Abstract
Plant secondary metabolites are well-recognized medicinally important compounds. Gossypol is an important plant secondary metabolite with several medicinal properties. Carbon nanotubes (CNTs) are allotropes of carbon with diverse applicability in chemical, physical, and biological sciences due to their high surface area. The current study demonstrates the enhancement of gossypol production in cotton cell suspension culture in culture media supplemented with water-soluble carbon nanotubes. The fresh and dry weights of cotton cell suspension culture grown in MS media with 20 µg/mL CNTs were, respectively, 1.9 and 2.13 fold higher than in control MS media after one month. The net enhancement of gossypol production in MS media supplemented with 20 µg/mL CNTs was 2.47 fold higher than the control. Confocal and SEM imaging showed the presence CNTs on the cell surface, which mediated the formation of extra channels that resulted in high biomass production in cotton cell suspension culture. The gossypol produced by this cell suspension culture showed antiproliferative activity against the prostate cancer cell line. Thus, this study demonstrated a new method for enhanced gossypol production, which can prove beneficial for the production of other plant-based biological active compounds.
1. Introduction
Plants produce a wide range of secondary metabolites. These bioactive molecules have many applications in pharmaceutical, food, and other industries [1]. The plant-metabolite-derived pharmaceutical industry is estimated to reach USD 5 trillion by the year 2050 [2,3]. However, the limited production of plant secondary metabolites using conventional methods remains a considerable challenge due to the over-exploitation of natural resources, societal., and regional restrictions [4]. Therefore, the development of alternative strategies is highly desired. Plant cell/tissue culture, especially the cell suspension culture, has been established as a promising alternative for the continuous production of plant metabolites within a limited cultivation time [5].
Plant structures such as shoots, roots, callus, cell suspension, etc., are being widely employed to produce secondary metabolites in less time compared with conventional approaches [6]. Consistent production and high yields of bioactive metabolites depend on many biotic as well as abiotic factors such as the culture medium, growth, and physiology of plant tissue cultures [2]. Various strategies such as the use of elicitors, metabolic/genetic engineering, and nanoparticles have been explored to stimulate plant tissue cultures to meet the growing demand for bioactive metabolites [2,7,8,9].
Nanoparticles revolutionized biological research. Their small size enables them to be suitable for biomedical applications such as fluorescence imaging, drug delivery, gene editing, and many more [10,11]. Several reports have shown in vitro/in vivo application of nanoparticles in the modulation of the biological and physiological processes of plants with factors such as germination efficiency, enhancing growth, and enriching secondary metabolites in plants [12,13,14]. Carbon nanotubes (CNTs) are carbon allotropes with a well-ordered, hollow cylindrical shape and length ranging from several hundred nanometers to micrometers [15]. CNTs have been reported to boost callus formation and the biosynthesis of secondary metabolites [14]. Hence, they can serve as promising elicitors for plant tissue culture, promoting plant growth and secondary metabolism, as well as protecting against stress conditions. However, the role of CNTs as potential plant tissue culture supplements is less investigated.
Therefore, in the present study, we explored the role of water-soluble CNTs to stimulate plant tissue culture growth and the induction of bioactive metabolite production. We used cotton cell suspension culture and explored a proficient method of enhanced metabolite, gossypol production. Through cytotoxicity analysis of the prostate cancer cell line, we also confirmed that the gossypol produced by the CNT-induced cell suspension culture is bioactive.
2. Materials and Methods
2.1. Chemicals
Carboxylic acid functionalized, single-walled water-soluble carbon nanotube (652490) and gossypol for HPLC standard (G8761) were purchased from Sigma-Aldrich, Merck, NY, USA. The physical properties of the used CNTs were >90% carbon basis, D × L 4–5 nm × 0.5–1.5 μm, and with bundle dimensions as provided by the manufacturer. All the tissue culture media and supplements were purchased from Himedia laboratories.
2.2. Induction and Maintenance of Cotton Callus
Hypocotyl (1 cm) and cotyledonary leaf (1 cm2) were used as explants from 7–10 days old in vitro grown cotton plants. The explants were transferred to Petri dishes having a basal medium that comprised an MS medium supplemented with B5 vitamins, 3% glucose, 0.22% phytagel, 0.5 mg/L auxins (2,4-D), and 0.2 mg/L cytokinins (6-benzylaminopurine). The Petri dishes were placed at 28 ± 2 °C, under 2000 lux. To estimate the growth response and gossypol content of calluses from different cultivars, the fresh weight of callus per explant was measured after 30, 45, and 60 days. A highly proliferative callus was chosen for cell suspension culture development.
2.3. Cell Suspension Culture
Calluses were excised and transferred to a liquid basal medium. The liquid basal medium was the same as the basal medium except that no phytagel was added. The pH was adjusted to 5.6 before autoclaving. Approximately 500 mg of the callus tissue from each type of explant was inoculated into 50 mL of the liquid basal medium in a 250 mL Erlenmeyer flask closed with cellulosic steri-stoppers (Riviera, India). The flask was placed on an illuminated continuous rotary shaker (Innova 4340, New Brunswick Scientific, Edison Township, NJ, USA) at 28 ± 2 °C. After 20 days of growth, the cultures were filtered through three screens of mesh size 100, 40, and 10 microns (Sigma-Aldrich, USA). The friable mass collected with a filter mesh of 40 microns was resuspended in 50 mL of the fresh medium. The cell masses were then subcultured with a fresh, liquid basal medium every 10 days.
2.4. Suspension Culture with CNT
To analyze the effect of CNTs on cell suspension culture, the spent medium (along with freely suspended cells) was first removed, and then a small portion of tissue from the parent culture was distributed to the fresh conical flasks containing the liquid basal medium. Two concentrations of CNTs (20 and 10 µg/mL) and 20 µg/mL of activated charcoal (20 µg/mL) were separately added to culture flasks. The culture flask containing no additions was considered blank. After one month of culture, fresh weight was determined for each treatment. The cells were dried for 4 days at 37 °C for dry mass and gossypol analysis.
2.5. Extraction and Quantification of Gossypol from Cotton Cell Suspension
Gossypol was extracted and quantified from cotton cell suspension culture as described previously [16], with some modifications. Briefly, the cells were filtered and incubated at 37 °C until they dried completely. The dried mass was powdered in liquid nitrogen, and 10 mg of each sample was taken into separate tubes containing 1 mL acetonitrile and a 0.1% aqueous TFA solution (v/v 80:20). The samples were sonicated for 10 min at 5 s pulse and stirred for 1 h, enabling proper mixing. After centrifugation, the supernatant containing gossypol was collected in a fresh tube and vacuum-dried, followed by resuspension in 100% acetonitrile. A Shimadzu 10 AVP HPLC binary gradient system connected with a 4.6 mm × 25 cm, 5 μm Zorbax SB-C18 column was used for gossypol quantification. The samples were filtered with a 0.2 µm filter and injected (20 µL) into the system using a Shimadzu auto-injector (Model SIL-10 ADVP). The column was eluted with 80:20 acetonitrile–0.1% aqueous TFA solution in isocratic conditions for 0–10 min, at a 1 mL/min flow rate. The concentration of acetonitrile was linearly increased to 100% over the period of 2 min and then kept steady for the next 1 min. A Shimadzu UV/VIS photodiode-array detector (ModelRF-10A XL Fluorescence Detector) was used for gossypol detection at 280 and 254 nm. Similar conditions were used for the preparation of standard curves with known concentrations (0.05, 0.625, 1.25, 2.5, 5, 10, 30, and 60 µg/mL) of commercially available gossypol. The peak corresponding to gossypol was detected at 10.72 min.
2.6. Peroxidase Assay of Cotton Cell Suspension Culture
CNT-treated cell suspension cultures were harvested via centrifugation after one month. The cells were lysed using glass beads (0.5 mm) and then homogenized in 100 mM phosphate buffer (pH 7.0), followed by centrifugation at 13,000 rpm for 10 min. A supernatant containing the total soluble protein (TSP) was used for analysis. Peroxidase activity was quantified using spectrophotometry and an in-gel activity assay in accordance with the earlier optimized method [17]. For the spectrophotometric assay, 100 ng TSP along with the standard peroxidase enzyme (Sigma) were coated on a microtiter plate through overnight incubation at 4 °C. The plate was washed with phosphate buffer after incubation, and 100 µL TMB (tetramethylbenzidine) and H2O2 were added to each well. The rate of peroxidase activity directly corresponds to the conversion of the substrate to a soluble blue substrate during the reaction. The reaction was stopped after 15 min by the addition of 50 µL of H2SO4 (100 mM) for 5 min, converting the blue product to a yellow product. The absorbance of this soluble yellow product was measured at 450 nm, and the peroxidase activity was estimated using a standard curve. An in-gel peroxidase assay was performed using SDS–polyacrylamide gel. TSP was mixed with protein-loading dye without DTT and resolved using 12% SDS–PAGE without heat denaturation. Subsequently, the gel was rinsed twice with 2.5% Triton X-100 and then twice with the 100 mM phosphate buffer for 5 min each. The peroxidase band was developed by using a diaminobenzidine/H2O2 (DAB) substrate, and the brown color corresponded to the peroxidase activity.
2.7. Confocal Imaging of Cotton Cell
The confocal imaging of cotton cells was performed as described earlier [18], with slight modifications. Briefly, a one-month-old cell suspension culture was taken, and the medium was removed through filtration. The cells were incubated in CdSO4 at room temperature with slow agitation and then washed several times with Milli-Q water. The washed cells were immersed in Milli-Q water pre-saturated with H2S gas for 10 min. The cells were again washed several times with Milli-Q water and observed under a confocal microscope (LSM 510 META) at 405 nm excitation and 475 nm emission.
2.8. SEM Imaging of Cotton Cell
For SEM analysis, the medium was removed from the cell suspension culture through filtration. The cells were washed twice with 0.1 M sodium cacodylate buffer and fixed in 2.5% glutaraldehyde (prepared in 0.1 M sodium cacodylate, 16 h, 4 °C). Thereafter, the cells were washed thrice with 0.1 M sodium cacodylate for 20 min each and incubated in osmium tetraoxide overnight. This was followed by washing the cells in the 0.1 M sodium cacodylate buffer twice. Subsequently, the cells were serially dehydrated in 15%, 30%, 60%, 90%, and three times in 100% alcohol for 20 min each till they reached a critical point. The dehydrated cells were coated with gold particles (2 coatings). The coated cells were finally visualized and imaged under the scanning electron microscope (FEG450 Quanta, FEI, Hillsboro, OR, USA).
2.9. Proliferation Assay
The antiproliferative activity of gossypol in PC-3 cell lines was determined with an MTT assay in triplicates as per the previously described protocol [19,20]. Briefly, PC-3 cells (5 × 104 cells/mL) were plated in 96-well cell culture plates and kept at 37 °C, under 5% CO2 and humified conditions. One day post-plating, the cells were treated with 1.2, 2.5, 5, and 10 µg/mL gossypol for 72 h. After treatment, 20 μL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT solution, 5 mg/mL) was added to each well. The plates were incubated in the dark for 4 h. The culture medium was replaced with 100 μL of DMSO in each well to dissolve the formazan crystals. The absorbance was measured using a microplate reader at 570 nm.
2.10. Statistical Analysis
All the experiments were conducted in triplicates on biological duplicates. The data were analyzed with a one-way ANOVA (p < 0.05), and the means were compared using Duncan’s multiple-range test (DMRT) by using SPSS 16.0 software.
3. Results
3.1. Callus Induction and Estimation of Gossypol Production
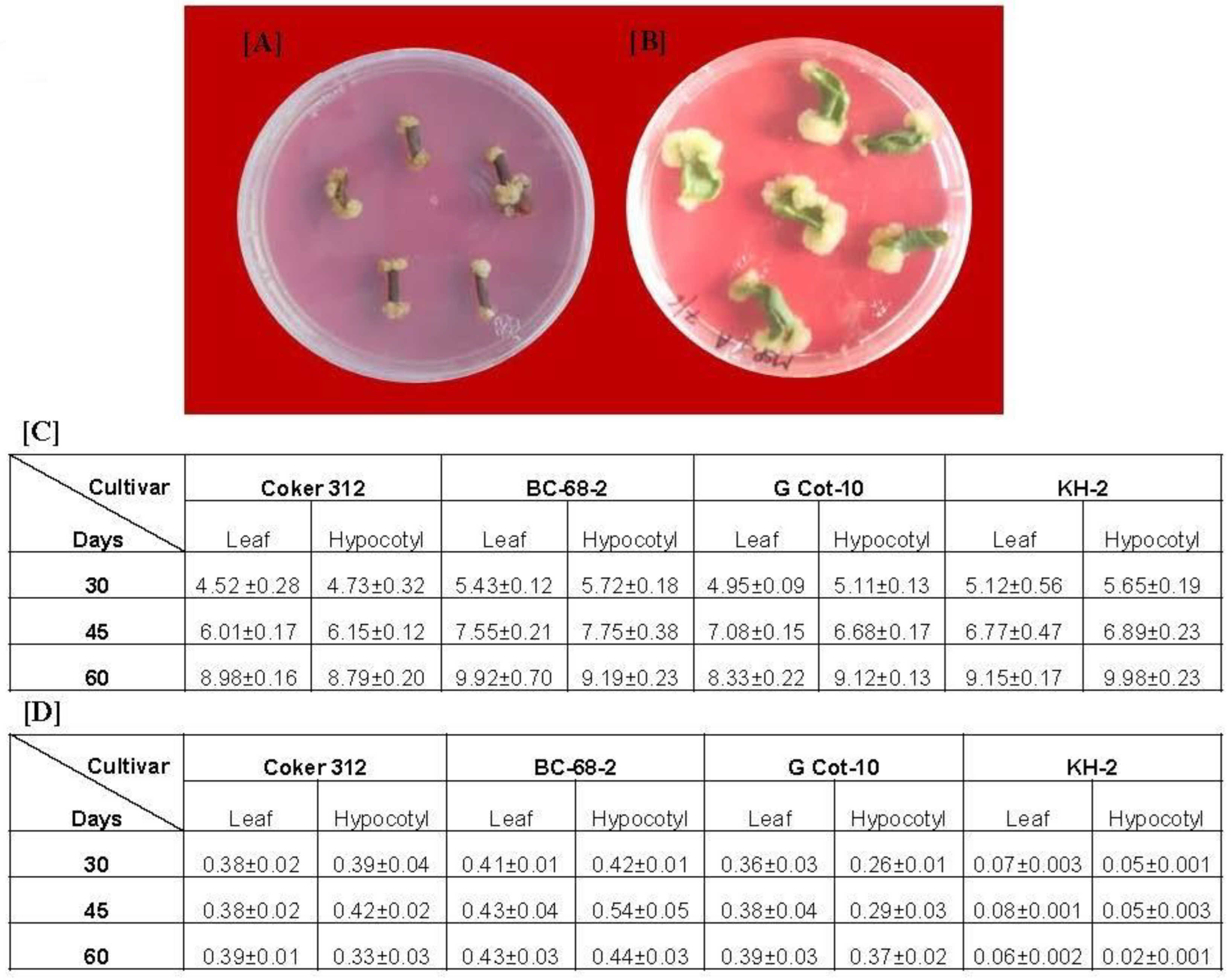
Gossypol is the main bioactive metabolite in Gossypium hirsutum; however, previous reports suggest that its concentration varies among cultivars [16]. Hence, we looked for a suitable callus to be used for the generation of cell suspension culture. For this, we initially analyzed four cultivars (Coker-312, G-Cot-10, BC-68-2, and KH-2) for callus induction and quantitative gossypol estimation. We used hypocotyl and leaves as explants from 7- to 10-day-old in vitro grown plants. The explants of each cultivar were transferred to Petri dishes with an MS medium individually supplemented with B5 vitamins. Callusing was initiated at the cut ends of each explant (Figure 1A,B). The hypocotyledonary explants started forming the calluses earlier than the leaf explants, which produced more friable calluses than the leaf explants (Figure 1A,B). The callus produced from the hypocotyledonary explants was also more friable than that of the leaf explants. We did not observe any significant difference in callus biomass (fresh weight) obtained at different time intervals (30, 45, and 60 days) from the leaf and hypocotyls of the same cultivars (Figure 1C). After 60 days of culture, the maximum callus biomass was obtained in the hypocotyls of KH-2 (9.98 g), followed by the leaf of BC-68-2 (9.92 g), while the minimum biomass was obtained with G-Cot-10 leaf callus (8.33 g). The callus thus obtained was further utilized for gossypol determination. The gossypol content was estimated after 30, 45, and 60 days of culture. We observed that the level of gossypol in all callus cultures was increased till 45 days and then subsequently decreased. The maximum gossypol was obtained from BC-68-2, while the minimum was obtained from KH-2 at each time interval (Figure 1D). Both the hypocotyl (0.543 mg/g DW) and leaf calluses (0.433 mg/g DW) of BC-68-2 accumulated the maximum gossypol content, while the lowest concentration of gossypol was estimated in KH-2 (Figure 1D). As the hypocotyledonary callus obtained from BC-68-2 had the maximum gossypol concentration, we used this callus to assess the effect of CNTs on the production of cell suspension culture and gossypol content.
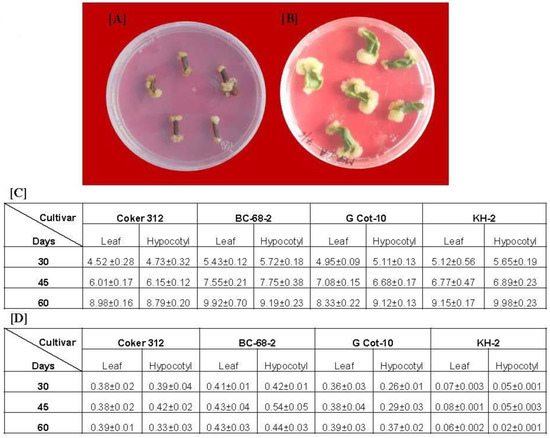
Figure 1.
Induction of callus on MS plate: hypocotyl (A) and leaf (B) explants showing callusing started at the cut ends; (C) biomass (fresh weight in grams) of callus from both explants of each cultivar at different time intervals; (D) gossypol content (mg/g of dry weight) of callus from both explants of each cultivar at different time intervals.
3.2. Effect of CNTs on Cotton Cell Suspension Culture and Gossypol Production
Cell suspension culture from the selected hypocotyl callus of BC-68-2 was treated with different CNT concentrations. After one month, the cells from the treated and untreated suspension cultures were filtered, and fresh weight was analyzed.
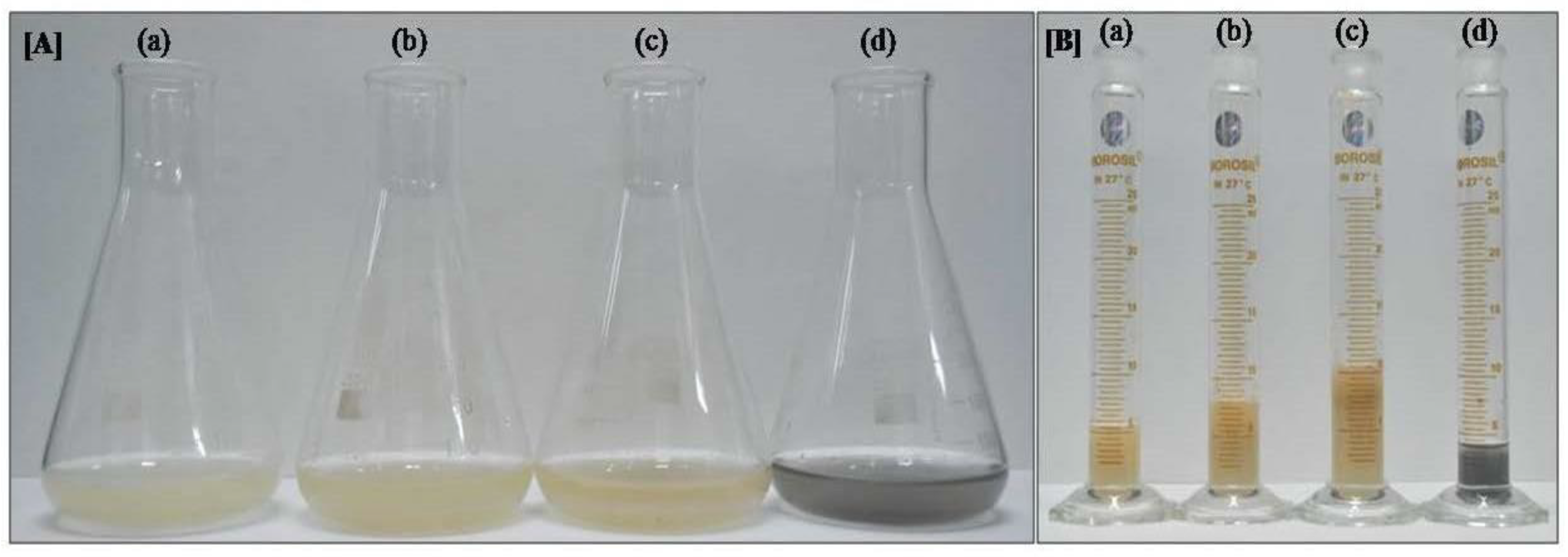
Both CNT concentrations significantly enhanced cell biomass in the suspension culture (Figure 2A,B and Table 1). The MS media supplemented with 20 and 10 µg/mL CNTs increased cell biomass by, respectively, 1.9 (10.42 ± 0.60 g) and 1.46 fold (7.98 ± 0.30 g) than the media without CNT supplementation (5.46 ± 0.45 g; Table 1). No significant difference was observed in the fresh weight of the cells grown in MS media with charcoal (5.00 ± 0.45 g) and those in the control MS media.
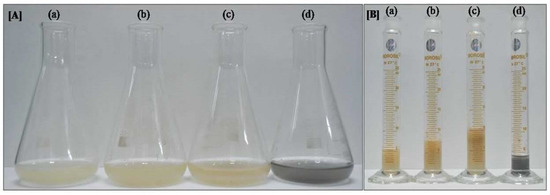
Figure 2.
Growth parameter of cotton cell suspension culture after one month: (A) biomass accumulation in MS supplemented with (a) no supplements, (b) 10 µg/mL CNT, (c) 20 µg/mL CNT, and (d) charcoal; (B) image was captured after removing media through filtration with filter paper, with (a) no supplements, (b) 10 µg/mL CNT, (c) 20 µg/mL CNT, and (d) charcoal. The image clearly shows the enhancement of biomass accumulation in CNT media.

Table 1.
Growth parameter and gossypol content of cell suspension culture after one month.
The dry weight (DW) of the cell suspension culture grown in the MS media supplemented with 20 and 10 µg/mL CNTs was 3.41 ± 0.30 g and 2.26 ± 0.45 g, respectively (Table 1). It was 2.13 and 1.53 fold greater than the DW of cells in the control MS media (1.47 ± 0.30 g). This dried mass of cells was used to extract gossypol. The concentrations of gossypol in the control MS and MS media with 20 µg/mL CNTs were 0.46 mg/g and 0.54 mg/g of DW, respectively (Table 1, Supplementary Figure S1). Our results showed that the MS media with 20 µg/mL CNTs had 1.16 fold (per g of DW) higher gossypol content. Since the dry weight of cells from MS media with 20 µg/g CNTs was 2.13 fold greater than the control MS media, the net enhancement of gossypol production was 2.47 fold (Table 1).
3.3. Peroxidase Assay of Cotton Cell
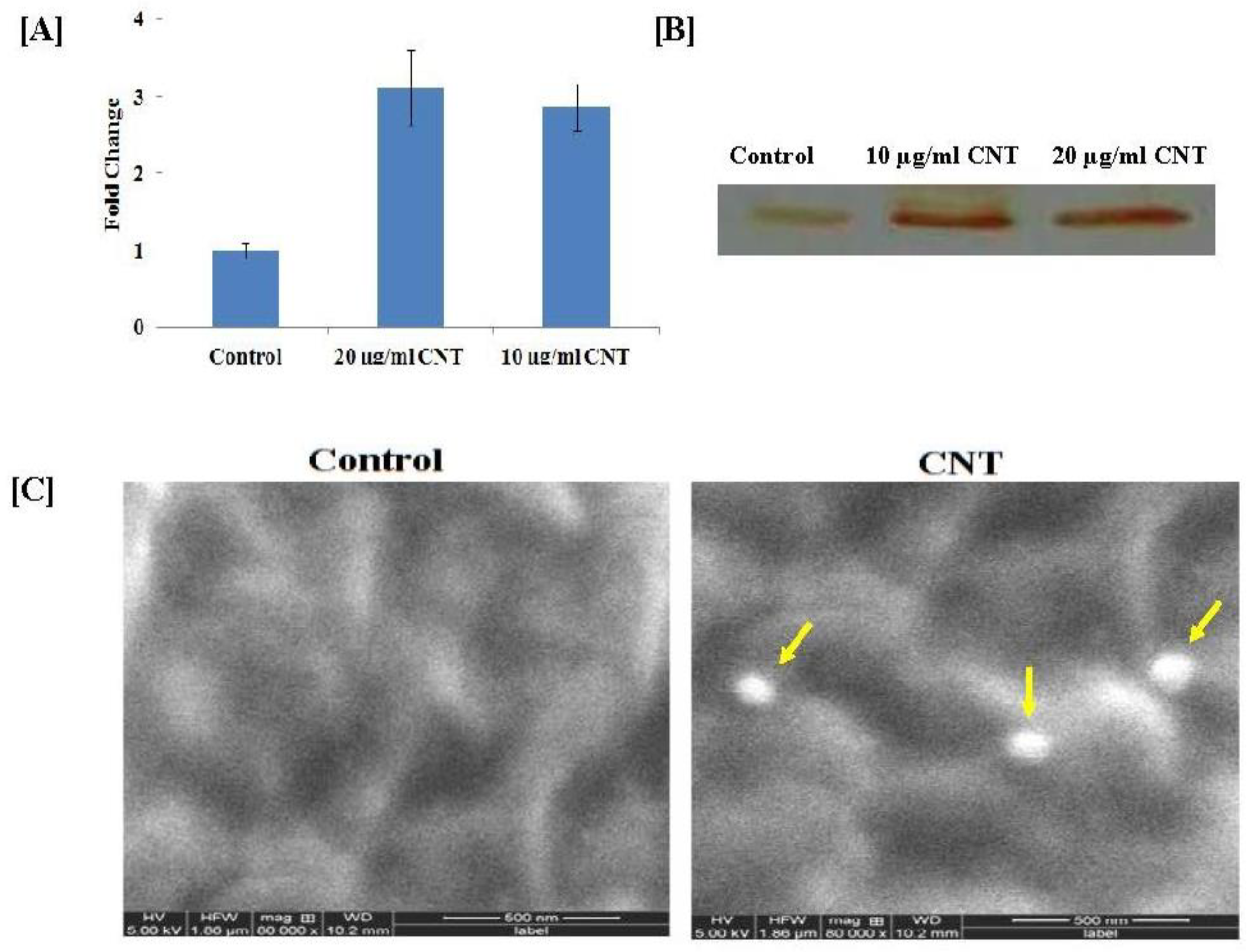
To observe the stress response of CNTs on the cotton cell suspension culture, we analyzed the peroxidase activity with two independent assays. The spectrophotometric assay showed that the peroxidase activity levels in 20 and 10 µg/mL CNT-treated cells were 3.12 ± 0.49 and 2.87 ± 0.3 fold higher than the untreated control cells (Figure 3A). This result was further confirmed by the in-gel peroxidase assay, according to which 20 and 10 µg/mL CNT-treated cells showed more intense brown peroxidase activity bands in comparison to the control cells (Figure 3B).
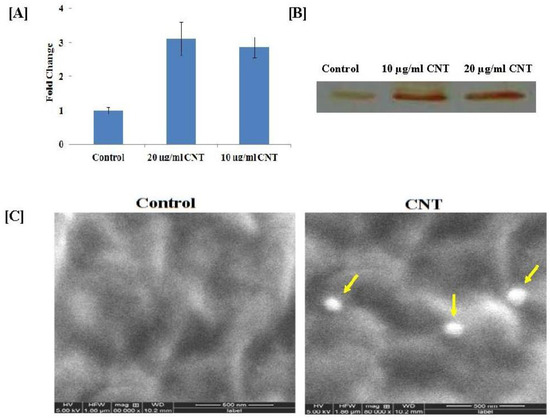
Figure 3.
Peroxidase activity of CNT-treated cotton cell suspension culture after one month: (A) quantitative (spectrophotometer); (B) qualitative (in-gel activity) method; (C) SEM imaging of cotton cell. Pores formed by CNTs on the surface of cells grown in 20 µg/g CNTs (shown with yellow arrows) were observed clearly, which were absent in the surface of the control cell. The image was captured at a very high resolution (160,000×).
3.4. Microscopic Image Analysis of Cotton Cells
To confirm the presence of CNTs on cotton cell surfaces, we visualized the CNT-treated cotton cells under a confocal microscope. The cells grown in 20 µg/mL CNTs supplemented with the MS medium showed green dots on the surface (indicated by the red arrow), which was not observed in the cells grown in the control MS medium (Supplementary Figure S2). These green dots were due to CdS, which confirmed the presence of CNTs on the cell surface. The results obtained from confocal imaging were further validated with scanning electron microscopy (SEM). The cells were visualized in SEM at high resolution (160,000×) such that the pores formed by CNTs on the surface of the cells were clearly observed, which were absent on the surface of the control cells (Figure 3C).
3.5. Antiproliferative Activity of Gossypol Produced from CNT-Induced Cotton Cell Suspension Culture
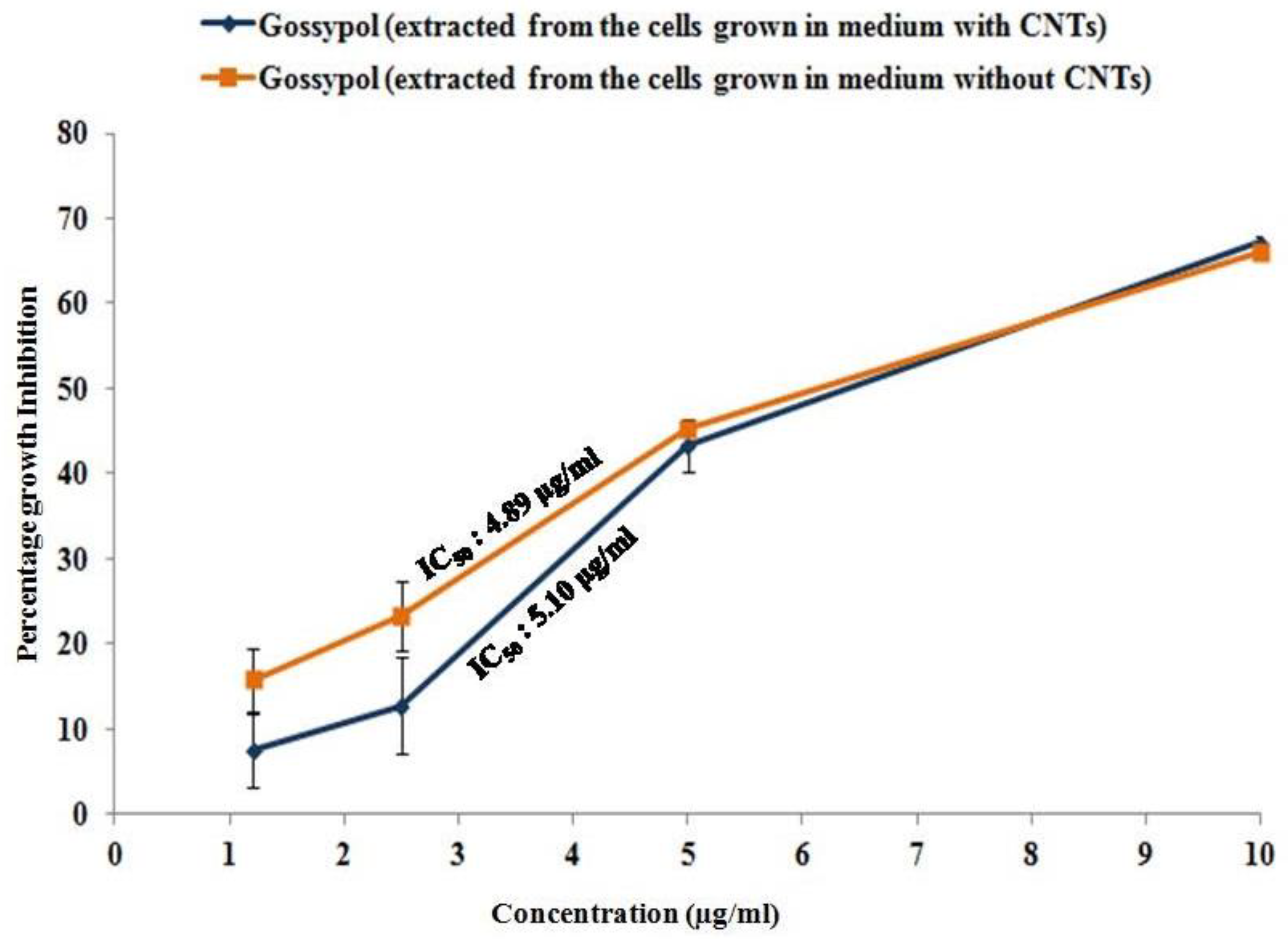
Gossypol is well-known for its antiproliferative activity against several cancer cell lines, including prostate cancer cell lines, one of which is PC-3 [19]. Therefore, we performed an MTT assay to confirm the biological activity of the gossypol produced in the CNT-induced cotton cell suspension culture. The gossypol produced by the cell suspension culture showed the inhibition of PC-3 growth in a dose-dependent manner. PC-3 cells showed 66.1%, 43.2%, 23.3%, and 15.71% growth inhibition at 10, 5, 2.5, and 1.2 μg/mL concentrations of gossypol (extracted from the cells grown in the control medium, without CNTs). The IC50 was 4.89 μg/mL (Figure 4). Similarly, 67.2%, 43.32%, 12.75%, and 7.52% growth inhibition was observed in the PC-3 cells treated with the same concentrations of gossypol extracted from the cells grown in the medium supplemented with 20 µg/mL CNTs. Its IC50 was 5.1 μg/mL in PC-3 cells (Figure 4). This suggests that the gossypol produced from our method was biologically active, as it showed antiproliferative activity and growth inhibition against PC-3 cells.
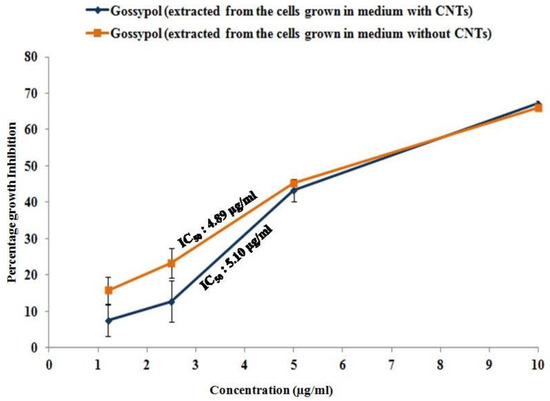
Figure 4.
Antiproliferative activity of gossypol produced by cell suspension culture against prostate cancer cell line PC-3. Graph showing the percentage growth inhibition of cancerous cells.
4. Discussion
Plant tissue culture is directed toward the propagation of plant cells or parts under controlled and sterile conditions. It has emerged as a beneficial method for large-scale clonal propagation and the production of useful bioactive metabolites. Cell suspension culture is a valuable platform for the biosynthesis of secondary metabolites.
In the present investigation, the gossypol production potential of the cell suspension culture was evaluated, and the effect of water-soluble carbon nanotubes was analyzed on cell growth as well as gossypol production. We found that callusing started after 10–15 days at the cut ends of the explants (Figure 1A,B), which was consistent with previous reports [21]. We found that callus initiation was higher in the hypocotyl explants than in the cotyledonary explants, but no significant difference was observed in the final callus biomass (Figure 1C). Furthermore, the callus produced by hypocotyl was more friable than the cotyledonary calluses, which were waterier because of their soft texture. This observation was in agreement with earlier reports where hypocotyl was found to be a better explant with higher callus formation efficiency than the cotyledonary and other plant tissue/explants [21,22].
Further, gossypol production was found at its highest in the hypocotyl callus of BC-68-2 (Figure 1D). The gossypol concentration increased with the age of the callus, but after 45 days, it was either constant or decreased; this may be due to the leaching of some gossypol in the medium (since the medium color turns brown with time). Furthermore, our results also suggested that the gossypol content varies among the different cultivars; this may be due to environmental adaptation and the geographical location of these cultivars. Moreover, we observed that CNTs drastically enhanced the biomass of the cotton cell suspension culture. This may be the reason for the overall increase in gossypol production by 1.6 fold, compared with the untreated control suspension culture (Table 1). Our results are consistent with earlier reports, which showed that CNTs induced growth enhancement in tobacco cells by the upregulation of aquaporin and the marker genes of cell division and cell wall extension [23]. Although we could not check the expression of these genes, it could be the reason, as the biomass of the cell suspension culture corresponds to the number of cells in the culture. There are many factors that regulate the growth of the cells in suspension cultures such as media composition, pH, temperature, illuminance, agitation, and aeration [24]. Carbon and nitrogen sources also play major roles in the optimum growth of the culture [25,26]. In our study, no significant difference was observed between the fresh weight of the cells grown in the MS media and those of charcoal and the control MS media (Figure 2), indicating that CNTs could not work as a carbon source for cells.
The cells extracted from the CNT-supplemented cotton cell suspension culture showed higher peroxidase activity (Figure 3A,B). Elevated peroxidase activity corresponds to tissue browning and stress response, as reported in the callus culture of Satureja khuzestanica supplemented with 500 µg/mL multi-walled CNTs [14]. This suggests that CNTs may cause a stress response in cotton cell suspension cultures. However, we did not observe tissue browning in our culture, which may be due to the less concentration of the CNTs used. By contrast, the overall gossypol production was increased, which supports the previous studies according to which high peroxidase activity increases phenolic production [27].
CNTs can penetrate the plant cell wall and have been reported to form channels from root to shoot, promoting growth in C. arietinum [18,23]. Our confocal images showed the presence of CNTs on the cell surface (Figure S2). The same can be inferred from SEM images, which clearly showed the presence of small pores on the surface of CNT-treated cells (Figure 3C). These pores were totally absent in the control cells. This shows that the CNTs present on the cell surface might have formed channels for nutrient absorption, which could have increased the cell growth in the suspension culture observed in our study.
Biologically active plant products are known to modulate mammalian cell proliferation, death, and motility [28]. Gossypol has been reported to inhibit cancer cell proliferation, where the IC50 of gossypol was 4.74 µg/mL in PC-3 cells [19]. Our data show that the gossypol produced by the present method is biologically active because it retains its antiproliferative activity against the PC-3 cancer cell line. The IC50 of gossypol produced from CNT-treated cotton cell suspension culture was 5.1 µg/mL in PC-3 cells, which is consistent with the previous findings (Figure 5). The IC50 of the gossypol produced from the untreated control cotton cell suspension culture was 4.9 µg/mL in the same cell line. In our study, we found that CNTs drastically enhance the growth of cells in the suspension culture corresponding to the overall increased gossypol production, indicating that CNTs play no role in the gossypol synthesis pathway. This might be the reason why CNTs do not affect the biological activity of the gossypol produced from the cell suspension culture.
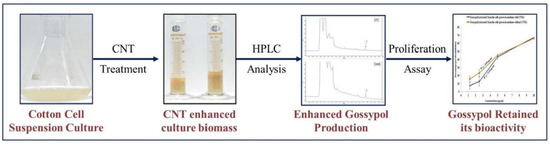
Figure 5.
Schematic representation of CNT-induced gossypol production in cotton cell suspension culture.
Collectively, our study explored a new method for increasing the growth of the cotton cell suspension culture for enhanced gossypol production without compromising its biological activity (Figure 5). These findings open a new pursuit for the production of biologically active plant-based compounds by using CNTs. Thus, this can be a method of choice for enhancing the production of several useful secondary metabolites in cell suspension cultures.
5. Conclusions
The cell suspension culture platform provides rapid and extensive production of bioactive metabolites in industrial setups. This method serves as a vital and consistent source to produce bioactive metabolites [29]. However, the production capacity of suspension cultures decreases with time due to many factors. The rising demand to improve this approach is highly desired for the sustainable and enhanced production of bioactive metabolites [2].
Our study demonstrates an alternate method to enhance the growth of plant-based cell suspension cultures using carbon nanotubes. CNTs work as elicitors and enhance the overall suspension culture biomass, thereby increasing the production of bioactive gossypol. Thus, this method has the potential to upscale the production of several useful secondary metabolites in cell suspension cultures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijtm2040046/s1, Figure S1: HPLC profile of extract of cell grown in MS with CNTs and without CNTs. Arrow on image shows the gossypol peak; Figure S2: Confocal Imaging of cotton cells. Cell grown in 20 µg/g CNTs showed green dots on surface (lower panel, showed by red marks), which was not observed on cells grown in control MS (upper panel). These green dots confirmed the presence of CNTs on cell surface. The image was captured at excitation 405 and emission LP 475 at 63X optical with additional digital zoom.
Author Contributions
S.D. and P.C.V. conceptualized the idea of this study. P.C.V., S.K.U. and S.D. designed the experiments. S.D. performed all the experiments in the P.C.V. lab except the peroxidase assay, which was performed in the S.K.U. lab with his help. A.S. maintained the PC-3 cell line, performed the proliferation assay, and wrote this section in the manuscript. S.D. and S.K.U. analyzed the results and prepared the original manuscript. P.C.V. and S.K.U. prepared the revised version of the manuscript and also arranged the funding. The institutional manuscript ID No. is CSIR-NBRI_MS/2022/22/03. All authors have read and agreed to the published version of the manuscript.
Funding
SERB-Department of Science & Technology, Government of India, Grant/Award Number: CRG/2021/005998.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
S.D. thanks DBT for the M.K. Bhan-YRFP fellowships. PCV is grateful for the financial support under Core Research Grant of SERB, New Delhi, India. S.K.U. is grateful to the Department of Science and Technology, Government of India, for partial financial support under the Promotion of University Research and Scientific Excellence (PURSE) grant scheme. The authors would also like to thank P.L. Saxena and Nidhi Arjaria for the SEM analysis.
Conflicts of Interest
The authors declare that they have no conflict of interest concerning this publication.
References
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019, 30, 1. [Google Scholar]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Shukla, A.; Singh, V.; Upadhyay, S.K. Bioprospecting of natural compounds for industrial and medical applications: Current scenario and bottleneck. Bioprospecting Plant Biodivers. Ind. Mol. 2021, 19, 53–71. [Google Scholar]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Verpoorte, R.; Alfermann, A.W. (Eds.) Metabolic Engineering of Plant Secondary Metabolism; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Khan, T.; Khan, M.A.; Karam, K.; Ullah, N.; Mashwani, Z.U.; Nadhman, A. Plant in vitro culture technologies; a promise into factories of secondary metabolites against COVID-19. Front. Plant Sci. 2021, 12, 356. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.H. Advantage of nanotechnology-based genome editing system and its application in crop improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Terrones, M. Science and technology of the twenty-first century: Synthesis, properties, and applications of carbon nanotubes. Annu. Rev. Mater. Res. 2003, 33, 419–501. [Google Scholar] [CrossRef]
- Verma, P.C.; Trivedi, I.; Singh, H.; Shukla, A.K.; Kumar, M.; Upadhyay, S.K.; Pandey, P.; Hans, A.L.; Singh, P.K. Efficient production of gossypol from hairy root cultures of cotton (Gossypium hirsutum L.). Curr. Pharm. Biotechnol. 2009, 10, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dixit, S.; Verma, P.C.; Singh, P.K. Differential peroxidase activities in three different crops upon insect feeding. Plant Signal. Behav. 2013, 8, e25615. [Google Scholar] [CrossRef]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Huang, X.F.; Mu, S.J.; An, Q.X.; Xia, A.J.; Chen, R.; Wu, D.C. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (−)-gossypol. Asian J. Androl. 2010, 12, 390. [Google Scholar] [CrossRef]
- Shukla, A.; Rajawat, J.; Dixit, S.; Mishra, S. Assays to evaluate tumor angiogenesis. In Protocol Handbook for Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; pp. 43–67. [Google Scholar]
- Kumar, M.; Singh, H.; Shukla, A.K.; Verma, P.C.; Singh, P.K. Induction and establishment of somatic embryogenesis in elite Indian cotton cultivar (Gossypium hirsutum L. cv Khandwa-2). Plant Signal. Behav. 2013, 8, e26762. [Google Scholar] [CrossRef][Green Version]
- Wilkins, T.A.; Mishra, R.; Trolinder, N.L. Agrobacterium mediated transformation and regeneration of cotton. J. Food Agric. Environ. 2004, 2, 179–187. [Google Scholar]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar]
- Nigra, H.M.; Alvarez, M.A.; Giulietti, A.M. Effect of carbon and nitrogen sources on growth and solasodine production in batch suspension cultures of Solanum eleagnifolium Cav. Plant Cell Tissue Organ Cult. 1990, 21, 55–60. [Google Scholar] [CrossRef]
- Bao Do, C.; Cormier, F. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Rep. 1991, 9, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress; Springer: Boston, MA, USA, 1984; pp. 273–320. [Google Scholar]
- Khan, A.W.; Farooq, M.; Haseeb, M.; Choi, S. Role of plant-derived active constituents in cancer treatment and their mechanisms of action. Cells 2022, 11, 1326. [Google Scholar] [CrossRef]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In vitro strategies for the enhancement of secondary metabolite production in plants: A review. Bull. Natl. Res. Cent. 2022, 46, 35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).