Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy
Abstract
1. Introduction
2. Characteristics of Marine-Derived Bioactives
3. Marine Sources of Bioactives
4. Marine Bioactives Towards Cancer Therapy
4.1. Alkaloids
4.2. Flavonoids
4.3. Polysaccharides
4.4. Terpenoids
4.5. Steroids and Glycosides
4.6. Peptides
5. Molecular Mechanisms of Marine-Derived Bioactives Towards Cancer Treatment
5.1. Algae
5.1.1. Brown Algae
5.1.2. Green Algae
5.1.3. Red Algae
5.1.4. Microalgae
5.2. Fungi
5.3. Tunicates (Ascidians) and Mollusks
5.4. Sponges
5.5. Bacteria and Actinomycetes
5.6. Echinodermata
6. Recent Clinical Trials and FDA Approvals on Some Marine-Derived Bioactives
7. Discussion and Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malekhayati, H.; Bargahi, A.; Khorami, S.; Khataminejad, M.; Fouladvand, M. Anti-Trichomonas Vaginalis Activity of Marine Ascidians (Tunicates; Ascidiacea) from the Bushehr Province, Iran. Turk. Parazitoloji Derg. 2024, 48, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, P.; Setiawan, A.; Tanaka, J. Coral Reef Organisms as a Potential Source of Anticancer Agents. In Proceedings of the 4th International Conference on Applied Sciences, Mathematics, and Informatics: Icasmi2022, Bandar Lampung, Indonesia, 8–9 September 2022; Volume 2970, p. 060011. Available online: https://pubs.aip.org/aip/acp/article-abstract/2970/1/060011/3311478/Coral-reef-organisms-as-a-potential-source-of?redirectedFrom=fulltext (accessed on 4 December 2024).
- Okeke, E.S.; Okagu, I.U.; Chukwudozie, K.; Ezike, T.C.; Ezeorba, T.P.C. Marine-Derived Bioactive Proteins and Peptides: A Review of Current Knowledge on Anticancer Potentials, Clinical Trials, and Future Prospects. Nat. Prod. Commun. 2024, 19, 3. [Google Scholar] [CrossRef]
- Nagy, N.S.; Essawy, A.E.; Al-Sherif, S.S.; Ali, M.M.; Alsawy, E.S.; Helal, M. Characterization and Biological Applications of Gonadal Extract of Paracentrotus Lividus Collected along the Mediterranean Coast of Alexandria, Egypt. PLoS ONE 2024, 19, e0296312. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Sivakumar, A.S.; Govindasamy, C.; El Newehy, A.S.; Rajathy Port Louis, L.; Sivanandham, M.; Rangarajalu, K.; Sangeetha, C.C.; Ghidan, A.Y.; Yousef Ghidan, A. Molecular Perspective on Starfish Tissue Extracts: Targeting Human Carcinoma KB Cells for Anticancer Therapy. J. King Saud Univ. Sci. 2024, 36, 103035. [Google Scholar] [CrossRef]
- Cai, C.; Yang, D.; Cao, Y.; Peng, Z.; Wang, Y.; Xi, J.; Yan, C.; Li, X. Anticancer Potential of Active Alkaloids and Synthetic Analogs Derived from Marine Invertebrates. Eur. J. Med. Chem. 2024, 279, 116850. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Wu, W.; Wang, P. Extraction, Characterization and Osteogenic Activity of a Type I Collagen from Starfish (Asterias Amurensis). Mar. Drugs 2023, 21, 274. [Google Scholar] [CrossRef]
- Kaliaperumal, K.; Salendra, L.; Liu, Y.; Ju, Z.; Sahu, S.K.; Elumalai, S.; Subramanian, K.; Alotaibi, N.M.; Alshammari, N.; Saeed, M.; et al. Isolation of Anticancer Bioactive Secondary Metabolites from the Sponge-Derived Endophytic Fungi Penicillium sp. and in-Silico Computational Docking Approach. Front. Microbiol. 2023, 14, 1216928. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.G.; Elmezain, W.A.; Baraka, D.M.; AboElmaaty, S.A.; Elhassanein, A.; Ibrahim, R.M.; Hamed, A.A. Anti-Cancer and Anti-Oxidant Bioactive Metabolites from Aspergillus Fumigatus WA7S6 Isolated from Marine Sources: In Vitro and In Silico Studies. Microorganisms 2024, 12, 127. [Google Scholar] [CrossRef]
- Kalyani, B.S.; Krishna, P.S.; Laxminarayana, E.; Sreenivasulu, K. Novel Bioactive Compounds from Streptomyces sp. NLKPB45 Isolated from Marine Soil Sediment. Pharm. Chem. J. 2023, 57, 834–841. [Google Scholar] [CrossRef]
- Esim, N.; Dawar, P.; Arslan, N.P.; Orak, T.; Doymus, M.; Azad, F.; Ortucu, S.; Albayrak, S.; Taskin, M. Natural Metabolites with Antioxidant Activity from Micro-and Macro-Algae. Food Biosci. 2024, 62, 105089. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Proteins and Bioactive Peptides from Algae: Insights into Antioxidant, Anti-Hypertensive, Anti-Diabetic and Anti-Cancer Activities. Trends Food Sci. Technol. 2024, 145, 104352. [Google Scholar] [CrossRef]
- Visuddho, V.; Halim, P.; Helen, H.; Muhar, A.M.; Iqhrammullah, M.; Mayulu, N.; Surya, R.; Tjandrawinata, R.R.; Ribeiro, R.I.M.A.; Tallei, T.E.; et al. Modulation of Apoptotic, Cell Cycle, DNA Repair, and Senescence Pathways by Marine Algae Peptides in Cancer Therapy. Mar. Drugs 2024, 22, 338. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Alkafaas, S.S.; Rady, H.A.; Abdelmoaty, B.E.; Bedair, H.M.; Ahmed, A.A.; El-Saadony, M.T.; Abuqamar, S.F.; El-Tarabily, K.A. How Synthesis of Algal Nanoparticles Affects Cancer Therapy?—A Complete Review of the Literature. Int. J. Nanomed. 2023, 18, 6601–6638. [Google Scholar] [CrossRef]
- FDA Approves Trabectedin for Sarcoma—NCI. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2015/fda-trabectedin-sarcoma (accessed on 21 November 2024).
- Eribulin|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/eribulin (accessed on 21 November 2024).
- Haggag, Y.A.; Abd Elrahman, A.A.; Ulber, R.; Zayed, A. Fucoidan in Pharmaceutical Formulations: A Comprehensive Review for Smart Drug Delivery Systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and Development of the Anticancer Agent Salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2008, 17, 2175. [Google Scholar] [CrossRef]
- Jeong, G.J.; Khan, S.; Tabassum, N.; Khan, F.; Kim, Y.M. Marine-Bioinspired Nanoparticles as Potential Drugs for Multiple Biological Roles. Mar. Drugs 2022, 20, 527. [Google Scholar] [CrossRef]
- Thompson, R.E.; Tuchman, A.J.; Alkon, D.L. Bryostatin Placebo-Controlled Trials Indicate Cognitive Restoration Above Baseline for Advanced Alzheimer’s Disease in the Absence of Memantine1. J. Alzheimers Dis. 2022, 86, 1221–1229. [Google Scholar] [CrossRef]
- Jørgensen, N.G.; Klausen, U.; Grauslund, J.H.; Helleberg, C.; Aagaard, T.G.; Do, T.H.; Ahmad, S.M.; Olsen, L.R.; Klausen, T.W.; Breinholt, M.F.; et al. Peptide Vaccination Against PD-L1 With IO103 a Novel Immune Modulatory Vaccine in Multiple Myeloma: A Phase I First-in-Human Trial. Front. Immunol. 2020, 11, 595035. [Google Scholar] [CrossRef] [PubMed]
- Thaman, J.; Saxena Pal, R.; Chaitanya, M.V.N.L.; Yanadaiah, P.; Thangavelu, P.; Sharma, S.; Amoateng, P.; Arora, S.; Sivasankaran, P.; Pandey, P.; et al. Reconciling the Gap between Medications and Their Potential Leads: The Role of Marine Metabolites in the Discovery of New Anticancer Drugs: A Comprehensive Review. Curr. Pharm. Des. 2023, 29, 3137–3153. [Google Scholar] [CrossRef]
- Cooreman, K.; De Spiegeleer, B.; Van Poucke, C.; Vanavermaete, D.; Delbare, D.; Wynendaele, E.; De Witte, B. Emerging Pharmaceutical Therapies of Ascidian-Derived Natural Products and Derivatives. Environ. Toxicol. Pharmacol. 2023, 102, 104254. [Google Scholar] [CrossRef]
- Popov, A.; Kozlovskaya, E.; Rutckova, T.; Styshova, O.; Vakhrushev, A.; Kupera, E.; Tekutyeva, L. Antitumor Properties of Matrikines of Different Origins: Prospects and Problems of Their Application. Int. J. Mol. Sci. 2023, 24, 9502. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Garima; Bharadvaja, N. A Comprehensive Review on Algal Nutraceuticals as Prospective Therapeutic Agent for Different Diseases. 3 Biotech 2023, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Tao, J.; Xu, S.; Bai, X.; Zhang, H. Marine Organisms as a Prolific Source of Bioactive Depsipeptides. Mar. Drugs 2023, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.H.; Wong, S.R.; Lee, S.H. The Therapeutic Anticancer Potential of Marine-Derived Bioactive Peptides: A Highlight on Pardaxin. Int. J. Pept. Res. Ther. 2023, 29, 90. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Mar. Drugs 2024, 22, 433. [Google Scholar] [CrossRef]
- Rachman, F.; Wibowo, J.T. Exploring Marine Rare Actinomycetes: Untapped Resources of Bioactive Compounds in Clinical Development. BIO Web Conf. 2024, 92, 02012. [Google Scholar] [CrossRef]
- Rudayni, H.A.; Rabie, A.M.; Aladwani, M.; Alneghery, L.M.; Abu-Taweel, G.M.; Al Zoubi, W.; Allam, A.A.; Abukhadra, M.R.; Bellucci, S. Biological Activities of Sargassum Algae Mediated ZnO and Co Doped ZnO Nanoparticles as Enhanced Antioxidant and Anti-Diabetic Agents. Molecules 2023, 28, 3692. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Lin, R.; Cao, X.; Yuan, L. Anti-Tumor Target Screening of Sea Cucumber Saponin Frondoside A: A Bioinformatics and Molecular Docking Analysis. Front. Oncol. 2023, 13, 1307838. [Google Scholar] [CrossRef]
- Venkatachalam, J.; Jeyadoss, V.S.; Bose, K.S.C.; Subramanian, R. Marine Seaweed Endophytic Fungi-Derived Active Metabolites Promote Reactive Oxygen Species-Induced Cell Cycle Arrest and Apoptosis in Human Breast Cancer Cells. Mol. Biol. Rep. 2024, 51, 611. [Google Scholar] [CrossRef]
- Lin, X.; Dong, L.; Miao, Q.; Huang, Z.; Wang, F. Cycloheptylprodigiosin from Marine Bacterium Spartinivicinus Ruber MCCC 1K03745T Induces a Novel Form of Cell Death Characterized by Golgi Disruption and Enhanced Secretion of Cathepsin D in Non-Small Cell Lung Cancer Cell Lines. Eur. J. Pharmacol. 2024, 974, 176608. [Google Scholar] [CrossRef]
- Xu, S.; Li, Z.; Xin, X.; An, F. Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells. Mar. Drugs 2024, 22, 227. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Huang, T.-C.; Muthusamy, S.; Lee, J.-F.; Duann, Y.-F.; Lin, C.-H. Piscidin-1, an Antimicrobial Peptide from Fish (Hybrid Striped Bass Morone saxatilis x M. chrysops), Induces Apoptotic and Necrotic Activity in HT1080 Cells. Zool. Sci. 2012, 29, 327–332. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Kuo, H.-M.; Yu, C.-L.; Selvam, P.; Su, I.-L.; Tseng, C.-C.; Yuan, C.-H.; Wen, Z.-H. Marine-Derived Antimicrobial Peptide Piscidin-1 Triggers Extrinsic and Intrinsic Apoptosis in Oral Squamous Cell Carcinoma through Reactive Oxygen Species Production and Inhibits Angiogenesis. Free Radic. Biol. Med. 2024, 220, 28–42. [Google Scholar] [CrossRef]
- Liu, M.; Li, F.; Feng, S.; Guo, J.; Yu, J.; Zou, S.; Gao, X.; Wei, Y. Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes Philippinarum. Foods 2024, 13, 3552. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-W.; Hu, X.-M.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, PH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef] [PubMed]
- Suo, S.-K.; Zhao, Y.-Q.; Wang, Y.-M.; Pan, X.-Y.; Chi, C.-F.; Wang, B. Seventeen Novel Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from the Protein Hydrolysate of Mytilus edulis: Isolation, Identification, Molecular Docking Study, and Protective Function on HUVECs. Food Funct. 2022, 13, 7831–7846. [Google Scholar] [CrossRef] [PubMed]
- Pavlicevic, M.; Maestri, E.; Marmiroli, M. Marine Bioactive Peptides—An Overview of Generation, Structure and Application with a Focus on Food Sources. Mar. Drugs 2020, 18, 424. [Google Scholar] [CrossRef]
- Shinnar, A.E.; Butler, K.L.; Park, H.J. Cathelicidin Family of Antimicrobial Peptides: Proteolytic Processing and Protease Resistance. Bioorg. Chem. 2003, 31, 425–436. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Peptides: Determination of Absolute Configuration Using Liquid Chromatography Methods and Evaluation of Bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef]
- Arai, M.; Yamano, Y.; Fujita, M.; Setiawan, A.; Kobayashi, M. Stylissamide X, a New Proline-Rich Cyclic Octapeptide as an Inhibitor of Cell Migration, from an Indonesian Marine Sponge of Stylissa sp. Bioorg. Med. Chem. Lett. 2012, 22, 1818–1821. [Google Scholar] [CrossRef]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, Peptidomimetics, and Polypeptides from Marine Sources: A Wealth of Natural Sources for Pharmaceutical Applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Zagon, J.; Dehne, L.-I.; Bögl, K.-W. D-Amino Acids in Organisms and Food. Nutr. Res. 1994, 14, 445–463. [Google Scholar] [CrossRef]
- Jimenez, E.C. Bromotryptophan and Its Analogs in Peptides from Marine Animals. Protein Pept. Lett. 2019, 26, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Caer, J.-P.; Mourier, G.; Thai, R.; Lamthanh, H.; Servent, D.; Benoit, E.; Molgó, J. Characterization of a Novel Conus Bandanus Conopeptide Belonging to the M-Superfamily Containing Bromotryptophan. Mar. Drugs 2014, 12, 3449–3465. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Yang, X.; Li, M.; Yip, R.C.S.; Li, Y.; Chen, H. Current Application and Modification Strategy of Marine Polysaccharides in Tissue Regeneration: A Review. Biomater. Adv. 2023, 154, 213580. [Google Scholar] [CrossRef]
- Ren, X.; Xie, X.; Chen, B.; Liu, L.; Jiang, C.; Qian, Q. Marine Natural Products: A Potential Source of Anti-Hepatocellular Carcinoma Drugs. J. Med. Chem. 2021, 64, 7879–7899. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Huang, C.; Shi, C.; Xiang, X. Biomedical Potency and Mechanisms of Marine Polysaccharides and Oligosaccharides: A Review. Int. J. Biol. Macromol. 2024, 265, 131007. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, Z.; Liang, Y.; Peng, T.; Hu, Z. Insights into Algal Polysaccharides: A Review of Their Structure, Depolymerases, and Metabolic Pathways. J. Agric. Food Chem. 2022, 70, 1749–1765. [Google Scholar] [CrossRef]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, Biosynthesis and Biological Activity of Terpenoids and Meroterpenoids in Bacteria and Fungi Isolated from Different Marine Habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, T.; Li, R.; Xu, R.; Baranenko, D.; Yang, L.; Xiao, D. Discovery of Jaspamycin from Marine-Derived Natural Product Based on MTA3 to Inhibit Hepatocellular Carcinoma Progression. Sci. Rep. 2024, 14, 25294. [Google Scholar] [CrossRef]
- Saini, N.; Sirohi, R.; A., A.; Saini, N.; Wadhwa, P.; Kaur, P.; Sharma, V.; Singh, G.; Singh, I.; Sahu, S.K. Marine-Derived Natural Products as Anticancer Agents. Med. Chem. 2023, 19, 538–555. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; da Cunha, N.B.; Carneiro, J.A.; Costa, R.A.d.; Alencar, S.A.d.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, M.; Cai, S.; Mou, H.; Li, D. Potential Application of Marine Fucosyl-Polysaccharides in Regulating Blood Glucose and Hyperglycemic Complications. Foods 2023, 12, 2600. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhai, Y.; Wang, X.; Fan, Q.; Dong, X.; Chen, M.; Han, T. Phosphorylation of Polysaccharides: A Review on the Synthesis and Bioactivities. Int. J. Biol. Macromol. 2021, 184, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Azha, N.u.; Farooq, R.; Sheikh, S.A.; Ganie, M.A.; Parray, M.N.; Mushtaq, H.; Hameed, I.; Lone, M.A. Exploring the Potential of Marine Natural Products in Drug Development: A Comprehensive Review. Phytochem. Lett. 2024, 59, 124–135. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Tenebro, C.P.; Sabido, E.M.; Suarez, A.F.L.; Paderog, M.J.V.; Reyes-Salarda, R.; Saludes, J.P. Marine-Derived Anticancer Agents Targeting Apoptotic Pathways: Exploring the Depths for Novel Cancer Therapies. Mar. Drugs 2024, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, A.; Salmen, S.H.; Al-Garadi, M.A.; Wainwright, M.; Ali Alharbi, S. Marine Actinomycetes: An Endless Source of Potentially Therapeutic Novel Secondary Metabolites and Other Bioactive Compounds. J. King Saud Univ. Sci. 2023, 35, 102931. [Google Scholar] [CrossRef]
- Ghareeb, A.; Fouda, A.; Kishk, R.M.; El Kazzaz, W.M. Unlocking the Therapeutic Potential of Bioactive Exopolysaccharide Produced by Marine Actinobacterium Streptomyces Vinaceusdrappus AMG31: A Novel Approach to Drug Development. Int. J. Biol. Macromol. 2024, 276, 133861. [Google Scholar] [CrossRef]
- Agena, R.; de Jesús Cortés-Sánchez, A.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Pro-Apoptotic Activity of Bioactive Compounds from Seaweeds: Promising Sources for Developing Novel Anticancer Drugs. Mar. Drugs 2023, 21, 182. [Google Scholar] [CrossRef]
- Basha, A.N.; Akhir, F.N.M.; Othman, N.; Hara, H. Anticancer Potential of Bioactive Compounds from Microalgae. A Review. J. Adv. Res. Micro Nano Eng. 2024, 20, 1–9. [Google Scholar] [CrossRef]
- Fu, Y.; Jiao, H.; Sun, J.; Okoye, C.O.; Zhang, H.; Li, Y.; Lu, X.; Wang, Q.; Liu, J. Structure-Activity Relationships of Bioactive Polysaccharides Extracted from Macroalgae towards Biomedical Application: A Review. Carbohydr. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, M.; Ghelani, H.; Jan, R.K.; Adrian, T.E. Anti-Inflammatory Effects of Bioactive Compounds from Seaweeds, Bryozoans, Jellyfish, Shellfish and Peanut Worms. Mar. Drugs 2023, 21, 524. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef]
- Noman, E.; Al-Shaibani, M.M.; Bakhrebah, M.A.; Almoheer, R.; Al-Sahari, M.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Almulaiky, Y.Q.; Abdulaal, W.H. Potential of Anti-Cancer Activity of Secondary Metabolic Products from Marine Fungi. J. Fungi 2021, 7, 436. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive Diepoxy Metabolites and Highly Oxygenated Triterpenoids from Marine and Plant-Derived Bacteria and Fungi. Microbiol. Res. 2023, 15, 66–90. [Google Scholar] [CrossRef]
- Elgoud Said, A.A.; Mahmoud, B.K.; Attia, E.Z.; Abdelmohsen, U.R.; Fouad, M.A. Bioactive Natural Products from Marine Sponges Belonging to Family Hymedesmiidae. RSC Adv. 2021, 11, 16179–16191. [Google Scholar] [CrossRef]
- Esposito, R.; Federico, S.; Glaviano, F.; Somma, E.; Zupo, V.; Costantini, M. Bioactive Compounds from Marine Sponges and Algae: Effects on Cancer Cell Metabolome and Chemical Structures. Int. J. Mol. Sci. 2022, 23, 10680. [Google Scholar] [CrossRef]
- Barzkar, N.; Sukhikh, S.; Babich, O. Study of Marine Microorganism Metabolites: New Resources for Bioactive Natural Products. Front. Microbiol. 2024, 14, 1285902. [Google Scholar] [CrossRef]
- Rafieezadeh, D. Marine Bioactive Peptides with Anticancer Potential, a Narrative Review. Int. J. Biochem. Mol. Biol. 2024, 15, 118–126. [Google Scholar] [CrossRef]
- Rai, T.; Kaushik, N.; Malviya, R.; Sharma, P.K. A Review on Marine Source as Anticancer Agents. J. Asian Nat. Prod. Res. 2024, 26, 415–451. [Google Scholar] [CrossRef] [PubMed]
- Ortigosa-Palomo, A.; Quiñonero, F.; Ortiz, R.; Sarabia, F.; Prados, J.; Melguizo, C. Natural Products Derived from Marine Sponges with Antitumor Potential against Lung Cancer: A Systematic Review. Mar. Drugs 2024, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Elissawy, A.M.; Soleiman Dehkordi, E.; Mehdinezhad, N.; Ashour, M.L.; Pour, P.M. Biomolecules Cytotoxic Alkaloids Derived from Marine Sponges: A Comprehensive Review. Biomolecules 2021, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, P.; Aparna, V.; Sebastian, A.T.; Muneer, A.; Prabha, B.; Vipin, C.L.; Ijinu, T.P. Clinically Tested Marine Mollusk-Derived Anticancer Agents: Chemico-Pharmacological Aspects. Stud. Nat. Prod. Chem. 2024, 83, 95–131. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Coelho, A.V.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Avhad, A.B.; Bhangale, C.J. Marine Natural Products and Derivatives. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad008. [Google Scholar] [CrossRef]
- Wang, E.; Sorolla, M.A.; Krishnan, P.D.G.; Sorolla, A. From Seabed to Bedside: A Review on Promising Marine Anticancer Compounds. Biomolecules 2020, 10, 248. [Google Scholar] [CrossRef]
- Barzkar, N.; Sukhikh, S.; Babich, O. A Comprehensive Review of Marine Sponge Metabolites, with Emphasis on Neopetrosia sp. Int. J. Biol. Macromol. 2024, 280, 135823. [Google Scholar] [CrossRef]
- Son, K.; Takhaveev, V.; Mor, V.; Yu, H.; Dillier, E.; Zilio, N.; Püllen, N.J.L.; Ivanov, D.; Ulrich, H.D.; Sturla, S.J.; et al. Trabectedin Derails Transcription-Coupled Nucleotide Excision Repair to Induce DNA Breaks in Highly Transcribed Genes. Nat. Commun. 2024, 15, 1388. [Google Scholar] [CrossRef]
- Alsalmi, O.; Mashraqi, M.M.; Alshamrani, S.; Almasoudi, H.H.; Alharthi, A.A.; Gharib, A.F. Variolin B from Sea Sponge against Lung Cancer: A Multitargeted Molecular Docking with Fingerprinting and Molecular Dynamics Simulation Study. J. Biomol. Struct. Dyn. 2024, 42, 3507–3519. [Google Scholar] [CrossRef]
- Zayed, A.O.H.; Altarabeen, M.; AlShamaileh, E.; Zain, S.M. The Potential of Some Functional Group Compounds Substituted 8-Manzamine A as RSK1 Inhibitors: Molecular Docking and Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.I.A.; Ramzi, M.M.; Rawi, N.N.; Siong, J.Y.F.; Bakar, K.; Bhubalan, K.; Ariffin, F.; Saidin, J.; Azemi, A.K.; Ismail, N. Characterization of Antibiofilm Compound from Marine Sponge Stylissa Carteri. Environ. Sci. Pollut. Res. 2024, 31, 37552–37563. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, J.T.; Bayu, A.; Aryati, W.D.; Fernandes, C.; Yanuar, A.; Kijjoa, A.; Putra, M.Y. Secondary Metabolites from Marine-Derived Bacteria with Antibiotic and Antibiofilm Activities against Drug-Resistant Pathogens. Mar. Drugs 2023, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Sivaganesan, P.; VL, S.; Sahoo, A.; Elanchezhian, C.; Nataraj, G.; Chaudhuri, S. A Comprehensive Review of Synthetic Approaches Toward Lamellarin D and Its Analogous. ChemistrySelect 2024, 9, e202403112. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, D.; Mandal, R.D.; Das, A.R. Harnessing the Benzyne Insertion Consequence to Enable π-Extended Pyrido-Acridine and Quinazolino-Phenanthridine. Org. Biomol. Chem. 2024, 22, 5591–5602. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Parween, G.; Khator, R.; Monga, V. A Comprehensive Review of Apigenin a Dietary Flavonoid: Biological Sources, Nutraceutical Prospects, Chemistry and Pharmacological Insights and Health Benefits. Crit. Rev. Food Sci. Nutr. 2024, 1–37. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, J.; El-Seedi, H.R.; Woźniak, K.S.; Daglia, M.; Little, P.J.; Weng, J.; Xu, S. Kaempferol and Atherosclerosis: From Mechanism to Medicine. Crit. Rev. Food Sci. Nutr. 2024, 64, 2157–2175. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; Ponce González, X.P.; Hernández-Savedra, N.Y. Antimicrobial and Anticarcinogenic Activity of Bioactive Peptides Derived from Abalone Viscera (Haliotis fulgens and Haliotis corrugata). Sci. Rep. 2023, 13, 15185. [Google Scholar] [CrossRef]
- Zhu, X.; Ding, G.; Ren, S.; Xi, J.; Liu, K. The Bioavailability, Absorption, Metabolism, and Regulation of Glucolipid Metabolism Disorders by Quercetin and Its Important Glycosides: A Review. Food Chem. 2024, 458, 140262. [Google Scholar] [CrossRef]
- Yin, Q.; Qin, W.; Liu, T.; Song, W.; Yang, Y.; Shan, W.; Kuang, J.; Chen, J.; Lu, W. MaMYB4 Is Involved in the Accumulation of Naringenin Chalcone, Phloretin and Dihydrokaempferol in the Peels of Banana Fruit under Chilling Injury. Postharvest Biol. Technol. 2024, 212, 112844. [Google Scholar] [CrossRef]
- Mod Razif, M.R.F.; Chan, S.Y.; Chew, Y.-L.; Hassan, M.; Ahmad Hisham, S.; Abdul Rahman, S.; Mai, C.-W.; Teo, M.Y.M.; Kee, P.E.; Khoo, K.S.; et al. Recent Developments in Luteolin-Loaded Nanoformulations for Enhanced Anti-Carcinogenic Activities: Insights from In Vitro and In Vivo Studies. Sci 2024, 6, 68. [Google Scholar] [CrossRef]
- Trivedi, M.; Vasani, P.; Sonowal, A.; Mukherjee, R.; Chatterjee, A.; Banerjee, D. Marine Biotherapeutics: Delving Into The Vast Untapped Resource Of Therapeutic Molecules Showing Better Outcomes Against Diseases. Educ. Adm. Theory Pract. 2024, 30, 01–07. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Farooqi, A.A.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Opportunities and Challenges of Fucoidan for Tumors Therapy. Carbohydr. Polym. 2024, 324, 121555. [Google Scholar] [CrossRef] [PubMed]
- Unger-Manhart, N.; Morokutti-Kurz, M.; Zieglmayer, P.; Lemell, P.; Savli, M.; Zieglmayer, R.; Prieschl-Grassauer, E. Carrageenan-Containing Nasal Spray Alleviates Allergic Symptoms in Participants with Grass Pollen Allergy: A Randomized, Controlled, Crossover Clinical Trial. Int. J. Gen. Med. 2024, 17, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.D.; Donado-Pestana, C.M.; More, T.H.; Duarte, G.B.S.; Duarte, S.G.; Dias, C.G.; Rodrigues, L.; Hernandez, G.N.; Fock, R.; Hiller, K.; et al. D-Limonene Supplementation Does Not Alter Postprandial Metabolism of Postmenopausal Women Challenged with a Mixed Macronutrient Tolerance Test: A Pilot Study. Food Prod. Process. Nutr. 2024, 6, 26. [Google Scholar] [CrossRef]
- Nagarajan, P.; Louis, L.R.P.; Patil, S.J.; Adam, J.K.; Krishna, S.B.N. Therapeutic Potential of Biologically Active Peptides from Marine Organisms for Biomedical Applications. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2024; Volume 81, pp. 467–500. [Google Scholar]
- Malyarenko, T.V.; Kicha, A.A.; Kuzmich, A.S.; Malyarenko, O.S.; Kalinovsky, A.I.; Popov, R.S.; Dmitrenok, P.S.; Ivanchina, N.V.; Stonik, V.A. New Rare Triterpene Glycosides from Pacific Sun Star, Solaster Pacificus, and Their Anticancer Activity. Mar. Drugs 2024, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.S.; Dyshlovoy, S.A.; Venz, S.; Jacobsen, C.; Hauschild, J.; Rohlfing, T.; Silchenko, A.S.; Avilov, S.A.; Balabanov, S.; Bokemeyer, C.; et al. Anticancer Activity of the Marine Triterpene Glycoside Cucumarioside A2-2 in Human Prostate Cancer Cells. Mar. Drugs 2024, 22, 20. [Google Scholar] [CrossRef]
- Niu, F.; Xie, W.; Zhang, W.; Kawuki, J.; Yu, X. Vitamin C, Vitamin E, β-Carotene and Risk of Parkinson’s Disease: A Systematic Review and Dose–Response Meta-Analysis of Observational Studies. Nutr. Neurosci. 2024, 27, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Ngamcharungchit, C.; Chaimusik, N.; Panbangred, W.; Euanorasetr, J.; Intra, B. Bioactive Metabolites from Terrestrial and Marine Actinomycetes. Molecules 2023, 28, 5915. [Google Scholar] [CrossRef]
- Ramu, A.K.; Rajendran, R.; Sivalingam, A.M.; Seshadri, V.D.; Bakrudeen Ali Ahmed, A. Anticancer Potentiated Bioactive Compounds from Marine Flora. In Marine Antioxidants: Preparations, Syntheses, and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 421–432. ISBN 9780323950862. [Google Scholar]
- Goutzourelas, N.; Kevrekidis, D.P.; Barda, S.; Malea, P.; Trachana, V.; Savvidi, S.; Kevrekidou, A.; Assimopoulou, A.N.; Goutas, A.; Liu, M.; et al. Antioxidant Activity and Inhibition of Liver Cancer Cells’ Growth of Extracts from 14 Marine Macroalgae Species of the Mediterranean Sea. Foods 2023, 12, 1310. [Google Scholar] [CrossRef]
- Hsiao, H.H.; Wu, T.C.; Tsai, Y.H.; Kuo, C.H.; Huang, R.H.; Hong, Y.H.; Huang, C.Y. Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum Aquifolium. Mar. Drugs 2021, 19, 215. [Google Scholar] [CrossRef]
- Hussain, A.; Bourguet-Kondracki, M.L.; Majeed, M.; Ibrahim, M.; Imran, M.; Yang, X.W.; Ahmed, I.; Altaf, A.A.; Khalil, A.A.; Rauf, A.; et al. Marine Life as a Source for Breast Cancer Treatment: A Comprehensive Review. Biomed. Pharmacother. 2023, 159, 114165. [Google Scholar] [CrossRef]
- Dissanayake, D.S.; Nagahawatta, D.P.; Lee, J.-S.; Jeon, Y.-J. Immunomodulatory Effects of Halichondrin Isolated from Marine Sponges and Its Synthetic Analogs in Oncological Applications. Mar. Drugs 2024, 22, 426. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Olama, Z.A.; Abou-elela, G.M.; Ramadan, H.S.; Hegazy, G.E.; El Badan, D.E.S. Exploring the Antimicrobial, Antiviral, Antioxidant, and Antitumor Potentials of Marine Streptomyces Tunisiensis W4MT573222 Pigment Isolated from Abu-Qir Sediments, Egypt. Microb. Cell Fact. 2023, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; Araújo, R.G.; Rodríguez-Aguayo, A.A.; Guzmán-Román, M.; García-Venegas, J.C.; Nájera-Martínez, E.F.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldivar, R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants 2023, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Elshiekh, R.M.; Elissawy, A.M.; Nasser, A.; Singab, B. Unearthing the Potential Fungal Bioactive Secondary Metabolites from the Red Sea: A Comprehensive Review. Arch. Pharm. Sci. Ain Shams Univ. 2023, 7, 254–273. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. The Aromatic Ketone 4′-Hydroxychalcone Inhibits TNFα-Induced NF-ΚB Activation via Proteasome Inhibition. Biochem. Pharmacol. 2011, 82, 620–631. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB P65 and Strategies for Therapeutic Manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Teng, F.; Li, L.; Zhong, S.; Luo, H.; Huang, Z. Anticancer Effects of Marine Compounds Blocking the Nuclear Factor Kappa B Signaling Pathway. Mol. Biol. Rep. 2022, 49, 9975–9995. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.S.; Hay, N. The Regulation and Activities of the Multifunctional Serine/Threonine Kinase Akt/PKB. Exp. Cell Res. 1999, 253, 210–229. [Google Scholar] [CrossRef]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt Pathway in Human Cancer. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Hay, N. The Akt-MTOR Tango and Its Relevance to Cancer. Cancer Cell 2005, 8, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The Phosphatidylinositol 3-Kinase–AKT Pathway in Human Cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Wei, J.; Gou, Z.; Wen, Y.; Luo, Q.; Huang, Z. Marine Compounds Targeting the PI3K/Akt Signaling Pathway in Cancer Therapy. Biomed. Pharmacother. 2020, 129, 110484. [Google Scholar] [CrossRef]
- Parate, S.; Kumar, V.; Lee, G.; Rampogu, S.; Hong, J.C.; Lee, K.W. Marine-Derived Natural Products as ATP-Competitive MTOR Kinase Inhibitors for Cancer Therapeutics. Pharmaceuticals 2021, 14, 282. [Google Scholar] [CrossRef]
- Sabatini, D.M. MTOR and Cancer: Insights into a Complex Relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.T.; Gibbons, J.J. The Mammalian Target of Rapamycin Signaling Pathway: Twists and Turns in the Road to Cancer Therapy. Clin. Cancer Res. 2007, 13, 3109–3114. [Google Scholar] [CrossRef]
- Kim, L.C.; Cook, R.S.; Chen, J. MTORC1 and MTORC2 in Cancer and the Tumor Microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The Mitochondrial Membrane Potential (Δψm) in Apoptosis; an Update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Saelens, X.; Festjens, N.; Walle, L.V.; Gurp, M.v.; Van Loo, G.; Vandenabeele, P. Toxic Proteins Released from Mitochondria in Cell Death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a Mitochondrial Protein That Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- van Loo, G.; van Gurp, M.; Depuydt, B.; Srinivasula, S.M.; Rodriguez, I.; Alnemri, E.S.; Gevaert, K.; Vandekerckhove, J.; Declercq, W.; Vandenabeele, P. The Serine Protease Omi/HtrA2 Is Released from Mitochondria during Apoptosis. Omi Interacts with Caspase-Inhibitor XIAP and Induces Enhanced Caspase Activity. Cell Death Differ. 2002, 9, 20–26. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial Control of Apoptosis: The Role of Cytochrome c. Biochim. Biophys. Acta (BBA)-Bioenerg. 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome c Release from Mitochondria. Cell Death Differ 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M. The Apoptosome: Heart and Soul of the Cell Death Machine. Neoplasia 1999, 1, 5–15. [Google Scholar] [CrossRef]
- Hill, M.M.; Adrain, C.; Duriez, P.J.; Creagh, E.M.; Martin, S.J. Analysis of the Composition, Assembly Kinetics and Activity of Native Apaf-1 Apoptosomes. EMBO J. 2004, 23, 2134–2145. [Google Scholar] [CrossRef]
- Schimmer, A.D. Inhibitor of Apoptosis Proteins: Translating Basic Knowledge into Clinical Practice. Cancer Res. 2004, 64, 7183–7190. [Google Scholar] [CrossRef]
- Joza, N.; Susin, S.A.; Daugas, E.; Stanford, W.L.; Cho, S.K.; Li, C.Y.J.; Sasaki, T.; Elia, A.J.; Cheng, H.-Y.M.; Ravagnan, L.; et al. Essential Role of the Mitochondrial Apoptosis-Inducing Factor in Programmed Cell Death. Nature 2001, 410, 549–554. [Google Scholar] [CrossRef]
- Susin, S.A.; Daugas, E.; Ravagnan, L.; Samejima, K.; Zamzami, N.; Loeffler, M.; Costantini, P.; Ferri, K.F.; Irinopoulou, T.; Prévost, M.-C.; et al. Two Distinct Pathways Leading to Nuclear Apoptosis. J. Exp. Med. 2000, 192, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G Is an Apoptotic DNase When Released from Mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. A Caspase-Activated DNase That Degrades DNA during Apoptosis, and Its Inhibitor ICAD. Nature 1998, 391, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 Family: Regulators of the Cellular Life-or-Death Switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Schuler, M.; Green, D.R. Mechanisms of P53-Dependent Apoptosis. Biochem. Soc. Trans. 2001, 29, 684. [Google Scholar] [CrossRef]
- Nova, P.; Gomes, A.M.; Costa-Pinto, A.R. It Comes from the Sea: Macroalgae-Derived Bioactive Compounds with Anti-Cancer Potential. Crit. Rev. Biotechnol. 2024, 44, 462–476. [Google Scholar] [CrossRef]
- Delma, C.R.; Somasundaram, S.T.; Srinivasan, G.P.; Khursheed, M.; Bashyam, M.D.; Aravindan, N. Fucoidan from Turbinaria Conoides: A Multifaceted ‘Deliverable’ to Combat Pancreatic Cancer Progression. Int. J. Biol. Macromol. 2015, 74, 447–457. [Google Scholar] [CrossRef]
- Delma, C.R.; Thirugnanasambandan, S.; Srinivasan, G.P.; Raviprakash, N.; Manna, S.K.; Natarajan, M.; Aravindan, N. Fucoidan from Marine Brown Algae Attenuates Pancreatic Cancer Progression by Regulating P53–NFκB Crosstalk. Phytochemistry 2019, 167, 112078. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of Apoptosis by Phloroglucinol Derivative from Ecklonia Cava in MCF-7 Human Breast Cancer Cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Jin, L.; Zhao, Y.; Zhu, G.; Shen, W. Dieckol Inhibits Non-small–Cell Lung Cancer Cell Proliferation and Migration by Regulating the PI3K/AKT Signaling Pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22346. [Google Scholar] [CrossRef]
- Xu, J.W.; Yan, Y.; Wang, L.; Wu, D.; Ye, N.K.; Chen, S.H.; Li, F. Marine Bioactive Compound Dieckol Induces Apoptosis and Inhibits the Growth of Human Pancreatic Cancer Cells PANC-1. J. Biochem. Mol. Toxicol. 2021, 35, e22648. [Google Scholar] [CrossRef]
- Wu, T.-C.; Hong, Y.-H.; Tsai, Y.-H.; Hsieh, S.-L.; Huang, R.-H.; Kuo, C.-H.; Huang, C.-Y. Degradation of Sargassum Crassifolium Fucoidan by Ascorbic Acid and Hydrogen Peroxide, and Compositional, Structural, and In Vitro Anti-Lung Cancer Analyses of the Degradation Products. Mar. Drugs 2020, 18, 334. [Google Scholar] [CrossRef]
- Eo, H.J.; Kwon, T.-H.; Park, G.H.; Song, H.M.; Lee, S.-J.; Park, N.-H.; Jeong, J.B. In Vitro Anticancer Activity of Phlorofucofuroeckol A via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells. Mar. Drugs 2016, 14, 69. [Google Scholar] [CrossRef]
- Ku, H.-C.; Cheng, C.-F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Liao, W.; Chen, Y.; Shan, S.; Chen, Z.; Wen, Y.; Chen, W.; Zhao, C. Marine Algae-derived Characterized Bioactive Compounds as Therapy for Cancer: A Review on Their Classification, Mechanism of Action, and Future Perspectives. Phytother. Res. 2024, 38, 4053–4080. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Luque-Ortega, J.R.; Rivas, L.; Albericio, F. Kahalalide F, an Antitumor Depsipeptide in Clinical Trials, and Its Analogues as Effective Antileishmanial Agents. Mol. Pharm. 2009, 6, 813–824. [Google Scholar] [CrossRef] [PubMed]
- García-Rocha, M.; Bonay, P.; Avila, J. The Antitumoral Compound Kahalalide F Acts on Cell Lysosomes. Cancer Lett. 1996, 99, 43–50. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Luo, H.; Yang, Y. Selenium-Containing Polysaccharide-Protein Complex in Se-Enriched Ulva Fasciata Induces Mitochondria-Mediated Apoptosis in A549 Human Lung Cancer Cells. Mar. Drugs 2017, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Kim, I.-H.; Nam, T.-J. Inhibition of AGS Human Gastric Cancer Cell Invasion and Proliferation by Capsosiphon Fulvescens Glycoprotein. Mol. Med. Rep. 2013, 8, 11–16. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2009, 26, 170. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Z.-J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and Potential Antitumor Activity of Polysaccharide from Gracilariopsis Lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- S.M., F.M.B.; Chitra, K.; Joseph, B.; Sundararajan, R.; S., H. Gelidiella Acerosa Inhibits Lung Cancer Proliferation. BMC Complement. Altern. Med. 2018, 18, 104. [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2006, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Tarhouni-Jabberi, S.; Zakraoui, O.; Ioannou, E.; Riahi-Chebbi, I.; Haoues, M.; Roussis, V.; Kharrat, R.; Essafi-Benkhadir, K. Mertensene, a Halogenated Monoterpene, Induces G2/M Cell Cycle Arrest and Caspase Dependent Apoptosis of Human Colon Adenocarcinoma HT29 Cell Line through the Modulation of ERK-1/-2, AKT and NF-ΚB Signaling. Mar. Drugs 2017, 15, 221. [Google Scholar] [CrossRef]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-Inhibitory Effects of the Astaxanthin-Rich Alga Haematococcus Pluvialis in Human Colon Cancer Cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-Sitosterol Induces G1 Arrest and Causes Depolarization of Mitochondrial Membrane Potential in Breast Carcinoma MDA-MB-231 Cells. BMC Complement. Altern. Med. 2013, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Laube, T.; Beil, W.; Seifert, K. Total Synthesis of Two 12-Nordrimanes and the Pharmacological Active Sesquiterpene Hydroquinone Yahazunol. Tetrahedron 2005, 61, 1141–1148. [Google Scholar] [CrossRef]
- Velatooru, L.R.; Baggu, C.B.; Janapala, V.R. Spatane Diterpinoid from the Brown Algae, Stoechospermum Marginatum Induces Apoptosis via ROS Induced Mitochondrial Mediated Caspase Dependent Pathway in Murine B16F10 Melanoma Cells. Mol. Carcinog. 2016, 55, 2222–2235. [Google Scholar] [CrossRef]
- Geisen, U.; Zenthoefer, M.; Peipp, M.; Kerber, J.; Plenge, J.; Managò, A.; Fuhrmann, M.; Geyer, R.; Hennig, S.; Adam, D.; et al. Molecular Mechanisms by Which a Fucus Vesiculosus Extract Mediates Cell Cycle Inhibition and Cell Death in Pancreatic Cancer Cells. Mar. Drugs 2015, 13, 4470–4491. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Guo, S.; Su, H.; Fan, X. The Antitumor Effect of Bromophenol Derivatives in Vitro and Leathesia Nana Extract in Vivo. Chin. J. Oceanol. Limnol. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl Bromophenols with Diverse Dimerization Patterns from the Brown Alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and Significance of C-KIT Receptor Tyrosine Kinase in Cancer: A Review. Bosn. J. Basic Med. Sci. 2022, 22, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.Y.; Lee, J.-Y.; Park, J.H.Y. Fucoidan Present in Brown Algae Induces Apoptosis of Human Colon Cancer Cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The Anti-Cancer Effects of Fucoidan: A Review of Both in Vivo and in Vitro Investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Chiu, Y.-H.; Chan, Y.-L.; Chiu, Y.-H.; Wang, H.; Huang, K.-C.; Li, T.-L.; Hsu, K.-H.; Wu, C.-J. Prophylactic Administration of Fucoidan Represses Cancer Metastasis by Inhibiting Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMPs) in Lewis Tumor-Bearing Mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Pan, H.-F.; Shao, S.-L.; Xu, X.-M. Anti-Tumor and Anti-Angiogenic Effects of Fucoidan on Prostate Cancer: Possible JAK-STAT3 Pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Karpiniec, S.S.; Dickinson, J.L. Fucoidan Suppresses the Growth of Human Acute Promyelocytic Leukemia Cells In Vitro and In Vivo. J. Cell. Physiol. 2016, 231, 688–697. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-ΚB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Silke, J.; O’Reilly, L.A. NF-ΚB and Pancreatic Cancer; Chapter and Verse. Cancers 2021, 13, 4510. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, B.; Wang, Z.; Yang, R. Alginic Acid Inhibits Non-Small Cell Lung Cancer-Induced Angiogenesis via Activating MiR-506 Expression. J. Nat. Med. 2021, 75, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin Inhibits Tumour-related Lymphangiogenesis and Growth of Breast Cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Imbs, T.I.; Ermakova, S.P. In Vitro Anticancer and Radiosensitizing Activities of Phlorethols from the Brown Alga Costaria Costata. Molecules 2020, 25, 3208. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2005, 22, 15. [Google Scholar] [CrossRef]
- López-Macià, À.; Jiménez, J.C.; Royo, M.; Giralt, E.; Albericio, F. Synthesis and Structure Determination of Kahalalide F 1,2. J. Am. Chem. Soc. 2001, 123, 11398–11401. [Google Scholar] [CrossRef]
- Pardo, B.; Paz-Ares, L.; Tabernero, J.; Ciruelos, E.; García, M.; Salazar, R.; López, A.; Blanco, M.; Nieto, A.; Jimeno, J.; et al. Phase I Clinical and Pharmacokinetic Study of Kahalalide F Administered Weekly as a 1-Hour Infusion to Patients with Advanced Solid Tumors. Clin. Cancer Res. 2008, 14, 1116–1123. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; Horenblas, S.; Meinhardt, W.; Stokvis, E.; de Reijke, T.M.; Jimeno, J.M.; Lopez-Lazaro, L.; Lopez Martin, J.A.; Beijnen, J.H.; Schellens, J.H.M. Phase I Clinical and Pharmacokinetic Study of Kahalalide F in Patients with Advanced Androgen Refractory Prostate Cancer. Clin. Cancer Res. 2005, 11, 1854–1862. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, G.; Wu, D.; Liu, D.; You, L.; Högger, P.; Simal-Gandara, J.; Wang, M.; da Costa, J.G.M.; Marunaka, Y.; et al. The Algal Polysaccharide Ulvan Suppresses Growth of Hepatoma Cells. Food Front. 2020, 1, 83–101. [Google Scholar] [CrossRef]
- Wang, Y.; An, E.-K.; Kim, S.-J.; You, S.; Jin, J.-O. Intranasal Administration of Codium Fragile Polysaccharide Elicits Anti-Cancer Immunity against Lewis Lung Carcinoma. Int. J. Mol. Sci. 2021, 22, 10608. [Google Scholar] [CrossRef]
- Surayot, U.; You, S. Structural Effects of Sulfated Polysaccharides from Codium Fragile on NK Cell Activation and Cytotoxicity. Int. J. Biol. Macromol. 2017, 98, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.L.; Jeong, S.; Kim, B.R.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; Kim, D.Y.; Kim, B.G.; et al. Codium Fragile F2 Sensitize Colorectal Cancer Cells to TRAIL-Induced Apoptosis via c-FLIP Ubiquitination. Biochem. Biophys. Res. Commun. 2019, 508, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Morgan, J.B.; Coothankandaswamy, V.; Liu, R.; Jekabsons, M.B.; Mahdi, F.; Nagle, D.G.; Zhou, Y.-D. The Caulerpa Pigment Caulerpin Inhibits HIF-1 Activation and Mitochondrial Respiration. J. Nat. Prod. 2009, 72, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Renju, G.L.; Muraleedhara Kurup, G.; Bandugula, V.R. Effect of Lycopene Isolated from Chlorella Marina on Proliferation and Apoptosis in Human Prostate Cancer Cell Line PC-3. Tumor Biol. 2014, 35, 10747–10758. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2010, 27, 165. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Roch, K.L.; Aalbersberg, W.; Kubanek, J. Antineoplastic Unsaturated Fatty Acids from Fijian Macroalgae. Phytochemistry 2008, 69, 2495–2500. [Google Scholar] [CrossRef]
- Iliopoulou, D.; Mihopoulos, N.; Vagias, C.; Papazafiri, P.; Roussis, V. Novel Cytotoxic Brominated Diterpenes from the Red Alga Laurencia o Btusa. J. Org. Chem. 2003, 68, 7667–7674. [Google Scholar] [CrossRef]
- Alarif, W.M.; Al-Lihaibi, S.S.; Ayyad, S.-E.N.; Abdel-Rhman, M.H.; Badria, F.A. Laurene-Type Sesquiterpenes from the Red Sea Red Alga Laurencia Obtusa as Potential Antitumor–Antimicrobial Agents. Eur. J. Med. Chem. 2012, 55, 462–466. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2014, 31, 160. [Google Scholar] [CrossRef]
- Pec, M.K.; Aguirre, A.; Moser-Thier, K.; Fernández, J.J.; Souto, M.L.; Dorta, J.; Diáz-González, F.; Villar, J. Induction of Apoptosis in Estrogen Dependent and Independent Breast Cancer Cells by the Marine Terpenoid Dehydrothyrsiferol. Biochem. Pharmacol. 2003, 65, 1451–1461. [Google Scholar] [CrossRef]
- Zaleta-Pinet, D.A.; Holland, I.P.; Muñoz-Ochoa, M.; Murillo-Alvarez, J.I.; Sakoff, J.A.; van Altena, I.A.; McCluskey, A. Cytotoxic Compounds from Laurencia Pacifica. Org. Med. Chem. Lett. 2014, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-M.; Mendis, E.; Kim, S.-K. Laurencia Okamurai Extract Containing Laurinterol Induces Apoptosis in Melanoma Cells. J. Med. Food 2008, 11, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sae-lao, T.; Luplertlop, N.; Janvilisri, T.; Tohtong, R.; Bates, D.O.; Wongprasert, K. Sulfated Galactans from the Red Seaweed Gracilaria Fisheri Exerts Anti-Migration Effect on Cholangiocarcinoma Cells. Phytomedicine 2017, 36, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2008, 25, 35. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J.; Fan, X.; He, L. Bromophenols Coupled with Methyl γ-Ureidobutyrate and Bromophenol Sulfates from the Red Alga Rhodomela confervoides. J. Nat. Prod. 2006, 69, 206–210. [Google Scholar] [CrossRef]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wheelhouse, R.T.; Wright, C.W. In-Vitro Cytotoxic Activities of the Major Bromophenols of the Red Alga Polysiphonia lanosa and Some Novel Synthetic Isomers. J. Nat. Prod. 2004, 67, 1445–1449. [Google Scholar] [CrossRef]
- Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine Bromophenol Bis (2,3-Dibromo-4,5-Dihydroxy-Phenyl)-Methane Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Modulating Β1-Integrin/FAK Signaling. Mar. Drugs 2015, 13, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2007, 24, 31. [Google Scholar] [CrossRef]
- Park, C.; Moon, D.-O.; Rhu, C.-H.; Choi, B.T.; Lee, W.H.; Kim, G.-Y.; Choi, Y.H. β-Sitosterol Induces Anti-Proliferation and Apoptosis in Human Leukemic U937 Cells through Activation of Caspase-3 and Induction of Bax/Bcl-2 Ratio. Biol. Pharm. Bull. 2007, 30, 1317–1323. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a Major Carotenoid in Isochrysis Aff. Galbana: Characterization of Extraction for Commercial Application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The Allenic Carotenoid Fucoxanthin, a Novel Marine Nutraceutical from Brown Seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Ahn, G.; Heo, S.-J.; Kang, S.-M.; Kang, M.-C.; Yang, H.-M.; Kim, D.; Roh, S.W.; Kim, S.-K.; Jeon, B.-T.; et al. Inhibition of Tumor Growth in Vitro and in Vivo by Fucoxanthin against Melanoma B16F10 Cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Karimian, H.; Khanabdali, R.; Razavi, M.; Firoozinia, M.; Zandi, K.; Abdul Kadir, H. Anticancer and Antitumor Potential of Fucoidan and Fucoxanthin, Two Main Metabolites Isolated from Brown Algae. Sci. World J. 2014, 2014, 768323. [Google Scholar] [CrossRef]
- Martin, L. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine Algal Carotenoids Inhibit Angiogenesis by Down-Regulating FGF-2-Mediated Intracellular Signals in Vascular Endothelial Cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In Vitro Anticancer Activity of Natural β-Carotene from Dunaliella Salina EU5891199 in PC-3 Cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Shamsuddin Ahmad, A.; Julius Siong, Y.F.; Fitrya Syamsumir, D.; Atikah Mohamed Zin, N.; Aisha Mohd Radzi, S.; Nur Islamiah Kassim, M.; Ariff Muzamel, M.; Ridzuan Yusof, M.; Chandra Segaran, T. The potential of carotenoids from marine tropical microalgae in the healing process of gastritis. J. Sustain. Sci. Manag. 2015, 10, 92–106. [Google Scholar]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary Lutein Modulates Growth and Survival Genes in Prostate Cancer Cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.; Swanson, H.; Rubin, L. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein Inhibits Breast Cancer Cell Growth by Suppressing Antioxidant and Cell Survival Signals and Induces Apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 515–527. [Google Scholar]
- Rybczyńska-Tkaczyk, K.; Grenda, A.; Jakubczyk, A.; Krawczyk, P. Natural Bacterial and Fungal Peptides as a Promising Treatment to Defeat Lung Cancer Cells. Molecules 2023, 28, 4381. [Google Scholar] [CrossRef]
- Kim, S.; Duncan, P.W.; Groban, L.; Segal, H.; Abbott, R.M.; Williamson, J.D. Patient-Reported Outcome Measures (PROM) as A Preoperative Assessment Tool. J. Anesth. Perioper. Med. 2017, 4, 274–281. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Katada, J.; Takahashi, J.; Uno, I.; Hayashi, Y. Synthesis and Biological Activities of Phenylahistin Derivatives. Bioorg. Med. Chem. 1999, 7, 1451–1457. [Google Scholar] [CrossRef]
- Ashok, A.; Doriya, K.; Rao, J.V.; Qureshi, A.; Tiwari, A.K.; Kumar, D.S. Microbes Producing L-Asparaginase Free of Glutaminase and Urease Isolated from Extreme Locations of Antarctic Soil and Moss. Sci. Rep. 2019, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Malhão, F.; Ramos, A.A.; Buttachon, S.; Dethoup, T.; Kijjoa, A.; Rocha, E. Cytotoxic and Antiproliferative Effects of Preussin, a Hydroxypyrrolidine Derivative from the Marine Sponge-Associated Fungus Aspergillus Candidus KUFA 0062, in a Panel of Breast Cancer Cell Lines and Using 2D and 3D Cultures. Mar. Drugs 2019, 17, 448. [Google Scholar] [CrossRef]
- Patra, S.; Praharaj, P.P.; Panigrahi, D.P.; Panda, B.; Bhol, C.S.; Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Jena, M.; Sethi, G.; et al. Bioactive Compounds from Marine Invertebrates as Potent Anticancer Drugs: The Possible Pharmacophores Modulating Cell Death Pathways. Mol. Biol. Rep. 2020, 47, 7209–7228. [Google Scholar] [CrossRef]
- Spicka, I.; Ocio, E.M.; Oakervee, H.E.; Greil, R.; Banh, R.H.; Huang, S.-Y.; D’Rozario, J.M.; Dimopoulos, M.A.; Martínez, S.; Extremera, S.; et al. Randomized Phase III Study (ADMYRE) of Plitidepsin in Combination with Dexamethasone vs. Dexamethasone Alone in Patients with Relapsed/Refractory Multiple Myeloma. Ann. Hematol. 2019, 98, 2139–2150. [Google Scholar] [CrossRef]
- Erba, E.; Bassano, L.; Di Liberti, G.; Muradore, I.; Chiorino, G.; Ubezio, P.; Vignati, S.; Codegoni, A.; Desiderio, M.A.; Faircloth, G.; et al. Cell Cycle Phase Perturbations and Apoptosis in Tumour Cells Induced by Aplidine. Br. J. Cancer 2002, 86, 1510–1517. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J.C. Other Approaches to Targeted Therapy. In Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2008; pp. 307–349. [Google Scholar]
- Song, F.-Q.; Liu, Y.; Kong, X.-S.; Chang, W.; Song, G. Progress on Understanding the Anticancer Mechanisms of Medicinal Mushroom: Inonotus Obliquus. Asian Pac. J. Cancer Prev. 2013, 14, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Rybczyńska-Tkaczyk, K.; Tobiasz, J.; Yelnytska-Stawasz, V.; Pomianek, T.; Gmiński, J. Biological and Anticancer Properties of Inonotus Obliquus Extracts. Process Biochem. 2018, 73, 180–187. [Google Scholar] [CrossRef]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal Metabolites with Anticancer Activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Greiner, J.F.W.; Kadhim, H.M.; Kaltschmidt, C. Subunit-Specific Role of NF-ΚB in Cancer. Biomedicines 2018, 6, 44. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Fernandez-Rodriguez, L.; Zhao, Y.; Monaco, G.; Trefny, M.P.; Yoshida, N.; Martin, K.; Sharma, A.; Olieric, N.; Shah, P.; et al. GEF-H1 Signaling upon Microtubule Destabilization Is Required for Dendritic Cell Activation and Specific Anti-Tumor Responses. Cell Rep. 2019, 28, 3367–3380.e8. [Google Scholar] [CrossRef]
- Matikas, A.; Georgoulias, V.; Kotsakis, A. Emerging Agents for the Prevention of Treatment Induced Neutropenia in Adult Cancer Patients. Expert Opin. Emerg. Drugs 2016, 21, 157–166. [Google Scholar] [CrossRef]
- Tonra, J.R.; Lloyd, G.K.; Mohanlal, R.; Huang, L. Plinabulin Ameliorates Neutropenia Induced by Multiple Chemotherapies through a Mechanism Distinct from G-CSF Therapies. Cancer Chemother. Pharmacol. 2020, 85, 461–468. [Google Scholar] [CrossRef]
- Gederaas, O.A.; Søgaard, C.D.; Viset, T.; Bachke, S.; Bruheim, P.; Arum, C.-J.; Otterlei, M. Increased Anticancer Efficacy of Intravesical Mitomycin C Therapy When Combined with a PCNA Targeting Peptide. Transl. Oncol. 2014, 7, 812–823. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Elshafei, A.; El-Ghonemy, D. Screening and Media Optimization for Enhancing L-Asparaginase Production, an Anticancer Agent, from Different Filamentous Fungi in Solid State Fermentation. Br. Biotechnol. J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Li, P.; Fan, Y.; Chen, H.; Chao, Y.; Du, N.; Chen, J. Phenylquinolinones with Antitumor Activity from the Indian Ocean-Derived Fungus Aspergillus Versicolor Y31-2. Chin. J. Oceanol. Limnol. 2016, 34, 1072–1075. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Zhou, T.; Zhao, Y.-Y.; Chen, L.; Gong, M.-W.; Xia, Q.-W.; Ying, M.-G.; Zheng, Q.-H.; Zhang, Q.-Q. Antitumor Effects and Related Mechanisms of Penicitrinine A, a Novel Alkaloid with a Unique Spiro Skeleton from the Marine Fungus Penicillium Citrinum. Mar. Drugs 2015, 13, 4733–4753. [Google Scholar] [CrossRef] [PubMed]
- Sirimangkalakitti, N.; Lin, J.; Harada, K.; Setiawan, A.; Arisawa, M.; Arai, M. Chemical Constituents and Anticancer Activities of Marine-Derived Fungus Trichoderma Lixii. Molecules 2024, 29, 2048. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.M.; Huyen, V.T.T.; Quyen, V.T.; Dao, P.T.; Quynh, D.T.; Huong, D.T.M.; Van Cuong, P.; Dat, T.T.H.; Minh, L.T.H. Antimicrobial and Cytotoxic Secondary Metabolites from a Marine-Derived Fungus Penicillium Citrinum VM6. Curr. Microbiol. 2024, 81, 32. [Google Scholar] [CrossRef] [PubMed]
- Virués-Segovia, J.R.; Pinedo, C.; Zorrilla, D.; Sánchez-Márquez, J.; Sánchez, P.; Ramos, M.C.; de la Cruz, M.; Aleu, J.; Durán-Patrón, R. Discovery of New Eremophilanes from the Marine-Derived Fungus Emericellopsis Maritima BC17 by Culture Conditions Changes: Evaluation of Cytotoxic and Antimicrobial Activities. Front. Mar. Sci. 2024, 11, 1386175. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Wang, H.; Xu, W.; Wang, C.; Ma, H.; Zhong, F.; Ou, J.; Luo, Z.; Luo, H.-B.; et al. Ergone Derivatives from the Deep-Sea-Derived Fungus Aspergillus Terreus YPGA10 and 25,28-Dihydroxyergone-Induced Apoptosis in Human Colon Cancer SW620 Cells. J. Nat. Prod. 2024, 87, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Tiwary, B.N. An Investigation on the Acrylamide Mitigation Potential of L-Asparaginase from BV-C Strain. Biocatal. Agric. Biotechnol. 2020, 27, 101677. [Google Scholar] [CrossRef]
- Jans, P.E.; Mfuh, A.M.; Arman, H.D.; Shaffer, C.V.; Larionov, O.V.; Mooberry, S.L. Cytotoxicity and Mechanism of Action of the Marine-Derived Fungal Metabolite Trichodermamide B and Synthetic Analogues. J. Nat. Prod. 2017, 80, 676–683. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Qi, X.; Li, D.; Zhu, T.; Mo, X. Anticancer Efficacy and Absorption, Distribution, Metabolism, and Toxicity Studies of Aspergiolide A in Early Drug Development. Drug Des. Dev. Ther. 2014, 8, 1965–1977. [Google Scholar] [CrossRef]
- Ankisetty, S.; Khan, S.; Avula, B.; Gochfeld, D.; Khan, I.; Slattery, M. Chlorinated Didemnins from the Tunicate Trididemnum Solidum. Mar. Drugs 2013, 11, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Almagthali, H.; Shaala, L.A.; Schmidt, E.W. Secondary Metabolites of the Genus Didemnum: A Comprehensive Review of Chemical Diversity and Pharmacological Properties. Mar. Drugs 2020, 18, 307. [Google Scholar] [CrossRef]
- Maroun, J.A.; Stewart, D.; Verma, S.; Eisenhauer, E. Phase I Clinical Study of Didemnin B. Investig. New Drugs 1998, 16, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Young, M.L.; Habermann, T.M.; Wolf, B.C.; Jimeno, J.; Cassileth, P.A. Phase II Trial of Didemnin B in Previously Treated Non-Hodgkin’s Lymphoma. Am. J. Clin. Oncol. Cancer Clin. Trial 2000, 23, 273–277. [Google Scholar] [CrossRef]
- Benvenuto, J.A.; Newman, R.A.; Bignami, G.S.; Raybould, T.J.G.; Raber, M.N.; Esparza, L.; Walters, R.S. Phase II Clinical and Pharmacological Study of Didemnin B in Patients with Metastatic Breast Cancer. Investig. New Drugs 1992, 10, 113–117. [Google Scholar] [CrossRef]
- Weiss, G.R.; Liu, P.Y.; O’Sullivan, J.; Alberts, D.S.; Brown, T.D.; Neefe, J.R.; Hutchins, L.F. A Randomized Phase II Trial of Trimetrexate or Didemnin B for the Treatment of Metastatic or Recurrent Squamous Carcinoma of the Uterine Cervix: A Southwest Oncology Group Trial. Gynecol. Oncol. 1992, 45, 303–306. [Google Scholar] [CrossRef]
- Vervoort, H.; Fenical, W.; Epifanio, R.d.A. Tamandarins A and B: New Cytotoxic Depsipeptides from a Brazilian Ascidian of the Family Didemnidae. J. Org. Chem. 2000, 65, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive Peptides from Marine Ascidians and Future Drug Development—A Review. Int. J. Pept. Res. Ther. 2018, 24, 13–18. [Google Scholar] [CrossRef]
- van Andel, L.; Fudio, S.; Rosing, H.; Munt, S.; Miguel-Lillo, B.; González, I.; Tibben, M.M.; de Vries, N.; de Vries Schultink, A.H.M.; Schellens, J.H.M.; et al. Pharmacokinetics and Excretion of 14C–Plitidepsin in Patients with Advanced Cancer. Investig. New Drugs 2017, 35, 589–598. [Google Scholar] [CrossRef]
- Broggini, M.; Marchini, S.V.; Galliera, E.; Borsotti, P.; Taraboletti, G.; Erba, E.; Sironi, M.; Jimeno, J.; Faircloth, G.T.; Giavazzi, R.; et al. Aplidine, a New Anticancer Agent of Marine Origin, Inhibits Vascular Endothelial Growth Factor (VEGF) Secretion and Blocks VEGF-VEGFR-1 (Flt-1) Autocrine Loop in Human Leukemia Cells MOLT-4. Leukemia 2003, 17, 52–59. [Google Scholar] [CrossRef]
- Ngandjui, Y.A.T.; Kereeditse, T.T.; Kamika, I.; Madikizela, L.M.; Msagati, T.A.M. Nutraceutical and Medicinal Importance of Marine Molluscs. Mar. Drugs 2024, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Kigoshi, H.; Kita, M. Antitumor Effects of Sea Hare-Derived Compounds in Cancer. In Handbook of Anticancer Drugs from Marine Origin; Springer International Publishing: Cham, Switzerland, 2015; pp. 701–739. [Google Scholar]
- Mir, R.; Karim, S.; Amjad Kamal, M.; Wilson, C.M.; Mirza, Z. Conotoxins: Structure, Therapeutic Potential and Pharmacological Applications. Curr. Pharm. Des. 2016, 22, 582–589. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Y.; Hua, H.; Li, D.; Tang, C. Marine Antitumor Peptide Dolastatin 10: Biological Activity, Structural Modification and Synthetic Chemistry. Mar. Drugs 2021, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Wesson, K.J.; Hamann, M.T. Keenamide A, a Bioactive Cyclic Peptide from the Marine Mollusk Pleurobranchus Forskalii. J. Nat. Prod. 1996, 59, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-Derived Drugs: Recent Advances in Cancer Therapy and Immune Signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Fontana, A.; Cavaliere, P.; Wahidulla, S.; Naik, C.G.; Cimino, G. A New Antitumor Isoquinoline Alkaloid from the Marine Nudibranch Jorunna Funebris. Tetrahedron 2000, 56, 7305–7308. [Google Scholar] [CrossRef]
- Lane, J.W.; Chen, Y.; Williams, R.M. Asymmetric Total Syntheses of (−)-Jorumycin, (−)-Renieramycin G, 3-epi-Jorumycin, and 3-epi-Renieramycin G. J. Am. Chem. Soc. 2005, 127, 12684–12690. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine Microorganisms as a Promising and Sustainable Source of Bioactive Molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef]
- Petsri, K.; Chamni, S.; Suwanborirux, K.; Saito, N.; Chanvorachote, P. Renieramycin T Induces Lung Cancer Cell Apoptosis by Targeting Mcl-1 Degradation: A New Insight in the Mechanism of Action. Mar. Drugs 2019, 17, 301. [Google Scholar] [CrossRef]
- Zovko, A.; Viktorsson, K.; Hååg, P.; Kovalerchick, D.; Färnegårdh, K.; Alimonti, A.; Ilan, M.; Carmeli, S.; Lewensohn, R. Marine Sponge Cribrochalina Vasculum Compounds Activate Intrinsic Apoptotic Signaling and Inhibit Growth Factor Signaling Cascades in Non–Small Cell Lung Carcinoma. Mol. Cancer Ther. 2014, 13, 2941–2954. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Huang, H.-L.; Lin, Y.-Y.; Tsui, K.-H.; Chen, P.-C.; Cheng, S.-Y.; Chong, I.-W.; Sung, P.-J.; Tai, M.-H.; Wen, Z.-H.; et al. BA6 Induces Apoptosis via Stimulation of Reactive Oxygen Species and Inhibition of Oxidative Phosphorylation in Human Lung Cancer Cells. Oxidative Med. Cell Longev. 2019, 2019, 6342104. [Google Scholar] [CrossRef]
- Roskelley, C.D.; Williams, D.E.; McHardy, L.M.; Leong, K.G.; Troussard, A.; Karsan, A.; Andersen, R.J.; Dedhar, S.; Roberge, M. Inhibition of Tumor Cell Invasion and Angiogenesis by Motuporamines. Cancer Res. 2001, 61, 6788–6794. [Google Scholar]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-Small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/MTOR Pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef]
- Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Echinoderms: A Review of Bioactive Compounds with Potential Health Effects. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 1–54. [Google Scholar]
- Thao, N.P.; Luyen, B.T.T.; Kim, E.J.; Kang, J.I.; Kang, H.K.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Van Minh, C.; Kim, Y.H. Steroidal Constituents from the Edible Sea Urchin Diadema savignyi Michelin Induce Apoptosis in Human Cancer Cells. J. Med. Food 2015, 18, 45–53. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lee, S.H. Therapeutic Application of Diverse Marine-Derived Natural Products in Cancer Therapy. Anticancer Res. 2019, 39, 5261–5284. [Google Scholar] [CrossRef]
- Wargasetia, T.; Permana, S.; Widodo, N. Potential Use of Compounds from Sea Cucumbers as MDM2 and CXCR4 Inhibitors to Control Cancer Cell Growth. Exp. Ther. Med. 2018, 6, 2985–2991. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Wätjen, W.; Ebada, S.S.; Bergermann, A.; Chovolou, Y.; Totzke, F.; Kubbutat, M.H.G.; Lin, W.; Proksch, P. Cytotoxic Effects of the Anthraquinone Derivatives 1′-Deoxyrhodoptilometrin and (S)-(−)-Rhodoptilometrin Isolated from the Marine Echinoderm Comanthus sp. Arch. Toxicol. 2017, 91, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.S.; Chingizova, E.A.; Pislyagin, E.A.; Yurchenko, E.A.; Klimovich, A.A.; Zelepuga, E.A.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S. Mechanisms of Action of Sea Cucumber Triterpene Glycosides Cucumarioside A0-1 and Djakonovioside A Against Human Triple-Negative Breast Cancer. Mar. Drugs 2024, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Menis, J.; Twelves, C. Eribulin (Halaven): A New, Effective Treatment for Women with Heavily Pretreated Metastatic Breast Cancer. Breast Cancer Targets Ther. 2011, 3, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Uemura, D. Halichondrins-Antitumor Polyether Macrolides from a Marine Sponge. Pure Appl. Chem. 1986, 58, 701–710. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ito, T.; Win, N.N.; Vo, H.Q.; Nguyen, H.T.; Morita, H. A New Sterol from the Vietnamese Marine Sponge Xestospongia Testudinaria and Its Biological Activities. Nat. Prod. Res. 2019, 33, 1175–1181. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Habib, E.S.; Eltahawy, N.A.; Hassanean, H.A.; Ibrahim, A.K.; Mohammed, A.F.; Fayez, S.; Hayallah, A.M.; Yamada, K.; Behery, F.A.; et al. New Cytotoxic Natural Products from the Red Sea Sponge Stylissa Carteri. Mar. Drugs 2020, 18, 241. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A.; et al. Bioactive Brominated Oxindole Alkaloids from the Red Sea Sponge Callyspongia Siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef]
- Olsen, E.K.; Søderholm, K.L.; Isaksson, J.; Andersen, J.H.; Hansen, E. Metabolomic Profiling Reveals the N -Acyl-Taurine Geodiataurine in Extracts from the Marine Sponge Geodia macandrewii (Bowerbank). J. Nat. Prod. 2016, 79, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, Y.-L.; Yu, X.-Y.; Pan, J.-Y.; Liu, X.-L.; Hong, L.-L.; Wang, B. Meroterpenoids from Marine Sponge Hyrtios sp. and Their Anticancer Activity against Human Colorectal Cancer Cells. Mar. Drugs 2024, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.T.; Hirano, S.; Fukuhara, S.; Watanabe, T.; Kanoh, N.; Iwabuchi, Y.; Usui, T.; Kataoka, T. Irciniastatin A Induces Potent and Sustained Activation of Extracellular Signal-Regulated Kinase and Thereby Promotes Ectodomain Shedding of Tumor Necrosis Factor Receptor 1 in Human Lung Carcinoma A549 Cells. Biol. Pharm. Bull. 2015, 38, 941–946. [Google Scholar] [CrossRef]
- Kashman, Y.; Hirsh, S.; McConnell, O.J.; Ohtani, I.; Kusumi, T.; Kakisawa, H. Ptilomycalin A: A Novel Polycyclic Guanidine Alkaloid of Marine Origin. J. Am. Chem. Soc. 1989, 111, 8925–8926. [Google Scholar] [CrossRef]
- Ye, X.; Anjum, K.; Song, T.; Wang, W.; Liang, Y.; Chen, M.; Huang, H.; Lian, X.-Y.; Zhang, Z. Antiproliferative Cyclodepsipeptides from the Marine Actinomycete Streptomyces sp. P11-23B Downregulating the Tumor Metabolic Enzymes of Glycolysis, Glutaminolysis, and Lipogenesis. Phytochemistry 2017, 135, 151–159. [Google Scholar] [CrossRef]
- Rettori, D.; Durán, N. Production, Extraction and Purificationof Violacein: An Antibiotic Pigment Producedby Chromobacterium Violaceum. World J. Microbiol. Biotechnol. 1998, 14, 685–688. [Google Scholar] [CrossRef]
- Sánchez, C.; Braña, A.F.; Méndez, C.; Salas, J.A. Reevaluation of the Violacein Biosynthetic Pathway and Its Relationship to Indolocarbazole Biosynthesis. ChemBioChem 2006, 7, 1231–1240. [Google Scholar] [CrossRef]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, A.; Hosokawa, K.; Soliev, A.B.; Osaka, I.; Arakawa, R.; Enomoto, K. Cytotoxic Prodigiosin Family Pigments from Pseudoalteromonas sp. 1020R Isolated from the Pacific Coast of Japan. Biosci. Biotechnol. Biochem. 2012, 76, 1229–1232. [Google Scholar] [CrossRef]
- Gerber, N.N.; Gauthier, M.J. New Prodigiosin-like Pigment from Alteromonas Rubra. Appl. Environ. Microbiol. 1979, 37, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Fehér, D.; Barlow, R.S.; Lorenzo, P.S.; Hemscheidt, T.K. A 2-Substituted Prodiginine, 2-(p-Hydroxybenzyl)Prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008, 71, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Riera-Romo, M.; Wilson-Savón, L.; Hernandez-Balmaseda, I. Metabolites from Marine Microorganisms in Cancer, Immunity, and Inflammation: A Critical Review. J. Pharm. Pharmacogn. Res. 2020, 8, 368–391. [Google Scholar] [CrossRef]
- Choi, S.Y.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. Biomed. Res. Int. 2015, 2015, 465056. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.-S.; Park, S.; Kim, J.; Kang, S.-J.; Lee, M.-H.; Ryu, S.; Choi, J.M.; Oh, T.-K.; Yoon, J.-H. Exceptional Production of Both Prodigiosin and Cycloprodigiosin as Major Metabolic Constituents by a Novel Marine Bacterium, Zooshikella Rubidus S1-1. Appl. Environ. Microbiol 2011, 77, 4967–4973. [Google Scholar] [CrossRef]
- Takahashi, Y.; Omura, S. Isolation of New Actinomycete Strains for the Screening of New Bioactive Compounds. J. Gen. Appl. Microbiol. 2003, 49, 141–154. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Kim, S.-K. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef]
- Choi, I.-K.; Shin, H.J.; Lee, H.-S.; Kwon, H.J. Streptochlorin, a Marine Natural Product, Inhibits NF-KappaB Activation and Suppresses Angiogenesis in Vitro. J. Microbiol. Biotechnol. 2007, 17, 1338–1343. [Google Scholar]
- Hughes, C.C.; MacMillan, J.B.; Gaudêncio, S.P.; Jensen, P.R.; Fenical, W. The Ammosamides: Structures of Cell Cycle Modulators from a Marine-Derived Streptomyces Species. Angew. Chem. Int. Ed. 2009, 48, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Ma, M.; Wang, N.; Zhou, Y.; Zhang, Z. Antiproliferative Metabolites against Glioma Cells from the Marine-Associated Actinomycete Streptomyces sp. ZZ735. Fitoterapia 2024, 178, 106176. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, W.; Liu, X.; Zaleta-Pinet, D.A.; Clark, B.R. Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018. Mar. Drugs 2019, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Nguyen, H.T.; Yu, N.H.; Yu, W.-J.; Kwon, Y.M.; Bae, S.S.; Choi, G.; Kim, J.-C. In Vitro and in Vivo Antimicrobial Activity of the Fungal Metabolite Toluquinol against Phytopathogenic Bacteria. Front. Microbiol. 2023, 14, 1221865. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Firli, L.N.; Rani, D.M.; Hidayatiningsih, A.; Lestari, N.D.; Wongso, H.; Tarman, K.; Rahaweman, A.C.; Manurung, J.; Ariantari, N.P.; et al. Indonesian Marine and Its Medicinal Contribution. Nat. Prod. Bioprospect. 2023, 13, 38. [Google Scholar] [CrossRef]
- Dou, Q.; Zonder, J. Overview of Proteasome Inhibitor-Based Anti-Cancer Therapies: Perspective on Bortezomib and Second Generation Proteasome Inhibitors versus Future Generation Inhibitors of Ubiquitin-Proteasome System. Curr. Cancer Drug Targets 2014, 14, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Orlowski, R.Z. The proteasome and proteasome inhibitors in cancer therapy. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 189–213. [Google Scholar] [CrossRef]
- Seyed, M.A.; Ayesha, S. Marine-Derived Pipeline Anticancer Natural Products: A Review of Their Pharmacotherapeutic Potential and Molecular Mechanisms. Futur. J. Pharm. Sci. 2021, 7, 203. [Google Scholar] [CrossRef]
- Twombly, R. First Proteasome Inhibitor Approved for Multiple Myeloma. JNCI J. Natl. Cancer Inst. 2003, 95, 845. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.M.; Tepper, J.E.; Baldwin, A.S.; Liu, R.; Adams, J.; Elliott, P.; Cusack, J.C. Enhancement of Radiosensitivity by Proteasome Inhibition: Implications for a Role of NF-ΚB. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Richardson, P.; Mitsiades, C.; Mitsiades, N.; Hayashi, T.; Munshi, N.; Dang, L.; Castro, A.; Palombella, V.; et al. NF-ΚB as a Therapeutic Target in Multiple Myeloma. J. Biol. Chem. 2002, 277, 16639–16647. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, O. Targeting of NF-KappaB Signaling Pathway, Other Signaling Pathways and Epigenetics in Therapy of Multiple Myeloma. Cardiovasc. Hematol. Disord.-Drug Targets 2013, 13, 16–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Schrader, K.K.; Elsohly, H.N.; Takamatsu, S. New Cell-Cell Adhesion Inhibitors from Streptomyces sp. UMA-044. J. Antibiot. 2003, 56, 673–681. [Google Scholar] [CrossRef]
- Zimmerman, T.; Blanco, F. Inhibitors Targeting the LFA-1/ICAM-1 Cell-Adhesion Interaction: Design and Mechanism of Action. Curr. Pharm. Des. 2008, 14, 2128–2139. [Google Scholar] [CrossRef]
- Cossío, F.P.; Mendoza, M.L.; Zubia, A.; Valcárcel, M.; Vara, Y.I.; Solaun, M.S.; López, J.J.; San Sebastian, E. Novel Inhibitors of the Lfa-1/Icam-1 Interaction and Uses Thereof. WIPO Patent WO/2007, 2007. p. 54128. [Google Scholar]
- Park, C.; Shin, H.J.; Kim, G.-Y.; Kwon, T.K.; Nam, T.-J.; Kim, S.-K.; Cheong, J.; Choi, I.-W.; Choi, Y.H. Induction of Apoptosis by Streptochlorin Isolated from Streptomyces sp. in Human Leukemic U937 Cells. Toxicol. In Vitro 2008, 22, 1573–1581. [Google Scholar] [CrossRef]
- Shin, D.Y.; Shin, H.J.; Kim, G.-Y.; Cheong, J.; Choi, I.-W.; Kim, S.-K.; Moon, S.-K.; Kang, H.S.; Choi, Y.H. Streptochlorin Isolated from Streptomyces sp. Induces Apoptosis in Human Hepatocarcinoma Cells through a Reactive Oxygen Species-Mediated Mitochondrial Pathway. J. Microbiol. Biotechnol. 2008, 18, 1862–1868. [Google Scholar] [CrossRef]
- Hawas, U.W.; Shaaban, M.; Shaaban, K.A.; Speitling, M.; Maier, A.; Kelter, G.; Fiebig, H.H.; Meiners, M.; Helmke, E.; Laatsch, H. Mansouramycins A−D, Cytotoxic Isoquinolinequinones from a Marine Streptomycete. J. Nat. Prod. 2009, 72, 2120–2124. [Google Scholar] [CrossRef]
- Nalini, S.; Sandy Richard, D.; Mohammed Riyaz, S.U.; Kavitha, G.; Inbakandan, D. Antibacterial Macro Molecules from Marine Organisms. Int. J. Biol. Macromol. 2018, 115, 696–710. [Google Scholar] [CrossRef]
- Kareh, M.; El Nahas, R.; Al-Aaraj, L.; Al-Ghadban, S.; Naser Al Deen, N.; Saliba, N.; El-Sabban, M.; Talhouk, R. Anti-Proliferative and Anti-Inflammatory Activities of the Sea Cucumber Holothuria polii Aqueous Extract. SAGE Open Med. 2018, 6. [Google Scholar] [CrossRef]
- Han, H.; Yi, Y.-H.; Li, L.; Liu, B.-S.; Pan, M.-X.; Yan, B.; Wang, X.-H. Triterpene Glycosides from Sea Cucumber Holothuria leucospilota. Chin. J. Nat. Med. 2010, 7, 346–350. [Google Scholar] [CrossRef]
- Han, H.; Zhang, W.; Yi, Y.; Liu, B.; Pan, M.; Wang, X. A Novel Sulfated Holostane Glycoside from Sea Cucumber Holothuria leucospilota. Chem. Biodivers. 2010, 7, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A Comparison between Two Brine Shrimp Assays to Detect in Vitrocytotoxicity in Marine Natural Products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.; Jia, H.; Zhou, Y.-D.; Nagle, D.G. Benzochromenones from the Marine Crinoid Comantheria Rotula Inhibit Hypoxia-Inducible Factor-1 (HIF-1) in Cell-Based Reporter Assays and Differentially Suppress the Growth of Certain Tumor Cell Lines. J. Nat. Prod. 2007, 70, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.; Mohammed, A.; Rao, C. Sea Cucumbers Metabolites as Potent Anti-Cancer Agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.X.; Vien, L.T.; Hoang, L.; Hanh, T.T.H.; Thao, D.T.; Van Thanh, N.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C. Van Cytotoxic Triterpene Diglycosides from the Sea Cucumber Stichopus Horrens. Bioorg. Med. Chem. Lett. 2017, 27, 2939–2942. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Wang, J.; Li, H.; Long, T.; Li, Z.; Wang, Y.; Dong, P.; Xue, C. In Vitro and in Vivo Anti-tumour Activities of Echinoside A and Ds -echinoside A from Pearsonothuria graeffei. J. Sci. Food Agric. 2012, 92, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Z.; Xue, Y.; Wang, J.; Li, H.; Tang, Q.; Wang, Y.; Dong, P.; Xue, C. Ds-Echinoside A, a New Triterpene Glycoside Derived from Sea Cucumber, Exhibits Antimetastatic Activity via the Inhibition of NF-ΚB-Dependent MMP-9 and VEGF Expressions. J. Zhejiang Univ. Sci. B 2011, 12, 534–544. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Stepanov, V.G.; Dong, Z.; Stonik, V.A. Constituents of the Sea Cucumber Cucumaria okhotensis. Structures of Okhotosides B1–B3 and Cytotoxic Activities of Some Glycosides from This Species. J. Nat. Prod. 2008, 71, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Roginsky, A.B.; Ding, X.-Z.; Woodward, C.; Collin, P.; Newman, R.A.; Bell, J.R.H.; Adrian, T.E. Review of the Apoptosis Pathways in Pancreatic Cancer and the Anti-apoptotic Effects of the Novel Sea Cucumber Compound, Frondoside A. Ann. New York Acad. Sci. 2008, 1138, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Madanchi, R.; Hauschild, J.; Otte, K.; Alsdorf, W.H.; Schumacher, U.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Honecker, F.; et al. The Marine Triterpene Glycoside Frondoside A Induces P53-Independent Apoptosis and Inhibits Autophagy in Urothelial Carcinoma Cells. BMC Cancer 2017, 17, 93. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Liu, Z.; Li, H.; Wang, J.; Li, Z.; Wang, Y.; Dong, P.; Xue, C. Differential Effects of Sulfated Triterpene Glycosides, Holothurin A 1, and 24-Dehydroechinoside A, on Antimetastasic Activity via Regulation of the MMP-9 Signal Pathway. J. Food Sci. 2010, 75, H285–H288. [Google Scholar] [CrossRef]
- Wang, W.; Hong, J.; Lee, C.-O.; Im, K.S.; Choi, J.S.; Jung, J.H. Cytotoxic Sterols and Saponins from the Starfish Certonardoa semiregularis. J. Nat. Prod. 2004, 67, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Russo, M.; Castellano, I.; Napolitano, A.; Palumbo, A. Ovothiol Isolated from Sea Urchin Oocytes Induces Autophagy in the Hep-G2 Cell Line. Mar. Drugs 2014, 12, 4069–4085. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hsieh, H.J.; Hwang, D. Cytotoxic and Apoptotic Activities of the Plancitoxin I from the Venom of Crown-of-thorns Starfish (Acanthaster planci) on A375.S2 Cells. J. Appl. Toxicol. 2015, 35, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Hsieh, H.J.; Hsieh, C.-H.; Hwang, D.-F. Plancitoxin I from the Venom of Crown-of-Thorns Starfish (Acanthaster planci) Induces Oxidative and Endoplasmic Reticulum Stress Associated Cytotoxicity in A375.S2 Cells. Exp. Mol. Pathol. 2015, 99, 7–15. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, X.; Tian, F.; Yi, Y.; Xu, Q.; Li, L.; Tong, L.; Lin, L.; Ding, J. Philinopside a, a Novel Marine-derived Compound Possessing Dual Anti-angiogenic and Anti-tumor Effects. Int. J. Cancer 2005, 114, 843–853. [Google Scholar] [CrossRef]
- Tian, F.; Zhu, C.; Zhang, X.; Xie, X.; Xin, X.; Yi, Y.; Lin, L.; Geng, M.; Ding, J. Philinopside E, a New Sulfated Saponin from Sea Cucumber, Blocks the Interaction between Kinase Insert Domain-Containing Receptor (KDR) and Alphavbeta3 Integrin via Binding to the Extracellular Domain of KDR. Mol. Pharmacol 2007, 72, 545–552. [Google Scholar] [CrossRef]
- Shin, D.M.; Holoye, P.Y.; Forman, A.; Winn, R.; Perez-Soler, R.; Dakhil, S.; Rosenthal, J.; Raber, M.N.; Hong, W.K. Phase II Clinical Trial of Didemnin B in Previously Treated Small Cell Lung Cancer. Investig. New Drugs 1994, 12, 243–249. [Google Scholar] [CrossRef]
- Maroun, J.A.; Belanger, K.; Seymour, L.; Matthews, S.; Roach, J.; Dionne, J.; Soulieres, D.; Stewart, D.; Goel, R.; Charpentier, D.; et al. Phase I Study of Aplidine in a Daily×5 One-Hour Infusion Every 3 Weeks in Patients with Solid Tumors Refractory to Standard Therapy. A National Cancer Institute of Canada Clinical Trials Group Study: NCIC CTG IND 115. Ann. Oncol. 2006, 17, 1371–1378. [Google Scholar] [CrossRef]
- Faivre, S.; Chièze, S.; Delbaldo, C.; Ady-vago, N.; Guzman, C.; Lopez-Lazaro, L.; Lozahic, S.; Jimeno, J.; Pico, F.; Armand, J.P.; et al. Phase I and Pharmacokinetic Study of Aplidine, a New Marine Cyclodepsipeptide in Patients With Advanced Malignancies. J. Clin. Oncol. 2005, 23, 7871–7880. [Google Scholar] [CrossRef]
- Kasper, B.; Reichardt, P.; Pink, D.; Sommer, M.; Mathew, M.; Rauch, G.; Hohenberger, P. Combination of Trabectedin and Gemcitabine for Advanced Soft Tissue Sarcomas: Results of a Phase I Dose Escalating Trial of the German Interdisciplinary Sarcoma Group (GISG). Mar. Drugs 2015, 13, 379–388. [Google Scholar] [CrossRef]
- Fabbroni, C.; Grignani, G.; Vincenzi, B.; Fumagalli, E.; De Pas, T.M.; Mazzocca, A.; Pantaleo, M.A.; Brunello, A.; Baldi, G.G.; Boglione, A.; et al. TRAbectedin in AdVanced REtroperitoneal Well Differentiated/Dedifferentiated Liposarcoma and Leiomyosarcoma (TRAVELL): Results of a Phase II Study from the Italian Sarcoma Group. ESMO Open 2024, 9, 103667. [Google Scholar] [CrossRef] [PubMed]
- Krasner, C.N.; Poveda, A.; Herzog, T.J.; Vermorken, J.B.; Kaye, S.B.; Nieto, A.; Claret, P.L.; Park, Y.C.; Parekh, T.; Monk, B.J. Patient-Reported Outcomes in Relapsed Ovarian Cancer: Results from a Randomized Phase III Study of Trabectedin with Pegylated Liposomal Doxorubicin (PLD) versus PLD Alone. Gynecol. Oncol. 2012, 127, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Kwok, H.F. Development of Marine-Derived Compounds for Cancer Therapy. Mar. Drugs 2021, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Mainwaring, P.; Price, T.; Millward, M.J.; Padrik, P.; Underhill, C.R.; Cannell, P.K.; Reich, S.D.; Trikha, M.; Spencer, A. Phase I Clinical Trial of Marizomib (NPI-0052) in Patients with Advanced Malignancies Including Multiple Myeloma: Study NPI-0052-102 Final Results. Clin. Cancer Res. 2016, 22, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Harrison, S.; Zonder, J.; Badros, A.; Laubach, J.; Bergin, K.; Khot, A.; Zimmerman, T.; Chauhan, D.; Levin, N.; et al. A Phase 1 Clinical Trial Evaluating Marizomib, Pomalidomide and Low-dose Dexamethasone in Relapsed and Refractory Multiple Myeloma NPI-0052-107): Final Study Results. Br. J. Haematol. 2018, 180, 41–51. [Google Scholar] [CrossRef]
- Ebbinghaus, S.; Rubin, E.; Hersh, E.; Cranmer, L.D.; Bonate, P.L.; Fram, R.J.; Jekunen, A.; Weitman, S.; Hammond, L.A. A Phase I Study of the Dolastatin-15 Analogue Tasidotin (ILX651) Administered Intravenously Daily for 5 Consecutive Days Every 3 Weeks in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2005, 11, 7807–7816. [Google Scholar] [CrossRef]
- Tamura, K.; Nakagawa, K.; Kurata, T.; Satoh, T.; Nogami, T.; Takeda, K.; Mitsuoka, S.; Yoshimura, N.; Kudoh, S.; Negoro, S.; et al. Phase I Study of TZT-1027, a Novel Synthetic Dolastatin 10 Derivative and Inhibitor of Tubulin Polymerization, Which Was Administered to Patients with Advanced Solid Tumors on Days 1 and 8 in 3-Week Courses. Cancer Chemother. Pharmacol. 2007, 60, 285–293. [Google Scholar] [CrossRef] [PubMed]
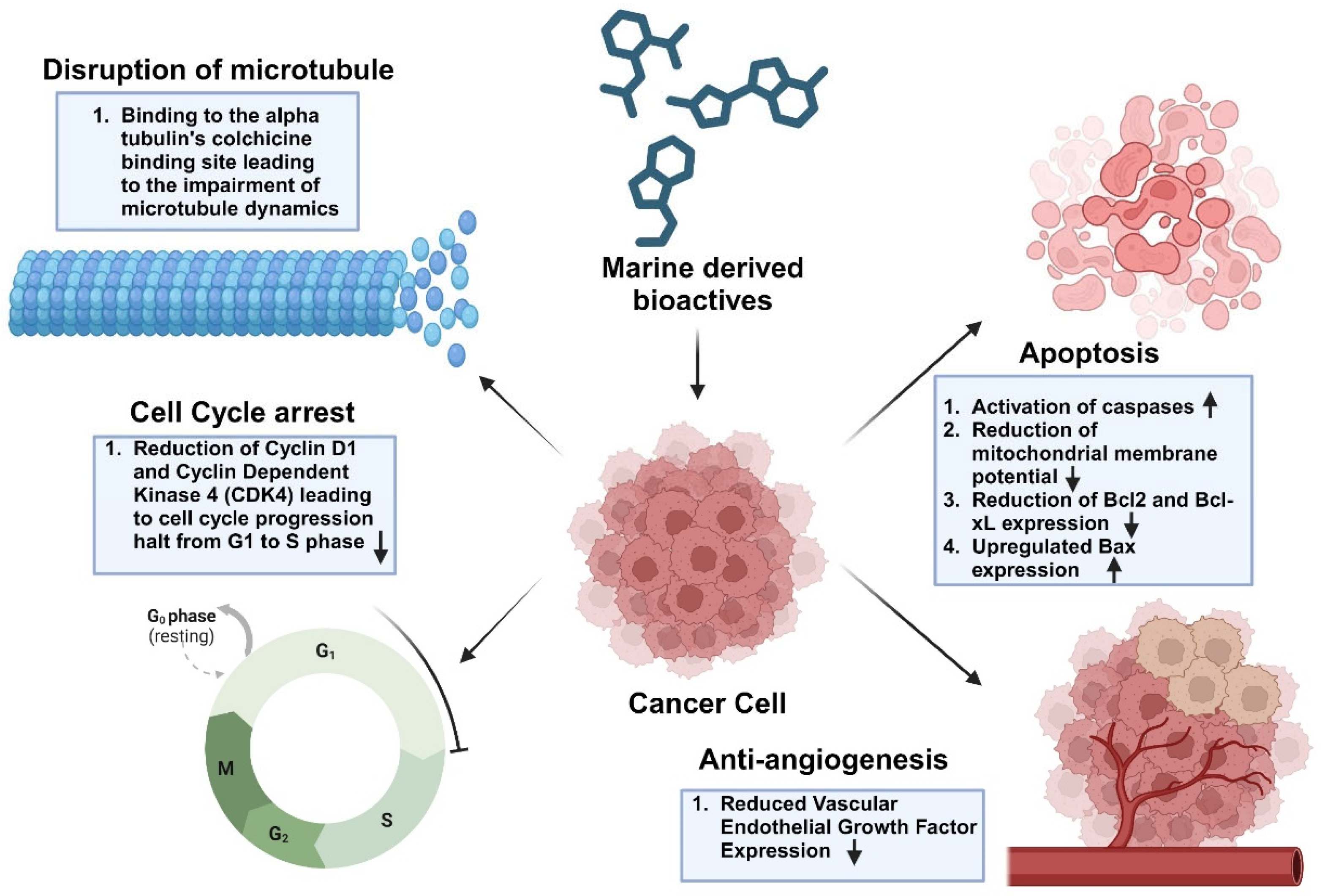
| Macroalgae | |||
|---|---|---|---|
| Marine-Derived Product | Source | Mechanism | References |
| Fucoidan extracted with water | Turbinaria conoides (Brown Algae) | Fucoidan significantly inhibited the proliferation of Mia PaCa-2 and PANC-1 human pancreatic cancer cell lines. Induced apoptotic cell death in both cell lines [143,144]. Demonstrated substantial anti-angiogenic properties (30). | [143,144] |
| Fucoidan-derived fractions | Turbinaria conoides (Brown algae) | Fractions showed anticancer activity towards PANC-1, MiaPaCa-2, Panc-3.27, and BxPC-3 human pancreatic cancer cell lines. The effect is initiated through the triggering of apoptosis, leading to the cleavage of poly-ADP ribose polymerase, a DNA repair enzyme, along with the activation of caspases 3, 8, and 9. This leads to apoptotic cell death. | [143,145] |
| Dioxinodehydroeckol 31, a derivative of phloroglucinol | Ecklonia cava (Brown algae) | The compound exhibited significant anticancer activity against MCF-7 and MDA-MB-231 breast cancer cell lines. This may be attributed to the induction of apoptosis driven by the NF-κB dependent pathway. | [146] |
| Dieckol | Ecklonia cava (Brown algae) | The compound inhibited cellular migration and invasion of A549 lung cancer cells. Additionally, it also induces apoptosis. The bioactives induce apoptosis through their blocking of the mTOR signaling pathway (34). | [143,147,148] |
| Fucoidan | Sargassum crassifolium (Brown algae) | Induces apoptosis in A549 lung cancer cells. The compound mediates activity through the reduction in mitochondrial membrane potential (Δψm), accompanied by a rise in cytochrome c release along with a rise in caspase 9 and 3 levels. There is also a reduction in Bcl-2 expression. | [143,149] |
| Phlorofucofuroeckol A | Eisenia bicyclis (brown algae) | Phlorofucofuroeckol A causes apoptosis and lowers cell viability in HCT116, SW480, LoVo, and HT-29 human colorectal cancer cells through a pathway involving ATF3. | [143,150,151] |
| Kahalalide F | Bryopsis sp. (Green algae) | The effect is mediated through alterations in the permeability of the plasma membrane leading to oncosis together with the changes in lysosomal morphology together with the inhibition of ErbB3 signaling pathways. The primary function of KF involves disrupting lysosome organization. This effect may arise from its hydrophobic characteristics, leading to its incorporation into lysosome membranes as an ionophore. This ultimately influences the exchange of protons for sodium, causing passive water influx into the cisternae, which in turn produces a swelling effect and the formation of large vacuoles. The development of these vacuoles seems to result from alterations in lysosomal membranes. | [152,153,154] |
| Serine-containing polysaccharide–protein complexes (Se-PPCs) | U. fasciata (Green algae) | Se-PPC drastically reduced the synthesis of cyclin D1 and cyclin-dependent kinase (CDK) 4, therefore impeding the transition of cells from the G1 phase to the S phase, thus leading to the proliferation of apoptotic sub-G1 phase cells. It also promotes the activation of the caspase-3 protein along with the upregulation of p53 protein. It induces a breakdown of the mitochondrial membrane potential (Δψm), leading to the release of cytochrome C into the cytoplasm of A549 cells. Additionally, it also inhibits caspase-9 activation by modulating the synthesis of pro-apoptotic proteins Bax and Bid, as well as anti-apoptotic proteins Bcl-2 and Bcl-XL protein. | [152,155] |
| Glycoprotein (Cf-GP) | Capsosiphon fulvescens (Green algae) | Inhibits the proliferation and metastasis of AGS human gastric cancer cells by reducing the expression of matrix metalloproteinase (MMPs) and tight junction proteins (TJPs). | [156] |
| Nigricanosides 10/11, ether-linked glycoglycerolipids | Avrainvillea nigrans (Green algae) | Promotes tubulin polymerization therefore inhibiting the growth of both MCF-7 and HCT-116 cell lines. | [152,157] |
| Polysaccharides | Gracilariopsis lemaneiformis (Red algae) | Demonstrate antitumor efficacy in human A549 lung, MKN28 gastric cancer together B16, mouse melanoma cell line by regulating cell shape and viability together with the Fas/Fas ligand pathway associated with apoptosis across all cell types. | [143,158] |
| Polyphenols and Flavonoids | Gelidiella acerosa (Red algae) | Inhibited cellular proliferation, migration, and colonization of A549 lung cancer cells. Additionally, it triggers apoptosis by increasing the production of Bcl-2 and Bcl-XL and activating caspase 3 and Bax protein. Furthermore, Glycogen synthase kinase-3 beta (GSK3b) was activated, accompanied by the downregulation of PI3K/Akt alongside decreased matrix metalloproteinase-2 (MMP2) expression in vitro, resulting in reduced tumor proliferation and enhanced anti-metastatic activity. | [143,159] |
| Diterpene laurenditerpenol | L. intricata (Red algae) | The compound exhibited significant inhibitory effects on the hypoxia-induced angiogenic factor (VEGF) and hypoxia-activated HIF-1 in human breast ductal cancer cells (T47D). | [152,160] |
| Halogenated monoterpene | Pterocladiella capillacea (Red algae) | Halogenated monoterpene causes cell-cycle arrest along with the production of apoptosis-linked proteins. It inhibits the proliferation of the HT-29 cell line by modulating the expression of ERK-1/-2, AKT, and NF-κB pathways. | [152,161] |
| Microalgae | |||
| Astaxanthin | Haematococcus pluvialis and Chlorella zofingiensis (Green microalgae) | Astaxanthin can inhibit NF-κB and Wnt signaling pathways while promoting apoptosis through the downregulation of key regulatory enzymes IKKβ and GSK-3β, thus mediating its anticancer effects. | [64,162] |
| β-sitosterol, a phytosterol derivative | Diacronema lutheri (syn. Pavlova lutheri), Tetraselmis sp., and Nannochloropsis sp | ß-sitosterol exerts its anticancer effects in leukemia cells by enhancing the Bax/Bcl-2 ratio and activating caspase-3. Treatment of human breast cancer cells with β-sitosterol can increase the Bax/Bcl-2 ratio as well as cause depolarization of mitochondrial membrane potential (Δψm). | [64,163] |
| Fungi | |||
|---|---|---|---|
| Plinabulin, a derivative of the diketopiperazine alkaloid halimide. | Aspergillus sp. | It binds to the alpha-tubulin’s colchicine-binding site, therefore impairing microtubule dynamics. This, in turn, leads to the disruption of tumor vasculature, which facilitates the dissemination of neoplasms, leading to the reduction in tumor growth. | [74,221,222] |
| Gliotoxin | Aspergillus spp. | Gliotoxin exhibits antitumor efficacy against human cervical cancer (HeLa) and chondrosarcoma cells through DNA fragmentation along with the activation of caspases (caspase-3, 8, and 9). It also downregulates Bcl-2 and upregulates Bax expression. | [68,223] |
| Physcion | Microsporum sp. | Physcion causes reactive oxygen species to develop, downregulated Bcl-2 expression, and upregulated Bax expression, leading to the death of HeLa cells. | [68,224]. |
| L-asparaginase (ASNase) | Fusarium oxysporum, Fusarium fujikuroi, Pyrenophora triticirepentis, and Aspergillus niger | ASNase degrades L-asparagine, therefore inhibiting the development of cancer cells. | [68] |
| Tunicates and Mollusks | |||
| Didemnin B | Trididemnum solidum (Tunicate) | Didemnin B hinders the proliferation of human prostate cancer cells by interfering with the synthesis of DNA, RNA, and proteins. | [225]. |
| Aplidine (Plitidepsin), a cyclic depsipeptide | Aplidium albicans (Tunicate) | Aplidine interacts with the transcription factor eEF1A2, modifying various pathways and consequently promoting cell-cycle arrest, inhibiting growth, and decreasing apoptosis. Also inhibits ornithine decarboxylase, an essential enzyme in tumor growth and development. Also inhibits the expression of genes that encode endothelial vascular growth factors and demonstrates anti-inflammatory properties. | [3,226,227] |
| Trabectedin | Ecteinascidia turbinate (Tunicate) | Trabectedin shows potential for treating tumors characterized by high TC-NER activity or, more broadly, those with functional DNA repair mechanisms. Trabectedin leads to persistent single-strand breaks (SSBs) in a TC-NER-dependent manner in cells exhibiting hypersensitivity to the drug. Furthermore, trabectedin-DNA adducts inhibit the incision activity of XPG endonuclease, leading to sustained XPF-mediated breaks. Furthermore, during TC-NER of trabectedin, the 3′ incision by XPG is inhibited, therefore hindering one of the two sequential NER incisions. | [82] |
| Kahalalide F | Elysia rufescens (Mollusk) | Kahalalide F impairs the activity of lysosomes and triggers cell death through intracellular acidification in prostate cancer cells. | [228] |
| Marine-Derived Product | Source | Mechanism | Reference |
|---|---|---|---|
| Sponges | |||
| Renieramycin T | Xestospongia sp. | Renieramycin T exhibits its cytotoxicity by triggering apoptosis in non-small cell lung cancer (NSCLC). It also induces the activation of p53 and caspases 9 and 3 while facilitating the degradation of Mcl-1, a pro-apoptotic protein within the Bcl-2 family, in the proteasome. | [75,268] |
| Acetylenic compounds such as (3S)-icos-4E-en-1-yn-3-ol and (3S)-14-methyldocos-4E-en-1-yn-3-ol | Cribrochalina vasculum | The compound exhibits antitumor efficacy against NSCLC cell line U-1810 along with the SCLC cell lines U-1285, H69, and H82. The primary mechanism of cytotoxicity is the cleavage of PARP, caspase-9, and caspase-3, which triggers apoptosis. Additionally, there are some conformational changes in Bak and Bax, resulting in the loss of mitochondrial potential together with the release of cytochrome C. The compounds initiate a decline in the phosphorylation of Akt, mTOR, and ERK while increasing the phosphorylation of JNK. | [269] |
| Sesterterpene BA6 (heteronemin) | Hyrtios erecta | BA6 primarily causes mitochondrial dysfunction by upregulating the generation of mitochondrial reactive oxygen species (mtROS). Additionally, BA6 stimulated cytochrome C release, triggered caspase-9 and -3 expression, and downregulated the anti-apoptotic protein Bcl-2, along with the elevation of the pro-apoptotic protein Bax. This leads to the induction of apoptosis in A549 lung cancer cells. | [270] |
| Motuporamines | Xestospongia exigua | Motuporamine C causes cytoskeletal alterations in cancer cells, therefore preventing ß1-integrin from activating, which is essential for cancer cell adhesion and invasion. This eventually suppresses angiogenesis and cell migration in PC-3 prostate cancer cells and MDA-231 breast carcinoma cells. | [81,271] |
| Stelletin B | Jaspis stellifera | It mediates its effects through targeting the PI3K/Akt/mTOR pathway. In addition to inducing apoptosis linked to an increase in ROS production and PARP cleavage, the drug was shown to cause G1 arrest, ascribed to a decrease in cyclin D1 and an increase in p27 expression. | [75,272] |
| Echinodermata | |||
| A novel steroid known as (cholest-8(14)-ene-3β,5a,6β,7a-tetraol) | Diadema savignyi | The steroid induces apoptosis followed by changes in the expression of proteins linked to apoptosis, such as reduced c-Myc expression along with the inactivation of extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) signaling cascade. | [273,274] |
| Triterpenoid glycoside Echinoside A and Ds-echinoside A | Pearsonothuria graeffei | The glycosides induce a cell cycle halt in hepatocellular carcinoma cells. Ds-echinoside A inhibits the expression of the mouse double minute 2 homolog (MDM2) and C-X-C chemokine receptor type 4 (CXCR4), therefore increasing apoptosis through p53 modulation as well as decreases cell growth and proliferation by means of protein tyrosine kinase 2 regulation. Furthermore, Ds-echinoside A has been demonstrated to impede the growth of human hepatocellular carcinoma cells together with the repression of angiogenesis, migration, adhesion, and invasion of cells by modulating the expression of TIMP-1, VEGF, and MMP9. | [275,276] |
| The two sulfated triterpene glycosides, holothurin A (HA) and dehydroechinoside A (DHEA) | Pearsonothuria graeffe | The glycosides affect metastasis by notably inhibiting MMP-9 and consequently elevating TIMP-1. HA and DHEA reduce the levels of NF-κB and VEGF, therefore restricting cell invasion and migration in HepG2 cells. | [277] |
| Anthraquinone derivatives such as rhodoptilometrin (SE16) and deoxyrhodoptilometrin (SE11) | Comanthus sp., | The compound demonstrates cytotoxicity against C6 glioma and HCT116 colon carcinoma cell lines by promoting both apoptotic and necrotic cell death. Both compounds hinder the expression of various protein kinases, including EGFR kinase, IGF1-receptor kinase, and focal adhesion kinase, which are linked to cell survival and the subsequent advancement of cancer cells. By reducing ERK phosphorylation, SE11 reduces the activity of EGF receptor kinase. | [225,278] |
| Cucumarioside A0-1 (Cuc A0-1) and djakonovioside A (Dj A) | Cucumaria djakonovi | The compound induces cell-cycle arrest, enhances reactive oxygen species (ROS) production, and reduces mitochondrial membrane potential (Δψm) in MDA-MB-231 cells. The depolarization of the mitochondrial membrane resulted in elevated levels of APAF-1 and cytochrome C. This results in the induction of caspase-9 and caspase-3, along with an increase in the levels of their cleaved forms. They also influenced the levels of Bax and Bcl-2 proteins, which are linked to mitochondria-mediated apoptosis in MDA-MB-231 cells. | [279] |
| Marine-Derived Product | Source | Mechanism | References |
|---|---|---|---|
| Streptodepsipeptides P11A and P11B | Streptomyces sp. | Inhibit the proliferation of glioma cell lines by inducing apoptosis and cell-cycle arrest at the G0/G1 phase. P11A reduces the expression of critical tumor metabolic enzymes: hexokinase 2 (HK2) (a key regulator of glycolysis), 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) (a regulator of the glycolytic flux), pyruvate kinase M2 (PKM2) (linked to aerobic glycolysis in cancer cells), lactate dehydrogenase (LLS), and fatty acid synthase (FASN) (involved in lipid biosynthesis essential for tumor cell survival). | [72,289] |
| Streptochlorin, an indole | Streptomyces, specifically 04DH110. | Demonstrates potent anticancer effects by inducing apoptosis in U937 (human leukemia) and Hep3B (hepatocarcinoma) cells. Mechanisms involve a significant reduction in mitochondrial membrane potential (Δψm), activation of executioner caspase-3, and downregulation of anti-apoptotic Bcl-2. Pro-apoptotic proteins such as Bax and FasL are upregulated, leading to apoptosis. In U937 cells, degradation of poly (ADP-ribose) polymerase (PARP) and phospholipase C-γ1 disrupts DNA repair and cell survival signaling. Anti-angiogenic effects are achieved through inhibition of VEGF-induced endothelial cell migration and tube formation, mediated via NF-κB pathway suppression. | [216,224,231,238] |
| Violacein, a violet pigment similar to indole | Chromobacterium violaceum | Induces apoptosis through mechanisms involving DNA fragmentation, chromatin condensation, and caspase activation. Violacein directly targets protein kinases involved in signal transduction, disrupting critical pathways for cancer cell proliferation. Studies in U937 (human leukemia) and HL 60 (human promyelocytic leukemia) cells indicate that violacein modulates signaling processes necessary for cell survival and tumor progression, particularly those involved in apoptosis and the cell cycle. | [290,291] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamzi, N.N.; Rahman, M.M.; Das, S. Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy. Int. J. Transl. Med. 2024, 4, 740-781. https://doi.org/10.3390/ijtm4040051
Tamzi NN, Rahman MM, Das S. Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy. International Journal of Translational Medicine. 2024; 4(4):740-781. https://doi.org/10.3390/ijtm4040051
Chicago/Turabian StyleTamzi, Nafisa Nawar, Md Motiur Rahman, and Subhadeep Das. 2024. "Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy" International Journal of Translational Medicine 4, no. 4: 740-781. https://doi.org/10.3390/ijtm4040051
APA StyleTamzi, N. N., Rahman, M. M., & Das, S. (2024). Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy. International Journal of Translational Medicine, 4(4), 740-781. https://doi.org/10.3390/ijtm4040051






