Effects of Phototherapy on Free Vitamin D Levels in Ten Patients with Atopic Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Phototherapy
2.3. Laboratory Analyses
2.4. Calculation of Percentage of Free 25(OH)D
2.5. Assessment of AD Severity
2.6. Comparative Statistics Regarding the Effect of Phototherapy on Vitamin D Levels
2.7. Statistical Analyses
2.8. Ethical Considerations
3. Results
3.1. Demographics
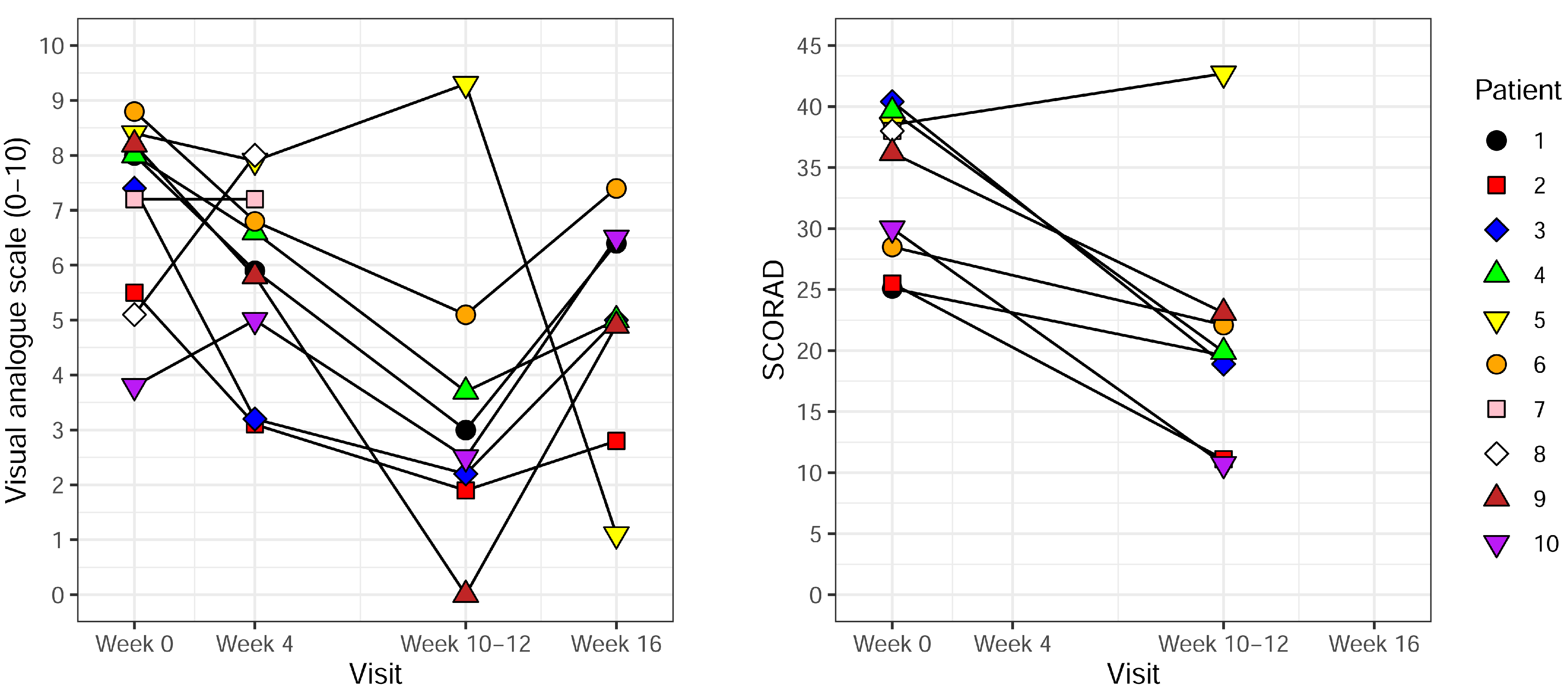
3.2. Effect of UVB Treatment
3.3. Vitamin D Status and Correlation to Disease Severity at Baseline
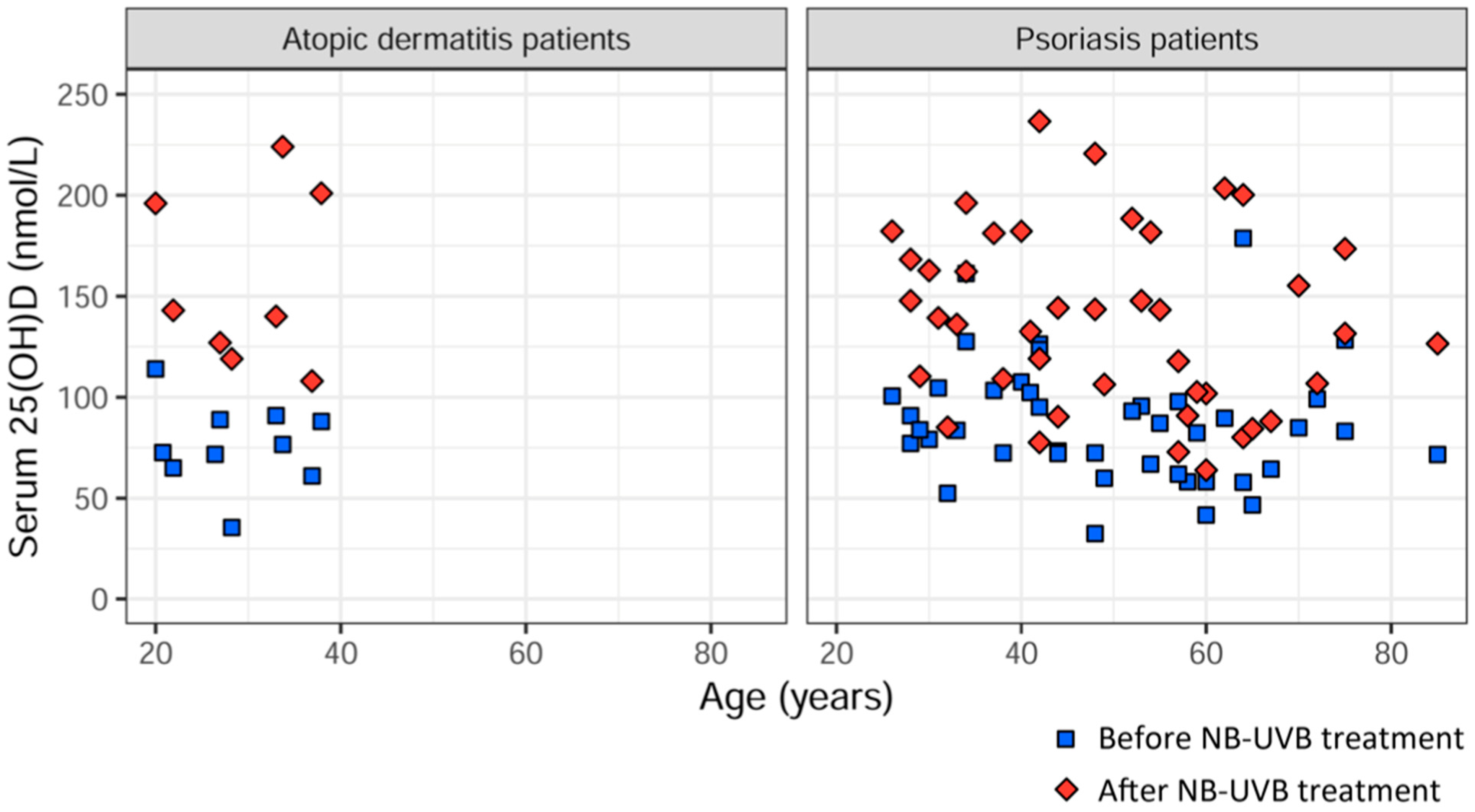
3.4. Comparison of Delta 25(OH)D between AD Patients and Psoriasis Patients after UVB Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet Lond. Engl. 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Weiland, S.K.; Hüsing, A.; Strachan, D.P.; Rzehak, P.; Pearce, N. ISAAC Phase One Study Group Climate and the Prevalence of Symptoms of Asthma, Allergic Rhinitis, and Atopic Eczema in Children. Occup. Environ. Med. 2004, 61, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Kim, S.; Park, G.-H.; Chang, S.E.; Bang, S.; Won, C.H.; Lee, M.W.; Choi, J.H.; Moon, K.C. Low Vitamin D Levels Are Associated with Atopic Dermatitis, but Not Allergic Rhinitis, Asthma, or IgE Sensitization, in the Adult Korean Population. J. Allergy Clin. Immunol. 2014, 133, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kim, J.H.; Kim, H.J.; Lee, J.-G.; Yoon, J.-H.; Kim, C.-H. Association of Serum 25-Hydroxyvitamin D with Serum IgE Levels in Korean Adults. Auris. Nasus. Larynx 2016, 43, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.-N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- El Taieb, M.A.; Fayed, H.M.; Aly, S.S.; Ibrahim, A.K. Assessment of Serum 25-Hydroxyvitamin d Levels in Children with Atopic Dermatitis: Correlation with SCORAD Index. Dermatitis 2013, 24, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-R.; Kim, Y.-N.; Lee, B.-H. Dietary Intakes and Lifestyle Patterns of Korean Children and Adolescents with Atopic Dermatitis: Using the Fourth and Fifth Korean National Health and Nutrition Examination Survey (KNHANES IV,V), 2007–2011. Ecol. Food Nutr. 2016, 55, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-Hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.R.; Shin, J.E.; Kim, Y.J.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Relationship between Serum 25-Hydroxyvitamin D and Interleukin-31 Levels, and the Severity of Atopic Dermatitis in Children. Korean, J. Pediatr. 2015, 58, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Baïz, N.; Dargent-Molina, P.; Wark, J.D.; Souberbielle, J.-C.; Annesi-Maesano, I. EDEN Mother-Child Cohort Study Group Cord Serum 25-Hydroxyvitamin D and Risk of Early Childhood Transient Wheezing and Atopic Dermatitis. J. Allergy Clin. Immunol. 2014, 133, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.E.; Havens, P.L.; Siegel, D.H.; Ali, O.; Wang, T.; Holland, K.E.; Galbraith, S.S.; Lyon, V.B.; Drolet, B.A. Serum 25-Hydroxyvitamin D Concentration Does Not Correlate with Atopic Dermatitis Severity. J. Am. Acad. Dermatol. 2013, 69, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.H.; Heede, N.G.; Tang, L.; Skaaby, T.; Thyssen, J.P.; Friedrich, N.; Linneberg, A. No Association between Vitamin D and Atopy, Asthma, Lung Function or Atopic Dermatitis: A Prospective Study in Adults. Allergy 2015, 70, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Farrell, C.-J.L.; Pusceddu, I.; Fabregat-Cabello, N.; Cavalier, E. Assessment of Vitamin D Status—A Changing Landscape. Clin. Chem. Lab. Med. 2017, 55, 3–26. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B. The Vitamin D3 Pathway in Human Skin and Its Role for Regulation of Biological Processes. Photochem. Photobiol. 2005, 81, 1246–1251. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal Expression of 25-Hydroxyvitamin d(3)-1 Alpha-Hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New Aspects of Vitamin D Metabolism and Action—Addressing the Skin as Source and Target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema Task Force 2020 Position Paper on Diagnosis and Treatment of Atopic Dermatitis in Adults and Children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Walters, I.B.; Ozawa, M.; Cardinale, I.; Gilleaudeau, P.; Trepicchio, W.L.; Bliss, J.; Krueger, J.G. Narrowband (312-Nm) UV-B Suppresses Interferon Gamma and Interleukin (IL) 12 and Increases IL-4 Transcripts: Differential Regulation of Cytokines at the Single-Cell Level. Arch. Dermatol. 2003, 139, 155–161. [Google Scholar] [CrossRef]
- Vähävihu, K.; Ala-Houhala, M.; Peric, M.; Karisola, P.; Kautiainen, H.; Hasan, T.; Snellman, E.; Alenius, H.; Schauber, J.; Reunala, T. Narrowband Ultraviolet B Treatment Improves Vitamin D Balance and Alters Antimicrobial Peptide Expression in Skin Lesions of Psoriasis and Atopic Dermatitis. Br. J. Dermatol. 2010, 163, 321–328. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized Trial of Vitamin D Supplementation for Winter-Related Atopic Dermatitis in Children. J. Allergy Clin. Immunol. 2014, 134, 831–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Coda, A.B.; Büchau, A.S.; Liu, P.T.; Kiken, D.; Helfrich, Y.R.; Kang, S.; Elalieh, H.Z.; Steinmeyer, A.; et al. Injury Enhances TLR2 Function and Antimicrobial Peptide Expression through a Vitamin D-Dependent Mechanism. J. Clin. Invest. 2007, 117, 803–811. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Langan, S.; Deckert, S.; Svensson, A.; von Kobyletzki, L.; Thomas, K.; Spuls, P. Harmonising Outcome Measures for Atopic Dermatitis (HOME) Initiative Assessment of Clinical Signs of Atopic Dermatitis: A Systematic Review and Recommendation. J. Allergy Clin. Immunol. 2013, 132, 1337–1347. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical Issues on Interpretation of Scoring Atopic Dermatitis: The SCORAD Index, Objective SCORAD and the Three-Item Severity Score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larkö, O.; Wennberg, A.-M.; Krogstad, A.L. Vitamin D Production in Psoriasis Patients Increases Less with Narrowband than with Broadband Ultraviolet B Phototherapy. Photodermatol. Photoimmunol. Photomed. 2009, 25, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kunz, B.; Oranje, A.P.; Labrèze, L.; Stalder, J.F.; Ring, J.; Taïeb, A. Clinical Validation and Guidelines for the SCORAD Index: Consensus Report of the European Task Force on Atopic Dermatitis. Dermatol. Basel Switz. 1997, 195, 10–19. [Google Scholar] [CrossRef]
- Williams, H.C.; Jburney, P.G.; Hay, R.J.; Archer, C.B.; Shipley, M.J.; Ahunter, J.J.; Bingham, E.A.; Finlay, A.Y.; Pembroke, A.C.; Cgraham-Brown, R.A.; et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis: I. Derivation of a Minimum Set of Discriminators for Atopic Dermatitis. Br. J. Dermatol. 1994, 131, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B.; Gallagher, J.C.; Jorde, R.; Berg, V.; Walsh, J.; Eastell, R.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; et al. Determination of Free 25(OH)D Concentrations and Their Relationships to Total 25(OH)D in Multiple Clinical Populations. J. Clin. Endocrinol. Metab. 2018, 103, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Buller, D.B.; Cokkinides, V.; Hall, H.I.; Hartman, A.M.; Saraiya, M.; Miller, E.; Paddock, L.; Glanz, K. Prevalence of Sunburn, Sun Protection, and Indoor Tanning Behaviors among Americans: Review from National Surveys and Case Studies of 3 States. J. Am. Acad. Dermatol. 2011, 65, S114.e1–S114.e11. [Google Scholar] [CrossRef]
- Samochocki, Z.; Bogaczewicz, J.; Jeziorkowska, R.; Sysa-Jędrzejowska, A.; Glińska, O.; Karczmarewicz, E.; McCauliffe, D.P.; Woźniacka, A. Vitamin D Effects in Atopic Dermatitis. J. Am. Acad. Dermatol. 2013, 69, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Moosbrugger-Martinz, V.; Schmuth, M.; Dubrac, S. A Mouse Model for Atopic Dermatitis Using Topical Application of Vitamin D3 or of Its Analog MC903. Methods Mol. Biol. Clifton NJ 2017, 1559, 91–106. [Google Scholar] [CrossRef]
- McCormack, P.L. Calcipotriol/Betamethasone Dipropionate: A Review of Its Use in the Treatment of Psoriasis Vulgaris of the Trunk, Limbs and Scalp. Drugs 2011, 71, 709–730. [Google Scholar] [CrossRef]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Polesie, S.; Gillstedt, M.; Osmancevic, A. Impact of Etanercept on Vitamin D Status and Vitamin D-Binding Protein in Bio-Naïve Patients with Psoriasis. Acta Derm. Venereol. 2021, 101, adv00604. [Google Scholar] [CrossRef]
- Oleröd, G.; Hultén, L.M.; Hammarsten, O.; Klingberg, E. The Variation in Free 25-Hydroxy Vitamin D and Vitamin D-Binding Protein with Season and Vitamin D Status. Endocr. Connect. 2017, 6, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Grigalavicius, M.; Juraleviciute, M.; Grant, W.B. Phototherapy and Vitamin D. Clin. Dermatol. 2016, 34, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.C.; Menegat, F.D.; Ferreira, C.E.S.; Faulhaber, A.C.L.; Campos, D.A.L.S.; Mangueira, C.L.P. Analytical and Clinical Validation of the New Roche Elecsys Vitamin D Total II Assay. Clin. Chem. Lab. Med. 2018, 56, e298–e301. [Google Scholar] [CrossRef]
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Female | Female | Male | Male | Female | Female | Female | Male | Male | Female |
| Age (years) | 33 | 20 | 34 | 22 | 27 | 38 | 26 | 21 | 28 | 37 |
| Duration of AD (years) | 33 | 15 | 13 | 18 | 23 | 36 | 15 | 19 | 25 | 33 |
| BMI (kg/m2) | 32 | 25 | 33 | 23 | 21 | 22 | 21 | 21 | 23 | 25 |
| Blood pressure, systolic/diastolic (mmHg) | 110/70 | 115/70 | 140/85 | 110/70 | 120/70 | 115/80 | 110/60 | 110/60 | 110/80 | 100/80 |
| Current smoking | No | No | No | No | No | No | No | Yes | No | No |
| Skin type (Fitzpatrick) | IV | III | III | III | III | II | II | III | III | II |
| Month of enrollment | September | November | December | December | January | February | March | January | February | October |
| Hours per day spent outdoors during summer (winter) | 3(3) | 5(1) | 8(3) | 6(1) | 5(4) | 8(1) | 5(1) | 8(3) | 7(3) | 5(1) |
| Mean number of fish meals/week | 4.5 | 1.5 | 2.5 | 1.0 | 0.5 | 2.0 | 1.0 | 0.0 | 0.0 | 2.5 |
| Use of medication that could affect vitamin D status | No | No | No | No | No | No | No | No | Yes | No |
| Number of treatments | 27 | 25 | 30 | 26 | 24 | 28 | 14 | 12 | 22 | 23 |
| Cumulative dose (J/cm2) | 37.4 | 15.65 | 37.55 | 40.6 | 32.75 | 9.0 | 2.7 | 3.5 | 16.5 | 14.5 |
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D (nmol/L) before treatment (visit 1) | 90.9 | 114.0 | 76.6 | 65.0 | 88.9 | 88.0 | 71.7 | 72.6 | 35.5 | 61.0 |
| 25(OH)D (nmol/L) after treatment (visit 3) | 140 | 196 | 224 | 143 | 127 | 201 | N/A | N/A | 119 | 108 |
| Free 25(OH)D (pmol/L) before treatment (visit 1) | 10.7 | 12.5 | 10.5 | 10.7 | 14.7 | 16.7 | 12.0 | 10.5 | 6.49 | 14.5 |
| Free 25(OH)D (pmol/L) after treatment (visit 3) | 18.2 | 23.7 | 34.4 | 23.0 | 21.2 | 50.2 | N/A | N/A | 18.5 | 24.7 |
| Percentage of free 25(OH)D (%) before treatment (visit 1) | 0.0118 | 0.0109 | 0.0137 | 0.0165 | 0.0166 | 0.0190 | 0.0167 | 0.0144 | 0.0183 | 0.0237 |
| Percentage of free 25(OH)D (%) after treatment (visit 3) | 0.0130 | 0.0121 | 0.0154 | 0.0161 | 0.0167 | 0.0250 | N/A | N/A | 0.0155 | 0.0229 |
| 1,25(OH)2D (pmol/L) before treatment (visit 1) | 158 | 100 | 158 | 142 | 84.0 | 139 | 110 | 48.0 | 56.0 | 94.0 |
| 1,25(OH)2D (pmol/L) after treatment (visit 3) | 145 | 138 | 234 | 168 | 150 | 116 | N/A | N/A | 116 | 39.0 |
| Objective SCORAD before treatment (visit 1) | 25.1 | 25.5 | 40.4 | 39.6 | 38.5 | 28.5 | 38.0 | 38.0 | 36.2 | 30.0 |
| Objective SCORAD after treatment (visit 3) | 19.6 | 11.1 | 18.9 | 19.9 | 42.7 | 22.1 | N/A | N/A | 23.1 | 10.7 |
| VAS before treatment (visit 1) | 8.0 | 5.5 | 7.4 | 8.0 | 8.4 | 8.8 | 7.2 | 5.1 | 8.2 | 3.8 |
| VAS during treatment (visit 2) | 5.9 | 3.1 | 3.2 | 6.6 | 7.9 | 6.8 | 7.2 | 8.0 | 5.8 | 5.0 |
| VAS after treatment (visit 3) | 3.0 | 1.9 | 2.2 | 3.7 | 9.3 | 5.1 | N/A | N/A | 0.0 | 2.5 |
| VAS 4–6 weeks after end of treatment (visit 4) | 6.4 | 2.8 | 5.0 | 5.0 | 1.1 | 7.4 | N/A | N/A | 4.9 | 6.5 |
| VAS pruritus before treatment (visit 1) | 8.5 | 5.0 | 6.3 | 6.9 | 6.0 | 5.0 | 6.0 | 4.0 | 7.0 | 7.0 |
| VAS pruritus after treatment (visit 3) | 3.0 | 2.0 | 3.0 | 2.5 | 7.0 | 5.0 | N/A | N/A | 7.0 | 2.0 |
| Before UVB | After UVB | Increase | |
|---|---|---|---|
| Total 25(OH)D (nmol/L) mean, median | 76.4 ± 21.1 | 157.3 ± 43.4 | 106% |
| 74.6 (66.7–88.7) | 141.5 (125.0–197.3) | ||
| Free 25(OH)D (pmol/L) mean, median | 11.9 ± 2.9 | 26.7 ± 10.7 | 124% |
| 11.4 (10.6–14.0) | 23.3 (20.5–27.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmelid, A.; Osmancevic, A.; Gillstedt, M.; Alsterholm, M. Effects of Phototherapy on Free Vitamin D Levels in Ten Patients with Atopic Dermatitis. Int. J. Transl. Med. 2022, 2, 586-596. https://doi.org/10.3390/ijtm2040044
Elmelid A, Osmancevic A, Gillstedt M, Alsterholm M. Effects of Phototherapy on Free Vitamin D Levels in Ten Patients with Atopic Dermatitis. International Journal of Translational Medicine. 2022; 2(4):586-596. https://doi.org/10.3390/ijtm2040044
Chicago/Turabian StyleElmelid, Andrea, Amra Osmancevic, Martin Gillstedt, and Mikael Alsterholm. 2022. "Effects of Phototherapy on Free Vitamin D Levels in Ten Patients with Atopic Dermatitis" International Journal of Translational Medicine 2, no. 4: 586-596. https://doi.org/10.3390/ijtm2040044
APA StyleElmelid, A., Osmancevic, A., Gillstedt, M., & Alsterholm, M. (2022). Effects of Phototherapy on Free Vitamin D Levels in Ten Patients with Atopic Dermatitis. International Journal of Translational Medicine, 2(4), 586-596. https://doi.org/10.3390/ijtm2040044







