Malaria and HIV Co-Infection among Pregnant Women in Africa: Prevalence, Effect on Immunity and Clinical Management: Review
Abstract
1. Introduction
2. Malaria and HIV co-infection during pregnancy
2.1. Epidemiological Evidence of Malaria and HIV Co-Infection among Pregnant Women in Africa
| Study Area | Study Design | Prevalence (%) | Conclusion | Ref. | ||
|---|---|---|---|---|---|---|
| Malaria | HIV | MHC | ||||
| HIV-Negative Pregnant Women | ||||||
| Cameroon | Prospective cohort | 25% | NR | NR | Primigravida single women, and women under 20 years had a higher incidence than multigravida married women, and women over 20 years. This study only saw Plasmodium falciparum. | [35] |
| Nigeria | Cross-sectional | 28.7% | NR | NR | Moderate malaria parasitemia was higher among HIV-positive pregnant women. All malaria preventive strategies should be intensified during pregnancy, as ITNs provided little protection. | [36] |
| Ghana | Cross-sectional | 11.0% | NR | NR | The prevalence of malaria and anemia in pregnancy among pregnant women in the Akatsi South District remains a source of concern. The high use of IPTp-SP and LLIN was observed, positively affecting malaria prevalence among pregnant women. | [37] |
| Ethiopia | Cross-sectional | 10.2% | NR | NR | Malaria is still a public health problem among pregnant women in the Sherkole district. Age, ITN-use, gravidity, gestational age, and health education had a significant association with malaria. | [38] |
| Malawi | Longitudinal | 5.0% | NR | NR | Increased bednet coverage explains changes in parasitemia and birth weight among pregnant women better than sulfadoxine-pyrimethamine use. | [39] |
| DR Congo | Cross-sectional | 14.97% | NR | NR | Malaria is common among Mwene Ditu’s pregnant women. The ANC attendance and an appropriate organization prove to be of paramount importance. | [40] |
| Burkina Faso | Cross-sectional | 15.7% | NR | NR | The prevalence of P. falciparum infection among pregnant women in Burkina Faso remains high, despite IPTp-SP and ITNs being shown to reduce the risk of disease. | [41] |
| Niger republic | Cross-sectional | 36.52% | NR | NR | Ignorance was discovered to be a factor in the epidemiology of malaria in the area, and a mass public-enlightenment program was proposed as a control measure. | [42] |
| Benin | Cross-sectional | 15.3% | NR | NR | This study demonstrates the higher performance of ultrasensitive RDT compared with conventional RDTs in detecting low parasite-density P. falciparum infections during pregnancy, particularly in the 1st trimester | [43] |
| Kenya | Cross-sectional | 12.9% | NR | NR | The majority of the women had anemia and asymptomatic malaria pregnancy. | [44] |
| HIV-positive pregnant women | ||||||
| Cameroon | Case study | 61.5% | 17.2% | 18.5% | Malaria and HIV research is lacking in many locations. The extent and effects of treatment interaction between these two diseases remain unknown. Malaria and HIV programs must cover the poor and vulnerable and integrate services whenever possible. | [45] |
| Seven sub-Saharan Africa | Cross-sectional | 31% | 1.3% | 0.52% | Malaria was associated with an increased prevalence of anemia during pregnancy. | [46] |
| Ethiopia | Cross-sectional | - | - | 22.2% | With increasing duration of ART use, there was a significant improvement in the mean CD4+ T cell count, Hb level, and parasite density in HIV/malaria co-infected pregnant women. | [47] |
| Nigeria | Cross-sectional | - | - | 56.3% | The prevalence of malaria recorded in this study is high, but with negative results for all socio-demographic variables of participants and malaria risk factors. | [48] |
2.2. Effect of Malaria and HIV Co-Infection on Mother-to-Child Transmission of Malaria and HIV
2.3. HIV and Malaria Co-Infection Affects Immune Modulation during Pregnancy
2.4. HIV Effect on the Humoral Immunity to Malaria
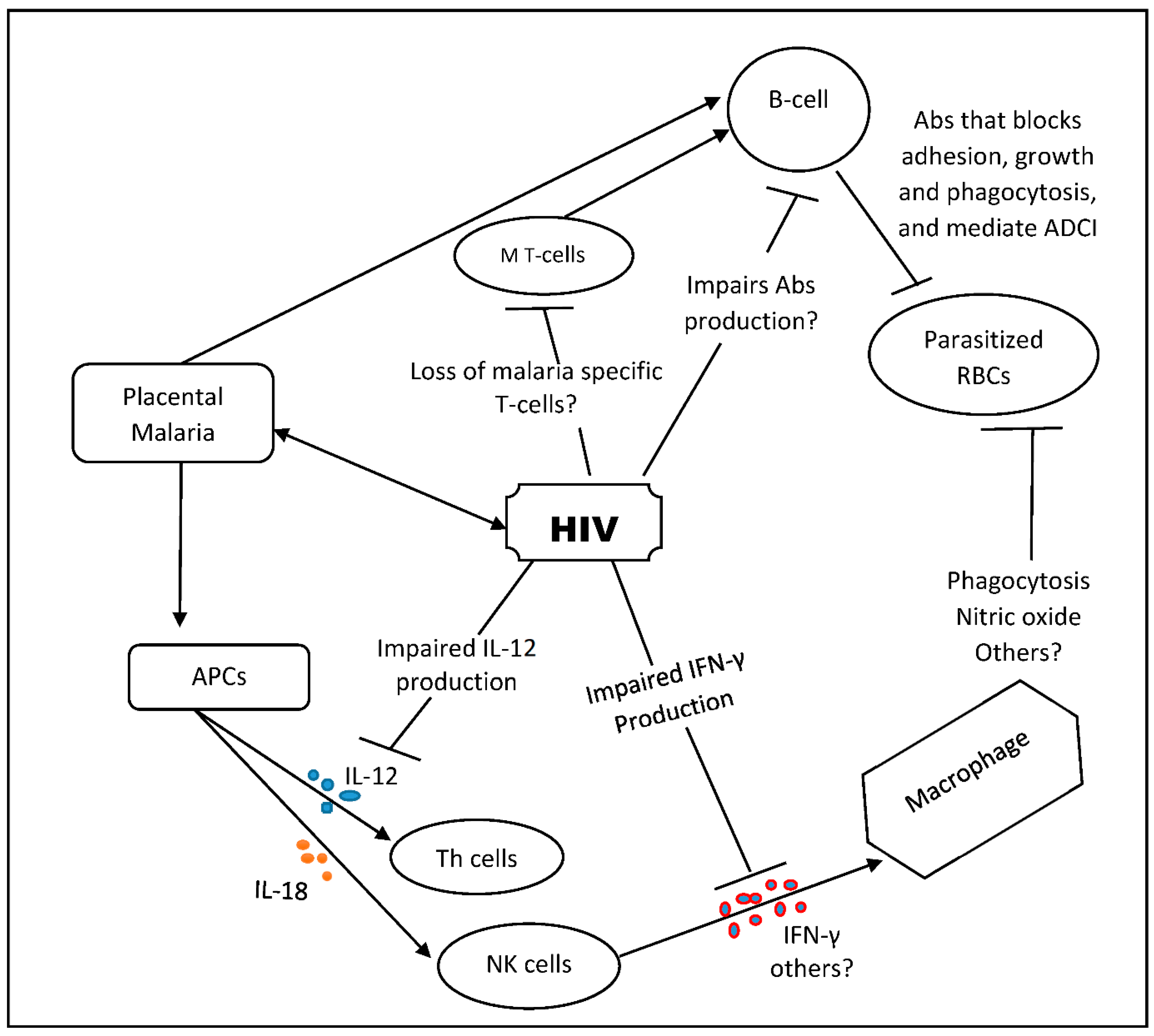
2.5. HIV Effect on the Cellular Immune Response to Malaria
3. Clinical Management of Malaria and HIV Co-Infection
3.1. The Clinical Management of Malaria
3.2. Role of Malaria Vaccine in the Management of Malaria during Pregnancy
3.3. Clinical Management of HIV-Infected Pregnant Women
3.4. Guidelines for Monitoring and Prophylaxis of HIV-Exposed Children
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwenti, T.E. Malaria and HIV coinfection in sub-Saharan Africa: Prevalence, impact, and treatment strategies. Res. Rep. Trop. Med. 2018, 9, 123–136. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Patnaik, P.; Kublin, J.G. Dual Infection with HIV and Malaria Fuels the Spread of Both Diseases in Sub-Saharan Africa. Science 2006, 314, 1603–1606. [Google Scholar] [CrossRef] [PubMed]
- Jamison, D.T.; World Bank (Eds.) Disease and mortality in Sub-Saharan Africa, 2nd ed.; World Bank: Washington, DC, USA, 2006; ISBN 978-0-8213-6397-3. [Google Scholar]
- Fried, M.; Duffy, P.E. Malaria during Pregnancy. Cold Spring Harb. Perspect. Med. 2017, 7, a025551. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; ter Kuile, F.O. Stillbirths: The hidden burden of malaria in pregnancy. Lancet Glob. Health 2017, 5, e1052–e1053. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Miles, D.J.; Crozier, S.; Waight, P.; Palmero, M.S.; Ojuola, O.; Touray, E.; van der Sande, M.; Whittle, H.; Rowland-Jones, S.; et al. Placental Malaria is associated with reduced early life weight development of affected children independent of low birth weight. Malar. J. 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.C.; Chongwe, G.; Chipukuma, H.; Jacobs, C.; Zgambo, J.; Michelo, C. Uptake of intermittent preventive treatment for malaria during pregnancy with Sulphadoxine-Pyrimethamine (IPTp-SP) among postpartum women in Zomba District, Malawi: A cross-sectional study. BMC Pregnancy Childbirth 2018, 18, 108. [Google Scholar] [CrossRef]
- World Health Organization. Preparing for Certification of Malaria Elimination; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000562-4. [Google Scholar]
- Wumba, R.D.; Zanga, J.; Aloni, M.N.; Mbanzulu, K.; Kahindo, A.; Mandina, M.N.; Ekila, M.B.; Mouri, O.; Kendjo, E. Interactions between malaria and HIV infections in pregnant women: A first report of the magnitude, clinical and laboratory features, and predictive factors in Kinshasa, the Democratic Republic of Congo. Malar. J. 2015, 14, 82. [Google Scholar] [CrossRef]
- Ibegu, M.I.; Hamza, K.L.; Umeokonkwo, C.D.; Numbere, T.-W.; Ndoreraho, A.; Dahiru, T. Use of long-lasting insecticidal nets among women attending antenatal clinic at a tertiary hospital in Bayelsa State, Nigeria 2019. Malar. J. 2020, 19, 455. [Google Scholar] [CrossRef]
- Manore, C.A.; Teboh-Ewungkem, M.I.; Prosper, O.; Peace, A.; Gurski, K.; Feng, Z. Intermittent Preventive Treatment (IPT): Its Role in Averting Disease-Induced Mortality in Children and in Promoting the Spread of Antimalarial Drug Resistance. Bull. Math. Biol. 2019, 81, 193–234. [Google Scholar] [CrossRef]
- Bouyou-Akotet, M.K.; Ionete-Collard, D.E.; Mabika, M.; Kendjo, E.; Matsiegui, P.-B.; Mavoungou, E.; Kombila, M. Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar. J. 2003, 7, 18. [Google Scholar] [CrossRef]
- Almaw, A.; Yimer, M.; Alemu, M.; Tegegne, B. Prevalence of malaria and associated factors among symptomatic pregnant women attending antenatal care at three health centers in north-west Ethiopia. PLoS ONE 2022, 17, e0266477. [Google Scholar] [CrossRef] [PubMed]
- Schantz-Dunn, J. Nour NM. Malaria and Pregnancy: A Global Health Perspective. Rev. Obstet. Gynecol. 2009, 2, 186–192. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2760896 (accessed on 29 September 2022). [CrossRef] [PubMed]
- Nyasa, R.B.; Fotabe, E.L.; Ndip, R.N. Trends in malaria prevalence and risk factors associated with the disease in Nkongho-mbeng; a typical rural setting in the equatorial rainforest of the South West Region of Cameroon. PLoS ONE 2021, 16, e0251380. [Google Scholar] [CrossRef] [PubMed]
- Touré, M.; Keita, M.; Kané, F.; Sanogo, D.; Kanté, S.; Konaté, D.; Diarra, A.; Sogoba, N.; Coulibaly, M.B.; Traoré, S.F.; et al. Trends in malaria epidemiological factors following the implementation of current control strategies in Dangassa, Mali. Malar. J. 2022, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- The Global Malaria Action Plan. Roll Back Malaria: For a Malaria Free World. Available online: https://www.unhcr.org/4afac5629 (accessed on 28 September 2022).
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004049-6. [Google Scholar]
- Agomo, C.O.; Oyibo, W.A.; Anorlu, R.I.; Agomo, P.U. Prevalence of Malaria in Pregnant Women in Lagos, South-West Nigeria. Korean J. Parasitol. 2009, 47, 179. [Google Scholar] [CrossRef]
- Kar, N.P.; Kumar, A.; Singh, O.P.; Carlton, J.M.; Nanda, N. A review of malaria transmission dynamics in forest ecosystems. Parasites Vectors 2014, 7, 265. [Google Scholar] [CrossRef]
- Nissan, H.; Ukawuba, I.; Thomson, M. Climate-proofing a malaria eradication strategy. Malar. J. 2021, 20, 190. [Google Scholar] [CrossRef]
- Craig, M.H.; Kleinschmidt, I.; Nawn, J.B.; Le Sueur, D.; Sharp, B.L. Exploring 30 years of malaria case data in KwaZulu-Natal, South Africa: Part I. The impact of climatic factors. Trop. Med. Int. Health 2004, 9, 1247–1257. [Google Scholar] [CrossRef]
- Christiansen-Jucht, C.; Parham, P.E.; Saddler, A.; Koella, J.C.; Basáñez, M.-G. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae. Parasites Vectors 2014, 7, 489. Available online: http://www.parasitesandvectors.com/content/7/1/489 (accessed on 28 September 2022). [CrossRef]
- Jetten, T.H.; Martens, W.J.M.; Takken, W. Model Simulations To Estimate Malaria Risk Under Climate Change. J. Med. Entomol. 1996, 33, 361–371. [Google Scholar] [CrossRef]
- Adugna, T.; Yewhelew, D.; Getu, E. Bloodmeal sources and feeding behavior of anopheline mosquitoes in Bure district, northwestern Ethiopia. Parasites Vectors 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.D.J. Preliminary review on the prevalence, proportion, geographical distribution, and characteristics of naturally acquired Plasmodium cynomolgi infection in mosquitoes, macaques, and humans: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Raja, T.N.; Hu, T.H.; Kadir, K.A.; Mohamad, D.S.A.; Rosli, N.; Wong, L.L.; Hii, K.C.; Simon Divis, P.C.; Singh, B. Naturally Acquired Human Plasmodium cynomolgi and P. knowlesi Infections, Malaysian Borneo. Emerg. Infect. Dis. 2020, 26, 1801–1809. [Google Scholar] [CrossRef]
- German Advisory Committee Blood (Arbeitskreis Blut), Subgroup ‘Assessment of Pathogens Transmissible by Blood’ Human Immunodeficiency Virus (HIV). Transfus. Med. Hemother 2016, 43, 203–222. [CrossRef]
- Parker, E.; Judge, M.A.; Macete, E.; Nhampossa, T.; Dorward, J.; Langa, D.C.; Schacht, C.D.; Couto, A.; Vaz, P.; Vitoria, M.; et al. HIV infection in Eastern and Southern Africa: Highest burden, largest challenges, greatest potential. South. Afr. J. HIV Med. 2021, 22, 8. [Google Scholar] [CrossRef]
- Kharsany, A.B.M.; Karim, Q.A. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016, 10, 34–48. [Google Scholar] [CrossRef]
- Shaw, G.M.; Hunter, E. HIV Transmission. Cold Spring Harb. Perspect. Med. 2012, 2, a006965. [Google Scholar] [CrossRef]
- Remera, E.; Mugwaneza, P.; Chammartin, F.; Mulindabigwi, A.; Musengimana, G.; Forrest, J.I.; Mwanyumba, F.; Kondwani, N.; Condo, J.U.; Riedel, D.J.; et al. Towards elimination of mother-to-child transmission of HIV in Rwanda: A nested case-control study of risk factors for transmission. BMC Pregnancy Childbirth 2021, 21, 339. [Google Scholar] [CrossRef]
- World Health Organization. Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission of HIV and Syphilis, 2nd ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151327-2. [Google Scholar]
- Asoba, G.N.; Ndamukong, K.J.N.; Achidi, E.A. Prevalence of malaria parasite infection in pregnant women in three towns of the South West Region of Cameroon. J. Cameroon Acad. Sci. 2009, 8, 2–9. [Google Scholar]
- Anne, N. Prevalence of malaria in HIV positive and HIV negative pregnant women attending antenatal clinics in south eastern Nigeria. Malawi Med. J. 2018, 30, 256. [Google Scholar] [CrossRef]
- Ahadzie-Soglie, A.; Addai-Mensah, O.; Abaka-Yawson, A.; Setroame, A.M.; Kwadzokpui, P.K. Prevalence and risk factors of malaria and anaemia and the impact of preventive methods among pregnant women: A case study at the Akatsi South District in Ghana. PLoS ONE 2022, 17, e0271211. [Google Scholar] [CrossRef] [PubMed]
- Gontie, G.B.; Wolde, H.F.; Baraki, A.G. Prevalence and associated factors of malaria among pregnant women in Sherkole district, Benishangul Gumuz regional state, West Ethiopia. BMC Infect. Dis. 2020, 20, 573. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Simpson, J.A.; Chaluluka, E.; Molyneux, M.E.; Rogerson, S.J. Decreasing Burden of Malaria in Pregnancy in Malawian Women and Its Relationship to Use of Intermittent Preventive Therapy or Bed Nets. PLoS ONE 2010, 5, e12012. [Google Scholar] [CrossRef] [PubMed]
- Jean-Claude, M.K.; Bienfait, M.M.; Simon, I.K.; Jean-Baptiste, K.S.Z. Epidemiological Aspects of Malaria in Pregnant Women: Prevalence and Risk Factors in Mwene Ditu, DR Congo. OALib 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Yaro, J.B.; Ouedraogo, A.; Diarra, A.; Sombié, S.; Ouedraogo, Z.A.; Nébié, I.; Drakeley, C.; Sirima, S.B.; Tiono, A.B.; Lindsay, S.W.; et al. Risk factors for Plasmodium falciparum infection in pregnant women in Burkina Faso: A community-based cross-sectional survey. Malar. J. 2021, 20, 362. [Google Scholar] [CrossRef]
- Banao, T.O.; Adamou, T.; Abubakar, U.; Ladan, M.J.; Bala, A.Y.; Mu’Awiyya, U.L. The prevalence of malaria among pregnant women in Maradi, Niger Republic. Niger. J. Parasitol. 2010, 31, 1. [Google Scholar] [CrossRef]
- Briand, V.; Cottrell, G.; Tuike Ndam, N.; Martiáñez-Vendrell, X.; Vianou, B.; Mama, A.; Kouwaye, B.; Houzé, S.; Bailly, J.; Gbaguidi, E.; et al. Prevalence and clinical impact of malaria infections detected with a highly sensitive HRP2 rapid diagnostic test in Beninese pregnant women. Malar. J. 2020, 19, 188. [Google Scholar] [CrossRef]
- Nyamu, G.W.; Kihara, J.H.; Oyugi, E.O.; Omballa, V.; El-Busaidy, H.; Jeza, V.T. Prevalence and risk factors associated with asymptomatic Plasmodium falciparum infection and anemia among pregnant women at the first antenatal care visit: A hospital based cross-sectional study in Kwale County, Kenya. PLoS ONE 2020, 15, e0239578. [Google Scholar] [CrossRef]
- Peter, M.N.; Gerard, E.F.; Julius, N.; Orock, B.; Daniel, M. Malaria and HIV/AIDS in Cameroon: Antenatal Care Attendees as Case Study in the Managemnt of Both Infections. Journal of the Cameroon Academy of Sciences. Available online: https://www.ajol.info/index.php/jcas/article/view/17707/16859 (accessed on 29 September 2022).
- Ssentongo, P.; Ba, D.M.; Ssentongo, A.E.; Ericson, J.E.; Wang, M.; Liao, D.; Chinchilli, V.M. Associations of malaria, HIV, and coinfection, with anemia in pregnancy in sub-Saharan Africa: A population-based cross-sectional study. BMC Pregnancy Childbirth 2020, 20, 379. [Google Scholar] [CrossRef]
- Heven, S. The Prevalence of HIV/Malaria Co-Infection during Pregnancy in Adama Hospital and ‘Awash Sebat Kilo’ Health Center, Ethiopia. 2009. Available online: http://etd.aau.edu.et/bitstream/handle/123456789/5521/Samuel%20Gemechu.pdf;jsessionid=F5CB9178E53A96A6B5418A6CA75DFD83?sequence=1 (accessed on 29 September 2022).
- Okechukwu, C.E.; Abdullahi, I.N.; Aliyu, D.; Kabiru, M.; Adekola, H.A.; Ikeh, E.I. Prevalence and risk factors of malaria and human immunodeficiency virus co-infec- tion among pregnant women at Sokoto, Nigeria. Rwanda Med. J. 2020, 77, 5. [Google Scholar]
- Ellington, S.R.; King, C.C.; Kourtis, A.P. Host factors that influence mother-to-child transmission of HIV-1: Genetics, coinfections, behavior and nutrition. Futur. Virol. 2011, 6, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Bulterys, M. Mother-to-Child Transmission of HIV: Pathogenesis, Mechanisms and Pathways. Clin. Perinatol. 2010, 37, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Zakama, A.K.; Ozarslan, N.; Gaw, S.L. Placental Malaria. Curr. Trop. Med. Rep. 2020, 7, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Bain, L.E.; Dobermann, D. Malaria, HIV and TB in the Democratic Republic of the Congo: Epidemiology, Disease Control Challenges and Interventions; Institute of Development Studies (IDS): East Sussex, UK, 2022; Available online: https://opendocs.ids.ac.uk/opendocs/bitstream/handle/20.500.12413/17290/1089_epidemiology_of_malaria_TB_and_HIV_in_DRC.pdf?sequence=1&isAllowed=y (accessed on 29 September 2022).
- Williams, H.A.; Bloland, P.B. Malaria Control during Mass Population Movements and Natural Disasters. 2002. 184p. ISBN 0-309-50362-0. Available online: http://www.nap.edu (accessed on 29 September 2022).
- Van geertruyden, J.-P.; Menten, J.; Colebunders, R.; Korenromp, E.; D’Alessandro, U. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar. J. 2008, 7, 134. [Google Scholar] [CrossRef]
- Msamanga, G.I.; Sharma, U.; Sinkala, M.; Brown, E.R.; Hoffman, I.F.; Goldenberg, R.L.; Taha, T.E.; Fawzi, W.W.; Young, A.M.; Read, J.S.; et al. Placental Malaria and Mother-to-Child Transmission of Human Immunodeficiency Virus-1. Am. J. Trop. Med. Hyg. 2009, 80, 508–515. [Google Scholar] [CrossRef]
- Babakhanyan, A.; Ekali, G.L.; Dent, A.; Kazura, J.; Nguasong, J.T.; Fodjo, B.A.Y.; Yuosembom, E.K.; Esemu, L.F.; Taylor, D.W.; Leke, R.G.F. Maternal Human Immunodeficiency Virus-Associated Hypergammaglobulinemia Reduces Transplacental Transfer of Immunoglobulin G to Plasmodium falciparum Antigens in Cameroonian Neonates. Open Forum Infect. Dis. 2016, 3, ofw092. [Google Scholar] [CrossRef]
- Hoffman, I.F.; Jere, C.S.; Taylor, T.E.; Munthali, P.; Dyer, J.R.; Wirima, J.J.; Rogerson, S.J.; Kumwenda, N.; Eron, J.J.; Fiscus, S.A.; et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS 1999, 13, 487–494. [Google Scholar] [CrossRef]
- Inion, I.; Mwanyumba, F.; Gaillard, P.; Chohan, V.; Verhofstede, C.; Claeys, P.; Mandaliya, K.; Marck, E.V.; Temmerman, M. Placental Malaria and Perinatal Transmission of Human Immunodeficiency Virus Type 1. J. Infect. Dis. 2003, 188, 1675–1678. [Google Scholar] [CrossRef]
- Mwapasa, V.; Rogerson, S.J.; Molyneux, M.E.; Abrams, E.T.; Kamwendo, D.D.; Lema, V.M.; Tadesse, E.; Chaluluka, E.; Wilson, P.E.; Meshnick, S.R. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS 2004, 18, 1051–1059. [Google Scholar] [CrossRef]
- Alemu, A.; Shiferaw, Y.; Addis, Z.; Mathewos, B.; Birhan, W. Effect of malaria on HIV/AIDS transmission and progression. Parasites Vectors 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Ned, R.M.; Moore, J.M.; Chaisavaneeyakorn, S.; Udhayakumar, V. Modulation of immune responses during HIV–malaria co-infection in pregnancy. Trends Parasitol. 2005, 21, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Mwapasa, V.; Rogerson, S.J. Protecting Pregnant Women from Malaria in Areas of High HIV Infection Prevalence. J Infect. Dis. 2006, 194, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired Immunity to Malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Mu, Y.; Unal, B.; Sundaresh, S.; Hirst, S.; Valdez, C.; Randall, A.; Molina, D.; Liang, X.; Freilich, D.A.; et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 2008, 8, 4680–4694. [Google Scholar] [CrossRef]
- Muema, D.K.; Ndungu, F.M.; Kinyanjui, S.M.; Berkley, J.A. Effect of HIV infection on the acute antibody response to malaria antigens in children: An observational study. Malar. J. 2011, 10, 55. [Google Scholar] [CrossRef]
- Nnedu, O.N.; O’Leary, M.P.; Mutua, D.; Mutai, B.; Kalantari-Dehaghi, M.; Jasinskas, A.; Nakajima-Sasaki, R.; John-Stewart, G.; Otieno, P.; Liang, X.; et al. Humoral immune responses to Plasmodium falciparum among HIV-1-infected Kenyan adults. Prot. Clin. Appl. 2011, 5, 613–623. [Google Scholar] [CrossRef]
- Ayisi, J.G.; Branch, O.H.; Rafi-Janajreh, A.; van Eijk, A.M.; ter Kuile, F.O.; Rosen, D.H.; Kager, P.A.; Lanar, D.E.; Barbosa, A.; Kaslow, D.; et al. Does Infection with Human Immunodeficiency Virus Affect the Antibody Responses to Plasmodium falciparum Antigenic Determinants in Asymptomatic Pregnant Women? J. Infect. 2003, 46, 164–172. [Google Scholar] [CrossRef]
- Andrews, K.; Lanzer, M. Maternal malaria: Plasmodium falciparum sequestration in the placenta. Parasitol. Res. 2002, 88, 715–723. [Google Scholar] [CrossRef]
- Michal, F.; Patrick, E. Duffy Adherence of plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996, 272, 1502–1504. [Google Scholar]
- Buffet, P.A.; Gamain, B.; Scheidig, C.; Baruch, D.; Smith, J.D.; Hernandez-Rivas, R.; Pouvelle, B.; Oishi, S.; Fujii, N.; Fusai, T.; et al. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: A receptor for human placental infection. Proc. Natl. Acad. Sci. USA. 1999, 96, 12743–12748. [Google Scholar] [CrossRef] [PubMed]
- Mount, A.M.; Mwapasa, V.; Elliott, S.R.; Beeson, J.G.; Tadesse, E.; Lema, V.M.; Molyneux, M.E.; Meshnick, S.R.; Rogerson, S.J. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet 2004, 363, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Diouf, I.; Fievet, N.; Doucouré, S.; Ngom, M.; Andrieu, M.; Mathieu, J.-F.; Gaye, A.; Thiaw, O.T.; Deloron, P. IL-12 producing monocytes and IFN-γ and TNF-α producing T-lymphocytes are increased in placentas infected by Plasmodium falciparum. J. Reprod. Immunol. 2007, 74, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chaisavaneeyakorn, S.; Moore, J.M.; Otieno, J.; Chaiyaroj, S.C.; Perkins, D.J.; Shi, Y.P.; Nahlen, B.L.; Lal, A.A.; Udhayakumar, V. Immunity to Placental Malaria. III. Impairment of Interleukin(IL)–12, not IL-18, and Interferon-Inducible Protein–10 Responses in the Placental Intervillous Blood of Human Immunodeficiency Virus/Malaria–Coinfected Women. J. Infect. Dis. 2002, 185, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Ayisi, J.; Nahlen, B.L.; Misore, A.; Lal, A.A.; Udhayakumar, V. Immunity to Placental Malaria. II. Placental Antigen–Specific Cytokine Responses Are Impaired in Human Immunodeficiency Virus–Infected Women. J. Infect. Dis. 2000, 182, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Douek, D.C.; Picker, L.J.; Koup, R.A. T Cell Dynamics in HIV-1 Infection. Annu. Rev. Immunol. 2003, 21, 265–304. [Google Scholar] [CrossRef]
- Djontu, J.C.; Siewe Siewe, S.; Mpeke Edene, Y.D.; Nana, B.C.; Chomga Foko, E.V.; Bigoga, J.D.; Leke, R.F.G.; Megnekou, R. Impact of placental Plasmodium falciparum malaria infection on the Cameroonian maternal and neonate’s plasma levels of some cytokines known to regulate T cells differentiation and function. Malar. J. 2016, 15, 561. [Google Scholar] [CrossRef]
- Suguitan, A., Jr. L.; Leke, R.G.F.; Fouda, G.; Zhou, A.; Thuita, L.; Metenou, S.; Fogako, J.; Megnekou, R.; Taylor, D.W. Changes in the Levels of Chemokines and Cytokines in the Placentas of Women with Plasmodium falciparum Malaria. J. Infect. Dis. 2003, 188, 1074–7082. [Google Scholar] [CrossRef]
- Chukwuagwu, I.U.; Ukibe, N.R.; Ogbu, I.I.; Ikimi, C.G.; Agu, V.O.; Kalu, O.A.; Ukibe, S.N.; Awalu, J.C. Evaluation of Serum Interleukin 6, Tumor Necrosis Factor-Alpha, and Interferon-Gamma Levels in Relation to Body Mass Index and Blood Pressure in HIV Seropositive Pregnant Women Coinfected with Malaria. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Ibitokou, S.A.; Denoeud-Ndam, L.; Cot, M.; Ezinmegnon, S.; Luty, A.J.F.; Ladékpo, R.; Ndam, N.T.; Massougbodji, A.; Zannou, D.-M.; Girard, P.-M. Insights Into Circulating Cytokine Dynamics During Pregnancy in HIV-Infected Beninese Exposed to Plasmodium falciparum Malaria. Am. J. Trop. Med. Hyg. 2015, 93, 287–292. [Google Scholar] [CrossRef]
- Chaisavaneeyakorn, S.; Moore, J.M.; Mirel, L.; Othoro, C.; Otieno, J.; Chaiyaroj, S.C.; Shi, Y.P.; Nahlen, B.L.; Lal, A.A.; Udhayakumar, V. Levels of Macrophage Inflammatory Protein 1α (MIP-1α) and MIP-1β in Intervillous Blood Plasma Samples from Women with Placental Malaria and Human Immunodeficiency Virus Infection. Clin. Diagn. Lab. Immunol. 2003, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Malaria Programme. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-Region in Africa. 2012. Available online: http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng (accessed on 27 September 2022).
- Basco, L.K.; Same-Ekobo, A.; Ngane, V.F.; Ndounga, M.; Metoh, T.; Ringwald, P.; Soula, G. Therapeutic efficacy of sulfadoxine–pyrimethamine, amodia- quine and the sulfadoxine–pyrimethamine–amodiaquine combination against uncomplicated Plasmodium falciparum malaria in young children in Cameroon. Bull. World Health Organ. 2002, 8, 538–545. [Google Scholar]
- Sayang, C.; Gausseres, M.; Vernazza-Licht, N.; Malvy, D.; Bley, D.; Millet, P. Treatment of malaria from monotherapy to artemisinin-based combination therapy by health professionals in urban health facilities in Yaoundé, central province, Cameroon. Malar. J. 2009, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Nkuo-Akenji, T.; Tevoufouet, E.E.; Nzang, F.; Fon, E.; Ebong, I.N. HIV/AIDS and malaria in pregnant women from Cameroon Short running title: HIV, malaria in pregnancy. Afr. J. Health Sci. 2011, 18, 5. [Google Scholar]
- González, R.; Desai, M.; Macete, E.; Ouma, P.; Kakolwa, M.A.; Abdulla, S.; Aponte, J.J.; Bulo, H.; Kabanywanyi, A.M.; Katana, A.; et al. Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Infected Women Receiving Cotrimoxazole Prophylaxis: A Multicenter Randomized Placebo-Controlled Trial. PLoS Med. 2014, 11, e1001735. [Google Scholar] [CrossRef]
- Houmsou, R.S.; Wama, B.E.; Elkanah, S.O.; Garba, L.C.; Hile, T.D.; Bingbeng, J.B.; Kela, S.L.; Amuta, E.U. Malarial Infection in HIV Infected Pregnant Women Attending a Rural Antenatal Clinic in Nigeria. Adv. Epidemiol. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Asante, K.P.; Adjei, G.; Enuameh, Y.; Owusu-Agyei, S. RTS,S malaria vaccine development: Progress and considerations for postapproval introduction. VDT 2016, 25, 25–32. [Google Scholar] [CrossRef]
- Aremu, T.O. Looking Beyond the Malaria Vaccine Approval to Acceptance and Adoption in Sub-Saharan Africa. Front. Trop. Dis. 2022, 3, 3. [Google Scholar] [CrossRef]
- Healy, S.A.; Fried, M.; Richie, T.; Bok, K.; Little, M.; August, A.; Riley, L.; Swamy, G.K.; Wylie, B.J.; Menendez, C.; et al. Malaria vaccine trials in pregnant women: An imperative without precedent. Vaccine 2019, 37, 763–770. [Google Scholar] [CrossRef]
- Coulibaly, D.; Kone, A.K.; Traore, K.; Niangaly, A.; Kouriba, B.; Arama, C.; Zeguime, A.; Dolo, A.; Lyke, K.E.; Plowe, C.V.; et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: A randomized, controlled phase 1 trial. eClinicalMedicine 2022, 52, 101579. [Google Scholar] [CrossRef]
- Gamain, B.; Chêne, A.; Viebig, N.K.; Tuikue Ndam, N.; Nielsen, M.A. Progress and Insights toward an Effective Placental Malaria Vaccine. Front. Immunol. 2021, 12, 634508. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.C.; Deye, G.A.; Sim, B.K.L.; Galbiati, S.; Kennedy, J.K.; Cohen, K.W.; Chakravarty, S.; Kc, N.; Abebe, Y.; James, E.R.; et al. PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: A randomized, placebo-controlled trial with controlled human malaria infection. PLoS Pathog. 2021, 17, e1009594. [Google Scholar] [CrossRef]
- Cutts, J.C.; Agius, P.A.; Lin, Z.; Powell, R.; Moore, K.; Draper, B.; Simpson, J.A.; Fowkes, F.J.I. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: A systematic review. BMC Med. 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Renn, J.P.; Doritchamou, J.Y.A.; Tentokam, B.C.N.; Morrison, R.D.; Cowles, M.V.; Burkhardt, M.; Ma, R.; Mahamar, A.; Attaher, O.; Diarra, B.S.; et al. Allelic variants of full-length VAR2CSA, the placental malaria vaccine candidate, differ in antigenicity and receptor binding affinity. Commun. Biol. 2021, 4, 1309. [Google Scholar] [CrossRef] [PubMed]
- Babakhanyan, A.; Fang, R.; Wey, A.; Salanti, A.; Sama, G.; Efundem, C.; Leke, R.J.I.; Chen, J.J.; Leke, R.G.F.; Taylor, D.W. Comparison of the specificity of antibodies to VAR2CSA in Cameroonian multigravidae with and without placental malaria: A retrospective case–control study. Malar. J. 2015, 14, 480. [Google Scholar] [CrossRef]
- Bompard, A.; Da, D.F.; Yerbanga, R.S.; Biswas, S.; Kapulu, M.; Bousema, T.; Lefèvre, T.; Cohuet, A.; Churcher, T.S. Evaluation of two lead malaria transmission blocking vaccine candidate antibodies in natural parasite-vector combinations. Sci. Rep. 2017, 7, 6766. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef]
- World Health Organization. Digital Adaptation Kit for HIV: Operational Requirements for Implementing WHO Recommendations in Digital Systems; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-005442-4. [Google Scholar]
- Bositis, C.M.; Gashongore, I.; Patel, D.M. Updates to the World Health Organization’s Recommendations for the Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Med. J. Zamb. 2010, 37, 111–117. [Google Scholar]
- World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants in Resource-Limited Settings: Towards Universal Access: Recommendations for a Public Health Approach; 2006 Version; World Health Organization: Geneva, Switzerland, 2006; ISBN 978-92-4-159466-0. [Google Scholar]
- for the Inserm U897 Modeling Infectious Diseases in Low-Income Countries Study Group; Nguefack, H.L.N.; Gwet, H.; Desmonde, S.; Oukem-Boyer, O.O.M.; Nkenfou, C.; Téjiokem, M.; Tchendjou, P.; Domkam, I.; Leroy, V.; et al. Estimating mother-to-child HIV transmission rates in Cameroon in 2011: A computer simulation approach. BMC Infect. Dis. 2015, 16, 11. [Google Scholar] [CrossRef]
- Fomulu, J.N.; Nana, P.N.; Nkwabong, E.; Wamba, F.M.; Foumane, P.; Mbu, R.; Tebeu, P.M. Efficacy of highly active triple antiretroviral therapy in preventing mother-to-child HIV transmission in the university teaching hospitals in Yaoundé, Cameroon. Clin. Mother Child Health 2009, 6, 1075–1080. [Google Scholar]
- The NAMSAL ANRS 12313 Study Group Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019, 381, 816–826. [CrossRef] [PubMed]
- Dorward, J.; Hamers, R.L. Dolutegravir in sub-Saharan Africa: Context is crucial. Lancet HIV 2019, 6, e72–e73. [Google Scholar] [CrossRef] [PubMed]
- Cameroon Ministry of Public Health: National guidelines on the prevention and managemnet of HIV infection in Cameroon. 2015. Available online: https://www.childrenandaids.org/sites/default/files/201805/Cameroon_Nat%20Guidelines%20HIV_2015. (accessed on 20 August 2022).
- Bauer, G.R.; Colgrove, R.C.; LaRussa, P.S.; Pitt, J.; Welles, S.L. Antiretroviral resistance in viral isolates from HIV-1-transmitting mothers and their infants. AIDS 2006, 20, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, N.; Kerin, T.; Ank, B.; Watts, D.H.; Camarca, M.; Joao, E.C.; Pilotto, J.H.; Veloso, V.G.; Bryson, Y.; Gray, G.; et al. Human Immunodeficiency Virus Antiretroviral Resistance and Transmission in Mother-Infant Pairs Enrolled in a Large Perinatal Study. Clin. Infect. Dis. 2018, 66, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Lippincott, W.; Wilkins. Perinatal transmission of multidrug-resistant HIV-1 despite viral suppression on an enfuvirtide-based treatment regimen. AIDS 2005, 19, 989–994. [Google Scholar]
- Rosebush, J.C.; Best, B.M.; Chadwick, E.G.; Butler, K.; Moye, J.; Smith, E.; Bradford, S.; Reding, C.A.; Mathiba, S.R.; Hanley, S.; et al. Pharmacokinetics and safety of maraviroc in neonates. AIDS 2021, 35, 419–427. [Google Scholar] [CrossRef] [PubMed]
| Main Aspect | Factors | |
|---|---|---|
| 1 | Genetic factors |
|
| ||
| ||
| 2 | Maternal or infant co-infection |
|
| ||
| ||
| ||
| ||
| ||
| 3 | Behavioral factors |
|
| ||
| ||
| ||
| 4 | Maternal nutritional status |
|
| ||
|
| Vaccine (Type) | Status | Target | Activity | Indication | Developer |
|---|---|---|---|---|---|
| RTS,S/Mosquirix (subunit) | Phase 3 complete | Sporozoite/liver stage | Prevent disease | Reduce childhood disease | GlaxoSmithKline |
| PfSPZ Vaccine (whole organism) | Phase 2 | Sporozoite/liver stage | Prevent infection | Elimination; travelers | Sanaria |
| VAR2CSA (subunit) | Phase 1 | Infected red cell | Control infection | Prevent placental disease | Various(EVI) |
| Pfs25/Pfs230 (subunit) | Phase 1 | Mosquito stages | Block transmission | Elimination | NIAID |
| DOSAGE | |
|---|---|
From birth to 6 weeks
|
|
| 1 mL = 10 mg NVP | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obase, B.N.; Bigoga, J.D.; Nsagha, D.S. Malaria and HIV Co-Infection among Pregnant Women in Africa: Prevalence, Effect on Immunity and Clinical Management: Review. Int. J. Transl. Med. 2023, 3, 187-202. https://doi.org/10.3390/ijtm3020014
Obase BN, Bigoga JD, Nsagha DS. Malaria and HIV Co-Infection among Pregnant Women in Africa: Prevalence, Effect on Immunity and Clinical Management: Review. International Journal of Translational Medicine. 2023; 3(2):187-202. https://doi.org/10.3390/ijtm3020014
Chicago/Turabian StyleObase, Bekindaka Ngemani, Jude Daiga Bigoga, and Dickson Shey Nsagha. 2023. "Malaria and HIV Co-Infection among Pregnant Women in Africa: Prevalence, Effect on Immunity and Clinical Management: Review" International Journal of Translational Medicine 3, no. 2: 187-202. https://doi.org/10.3390/ijtm3020014
APA StyleObase, B. N., Bigoga, J. D., & Nsagha, D. S. (2023). Malaria and HIV Co-Infection among Pregnant Women in Africa: Prevalence, Effect on Immunity and Clinical Management: Review. International Journal of Translational Medicine, 3(2), 187-202. https://doi.org/10.3390/ijtm3020014





