Simple Summary
A gastric gastrointestinal stromal tumor (GIST) is a rare type of tumor that originates in the stomach’s connective tissue. GISTs develop from specialized cells called interstitial cells of Cajal, which regulate digestive tract movements. These tumors can vary in size and may not always cause symptoms. Common symptoms include abdominal pain, gastrointestinal bleeding, or a feeling of fullness. Gastric GISTs are typically diagnosed through imaging tests like CT scans and are confirmed with a biopsy. Treatment options for GISTs depend on factors such as tumor size, location, and aggressiveness. Surgical removal is often the primary treatment, with medication (tyrosine kinase inhibitors) used before or after surgery to shrink the tumor or prevent recurrence. Regular monitoring is crucial because GISTs can grow slowly or aggressively. Overall, a multidisciplinary team is essential for managing localized gastric GISTs effectively.
Abstract
Gastric gastrointestinal stromal tumors (GIST) are rare, neuroectodermal tumors primarily residing in the stomach with characteristic genetic mutations. They are often identified using ultrasound and cross-sectional imaging, or they are noted during endoscopy. Localized gastric GISTs are commonly treated with surgical resection, with the possible use of neoadjuvant or adjuvant medical therapies as they are considered to have malignant potential. The use of tyrosine kinase inhibitors (TKI) such as imatinib has been shown to successfully reduce pre-operative tumor burden, recurrence, and disease progression. Surgical resection considerations vary depending on tumor size, location, and malignant potential. Neoadjuvant and adjuvant TKI therapy dosing varies in response to the type of GIST mutation present and greatly influences prognosis. Novel cooperative minimally invasive surgical techniques and targeted therapies are currently in development to address challenges in GIST treatment for tumors in challenging locations or with significant potential for progression. The management of localized gastric GISTs continues to rapidly evolve; each case should be managed individually, where care is taken in considering details, including tumor location, tumor size, and the molecular genetic profile, before embarking on a course of treatment.
1. Introduction
In 1983, Mazur and Clark reevaluated 28 gastric tumors classified to be of smooth muscle origin, including leiomyoma and leiomyosarcoma [1]. What they discovered instead were 9 tumors expressing the S-100 protein, suggesting cells derived from neuroectodermal tissue. These tumors were the first to be categorized as gastrointestinal stromal tumors (GIST), a type of mesenchymal tumor derived from the interstitial cells of Cajal (ICC) or ICC precursor cells. In fact, the landmark study describing the morphological and immunophenotypic similarities between GISTs and ICC proposed that the name of the tumor be gastrointestinal pacemaker cell tumor [2]. A retrospective cohort study from Canada from 2011–2021 showed the stomach as the most common site of primary GISTs (63%, n = 156) [3]. An analysis of the SEER-18 cancer registry in the US from 2000–2018 found a similar frequency of primary GISTs located in the stomach at 63.4% (n = 6868), with a large portion of tumors averaging between 6 to 10 cm (35.8%, n = 2865) [4]. A national analysis of GISTs of the upper GI tract from between 2000–2016 in Germany included 1361 patients, and an even higher proportion were found to be located in the stomach (91.2%, n = 1254) [5]. In that analysis, the gastric tumors were most commonly between 2 and 5 cm (40.7%) [5]. Gastric primary GISTs are less likely to have distant metastasis, and they have a longer median overall survival (130 months) when compared to those found in the esophagus (97 months) or those specifically at the gastroesophageal junction (72 months) [5].
According to the United States Surveillance, Epidemiology and End Results Program (SEER) registry, which includes data from among 74,520 patients diagnosed with gastric cancer located outside the gastric cardia, GISTs were found to be the third most common histology at 6.7% (n = 5345) behind gastric adenocarcinoma (66.2%) and non-MALT lymphomas (6.9%) [6]. The age-standardized incidence rates per 100,000 between men and women were found to be similar (0.49 vs. 0.45), and there were significantly higher rates between the age groups of 50+ compared to ages 20–49 (0.99 vs. 0.13) [6]. An analysis of the SEER registry showed a rise in the incidence of non-cardia gastric cancers, which were primarily due to increasing diagnoses of GISTs and neuroendocrine tumors rather than tumors associated with H. pylori, such as gastric adenocarcinoma [6]. Therefore, an appreciation for and solid understanding of both the pathophysiology and ever-evolving standard treatment of gastric GISTs is necessary for general surgeons and surgical oncologists alike.
2. Presentation
In an international systematic review of 15 epidemiological studies, 82% of GISTs were found to be symptomatic [7]. Patients have complained of vague symptomatology including nausea, abdominal pain, distension, or early satiety [8]. Although GISTs can develop throughout the intestinal tract, the majority are found in the stomach (60%), which is where this review will focus. However, 30% of GISTs are located in the small intestine, and significantly fewer arise in the colon and rectum (5%) and esophagus (1%) [9,10]. Gastric GISTs can often present with hematemesis, melena, and chronic anemia due to mucosal ulcerations, while small intestinal GISTs are more often associated with intestinal obstruction [11,12].
3. Diagnostic Modalities
Gastric GISTs are often identified first in computerized tomography (CT) scans, either incidentally or in the targeted workups of symptoms. Intravenous contrast use allows for improved detection, particularly of smaller masses, but it is also valuable for discerning gastric GISTs from other gastric tumors. While tumors measuring <5 cm can be homogeneous and well circumscribed, larger gastric GISTs (or those with aggressive phenotypes) have irregular borders. Masses of >5 cm may demonstrate necrosis, calcifications, or cystic portions (Figure 1) [13]. Given the relatively consistent nature of the characteristics of gastric GISTs in CT imaging, a practical scoring guideline was published to readily differentiate GISTs from other subepithelial tumors. In addition to the features noted above, a patient age greater than 49 years old, lower attenuation on unenhanced images (<43 HU), a non-cardia location, and the absence of enlarged lymph nodes were included in the algorithm [14]. After studying 64 patients and employing a validation cohort of 92 patients, the accuracy of diagnosing gastric GISTs in CT imaging alone using the scoring algorithm was 92% with a sensitivity of 100%, and a score of greater than or equal to 4 was found to accurately predict GISTs in all but one case [14].
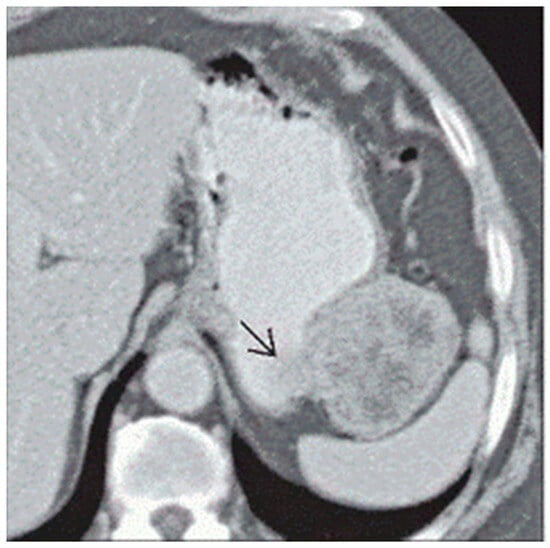
Figure 1.
Arrow pointing to a heterogeneous, irregular bordered gastric GIST located on the greater curvature of the stomach abutting the splenic hilum [13].
In patients presenting with upper gastrointestinal bleeding, an upper endoscopy may be performed ahead of cross-sectional imaging. In this subset of patients, endoscopic evaluation allows for diagnostic tissue sampling via fine needle aspiration and histopathologic confirmation of GISTs, as well as a potential for the therapeutic hemostasis of gastrointestinal bleeding secondary to associated mucosal erosion [15]. If patients are found to have a gastric tumor in imaging first, an upper endoscopy is routinely recommended for a better characterization of the mass and consideration of tissue diagnosis ahead of any treatment. Endoscopic ultrasound (EUS) allows for a more detailed evaluation of submucosal masses, particularly small gastric GISTs (<2 cm), which are often considered to be low risk lesions [16]. Through examining the borders, heterogeneity, and disruption of the surrounding layers of the stomach, EUS enables a better appreciation of the potentially aggressive nature or malignant potential of the mass [16]. However, a retrospective study of 49 GIST patients that underwent standard EUS found that EUS did not allow for the accurate prediction of the mitotic index or a malignant potential of 2 cm to 5 cm GISTs [17]. Given that biopsy carries an inherent risk of bleeding, insufficient sampling, false negatives, as well as the potential for tumor disruption and seeding, physicians and researchers have sought non-interventional techniques to facilitate more accurate diagnoses with less risk prior to resection. Recent advances in the technology of EUS have allowed for improved characterizations of submucosal masses. Contrast-enhanced harmonic endoscopic ultrasonography (CH-EUS) has demonstrated more success in real-time GIST differentiation and in the evaluation of malignant potential than conventional EUS alone [16]. In a systematic review and meta-analysis including 187 patients from 4 studies, CH-EUS had a pooled sensitivity of 89% and a specificity of 82% in discriminating between GISTs and other submucosal lesions [16]. In the same study, a separate meta-analysis of 143 patients from 5 studies demonstrated a pooled sensitivity of 96% and a specificity of 53% for CH-EUS predictions of the malignant potential of gastric GISTs [16].
CT and EUS have been compared to determine which imaging modality is more accurate in predicting prognosis and malignant potential. In a retrospective study of 53 patients who underwent laparoscopic wedge resections of gastric subepithelial tumors (SETs), the accuracy of EUS was higher (89.3%) than abdominopelvic CT (74.2%) in identifying gastric GISTs from other types of subepithelial tumors [18]. CT appears to perform better, however, than EUS in predicting prognosis and the mitotic index for GISTs of >2 cm. A retrospective, single-center study of 50 patients with primary gastric GISTs greater than 2 cm were evaluated for the association of CT and EUS findings with markers of malignancy [19]. In EUS, malignant tumors were associated with the presence of cystic spaces, while exophytic growth predicted malignant potential in CT [19]. In CT, an irregular shape and exophytic growth patterns also correlated with a higher mitotic index [19]. In a different study, CT findings were able to discriminate benign tumors from GISTs by evaluating intralesional necrosis and the long–short diameter ratio of the tumor [20].
Ultimately, clinicians should use their best judgement in ordering diagnostic imaging for gastric lesions, and they can expect CT with intravenous contrast to help differentiate gastric GISTs from other submucosal lesions. EUS can be ordered to examine the mass more closely for malignant features and to obtain tissue diagnosis if needed prior to treatment. The addition of contrast-enhanced harmonic EUS can differentiate truly high-risk lesions from more indolent lesions, potentially obviating the need for a biopsy before excision. Per the National Cancer Center Network GIST task force, if a biopsy is necessary, a core needle biopsy is preferred to fine needle aspiration as it allows for a more accurate depiction of the mitotic rate, which allows for more accurate prognostication [21].
The consensus approach for a classification of prognostic factors was first suggested in 2002, which was then supported by the Armed Forces Institute of Pathology (AFIP) in large retrospective studies by Miettinen et al. Size and mitotic rate (>5 mitoses per 50 high powered field) are considered to be the primary differentiating characteristics for gastric GISTs, and they are generally accepted as a lower risk than small bowel GISTs [16] (Table 1). Gastric GISTs greater than 10 cm with a mitotic rate less than or equal to 5 per HPF are considered intermediate risk. Gastric GISTs of 2–5 cm in size with a mitotic rate greater than 5 are also considered intermediate risk, while tumors larger than 5 cm with mitotic rates greater than 5 are considered high risk for recurrence after resection with curative intent in the AFIP model. Current guidelines recommend that all gastric GISTs of 2 cm and larger be resected if possible, while gastric GISTs that are less than 2 cm in size can be assessed for malignant features by EUS, where they are either resected or surveilled accordingly.

Table 1.
Evaluation of the malignant potential for gastric gastrointestinal stromal tumors (GISTs), based on NCCN Guidelines using size criteria and the mitotic rate [10,21,22,23].
4. Molecular Genetics of Gastric GISTs
Although tumor size and the mitotic rate are conventional indices for predicting the prognosis and malignant potential of GISTs, the discovery of genes implicated in GIST tumorigenesis and the subclassification of their alterations has opened another domain to inform clinical decision making. The growing catalog of molecular subtypes of GISTs and their prognostic and therapeutic implications have been thoroughly described [24,25,26]. This section will focus on genetic alterations germane to the gastric localization of the disease. Some of the alterations described may also be present in non-gastric manifestations of the tumor.
GISTs are largely sporadic, and 82–87% of all cases are characterized by gain-of-function mutations in either of the proto-oncogenes c-KIT or PDGFRA (Table 2) [24,27]. Activating mutations in these two genes are mutually exclusive, and they represent alternative pathways to GIST tumorigenesis [28,29]. These genes encode related receptor tyrosine kinases (RTKs) that signal upstream of the pathways involved in the proliferation and inhibition of apoptosis, including JAK-STAT3, PI3K-AKT-mTOR, and RAS-MAPK. Certain mutations lead to their constitutive activation and ligand-independent signaling of their downstream pathways. Up to 15% of adult GISTs do not possess mutations in KIT or PDGFRA, and they have been historically termed wild-type (WT) GISTs: a category that encompasses alterations in several other genes and a spectrum of tumor behaviors [24].

Table 2.
Molecular subtypes of gastric GISTs.
For GISTs in general, primary mutations have been described in the KIT functional domains encoded by exons 8, 9, 11, 13, and 17. Approximately 70% of GISTs harbor mutations in exon 11, and the vast majority of exon 11-mutated GISTs arise in the stomach [30]. Tumors characterized by deletions affecting codons 557/558 of KIT exon 11 have significantly higher mitotic indices and risk profiles when compared to tumors possessing other exon 11 alterations, including single-nucleotide substitutions and duplications [31]. Tumors typified by KIT exon 11 codon 557/558 deletions represent 23–28% of all GISTs, and they are among the more aggressive genotypes commonly found in the gastric location [32]. Mutations in exon 9 are almost exclusive to non-gastric GISTs and thus will not be discussed here [33]. It is worth noting that one study has claimed to observe a higher proportion of exon 9 mutations in gastric primaries, but it also deemed the mutations to not have clear prognostic implications [34]. Primary mutations in exons 13 and 17 are rare, where they are estimated to occur at a frequency of 1–2% among all GISTs, and though are they more common in the GISTs of the small bowel, they do also occur in gastric GISTs [35]. Gastric GISTs with exon 13 mutations have been shown to be slightly more aggressive than gastric GISTs on average [35].
PDGFRA mutations are generally understood to be indolent, are largely associated with the gastric localization of GISTs, and tend to have a good prognosis [9]. Due to the indolence of PDGFRA-mutated tumors, their frequency amongst all GISTs has been estimated as low as 1.6% in phase III clinical trials for advanced disease but as high as 14% in wider population studies [24]. Primary PDGFRA mutations have been identified in exons 12 and 18 and rarely in exon 14 [36]. The most frequently observed PDGFRA mutation is a D842V substitution in exon 18, which has been reported in as many as 75% of PDGFRA-mutated GISTs [24]. The exon 18 D842V mutation represents an important subtype of GISTs because of its atypical response to treatment, which is discussed further in the subsequent section on pharmacologic therapy.
KIT/PDGFRA WT GISTs are divided into two groups based on the presence of genetic or epigenetic alterations in one of the four subunits of the mitochondrial succinate dehydrogenase complex: an enzyme complex that catalyzes the oxidation of succinate to fumarate in both the citric acid cycle and electron transport chain [37]. Deficits in the SDH complex lead to an accumulation of succinate, which erroneously activates hypoxic signaling (which induces angiogenesis and eventually tumorigenesis). SDH mutations may be present in sporadic GISTs, but they also manifest in the non-familial Carney triad (CT) of endocrine tumors and the familial Carney–Stratakis syndrome (CSS) [38,39]. SDH-deficient GISTs are rare but predominantly occur in the stomach [40]. SDH-deficient GISTs behave atypically when compared to KIT/PDGFRA mutant GISTs, often metastasizing but then remaining indolent and stable [31].
SDH-competent GISTs arise from somatic or germline mutations in genes such as NF1 and BRAF; however, NF1-mutant GISTs usually arise in the small bowel while BRAF-mutant GISTs have been observed in the stomach in addition to the small bowel [41]. SDH-competent GISTs generally are presumed to behave like KIT/PDGFRA-mutant GISTs. It should be emphasized that both SDH-deficient and SDH-competent GISTs are rare and that numerous other subtypes of SDH-competent KIT/PDGFRA WT GISTs have been observed [24]. It is for this reason that generically associating all these subtypes under the title of “WT GIST” may be an unhelpful simplification. Further elucidation of the rare GIST subtypes is necessary, and mutational analysis and next generation sequencing should be a cornerstone of GIST management [42,43].
A recent study described the anatomic–genomic landscape of gastric GISTs, finding that tumors in the proximal stomach including the cardia and fundus were routinely KIT positive while distal gastric GISTs were more diverse in their mutational profiles [44]. The apparent anatomic–genomic heterogeneity of gastric GISTs highlights the importance of continuing to gather genomic data on these tumors.
5. Operative Management and Decision Making
Surgical resection is the mainstay for patients with gastric GISTs and no sign of metastatic disease on staging workup. The surgery-first approach is appropriate unless the patient’s comorbidities place them at substantial operative risk. Additionally, some lesions are in anatomical locations that are less favorable, and surgery may be better tolerated after neoadjuvant therapy, as described in the next chapter. For example, tumors near the gastroesophageal junction (GEJ), or large tumors on the lesser curve, could require resection that would compromise the GEJ or result in proximal or total gastrectomy (Figure 2). In these cases, neoadjuvant therapy would be reasonable to limit the morbidity of resection.
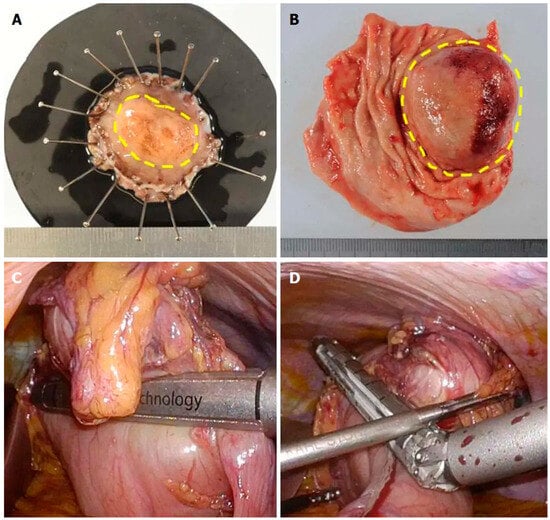
Figure 2.
Pathologic picture following a laparoscopic and endoscopic cooperative surgery removing a gastric GIST (A) compared to a specimen obtained via traditional wedge gastric resection (B). Note the intraoperative images (C,D) demonstrate a classic wedge resection using laparoscopic stapling devices. Comparing specimen (A) to (B), the reader should note a very close lateral margin on (B) with redundant benign tissue on the opposite side, whereas (A) has a precise circumferential radial margin [43].
Intraoperative tumor rupture is associated with a worse prognosis when compared to the status of surgical margins without rupture [45]. A randomized controlled trial defined rupture as “tumor spillage or fracture, piecemeal resection, and incisional biopsy occurring either before or at the time of the operation” [45]. Therefore, preoperative planning is critical. The ideal surgical margin status has been defined as no residual tumor (R0) without need for lymphadenectomy, as sarcomatous lesions rarely metastasize to nodes. There is little documented in the literature regarding the re-resection of microscopically positive margins, particularly in the setting of large tumors [23]. In fact, a study from the American College of Surgeons Oncology Group (ACOSOG) GIST working group demonstrated that there was no significant difference in recurrence-free survival with or without imatinib therapy in patients with an R1 compared to R0 resection [46].
The current NCCN guidelines recommend a minimally invasive approach for GISTs of <5 cm when available [22]. However, the current trend has leaned toward minimally invasive approaches, utilizing a laparoscopic or robotic platform for tumors up to 10 cm in size or even larger, as well as in the setting of neoadjuvant therapy (Figure 2 and Figure 3). A study of the National Cancer Database demonstrated a transition from majority open (71.5%) cases in 2010 to 9.6% robotic and 48.8% laparoscopic by 2016 [47]. Over this time, minimally invasive approaches were demonstrated to be associated with lower rates of readmission and lower rates of 90-day mortality compared to historical data. A separate non-inferiority study studying the National Cancer Database from 2010–2017 specifically evaluated outcomes of gastric GISTs measuring 5–10 cm [48]. The minimally invasive approach again showed improved postoperative morbidity, lower readmission rates, shorter length of stay, and improved 30- and 90-day mortality without a significant difference in overall survival when compared to the open approach [48]. The study also found no statistically significant difference in the achievement of R0 resection margins [48]. However, data are sparse regarding the difference in intraoperative tumor ruptures between the two techniques; moreover, further research is necessary, as this variable has been shown to be directly related to recurrence free survival [22].
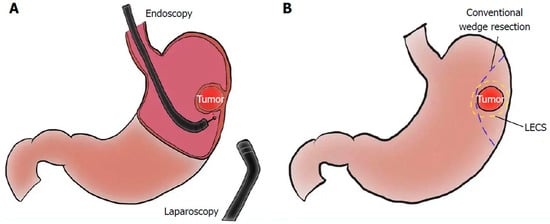
Figure 3.
Graphic representation of the setup (A) and anticipated margins from a laparoscopic and endoscopic cooperative surgery (B) [43].
The most common surgical technique for gastric GISTs is a gastric wedge resection, which can be achieved by an experienced surgeon during a minimally invasive approach with a laparoscopic linear stapler. However, the anatomical location of the tumor, the distance from the gastroesophageal junction for example, may change the surgeon’s preferred operative approach, i.e., whether it is minimally invasive or open (Figure 2 and Figure 3). As the recommended margin status is R0, rather than the multiple centimeter margins required for other tumors (such as in gastric adenocarcinoma), surgeons can complete a simple resection in anatomically challenging locations without needing to perform a total gastrectomy or esophagectomy (Figure 2 and Figure 3). A classification schema originally developed by Privette et al. (and expanded upon by Al-Thani et al.) to include the posterior gastric wall involves the consideration of a surgical technique for four different anatomically challenging locations when simple wedge resection may not be possible (Table 3) [49,50]. In tumors of the fundus and the greater curvature, wedge resection is often feasible and appropriate. However, for masses located at the pre-pyloric region or within the antrum, intraoperative endoscopy may demonstrate that laparoscopic gastrectomy causes significant narrowing at the gastric outlet, which is suboptimal. In these cases, a minimally invasive distal gastrectomy with a side-to-side gastrojejunostomy may be necessary [50]. Creative surgical maneuvers allow for less aggressive gastric resections for these challenging locations. The laparoscopic transgastric resection technique requires placement of three intragastric ports with endoscopic visualization. Traction is applied to the mass to expose normal gastric tissue at the base for intragastric use of a laparoscopic linear stapling device. Al-Thani’s approach to the posterior gastric wall adopted for robotic resection includes placement of two stay sutures to provide medial and lateral traction, resulting in organoaxial rotation to expose the posterior stomach wall, which is then followed by wedge resection and intracorporeal suturing for primary closure [50]. Smaller tumors of <3 cm can even be removed using an endoscopic snare device to prevent extending the gastrotomy into anatomically sensitive locations. The laparoscopic and endoscopic cooperative surgical approach has been shown to allow for a more precise resection margin with less unnecessary tissue resection (Figure 2 and Figure 3) [51].

Table 3.
Privette classification for gastric GISTs at anatomically challenging locations and possible surgical techniques [49,50].
A recent case series showed the additional benefit of a robotic approach to partial gastrectomy for localized gastric GISTs in difficult locations, with an average tumor size of 3.9 cm [52]. Of the patients that underwent the resection, 48% were classified as Privette 3 (near the GEJ), with a majority of these tumor burdens being intraluminal and on average only 2.5 cm away from the GEJ. These tumors did not require transgastric resection as proposed above. Approximately 60% of patients were able to undergo robotic stapled resection, while 36% required excision followed by intracorporeal suture closure of the defect. All patients in that study were able to preserve the pylorus and GEJ, with no conversion to open surgery necessary. The authors highlighted the higher risk of resection of the GISTs located at the gastroesophageal junction due to the lack of serosa, which can cause leaks after closure of the esophagogastric gastrotomy. One patient in this study required reoperation due to a leak located at this site. However, the total morbidity rate, classified as those with a Clavien–Dindo Grade 3 or above, was comparable to the reported literature. Lastly, it is important to note that upper endoscopy is an important visualization tool to minimize excessive gastric wall resection beyond the R0 status, and it should be routinely considered.
Recent updates in the field of the gastric GISTs include the advancement of cooperative surgery, defined as laparoscopic-guided or assisted endoscopic submucosal dissection [53]. Endoscopy is performed to confirm incision lines and perform mucosal/submucosal incision. The incision is then continued laparoscopically in the seromuscular direction, which is followed by laparoscopic stapling or suturing. This approach is typically performed for tumors of <5 cm, particularly for tumors closer to the esophagogastric junction as these can be particularly challenging to resect without narrowing the esophageal inlet.
6. Pharmacologic Therapy for Localized Gastric GISTs
Although surgical resection is the mainstay of treatment for localized gastric GISTs, adjuvant and neoadjuvant pharmacologic therapies are an important component to management for many patients depending on their risk of recurrence. Conventional chemotherapy is ineffective in treating GISTs, but the discovery of the targeted therapy imatinib drastically changed GIST management [24]. Imatinib is an orally available small molecule tyrosine kinase inhibitor (TKI) that targets receptor tyrosine kinases, including c-KIT and PDGFRA. Imatinib prevents activated tyrosine kinases from phosphorylating tyrosine residues on their substrates, thereby blocking downstream cell growth and proliferation signaling. Imatinib semi-competitively binds to its targets, locking them in a closed conformation [26].
At least three phase III clinical trials have evaluated the benefit of adjuvant imatinib for localized GISTs. In the randomized, double-blinded trial ACOSOG Z9001, 713 patients who had undergone resection of a primary GIST measuring at least 3 cm and expressing KIT were treated with either imatinib, at a dose of 400 mg daily, or placebo for a year [54]. Of note, gastric GISTs represented 58.2% and 66.4% of the patients enrolled in the treatment and placebo arms, respectively. Relapse-free survival (RFS) at 1 year was 98% in the imatinib arm versus 83% in the placebo arm [54]. Subsequent subgroup analysis showed particular benefit in high-risk tumors with relapse occurring in 19% and 47% of the imatinib and high-risk placebo subgroups, respectively. However, overall survival was not shown to be significantly improved in the imatinib arm [54].
In the EORTC 62024 trial, 908 patients with intermediate or high-risk GISTs, as defined by the 2002 NIH classification, were randomly assigned to either receive either 2 years of a daily oral administration of 400 mg of imatinib or undergo observation after resection of localized GISTs [55]. The aforementioned study originally utilized a primary endpoint of overall survival but was modified to imatinib failure-free survival (IFFS), which is defined as the time to start of a new systemic treatment with or without imatinib. Gastric GISTs comprised 55.7% and 55.1% of the observation and imatinib adjuvant arms, respectively. The selfsame study found modestly improved RFS in the imatinib treatment arm at 5- and 10-year follow-ups, as well as a trend toward higher IFFS and RFS (particularly in the subgroup of patients with high-risk disease) [45,56].
While ACOSOG Z9001 and EORTC 62024 suggested improved outcomes for patients treated with adjuvant imatinib, the only phase III study to show significant improvement in OS with imatinib was the Scandinavian Sarcoma Group (SSG) XVIII trial, which enrolled 400 patients with high-risk GISTs by the modified consensus NIH criteria and treated them with 400 mg of daily imatinib for either 12 months or 36 months [57,58]. All patients included had an activating mutation in either KIT or PDGFRA, and patients with PDGFRA D842V mutations were excluded. Furthermore, 49% of the patients in the 12-month treatment arm and 53% in the 36-month arm had gastric GISTs. The aforementioned study found that the RFS at 5 years was 71% and 53% for the 36 month and 12-month imatinib arms, respectively, while the OS was 92% and 86% [57]. This difference persisted at the 10-year update with RFS at 53% versus 42% and OS at 79% versus 65% in favor of longer adjuvant treatment [59]. These data established 3 years as the guideline duration for adjuvant imatinib therapy. Despite the benefits in OS observed in the study, the high rate of recurrence after cessation of therapy suggests that imatinib does not eliminate micrometastatic disease. Studies on longer duration adjuvant imatinib have been conducted but have not clearly demonstrated a benefit in treatment beyond the 3-year guideline. There are presently ongoing efforts, recently presented at the European Society of Medical Oncology, which are attempting to discern the difference in outcomes for a longer duration of administering imatinib compared to the current standard 3-year treatment. These data will be critical for determining treatment courses of imatinib for patients in the future as we continue to tailor management to individual patients.
Molecular subtype plays a role in tailoring adjuvant therapy for localized gastric GISTs. The PDGFRA D842V mutant GIST is known to be primarily resistant to imatinib, and patients with this mutation should not be treated with imatinib in the adjuvant setting [22,24]. Studies conducted in the metastatic setting have shown response to the alternative TKI avapritinib, but prospective study on its efficacy as an adjuvant treatment after resection of localized gastric GIST has not yet been conducted [22]. For patients with KIT/PDGFRA WT SDH-deficient gastric GISTs, there is no biologic basis for treating with a TKI, and these should be avoided in the adjuvant setting [22]. Although KIT exon 9 mutations are uncommon in gastric GISTs, studies conducted in the metastatic setting have shown improved response to a higher dose of 800 mg of imatinib for patients harboring these mutations [22]. A prospective study of high-dose imatinib for GISTs with KIT exon 9 mutations has not yet been conducted, and its benefit in this setting is uncertain.
Neoadjuvant therapy may be considered to reduce the morbidity of resection for patients with localized gastric GISTs if the tumor is particularly large. One recent phase II study on patients with a large (≥10 cm) gastric GIST achieved a high rate of R0 resection after 6–9 months of treatment with neoadjuvant imatinib dosed at 400 mg daily [60,61].
7. Future Novel Management Approaches
Due to the heterogeneity of GISTs and myriad subtypes, even within the gastric localization, clinicians face prognostic challenges when estimating the risk of recurrence and ambiguity when making treatment decisions. Several artificial intelligence models are being studied for their potential application to risk assessment and management of gastric GISTs. In one study, investigators trained interpretable AI methods known as optimal classification trees (OCTs) on the well-known predictors of recurrence (tumor size, mitotic count, and tumor site) in a cohort of patients with resected localized GISTs from the pre-imatinib era, and they tested the OCTs against a validation cohort with similar characteristics [62]. Of note, gastric GISTs represented 55.2% and 57.4% of the training and validation cohorts, respectively. These models utilized non-linear relationships between predictors of recurrence in the training dataset to inform their predictions, which differentiates them from statistical models that do not utilize artificial intelligence. The ability of the OCTs to predict the probability of recurrence was compared to the MSK nomogram, which is the only existing validated prognostic model that predicts a probability of recurrence for a given patient [63]. The investigators reported superior discrimination and calibration, i.e., the ability to separate predictive data into classes and the ability to predict accurately, respectively, in their OCT model [62]. In particular, the OCT model was superior at predicting recurrence for patients with a 25–50% risk and a >50% risk with a lower rate of overprediction [62]. Accurate prediction of recurrence is important to limit the financial cost and biologic toxicity of adjuvant treatments that may not be necessary.
Another recent study explored the application of deep learning to predictions of both outcome and mutation status by training models on digitized HES-stained whole slide images (WSIs) [64]. For their mutation classification model, gastric GISTs were represented in the training and validation sets at 54.9% and 53.4%, respectively. At the gene level, the investigators reported AUCs of 0.61, 0.91, and 0.71 in a cross-validation of the training set for a classification of samples as KIT-mutant, PDGFRA-mutant, and KIT/PDGFRA-WT, respectively, as well as AUCs of 0.76, 0.90, and 0.55 in the independent testing of the validation set. Further, at the codon level, the PDGFRA exon 18 D842V mutation was predicted with AUCs of 0.87 and 0.90 in the cross-validation and independent testing, respectively [64]. This is notable because, if exon 18 D842V mutations can be predicted from WSIs, it suggests the possibility of a future where appropriate treatment recommendations (e.g., avoidance of adjuvant imatinib) could be made without incurring the costs of genetic testing and delaying care.
Another recent study applied artificial intelligence to the prediction of malignant potential by conventional AFIP risk groups utilizing images from EUS [65]. The investigators trained their model on images from a retrospective cohort of 55 patients with gastric GISTs and known AFIP classification, and they tested it on a prospective dataset of 15 patients. The investigators reported an overall sensitivity, specificity, and accuracy for predicting malignancy risk of 75%, 73%, and 66%, respectively, in the validation cohort [65]. These data suggest that AI could possibly be leveraged to predict the malignant potential of a gastric GIST intra-procedurally during EUS.
8. Conclusions
Gastric GIST management continues to evolve rapidly as molecular genetics allows physicians to subclassify prognostic features and better leverage medical therapies in both the neoadjuvant and adjuvant settings. New diagnostic technologies allow clinicians to better prognosticate based on imaging criteria alone, limiting invasive biopsies that may be unindicated or dangerous. Choosing a treatment strategy based on mutation type, mass location, and patient characteristics can give surgeons a distinct advantage in the OR as they use more minimally invasive techniques. Surgical resection of localized gastric GISTs remains the cornerstone of treatment and is often sufficient for curative management. However, we now have molecular genetics to guide our estimation of disease phenotypes and can better anticipate which patients may need adjuvant treatment to maintain disease-free survival. Adjuvant therapy for even the most high-risk patients with aggressive disease is evolving rapidly and should be tailored to each individual case. Artificial intelligence may be leveraged in several aspects of gastric GIST management to optimize treatment outcomes.
A multidisciplinary group with dedicated gastroenterologists, medical oncologists, surgical oncologists, pathologic specialists, etc., is required to appropriately manage localized gastric GISTs with exceptional attention to detail, which is required in order to allow patients the best chance of disease-free and overall survival.
Author Contributions
Conceptualization, Z.L., D.M. and S.D.; methodology Z.L., D.M. and S.D.; software, Z.L., D.M. and S.D.; validation, Z.L., D.M., A.S., M.K. and S.D.; formal analysis, Z.L., D.M., A.S., M.K. and S.D.; investigation, Z.L., D.M., A.S., M.K. and S.D.; resources, S.D.; data curation, Z.L., D.M. and A.S.; writing—original draft preparation, Z.L., D.M., A.S., M.K. and S.D.; writing—review and editing, Z.L., D.M., A.S., M.K. and S.D.; visualization, Z.L., D.M., A.S. and S.D.; supervision, M.K. and S.D.; project administration, D.M. and S.D.; funding acquisition: not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazur, M.T.; Clark, H.B. Gastric stromal tumors. Reappraisal of histogenesis. Am. J. Surg. Pathol. 1983, 7, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Kindblom, L.G.; Remotti, H.E.; Aldenborg, F.; Meis-Kindblom, J.M. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am. J. Pathol. 1998, 152, 1259–1269. [Google Scholar] [PubMed]
- Alfagih, A.; AlJassim, A.; Alshamsan, B.; Alqahtani, N.; Asmis, T. Gastrointestinal Stromal Tumors: 10-Year Experience in Cancer Center-The Ottawa Hospital (TOH). Curr. Oncol. 2022, 29, 7148–7157. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Ullah, A.; Waheed, A.; Karki, N.R.; Adhikari, N.; Vemavarapu, L.; Belakhlef, S.; Bendjemil, S.M.; Mehdizadeh Seraj, S.; Sidhwa, F.; et al. Gastrointestinal Stromal Tumors (GIST): A Population-Based Study Using the SEER Database, including Management and Recent Advances in Targeted Therapy. Cancers 2022, 14, 3689. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, T.S.A.; Pieper, L.; Kist, M.; Thomaschewski, M.; Klinkhammer-Schalke, M.; Zeissig, S.R.; Tol, K.K.; Wellner, U.F.; Keck, T.; Hummel, R. Gastrointestinal stromal tumors of the upper GI tract: Population-based analysis of epidemiology, treatment and outcome based on data from the German Clinical Cancer Registry Group. J. Cancer Res. Clin. Oncol. 2023, 149, 7461–7469. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.D.; McKinley, M.; McBay, B.; Zylberberg, H.M.; Gomez, S.L.; Hur, C.; Kastrinos, F.; Gupta, S.; Kim, M.K.; Itzkowitz, S.H.; et al. Epidemiology of Gastric Malignancies 2000–2018 According to Histology: A Population-Based Analysis of Incidence and Temporal Trends. Clin. Gastroenterol. Hepatol 2023, 21, 3285–3295.e3288. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Scherübl, H.; Faiss, S.; Knoefel, W.T.; Wardelmann, E. Management of early asymptomatic gastrointestinal stromal tumors of the stomach. World J. Gastrointest. Endosc. 2014, 6, 266–271. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q. Prognostic Indicators for Gastrointestinal Stromal Tumors: A Review. Transl. Oncol. 2020, 13, 100812. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef]
- Yacob, M.; Inian, S.; Sudhakar, C.B. Gastrointestinal Stromal Tumours: Review of 150 Cases from a Single Centre. Indian J. Surg. 2015, 77, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Caterino, S.; Lorenzon, L.; Petrucciani, N.; Iannicelli, E.; Pilozzi, E.; Romiti, A.; Cavallini, M.; Ziparo, V. Gastrointestinal stromal tumors: Correlation between symptoms at presentation, tumor location and prognostic factors in 47 consecutive patients. World J. Surg. Oncol. 2011, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.B. Gastric GIST. Available online: https://radiologykey.com/gastric-gist/ (accessed on 19 December 2023).
- Ghanem, N.; Altehoefer, C.; Furtwängler, A.; Winterer, J.; Schäfer, O.; Springer, O.; Kotter, E.; Langer, M. Computed tomography in gastrointestinal stromal tumors. Eur. Radiol. 2003, 13, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Jin, E. Gastric sub-epithelial tumors: Identification of gastrointestinal stromal tumors using CT with a practical scoring method. Gastric Cancer 2019, 22, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ota, S.; Yamasaki, M.; Batsaikhan, B.; Furukawa, A.; Watanabe, Y. Gastrointestinal stromal tumors: A comprehensive radiological review. Jpn. J. Radiol. 2022, 40, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Tao, K.G.; Zhang, L.Y.; Wu, K.M.; Shi, J.; Zeng, X.; Lin, Y. Value of contrast-enhanced harmonic endoscopic ultrasonography in differentiating between gastrointestinal stromal tumors: A meta-analysis. J. Dig. Dis. 2019, 20, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Seven, G.; Arici, D.S.; Senturk, H. Correlation of Endoscopic Ultrasonography Features with the Mitotic Index in 2- to 5-cm Gastric Gastrointestinal Stromal Tumors. Dig. Dis. 2022, 40, 14–22. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shim, K.N.; Lee, J.H.; Lim, J.Y.; Kim, T.O.; Choe, A.R.; Tae, C.H.; Jung, H.K.; Moon, C.M.; Kim, S.E.; et al. Comparison of the Diagnostic Ability of Endoscopic Ultrasonography and Abdominopelvic Computed Tomography in the Diagnosis of Gastric Subepithelial Tumors. Clin. Endosc. 2019, 52, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, L.; Dong, X.; Li, Y.; Yu, J.; Xiong, W.; Li, G. The roles of CT and EUS in the preoperative evaluation of gastric gastrointestinal stromal tumors larger than 2 cm. Eur. Radiol. 2019, 29, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.W.; Jung, D.H.; Kim, J.S.; Shin, Y.R.; Choi, S.H.; Kim, B.-W. CT Versus Endoscopic Ultrasound for Differentiating Small (2–5 cm) Gastrointestinal Stromal Tumors from Leiomyomas. Am. J. Roentgenol. 2019, 213, 586–591. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Riedel, R.F.; Sicklick, J.K.; Pollack, S.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; et al. NCCN Guidelines® Insights: Gastrointestinal Stromal Tumors, Version 2.2022. J. Natl. Compr. Canc Netw. 2022, 20, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Antonescu, C.R.; DeMatteo, R.P.; Ganjoo, K.N.; Maki, R.G.; Pisters, P.W.; Raut, C.P.; Riedel, R.F.; Schuetze, S.; et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Canc Netw. 2010, 8 (Suppl. 2), S1–S41, quiz S42–S44. [Google Scholar] [CrossRef] [PubMed]
- Szucs, Z.; Thway, K.; Fisher, C.; Bulusu, R.; Constantinidou, A.; Benson, C.; van der Graaf, W.T.; Jones, R.L. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017, 13, 93–107. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Fanale, D.; Vincenzi, B.; De Luca, I.; Bartolotta, T.V.; Cannella, R.; Pantuso, G.; Cabibi, D.; Russo, A.; Bazan, V.; et al. Type and Gene Location of KIT Mutations Predict Progression-Free Survival to First-Line Imatinib in Gastrointestinal Stromal Tumors: A Look into the Exon. Cancers 2021, 13, 993. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Rutkowski, P.; Piskorz, A.; Ciwoniuk, M.; Osuch, C.; Bylina, E.; Sygut, J.; Chosia, M.; Rys, J.; Urbanczyk, K.; et al. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience. Ann. Oncol. 2012, 23, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.; Miettinen, M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs). Semin. Diagn. Pathol. 2006, 23, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Rubin, B.P.; Longley, B.J.; Fletcher, J.A. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum. Pathol. 2002, 33, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Martín-Broto, J.; Rubio, L.; Alemany, R.; López-Guerrero, J.A. Clinical implications of KIT and PDGFRA genotyping in GIST. Clin. Transl. Oncol. 2010, 12, 670–676. [Google Scholar] [CrossRef]
- Wozniak, A.; Rutkowski, P.; Schöffski, P.; Ray-Coquard, I.; Hostein, I.; Schildhaus, H.-U.; Le Cesne, A.; Bylina, E.; Limon, J.; Blay, J.-Y.; et al. Tumor Genotype Is an Independent Prognostic Factor in Primary Gastrointestinal Stromal Tumors of Gastric Origin: A European Multicenter Analysis Based on ConticaGIST. Clin. Cancer Res. 2014, 20, 6105–6116. [Google Scholar] [CrossRef]
- Kalfusova, A.; Linke, Z.; Kalinova, M.; Krskova, L.; Hilska, I.; Szabova, J.; Vicha, A.; Kodet, R. Gastrointestinal stromal tumors—Summary of mutational status of the primary/secondary KIT/PDGFRA mutations, BRAF mutations and SDH defects. Pathol. Res. Pract. 2019, 215, 152708. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Sommer, G.; Sarran, L.; Tschernyavsky, S.J.; Riedel, E.; Woodruff, J.M.; Robson, M.; Maki, R.; Brennan, M.F.; Ladanyi, M.; et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin. Cancer Res. 2003, 9, 3329–3337. [Google Scholar] [PubMed]
- Künstlinger, H.; Huss, S.; Merkelbach-Bruse, S.; Binot, E.; Kleine, M.A.; Loeser, H.; Mittler, J.; Hartmann, W.; Hohenberger, P.; Reichardt, P.; et al. Gastrointestinal stromal tumors with KIT exon 9 mutations: Update on genotype-phenotype correlation and validation of a high-resolution melting assay for mutational testing. Am. J. Surg. Pathol. 2013, 37, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.; Corless, C.L.; Heinrich, M.C.; Debiec-Rychter, M.; Sciot, R.; Wardelmann, E.; Merkelbach-Bruse, S.; Schildhaus, H.U.; Steigen, S.E.; Stachura, J.; et al. Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or exon 17 mutations: A multicenter study on 54 cases. Mod. Pathol. 2008, 21, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Martinez-Marín, V.; Serrano, C.; Hindi, N.; López-Guerrero, J.A.; Bisculoa, M.; Ramos-Asensio, R.; Vallejo-Benítez, A.; Marcilla-Plaza, D.; González-Cámpora, R. Gastrointestinal stromal tumors (GISTs): SEAP-SEOM consensus on pathologic and molecular diagnosis. Clin. Transl. Oncol. 2017, 19, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Smith, S.C.; Faber, A.C.; Trent, J.; Grossman, S.R.; Stratakis, C.A.; Boikos, S.A. Gastrointestinal Stromal Tumors: The GIST of Precision Medicine. Trends Cancer 2018, 4, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.A.; Stratakis, C.A. Familial paraganglioma and gastric stromal sarcoma: A new syndrome distinct from the Carney triad. Am. J. Med. Genet. 2002, 108, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Matyakhina, L.; Bei, T.A.; McWhinney, S.R.; Pasini, B.; Cameron, S.; Gunawan, B.; Stergiopoulos, S.G.; Boikos, S.; Muchow, M.; Dutra, A.; et al. Genetics of Carney Triad: Recurrent Losses at Chromosome 1 but Lack of Germline Mutations in Genes Associated with Paragangliomas and Gastrointestinal Stromal Tumors. J. Clin. Endocrinol. Metab. 2007, 92, 2938–2943. [Google Scholar] [CrossRef]
- Brčić, I.; Argyropoulos, A.; Liegl-Atzwanger, B. Update on Molecular Genetics of Gastrointestinal Stromal Tumors. Diagnostics 2021, 11, 194. [Google Scholar] [CrossRef]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; LaQuaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016, 2, 922–928. [Google Scholar] [CrossRef]
- Wei, C.H.; Pettersson, J.; Campan, M.; Chopra, S.; Naritoku, W.; Martin, S.E.; Ward, P.M. Gain of TP53 Mutation in Imatinib-treated SDH-Deficient Gastrointestinal Stromal Tumor and Clinical Utilization of Targeted Next-generation Sequencing Panel for Therapeutic Decision Support. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; Vivancos, A.; López-Pousa, A.; Matito, J.; Mancuso, F.M.; Valverde, C.; Quiroga, S.; Landolfi, S.; Castro, S.; Dopazo, C.; et al. Clinical value of next generation sequencing of plasma cell-free DNA in gastrointestinal stromal tumors. BMC Cancer 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; de la Torre, J.; NS, I.J.; Sutton, T.L.; Zhao, B.; Khan, T.M.; Banerjee, S.; Cui, C.; Nguyen, V.; Alkhuziem, M.; et al. Location of Gastrointestinal Stromal Tumor (GIST) in the Stomach Predicts Tumor Mutation Profile and Drug Sensitivity. Clin. Cancer Res. 2021, 27, 5334–5342. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Bonvalot, S.; Poveda Velasco, A.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.R.; Italiano, A.; Gelderblom, H.J.; et al. Quality of Surgery and Outcome in Localized Gastrointestinal Stromal Tumors Treated Within an International Intergroup Randomized Clinical Trial of Adjuvant Imatinib. JAMA Surg. 2020, 155, e200397. [Google Scholar] [CrossRef] [PubMed]
- McCarter, M.D.; Antonescu, C.R.; Ballman, K.V.; Maki, R.G.; Pisters, P.W.T.; Demetri, G.D.; Blanke, C.D.; von Mehren, M.; Brennan, M.F.; McCall, L.; et al. Microscopically positive margins for primary gastrointestinal stromal tumors: Analysis of risk factors and tumor recurrence. J. Am. Coll. Surg. 2012, 215, 53–59; discussion 59–60. [Google Scholar] [CrossRef] [PubMed]
- Gevorkian, J.; Le, E.; Alvarado, L.; Davis, B.; Tyroch, A.; Chiba, S.; Konstantinidis, I.T. Trends and outcomes of minimally invasive surgery for gastrointestinal stromal tumors (GIST). Surg. Endosc. 2022, 36, 6841–6850. [Google Scholar] [CrossRef] [PubMed]
- Crocker, A.B.; Vega, E.A.; Kutlu, O.C.; Salehi, O.; Mellado, S.; Li, M.; Kozyreva, O.; Conrad, C. Is minimally invasive surgery for large gastric GIST actually safe? A comparative analysis of short- and long-term outcomes. Surg. Endosc. 2022, 36, 6975–6983. [Google Scholar] [CrossRef]
- Privette, A.; McCahill, L.; Borrazzo, E.; Single, R.M.; Zubarik, R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg. Endosc. 2008, 22, 487–494. [Google Scholar] [CrossRef]
- Al-Thani, H.; El-Menyar, A.; Mekkodathil, A.; Elgohary, H.; Tabeb, A.H. Robotic management of gastric stromal tumors (GIST): A single Middle Eastern center experience. Int. J. Med. Robot. 2017, 13, e1729. [Google Scholar] [CrossRef]
- Aisu, Y.; Yasukawa, D.; Kimura, Y.; Hori, T. Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits. World J. Gastrointest. Oncol. 2018, 10, 381–397. [Google Scholar] [CrossRef]
- Lwin, T.M.; Fong, Z.V.; Narayan, R.R.; Wang, S.J.; Wang, J. Robotic Function-Preserving Resection of Gastric Gastrointestinal Stromal Tumor. J. Surg. Res. 2023, 290, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.Z.J.; Ishraq, F.; Chay, A.F.T.; Tay, K.V. Lap-Endo cooperative surgery (LECS) in gastric GIST: Updates and future advances. Surg. Endosc. 2023, 37, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; Ballman, K.V.; Antonescu, C.R.; Kolesnikova, V.; Maki, R.G.; Pisters, P.W.; Blackstein, M.E.; Blanke, C.D.; Demetri, G.D.; Heinrich, M.C.; et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: The ACOSOG Z9001 trial. J. Clin. Oncol. 2014, 32, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Przybył, J.; Zdzienicki, M. Extended adjuvant therapy with imatinib in patients with gastrointestinal stromal tumors: Recommendations for patient selection, risk assessment, and molecular response monitoring. Mol. Diagn. Ther. 2013, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Le Cesne, A.; Velasco, A.P.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.R.; Italiano, A.; Gelderblom, H.; et al. Final analysis of the randomized trial on imatinib as an adjuvant in localized gastrointestinal stromal tumors (GIST) from the EORTC Soft Tissue and Bone Sarcoma Group (STBSG), the Australasian Gastro-Intestinal Trials Group (AGITG), UNICANCER, French Sarcoma Group (FSG), Italian Sarcoma Group (ISG), and Spanish Group for Research on Sarcomas (GEIS). Ann. Oncol. 2021, 32, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Hartmann, J.T.; Pink, D.; Schütte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.E.; et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012, 307, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Reichardt, A.; Hermes, B.; Schütte, J.; Cameron, S.; Hohenberger, P.; Jost, P.J.; Al-Batran, S.E.; et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients With High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol. 2020, 6, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Yang, H.K.; Cho, H.; Ryu, M.H.; Masuzawa, T.; Park, S.R.; Matsumoto, S.; Lee, H.J.; Honda, H.; Kwon, O.K.; et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br. J. Cancer 2017, 117, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-H.; Kurokawa, Y.; Yook, J.-H.; Cho, H.; Kwon, O.-K.; Masuzawa, T.; Lee, K.H.; Matsumoto, S.; Park, Y.S.; Honda, H.; et al. Long-term outcomes of a phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumors of the stomach. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2023, 26, 775–787. [Google Scholar] [CrossRef]
- Bertsimas, D.; Margonis, G.A.; Tang, S.; Koulouras, A.; Antonescu, C.R.; Brennan, M.F.; Martin-Broto, J.; Rutkowski, P.; Stasinos, G.; Wang, J.; et al. An interpretable AI model for recurrence prediction after surgery in gastrointestinal stromal tumour: An observational cohort study. EClinicalMedicine 2023, 64, 102200. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.S.; Gönen, M.; Gutiérrez, A.; Broto, J.M.; García-del-Muro, X.; Smyrk, T.C.; Maki, R.G.; Singer, S.; Brennan, M.F.; Antonescu, C.R.; et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009, 10, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Karanian, M.; Perret, R.; Camara, A.; Le Loarer, F.; Jean-Denis, M.; Hostein, I.; Michot, A.; Ducimetiere, F.; Giraud, A.; et al. Deep learning predicts patients outcome and mutations from digitized histology slides in gastrointestinal stromal tumor. NPJ Precis. Oncol. 2023, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, J.; Hu, M.; Zhong, Q.; Er, L.; Shi, H.; Cheng, W.; Chen, K.; Liu, Y.; Qiu, B.; et al. Artificial Intelligence in the Prediction of Gastrointestinal Stromal Tumors on Endoscopic Ultrasonography Images: Development, Validation and Comparison with Endosonographers. Gut Liver 2023, 17, 874–883. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).