.; Zhou, Y. Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment. Front. Cell Dev. Biol. 2022, 10, 841523. [Google Scholar] [CrossRef] [PubMed]
- Josloff, K.; Beiriger, J.; Khan, A.; Gawel, R.J.; Kirby, R.S.; Kendrick, A.D.; Rao, A.K.; Wang, R.X.; Schafer, M.M.; Pearce, M.E.; et al. Comprehensive Review of Cardiovascular Disease Risk in Nonalcoholic Fatty Liver Disease. J. Cardiovasc. Dev. Dis. 2022, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Mandavia, C.H.; Sowers, J.R. Insulin resistance and heart failure: Molecular mechanisms. Heart Fail. Clin. 2012, 8, 609–617. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Gado, M.; Tsaousidou, E.; Bornstein, S.R.; Perakakis, N. Sex-based differences in insulin resistance. J. Endocrinol. 2024, 261, e230245. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Lin, F.; Grady, D.; Bittner, V.; Cauley, J.A.; Barrett-Connor, E. Glycemic effects of postmenopausal hormone therapy: The Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2003, 138, 1–9. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Walsh, J.M.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef]
- Rattanavichit, Y.; Chukijrungroat, N.; Saengsirisuwan, V. Sex differences in the metabolic dysfunction and insulin resistance of skeletal muscle glucose transport following high fructose ingestion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1200–R1212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, L.C.; Arthur, G.; de Carvalho Cruz, M.; Stec, D.E.; Badmus, O.O. Contribution of Sex Differences to Development of Cardiovascular Disease in Metabolic-Associated Steatotic Liver Disease (MASLD). Int. J. Transl. Med. 2024, 4, 782-809. https://doi.org/10.3390/ijtm4040052
Taylor LC, Arthur G, de Carvalho Cruz M, Stec DE, Badmus OO. Contribution of Sex Differences to Development of Cardiovascular Disease in Metabolic-Associated Steatotic Liver Disease (MASLD). International Journal of Translational Medicine. 2024; 4(4):782-809. https://doi.org/10.3390/ijtm4040052
Chicago/Turabian StyleTaylor, Lucy C., Gertrude Arthur, Marcella de Carvalho Cruz, David E. Stec, and Olufunto O. Badmus. 2024. "Contribution of Sex Differences to Development of Cardiovascular Disease in Metabolic-Associated Steatotic Liver Disease (MASLD)" International Journal of Translational Medicine 4, no. 4: 782-809. https://doi.org/10.3390/ijtm4040052
APA StyleTaylor, L. C., Arthur, G., de Carvalho Cruz, M., Stec, D. E., & Badmus, O. O. (2024). Contribution of Sex Differences to Development of Cardiovascular Disease in Metabolic-Associated Steatotic Liver Disease (MASLD). International Journal of Translational Medicine, 4(4), 782-809. https://doi.org/10.3390/ijtm4040052





