Advances in Ophthalmic Organ-on-a-Chip Models: Bridging Translational Gaps in Disease Modeling and Drug Screening
Abstract
1. Introduction
2. Corneal Organ-on-a-Chip Platforms and Their Translational Applications
2.1. Cornea Autonomy
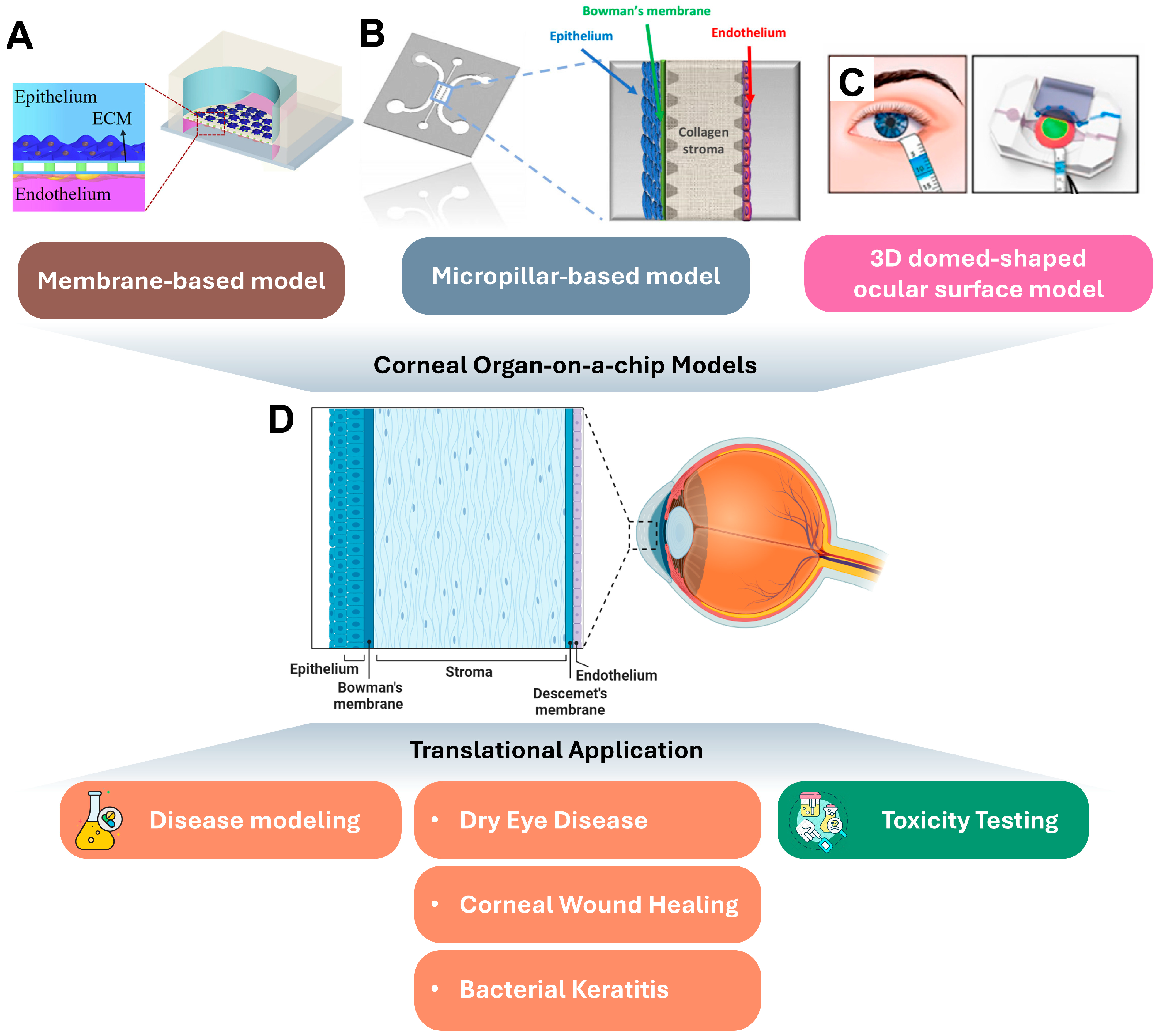
2.2. Corneal Organ-on-a-Chips
2.3. Translational Application of Corneal Organ-on-a-Chips
2.3.1. Disease Modeling and Drug Screening
2.3.2. Toxicity Testing
2.4. Current Limitations and Future Directions of Corneal Organ-on-a-Chips
3. Retinal Organ-on-a-Chip Platforms and Their Translational Applications
3.1. Retina Autonomy
3.2. Retinal Organ-on-a-Chip Platforms

3.3. Translational Application of Retinal Organ-on-a-Chips
3.3.1. Disease Modeling
3.3.2. Cell Therapy
3.3.3. Gene Therapy
3.3.4. Personalized Medicine
3.4. Current Limitations and Future Directions of Retinal Organ-on-a-Chips
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willoughby, C.E.; Ponzin, D.; Ferrari, S.; Lobo, A.; Landau, K.; Omidi, Y. Anatomy and physiology of the human eye: Effects of mucopolysaccharidoses disease on structure and function—A review. Clin. Exp. Ophthalmol. 2010, 38, 2–11. [Google Scholar] [CrossRef]
- O’Leary, F.; Campbell, M. The blood–retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Soden, P.A.; Lee, E. Tissue-Engineered Models for Glaucoma Research. Micromachines 2020, 11, 612. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Wu, A.; Lu, R.; Lee, E. Tissue engineering in age-related macular degeneration: A mini-review. J. Biol. Eng. 2022, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- De Cillà, S.; Farruggio, S.; Cocomazzi, G.; Mary, D.; Alkabes, M.; Rossetti, L.; Vujosevic, S.; Grossini, E. Aflibercept and Ranibizumab Modulate Retinal Pigment Epithelial Cells Function by Acting on Their Cross Talk with Vascular Endothelial Cells. Cell. Physiol. Biochem. 2020, 54, 161–179. [Google Scholar] [CrossRef]
- Nebel, C.; Aslanidis, A.; Rashid, K.; Langmann, T. Activated microglia trigger inflammasome activation and lysosomal destabilization in human RPE cells. Biochem. Biophys. Res. Commun. 2017, 484, 681–686. [Google Scholar] [CrossRef]
- Tamm, E.R.; Russell, P.; Epstein, D.L.; Johnson, D.H.; Piatigorsky, J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2577–2582. [Google Scholar]
- Van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, A.; Raîche-Marcoux, G.; Maranda, C.; Bertrand, N.; Boisselier, E. Animal Models in Eye Research: Focus on Corneal Pathologies. Int. J. Mol. Sci. 2023, 24, 16661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Inomata, T.; Shih, K.C.; Okumura, Y.; Fujio, K.; Huang, T.; Nagino, K.; Akasaki, Y.; Fujimoto, K.; Yanagawa, A.; et al. Application of Animal Models in Interpreting Dry Eye Disease. Front. Med. 2022, 9, 830592. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Kolarzyk, A.M.; Loy, C.; Lu, R.; Vlaminck, I.D.; Fowell, D.; Lee, E. Investigating lymphatic vessel remodeling and anti-tumor immunity in pancreatic cancer using tumor-on-chip and mouse models. Cancer Res. 2023, 83, 4614. [Google Scholar] [CrossRef]
- Kwak, T.J.; Lee, E. In vitro modeling of solid tumor interactions with perfused blood vessels. Sci. Rep. 2020, 10, 20142. [Google Scholar] [CrossRef]
- Yi, H.G.; Lee, H.; Cho, D.W. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef]
- He, Y.T.; Fu, Q.; Pang, Y.; Li, Q.; Li, J.; Zhu, X.; Lu, R.H.; Sun, W.; Liao, Q.; Schröder, U. Customizable design strategies for high-performance bioanodes in bioelectrochemical systems. iScience 2021, 24, 102163. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, W.; He, Y.; Zhang, S.; Fu, Q.; Pang, Y.; Sun, W. Ferric ion crosslinking-based 3D printing of a graphene oxide hydrogel and its evaluation as a bio-scaffold in tissue engineering. Biotechnol. Bioeng. 2021, 118, 1006–1012. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Gong, J.; Gong, Y.; Zou, T.; Zeng, Y.; Yang, C.; Mo, L.; Kang, J.; Fan, X.; Xu, H.; Yang, J. A controllable perfusion microfluidic chip for facilitating the development of retinal ganglion cells in human retinal organoids. Lab Chip 2023, 23, 3820–3836. [Google Scholar] [CrossRef] [PubMed]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-A-chip: A Guide to Biomaterial Choice and Fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Seo, J.; Byun, W.Y.; Alisafaei, F.; Georgescu, A.; Yi, Y.S.; Massaro-Giordano, M.; Shenoy, V.B.; Lee, V.; Bunya, V.Y.; Huh, D. Multiscale reverse engineering of the human ocular surface. Nat. Med. 2019, 25, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Ito, S.; Kai, H.; Nagamine, K.; Nagai, N.; Nishizawa, M.; Abe, T.; Kaji, H. Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Sci. Rep. 2017, 7, 3538. [Google Scholar] [CrossRef] [PubMed]
- Manafi, N.; Shokri, F.; Achberger, K.; Hirayama, M.; Mohammadi, M.H.; Noorizadeh, F.; Hong, J.; Liebau, S.; Tsuji, T.; Quinn, P.M.J.; et al. Organoids and organ chips in ophthalmology. Ocul. Surf. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Haderspeck, J.C.; Chuchuy, J.; Kustermann, S.; Liebau, S.; Loskill, P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert. Opin. Drug Discov. 2019, 14, 47–57. [Google Scholar] [CrossRef]
- Su, T.; Liang, L.; Zhang, L.; Wang, J.; Chen, L.; Su, C.; Cao, J.; Yu, Q.; Deng, S.; Chan, H.F.; et al. Retinal organoids and microfluidic chip-based approaches to explore the retinitis pigmentosa with USH2A mutations. Front. Bioeng. Biotechnol. 2022, 10, 939774. [Google Scholar] [CrossRef]
- Meek, K.M.; Dennis, S.; Khan, S. Changes in the refractive index of the stroma and its extrafibrillar matrix when the cornea swells. Biophys. J. 2003, 85, 2205–2212. [Google Scholar] [CrossRef]
- Nishida, T. Neurotrophic mediators and corneal wound healing. Ocul. Surf. 2005, 3, 194–202. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S. Biochemistry of human tear film: A review. Exp. Eye Res. 2022, 220, 109101. [Google Scholar] [CrossRef]
- Puleo, C.M.; McIntosh Ambrose, W.; Takezawa, T.; Elisseeff, J.; Wang, T.H. Integration and application of vitrified collagen in multilayered microfluidic devices for corneal microtissue culture. Lab Chip 2009, 9, 3221–3227. [Google Scholar] [CrossRef]
- Bennet, D.; Estlack, Z.; Reid, T.; Kim, J. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation. Lab Chip 2018, 18, 1539–1551. [Google Scholar] [CrossRef]
- Bai, J.; Fu, H.; Bazinet, L.; Birsner, A.E.; D’Amato, R.J. A Method for Developing Novel 3D Cornea-on-a-Chip Using Primary Murine Corneal Epithelial and Endothelial Cells. Front. Pharmacol. 2020, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hao, R.; Du, J.; Wu, X.; Chen, X.; Zhang, Y.; Li, W.; Gu, Z.; Yang, H. A human cornea-on-a-chip for the study of epithelial wound healing by extracellular vesicles. iScience 2022, 25, 104200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hao, R.; Chen, X.; Ma, L.; Zhang, Y.; Yang, H. Protocol to develop a microfluidic human corneal barrier-on-a-chip to evaluate the corneal epithelial wound repair process. STAR Protoc. 2023, 4, 102122. [Google Scholar] [CrossRef]
- Deng, Y.; Li, L.; Xu, J.; Yao, Y.; Ding, J.; Wang, L.; Luo, C.; Yang, W.; Li, L. A biomimetic human disease model of bacterial keratitis using a cornea-on-a-chip system. Biomater. Sci. 2024, 12, 5239–5252. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef]
- Bonneau, N.; Potey, A.; Vitoux, M.A.; Magny, R.; Guerin, C.; Baudouin, C.; Peyrin, J.M.; Brignole-Baudouin, F.; Réaux-Le Goazigo, A. Corneal neuroepithelial compartmentalized microfluidic chip model for evaluation of toxicity-induced dry eye. Ocul. Surf. 2023, 30, 307–319. [Google Scholar] [CrossRef]
- Cho, K.; Lee, J.; Kim, J. Integrated high-throughput drug screening microfluidic system for comprehensive ocular toxicity assessment. Toxicol. In Vitro 2024, 98, 105843. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, F.; Moghe, P.V.; Toner, M.; Yarmush, M.L. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: Hepatocytes cultured in a sandwich configuration. FASEB J. 1996, 10, 1471–1484. [Google Scholar] [CrossRef]
- Öztürk-Öncel, M.; Erkoc-Biradli, F.Z.; Rasier, R.; Marcali, M.; Elbuken, C.; Garipcan, B. Rose petal topography mimicked poly(dimethylsiloxane) substrates for enhanced corneal endothelial cell behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112147. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Shihabeddin, T.Z.; Awkal, J.A.; Najjar, A.M.; Miron-Mendoza, M.; Maruri, D.P.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces. J. Funct. Biomater. 2023, 14, 217. [Google Scholar] [CrossRef]
- Fabre, K.M.; Livingston, C.; Tagle, D.A. Organs-on-chips (microphysiological systems): Tools to expedite efficacy and toxicity testing in human tissue. Exp. Biol. Med. 2014, 239, 1073–1077. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Freitas, R.; Mohammed, I.; Ting, D.S.J.; Said, D.G. The pre-Descemet’s layer (Dua’s layer, also known as the Dua-Fine layer and the pre-posterior limiting lamina layer): Discovery, characterisation, clinical and surgical applications, and the controversy. Prog. Retin. Eye Res. 2023, 97, 101161. [Google Scholar] [CrossRef]
- Kate, A.; Basu, S. A Review of the Diagnosis and Treatment of Limbal Stem Cell Deficiency. Front. Med. 2022, 9, 836009. [Google Scholar] [CrossRef]
- Pan, W.W.; Wubben, T.J.; Besirli, C.G. Photoreceptor metabolic reprogramming: Current understanding and therapeutic implications. Commun. Biol. 2021, 4, 245. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Cheng, L.; Kuehn, M.H. Human Retinal Organoids in Therapeutic Discovery: A Review of Applications. Handb. Exp. Pharmacol. 2023, 281, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.T.; Gard, A.L.; Gaibler, R.; Mulhern, T.J.; Strelnikov, R.; Azizgolshani, H.; Cain, B.P.; Isenberg, B.C.; Haroutunian, N.J.; Raustad, N.E.; et al. A high-throughput microfluidic bilayer co-culture platform to study endothelial-pericyte interactions. Sci. Rep. 2021, 11, 12225. [Google Scholar] [CrossRef]
- Huang, C.P.; Lu, J.; Seon, H.; Lee, A.P.; Flanagan, L.A.; Kim, H.Y.; Putnam, A.J.; Jeon, N.L. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip 2009, 9, 1740–1748. [Google Scholar] [CrossRef]
- Chung, M.; Lee, S.; Lee, B.J.; Son, K.; Jeon, N.L.; Kim, J.H. Wet-AMD on a Chip: Modeling Outer Blood-Retinal Barrier In Vitro. Adv. Healthc. Mater. 2018, 7, 1700028. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Jeon, J.S. Microfluidic outer blood-retinal barrier model for inducing wet age-related macular degeneration by hypoxic stress. Lab Chip 2022, 22, 4359–4368. [Google Scholar] [CrossRef]
- Lu, R.; Lee, B.J.; Lee, E. Three-Dimensional Lymphatics-on-a-Chip Reveals Distinct, Size-Dependent Nanoparticle Transport Mechanisms in Lymphatic Drug Delivery. ACS Biomater. Sci. Eng. 2024, 10, 5752–5763. [Google Scholar] [CrossRef] [PubMed]
- Ilan, I.S.; Yslas, A.R.; Peng, Y.; Lu, R.; Lee, E. A 3D Human Lymphatic Vessel-on-Chip Reveals the Roles of Interstitial Flow and VEGF-A/C for Lymphatic Sprouting and Discontinuous Junction Formation. Cell. Mol. Bioeng. 2023, 16, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Park, E.; Choi, J.; Lu, R.; Yu, J.S.; Kim, C.; Zhao, L.; Yu, J.; Nakashima, B.; Lee, S.; et al. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat. Neurosci. 2024, 27, 913–926. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Kutys, M.L.; Tefft, J.B.; Chen, C.S. Microfabricated blood vessels for modeling the vascular transport barrier. Nat. Protoc. 2019, 14, 1425–1454. [Google Scholar] [CrossRef]
- Arık, Y.B.; Buijsman, W.; Loessberg-Zahl, J.; Cuartas-Vélez, C.; Veenstra, C.; Logtenberg, S.; Grobbink, A.M.; Bergveld, P.; Gagliardi, G.; den Hollander, A.I.; et al. Microfluidic organ-on-a-chip model of the outer blood-retinal barrier with clinically relevant read-outs for tissue permeability and vascular structure. Lab Chip 2021, 21, 272–283. [Google Scholar] [CrossRef]
- Zhang, H.; Rahman, T.; Lu, S.; Adam, A.P.; Wan, L.Q. Helical vasculogenesis driven by cell chirality. Sci. Adv. 2024, 10, eadj3582. [Google Scholar] [CrossRef] [PubMed]
- Jahagirdar, D.; Yadav, S.; Gore, M.; Korpale, V.; Mathpati, C.S.; Chidambaram, S.; Majumder, A.; Jain, R.; Dandekar, P. Compartmentalized microfluidic device for in vitro co-culture of retinal cells. Biotechnol. J. 2022, 17, e2100530. [Google Scholar] [CrossRef]
- Yeste, J.; García-Ramírez, M.; Illa, X.; Guimerà, A.; Hernández, C.; Simó, R.; Villa, R. A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood-retinal barrier. Lab Chip 2017, 18, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Masin, L.; Bergmans, S.; Van Dyck, A.; Farrow, K.; De Groef, L.; Moons, L. Local glycolysis supports injury-induced axonal regeneration. J. Cell Biol. 2024, 223, e202402133. [Google Scholar] [CrossRef]
- Boal, A.M.; McGrady, N.R.; Chamling, X.; Kagitapalli, B.S.; Zack, D.J.; Calkins, D.J.; Risner, M.L. Microfluidic Platforms Promote Polarization of Human-Derived Retinal Ganglion Cells That Model Axonopathy. Transl. Vis. Sci. Technol. 2023, 12, 1. [Google Scholar] [CrossRef]
- Kwon, H.; Kevala, K.; Xin, H.; Patnaik, S.; Marugan, J.; Kim, H.Y. Ligand-Induced GPR110 Activation Facilitates Axon Growth after Injury. Int. J. Mol. Sci. 2021, 22, 3386. [Google Scholar] [CrossRef]
- Nafian, F.; Kamali Doust Azad, B.; Yazdani, S.; Rasaee, M.J.; Daftarian, N. A lab-on-a-chip model of glaucoma. Brain Behav. 2020, 10, e01799. [Google Scholar] [CrossRef]
- Amos, G.; Ihle, S.J.; Clément, B.F.; Duru, J.; Girardin, S.; Maurer, B.; Delipinar, T.; Vörös, J.; Ruff, T. Engineering an in vitro retinothalamic nerve model. Front. Neurosci. 2024, 18, 1396966. [Google Scholar] [CrossRef]
- Achberger, K.; Cipriano, M.; Düchs, M.J.; Schön, C.; Michelfelder, S.; Stierstorfer, B.; Lamla, T.; Kauschke, S.G.; Chuchuy, J.; Roosz, J.; et al. Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors. Stem Cell Rep. 2021, 16, 2242–2256. [Google Scholar] [CrossRef]
- Spivey, E.C.; Yin, J.; Chaum, E.; Wikswo, J.P. A Microfluidic Platform for the Time-Resolved Interrogation of Polarized Retinal Pigment Epithelial Cells. Transl. Vis. Sci. Technol. 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Thakur, A.; Redenti, S.; Vazquez, M. A model microfluidics-based system for the human and mouse retina. Biomed. Microdevices 2015, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Peña, J.S.; Redenti, S.; Vazquez, M. A novel electro-chemotactic approach to impact the directional migration of transplantable retinal progenitor cells. Exp. Eye Res. 2019, 185, 107688. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mishra, S.; Pena, J.; Zhou, J.; Redenti, S.; Majeska, R.; Vazquez, M. Collective adhesion and displacement of retinal progenitor cells upon extracellular matrix substrates of transplantable biomaterials. J. Tissue Eng. 2018, 9, 2041731417751286. [Google Scholar] [CrossRef]
- Pena, C.D.; Zhang, S.; Majeska, R.; Venkatesh, T.; Vazquez, M. Invertebrate Retinal Progenitors as Regenerative Models in a Microfluidic System. Cells 2019, 8, 1301. [Google Scholar] [CrossRef]
- Mut, S.R.; Mishra, S.; Vazquez, M. A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells. Micromachines 2022, 13, 406. [Google Scholar] [CrossRef]
- Trapani, I.; Auricchio, A. Seeing the Light after 25 Years of Retinal Gene Therapy. Trends Mol. Med. 2018, 24, 669–681. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R. Advances in Ophthalmic Organ-on-a-Chip Models: Bridging Translational Gaps in Disease Modeling and Drug Screening. Int. J. Transl. Med. 2024, 4, 710-725. https://doi.org/10.3390/ijtm4040049
Lu R. Advances in Ophthalmic Organ-on-a-Chip Models: Bridging Translational Gaps in Disease Modeling and Drug Screening. International Journal of Translational Medicine. 2024; 4(4):710-725. https://doi.org/10.3390/ijtm4040049
Chicago/Turabian StyleLu, Renhao. 2024. "Advances in Ophthalmic Organ-on-a-Chip Models: Bridging Translational Gaps in Disease Modeling and Drug Screening" International Journal of Translational Medicine 4, no. 4: 710-725. https://doi.org/10.3390/ijtm4040049
APA StyleLu, R. (2024). Advances in Ophthalmic Organ-on-a-Chip Models: Bridging Translational Gaps in Disease Modeling and Drug Screening. International Journal of Translational Medicine, 4(4), 710-725. https://doi.org/10.3390/ijtm4040049





