Abstract
Patients with obstructive sleep apnea (OSA) have an increased risk of cardiovascular disease (CVD). Nitric oxide (NO) and heme oxygenase-1 (HO-1) affect vascular tone and are vasoprotective. Furthermore, hydrogen sulfide (H2S), an HO-1 inducer, is known to be a major effector molecule driving apneas. This study was conducted to examine the molecular relationships between these gasotransmitters and HO-1 in patients with OSA. Individuals who presented for evaluation for possible OSA were recruited and underwent overnight polysomnography. Individuals with an apnea-hypopnea index (AHI) of >5 per hour (OSA diagnosis) were considered cases (n = 19), while those with an AHI of <5 per hour (n = 6) were the controls. Blood samples were obtained before sleep and again from OSA cases prior to initiating treatment. H2S, NO, and HO-1 levels were assayed. Patients with OSA showed lower NO and H2S levels at baseline compared to controls. NO levels further decreased significantly from baseline in patients at the time of OSA diagnosis, while H2S levels largely showed an increasing trend, which was observed only when the subjects showing a baseline H2S level of >0.5 μM were excluded. Interestingly, analysis of HO-1 did not show a significant change from baseline, confirming the inverse relationship between the two gasotransmitters. The alterations in the bioavailability of endogenous H2S and its molecular interactions with NO and HO-1 regulating vascular tone may play a role in the pathogenesis of CVD in OSA patients.
1. Introduction
Obstructive sleep apnea (OSA) and cardiovascular disease (CVD) are common comorbidities. The estimated prevalence of OSA at AHI >= 15 events per hour ranges from 6 to 17% in the general population, up to 49% in older subjects and patients with established CVD [1]. Indeed, OSA has been implicated as an independent risk factor for CVD [2,3,4,5,6]. Endothelial dysfunction is one of the likely mechanisms contributing to the heightened CVD risk in OSA patients, in addition to other factors such as increased sympathetic activity, oxidative stress, and inflammation [7,8,9]. According to the World Health Organization (WHO), in 2016 almost 18 million deaths were related to CVD [10]. As a step towards reducing the risk of CVD in OSA patients, the underlying pathophysiological mechanisms linking CVD and OSA must be better elucidated [11].
Nitric oxide (NO) and heme oxygenase-1 (HO-1) have both been shown to modulate vascular tone [12]. Both NO and NO derivatives are potent stimulators of HO-1 production [13]. Reduced endothelial nitric oxide synthase (eNOS) expression leading to decreased NO levels is one of the earliest changes implicated in endothelial dysfunction [14,15]. This feature is consistently noted in patients with OSA and is an important predictor of atherosclerosis leading to CVD [6]. HO-1 is an anti-atherogenic enzyme that may be a promising therapeutic target in the treatment of vascular disease, due to its vasoprotective effects [16]. End products of HO-1 include a second gasotransmitter, carbon monoxide (CO), and the bile pigments bilirubin and biliverdin, which have anti-inflammatory, anti-oxidant, anti-apoptotic, and antithrombotic effects [17,18,19]. Overexpression of HO-1 in animal models has been shown to restore eNOS, increase the production of NO, and improve the endothelium-dependent vascular relaxation [20]. In contrast, a reduction in CO caused increased sleep apnea indices during rapid eye movement (REM) sleep [21]. Treating OSA increases endogenous levels of NO, potentially improving cardiovascular outcomes [22].
Hydrogen sulfide (H2S) is another gasotransmitter that has recently gained importance due to its anti-oxidative and cytoprotective properties and its ability to induce HO-1 [23,24]. H2S plays a potential role in several physiological mechanisms, including endothelial function, vasomotor tone regulation, and angiogenesis, which are implicated in CVD [25,26]. H2S is shown to have close interactions with the NO pathway that could potentially affect the endothelial function [27]. However, H2S is shown to have both pro-inflammatory and anti-inflammatory actions. H2S can cause either vasodilation or vasoconstriction in a concentration-dependent manner, thereby exerting a biphasic effect on the vascular endothelium [28]. Higher concentrations of H2S in the presence of NO can induce significant vasodilation by opening KATP channels, while this effect is attenuated with a reduction in NO levels. At lower concentrations, H2S has been shown to inhibit endothelial nitric oxide synthase (e-NOS), causing vasoconstriction [29].
Very little is known about the bioavailability of H2S in the context of OSA. Moreover, no studies have examined the molecular interaction of these gasotransmitters, specifically with respect to HO-1. We speculate that alterations in the bioavailability of endogenous H2S and the consequent adaptations in the molecular interactions between H2S, NO, and HO-1 regulating the vascular tone may play a role in the pathogenesis of CVD in OSA patients. This preliminary study was designed to examine such alterations in newly diagnosed OSA patients, with a long-term goal of not only gaining insights into the pathophysiological mechanisms leading to CVD but also developing clinically effective therapeutic modalities that target these gasotransmitters to reduce the CVD risk in these patients.
2. Methods
The study was approved by the Institutional Review Board at the University of Minnesota. Informed written consent was obtained from all the study subjects. The recruitment took place from September 2012 through April 2013.
2.1. Study Participants
A total of twenty-five patients who were referred to our academic sleep center for evaluation for possible OSA were recruited. The inclusion criteria included no previous diagnosis of OSA, naïve to CPAP treatment, ages 25–75 years, with BMI greater than 30 kg/m2. Patients using corticosteroids, oral contraceptives, and anti-inflammatory agents, and those with chronic obstructive pulmonary disease, neuromuscular disorders, recent or concomitant systemic infections, or upper or lower airway infections and autoimmune diseases were excluded. History, physical exam results, blood pressure measurements, and overnight polysomnography (PSG) were obtained for all subjects. The cases and controls were identified during the PSG, based on the apnea-hypopnea index (AHI), which is the average number of episodes of apneas and hypopneas per hour of sleep. The cases were subjects who qualified for the diagnosis of OSA with an AHI of more than 5 per hour (n = 19), while those without OSA, with an AHI of <5 per hour (n = 6), acted as controls.
2.2. Polysomnography (PSG)
All subjects underwent supervised overnight PSG for evaluation of OSA at the University of Minnesota Sleep Center. The PSG monitoring system (Excel-Tech Corp., Oakville, ON, Canada) included techniques for measuring standard physiologic parameters, such as electroencephalography, electrooculography, electromyography, electrocardiography, a thermistor nasal pressure transducer and instrumentation for oral airflow measurements. The respiratory parameters of chest and abdominal movements were recorded using respiratory inductance plethysmography. Oxygen saturation was recorded using pulse oximetry. All studies were manually scored by an experienced registered PSG technologist and were reviewed and interpreted by a board-certified sleep physician. Disordered breathing events were scored in accordance with guidelines from the American Academy of Sleep Medicine [30].
2.3. Sample Collection
Approximately 4 mL of venous blood was collected from each subject in an EDTA tube and a sodium heparin tube. Initial baseline blood samples were obtained from all the subjects before sleep. A second blood sample was obtained only from patients diagnosed with OSA, prior to initiation of treatment with continuous positive airway pressure (CPAP). CPAP was initiated if the AHI was greater than 10 per hour. The blood was processed immediately to separate the plasma in the sleep lab. Blood collected in heparin tubes was centrifuged immediately at 3000 rpm for 2 min to separate the plasma, and ~300 μL of plasma was added to the stabilizing solution provided for the H2S assay. All samples were frozen until analysis.
2.4. H2S Analysis
H2S analysis was performed using a modified methylene blue reaction coupled with high-performance liquid chromatography (HPLC) detection with slight modifications [31]. Shortly after collection, 0.5 mL of plasma was added to a stabilizing solution consisting of 350 µL of 1% zinc acetate and 50 µL of 1.5 M sodium hydroxide. The samples were then frozen at −80 °C until analysis. A 6-point standard curve (0.1–3 µM) was generated from an H2S stock solution (100 µM) that was prepared by spiking phosphate-buffered saline (PBS) with 1 mM sodium hydrogen sulfide (NaHS; Alfa Aesar, MA, USA) and processed identically to the plasma samples. The methylene blue color development was achieved by reaction with 20 mM N, N-dimethyl-p-phenylenediamine dihydrochloride (N,N-dpd; Sigma-Aldrich, St. Louis, MO, USA) in 7.2 N HCl and 30 mM ferric chloride (Fisher Scientific), in 1.2 N HCl. After separation of protein with 10% trichloroacetic acid (Ricca Chemical Company, Arlington, TX, USA), the clear supernatant was taken for HPLC analysis, performed in a series 1260 Infinity system (Agilent Technologies, Santa Clara, CA, USA) connected to a UV/Vis detector to measure absorbance at 667 nm. Peak separation was achieved using a mobile phase consisting of a 72% solution containing 0.15% v/v trifluoroacetic acid (Sigma-Aldrich) in ultra-pure water and 28% acetonitrile, using an Eclipse XDB-C18 column. The HPLC system was controlled, and data were processed, using ChemStation software (Agilent Technologies, Santa Clara, CA, USA). The retention time was observed to be ~5.6 min. GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) was used to create a standard curve and perform a nonlinear second-order polynomial (quadratic) regression. Acceptable r2 values were ≥0.995.
2.5. Nitric Oxide Analysis
Ultra-filtered plasma samples were analyzed using a nitrate/nitrite colorimetric assay kit (Cayman Chemicals, Ann Arbor, MI, USA) as per the manufacturer’s instructions. The sum of nitrates and nitrites, which are stable end products of NO metabolism, is considered an index of total NO production. Absorbance was measured using a Synergy™ 2 multi-detection microplate reader (BioTek, Winooski, VT, USA). The concentration of nitrates + nitrites in the patient samples was determined by plotting the absorbance readings as a function of NO concentration and performing a linear regression analysis. Values from the regression were substituted into the formula in the assay protocol to calculate the total NO (nitrate + nitrites in µM) in the sample. The lower limit of detection was 5 µM.
2.6. Heme Oxygenase-1 (HO-1) Analysis
Plasma HO-1 levels were determined using the enzyme-linked immunosorbent assay (ELISA) method (HO-1 [human] ELISA kit, Enzo Life Sciences, Farmingdale, NY, USA) as per the manufacturer’s protocol and as described previously [32].
2.7. Biostatistical Analysis
Descriptive statistics were shown as frequencies and percentages or as the mean ± standard error (SE), as appropriate. For continuous variables, p-values were calculated using a two-sample t-test or a Wilcoxon rank test, depending on the distribution of data for two-group comparison, a Wilcoxon signed rank test for paired comparison, or a Kruskal–Wallis test for three-group comparison. For categorical variables, the p-value was calculated using Fisher’s exact test. For bivariate association, Spearman’s correlation coefficient was calculated. All analyses were performed using the SAS system (v. 9.3; SAS Institute, Cary, NC, USA). The p-values were two-sided, and p < 0.05 was considered statistically significant.
3. Results
3.1. Participant Characteristics
The baseline characteristics of all participants are shown in Table 1. Of the 25 subjects recruited, we identified 19 patients with OSA (11 males and 8 females) and 6 controls without OSA (1 male and 5 females). Patients with OSA were older compared to controls, 51 ± 12 vs. 36 ± 11 years, respectively. BMI, height, and smoking history (current or former) were similar in both groups. The prevalence of medical comorbidities, including hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, hyperlipidemia, and atrial fibrillation were also similar in both groups.

Table 1.
Demographic and clinical characteristics of participants in this study.
3.2. Alterations in Plasma Gasotransmitter Levels following Obstructive Events
Baseline levels of both NO and H2S in patients with OSA were lower compared to controls, though there were no statistically significant differences (Table 2). The trend towards reduction of these gasotransmitters in OSA patients is evident when we calculate the ratio of H2S to the total NO products (H2S/NO). Notably, the baseline concentration of HO-1 was identical in both OSA patients and controls.

Table 2.
Baseline levels of the gasotransmitters and HO-1 in samples collected prior to sleep. All data are represented as mean (SE).
Subsequent analysis of the samples collected prior to the initiation of CPAP treatment in patients with OSA (n = 17) revealed a significant decrease in NO from the baseline mean levels after an average of 233 ± 60.5 min of untreated OSA. The mean reduction in NO was −3.6 μM ± 6.73 (p = 0.03). In contrast, we did not observe a clear difference in the levels of H2S between the two time points. However, it was interesting to note that when we excluded subjects with a baseline H2S level of >0.5 μM (n = 4), we observed a trend towards an increase in H2S levels from baseline to the time of diagnosis of OSA. The average increase in H2S levels was observed to be 0.125 ± 0.06 μM (p = 0.09) after obstructive events (Figure 1). We sought to understand if there were any differences in the co-variables among these four cases with high baseline H2S. Three out of the four cases had an AHI greater than 70 per hour but did not have REM sleep during the baseline. Two of the four cases showed evidence of sleep-associated hypoxemia, which is defined as time spent with oxygen saturation at or below 88% for more than 5 min [33], with no REM, in the baseline study. There were no other differences in the co-variables tested among the four cases.
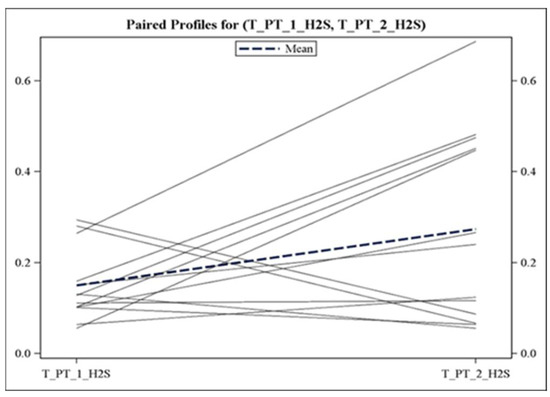
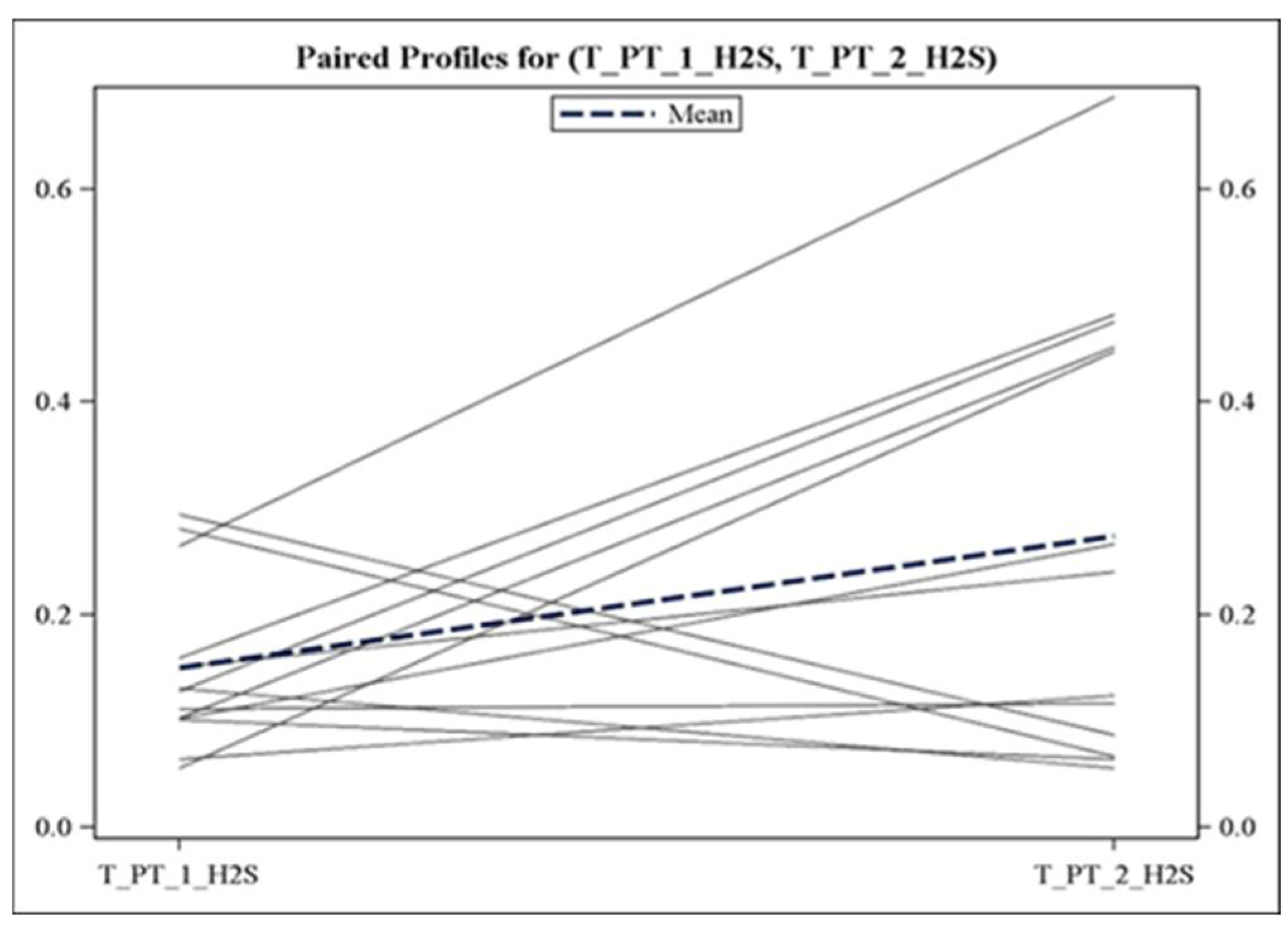
Figure 1.
Individual trajectories of H2S concentration in patients with OSA. H2S levels (in μM) were analyzed at baseline (before sleep, T_PT_1) and at the time of OSA diagnosis (T_PT_2). Data from patients with baseline H2S of >0.5 μM (n = 4) were not included.
3.3. Effect of Obstructive Events on Heme Oxygenase-1 (HO-1) Expression
In addition to the gaseous mediators, we measured the concentration of the antioxidant protein HO-1 in plasma samples. HO-1 mRNA is known to be induced both by NO and H2S [34].
However, we did not observe any difference in HO-1 levels in samples collected from 17 patients with OSA after obstructive events, compared to the mean baseline concentrations. The mean concentration of HO-1 at the second time point, prior to the initiation of CPAP treatment, was 2.92 ± 1.46 ng/mL. We further confirmed the relationship between HO-1 protein concentrations and the two gasotransmitter levels at the time of OSA diagnosis. Our analysis showed significant negative (r = −0.495, p = 0.04) and positive (r = 0.458, p = 0.06) correlations between HO-1 and NO and H2S, respectively, confirming the inverse relationship between the two gasotransmitters.
3.4. Effect of Severity of OSA on Baseline Levels of NO, H2S, and HO-1
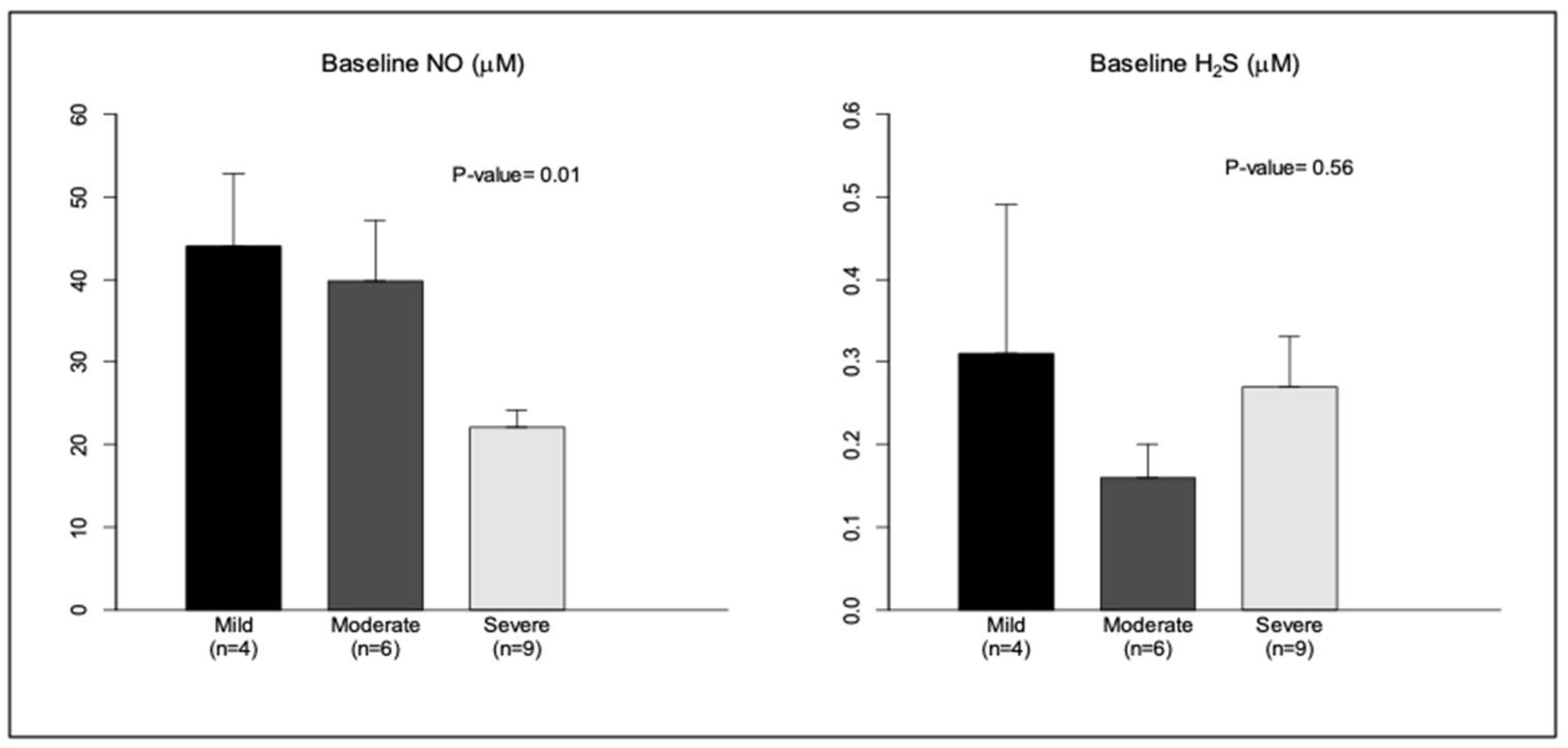
To further understand the interactions between these molecules, we compared baseline levels of NO, H2S, and HO-1 among patients with OSA in terms of OSA severity. The severity of OSA was classified based on the AHI into: mild OSA, if the AHI was >5 to <15 per hour, moderate OSA if the AHI was >15 to <30 per hour, and severe OSA (>30 per hour) [35]. Out of the 19 cases, there were 4 cases with mild OSA, 6 with moderate OSA, and 9 with severe OSA. NO levels were inversely proportional to the degree of severity of OSA, similarly to previous observations [36]. A similar analysis with baseline levels of H2S did not show a relationship with the degree of severity, probably due to higher variability in these values (Figure 2). Additionally, baseline HO-1 also showed no relationship with severity. However, correlation analysis of baseline NO and H2S levels revealed a moderate inverse relationship between these gasotransmitters (Spearman correlation coefficient r = −0.32; p = 0.11).
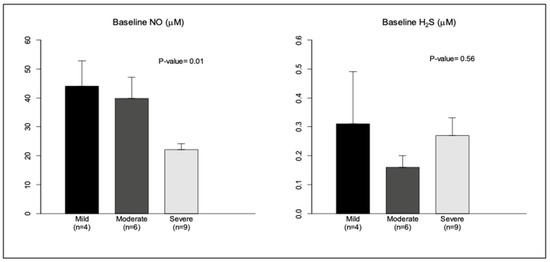
Figure 2.
Baseline NO and H2S levels (in μM) were analyzed in relation to severity of OSA (AHI > 5/h). 1: Mild OSA (AHI 5–15/h); 2: moderate OSA (AHI 15–30/h); 3: severe OSA (AHI > 30/h). Data are shown as mean ± SE.
3.5. A Trend towards an Increase in H2S and Decrease in HO-1 with Frequent Periodic Limb Movements
Periodic limb movements during sleep (PLMs) are episodes of periodic repetitive limb movements, commonly involving the lower extremities. PLMs and OSA have been associated with systemic inflammation and CVD. We categorized the patients based on the frequency of periodic limb movements index (PLMI) into two groups: those with a PLMI of >20 and those with a PLMI of <20 per hour. Of the 17 cases with OSA, 5 had a PLMI of >20 per hour.
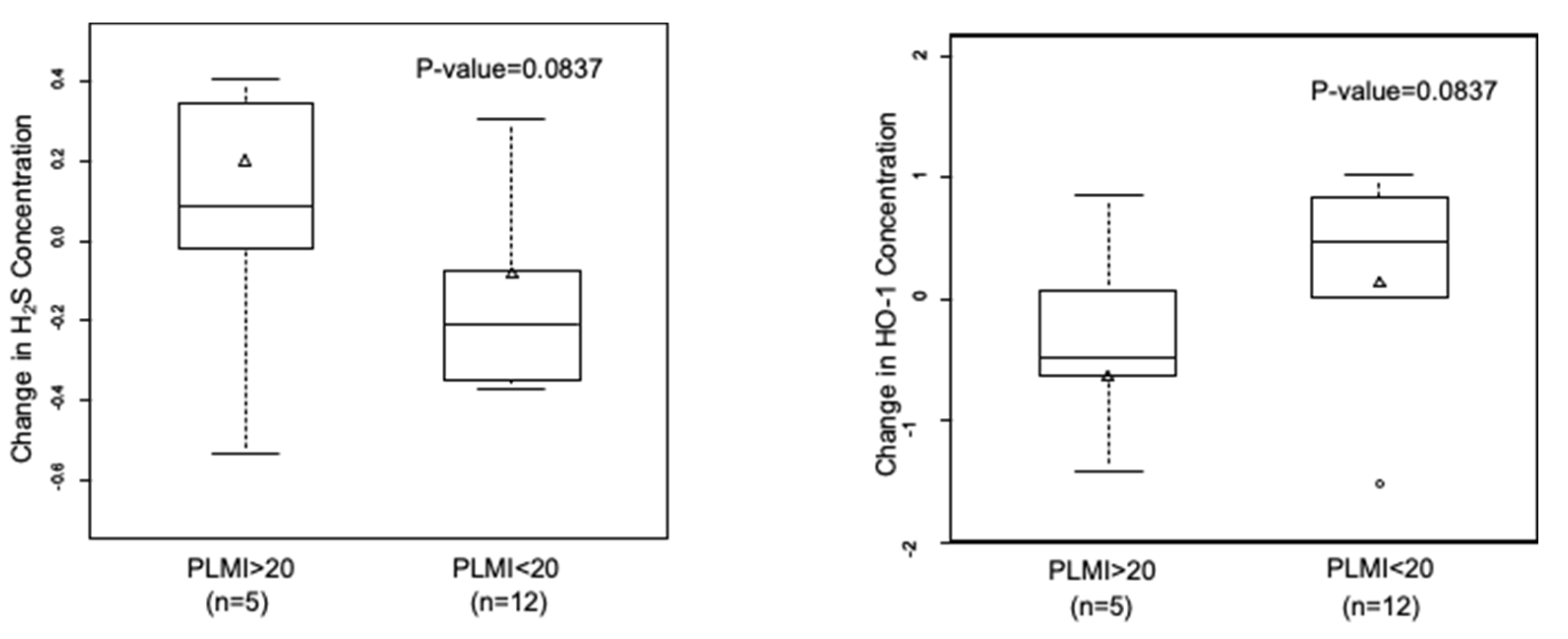
Subjects with PLMI > 20 showed higher NO and lower H2S levels compared to subjects with PLMI < 20. When we analyzed the changes in gasotransmitter levels between the two time points, we found that NO levels largely decreased in all OSA patients, irrespective of the PLMI (data not shown). However, OSA patients with a PLMI of >20 per hour showed an increase in H2S levels, and a decrease was observed in subjects with a PLMI of <20 per hour (0.20 ± 0.12 µM vs. −0.08 ± 0.08 µM; p = 0.08). On the other hand, we observed an inverse relationship between changes in H2S and HO-1 levels vs. PLM frequency (Figure 3). HO-1 levels decreased in subjects with a PLMI of >20 per hour and increased in those with a PLMI of <20 per hour (−0.64 ± 0.23 ng/mL vs. 0.14 ± 0.23 ng/mL; p = 0.08).
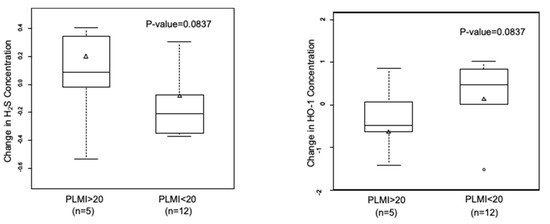
Figure 3.
Inverse correlation in changes in H2S and HO-1 levels in relation to PLMI. Subjects with OSA diagnosis (n = 17) were grouped based on PLMI. Data are shown as mean ± SE.
4. Discussion
The gasotransmitters H2S and NO and the mediator protein HO-1 all play a role in regulating the vascular tone. To the best of our knowledge, this is the first report of the molecular interactions between plasma H2S, NO, and HO-1 in OSA patients. Previously, Peng et al. demonstrated the complementary roles of CO and H2S in sleep apnea [21]. We demonstrated that for patients with OSA who have a baseline H2S concentration of <0.5 µM, there is a trend towards increasing its levels after a sufficient number of obstructive events. Furthermore, we showed that the functional consequence of the changes in gasotransmitter levels on downstream signaling is dependent on the relative magnitude of these alterations. Although HO-1 is a known downstream mediator protein of gasotransmitters, we showed that the level of this antioxidant is not altered in this cohort, probably due to the inverse correlation in H2S and NO activity.
OSA patients have recurrent episodes of hypoxemia followed by re-oxygenation, which can potentially cause rapid fluctuations in the levels of the gasotransmitters. Previous studies have shown that hypoxia induces H2S production [37]. It has also been shown that H2S enhances the sensory response of the carotid body to hypoxia, and the chemo-reflex mediates increased sympathetic nerve activity [38,39], supporting the potential role of H2S in CVD risk in OSA patients. Mice deficient in HO-2, an isoform of HO-1 responsible for CO production, showed carotid body hyperactivity and sleep apnea, which was normalized by genetic ablation of an H2S-producing enzyme [21]. This indicates that H2S is a major effector molecule driving apneas. We observed a trend towards an increase in the H2S levels in OSA patients. It is plausible that there may have been a significant increase in H2S levels immediately after the apnea episodes, but this would have been transient, with the levels becoming reduced with the resumption of normoxia. This can be proved only by measuring oxygen and H2S in real time, which can be challenging in a sleep laboratory setting.
Previous studies have demonstrated the molecular interactions of H2S and NO [40]. King et al. recently demonstrated that the cardioprotective action of H2S is mediated by the activation of the enzyme endothelial nitric oxide synthase (e-NOS), which catalyzes the conversion of L-arginine to NO [28]. However, we observed an inverse relationship between NO and H2S, which is in line with a recent report on plasma-free H2S levels in CVD [41]. The increase in H2S, especially in those with low baseline H2S levels, could also be an endogenous countermeasure in response to decreased NO levels, aimed at maintaining HO-1 levels at equilibrium. Additionally, NO levels were observed to be inversely correlated with the severity of OSA, as previously reported [42]. A similar analysis with baseline levels of H2S or HO-1 did not show any correlation with the degree of severity. This is likely to be due to potential skewing by one subject with high H2S (0.8) but with mild OSA (AHI = 14 per hour) affecting the trend in H2S levels.
We also stratified the subjects based on periodic limb movements (PLMs). PLMs are more prevalent in OSA patients compared to the general population and have been associated with systemic inflammation and CVD [43]. In our study, patients with a high PLM index showed a trend towards an increase in H2S levels and a decrease in levels of NO and HO-1. Moreover, we observed an inverse correlation between baseline NO and H2S levels vs. PLM frequency, indicating that the effect of PLMs on gasotransmitters is one of the potential mechanisms by which PLMs could increase the risk of CVD in OSA patients.
5. Limitations
This was a preliminary study investigating plasma H2S levels in patients with OSA. We acknowledge several limitations in this study. The most important limitation is the small sample size and lack of a third time point after CPAP treatment. In addition, although the patients with OSA and the controls were similar with respect to gender, BMI, height, and medical comorbidities, the patients with OSA were approximately 15 years older. Enrolling patients with severe OSA (AHI > 30 per hour) and including a third time point for venous blood sampling post CPAP treatment to analyze the effects of CPAP on levels of H2S, NO, and HO-1 would have strengthened our study. Lastly, screening for dietary factors such as garlic-containing diets and other herbal supplements may have been beneficial, since garlic-rich diets have been shown to increase the biological production of H2S [44]. However, in this preliminary investigation, we found that untreated OSA acutely lowers NO levels without concomitant reductions in HO-1 and a transient increase in H2S. Further studies with larger sample sizes, including the effects of CPAP on H2S, NO, and HO-1 in subjects with severe OSA, would be likely to provide further important information regarding the implications of these molecules and their interactions, leading to a better understanding of their implications for CVD risk in OSA patients.
6. Conclusions
Observations from our study showed that H2S could be a potential mediator contributing to CVD risk in OSA patients. Further studies with larger sample sizes, including subjects with severe hypoxemia, with measurements at various time points to understand the circadian variation, could elucidate the role of H2S in OSA.
Author Contributions
S.P., and R.V.K. designed the experiments; S.P., R.V.K., L.B.H. and J.L. performed the experiments; S.P., R.V.K., L.B.H., J.L., L.Z. and A.A. analyzed the data; R.V.K. contributed essential reagents/tools; S.P., R.V.K., L.B.H., J.L., L.Z. and A.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Minnesota Medical Foundation Research Grant (grant number: 4103-9216-12) to the authors (S.P. and R.V.K.). Statistical support was provided by a National Center for Advancing Translational Sciences of the National Institutes of Health Award (number UL1TR000114) to the UMN CTSI.
Informed Consent Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Minnesota (protocol #1208M18662 approved on 6 December 2014).
Acknowledgments
The authors acknowledge Virend Somers and Gregory Vercellotti for the careful review of this manuscript and helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
Competing Financial Interests
The authors declare no competing financial interests.
Abbreviations
| OSA | Obstructive sleep apnea |
| CVD | Cardiovascular disease |
| NO | Nitric oxide |
| H2S | Hydrogen sulfide |
| 3-MST | 3-mercaptopyruvate sulfurtransferase |
| e-NOS | Endothelial nitric oxide synthase |
| CPAP | Continuous positive airway pressure |
| PSG | Polysomnography |
| AHI | Apnea-hypopnea index |
| AASM | American Academy of Sleep Medicine |
| HPLC | High-performance liquid chromatography |
| CHF | Congestive heart failure |
| CAD | Coronary artery disease |
| Afib | Atrial fibrillation |
| REM | Rapid eye movement |
| PLMs | Periodic limb movements during sleep |
| PLMI | Periodic limb movement index |
| ROS | Reactive oxygen species |
References
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- May, A.M.; Mehra, R. Obstructive sleep apnea: Role of intermittent hypoxia and inflammation. Semin. Respir. Crit. Care Med. 2014, 35, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Peker, Y.; Kraiczi, H.; Hedner, J.; Loth, S.; Johansson, A.; Bende, M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur. Respir. J. 1999, 14, 179–184. [Google Scholar] [CrossRef]
- Drager, L.F.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: An emerging risk factor for atherosclerosis. Chest 2011, 140, 534–542. [Google Scholar] [CrossRef]
- Hartmann, G.; Tschop, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschop, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Montano, N.; Cogliati, C.; van de Borne, P.J.; Dyken, M.E.; Somers, V.K. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998, 98, 1071–1077. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann. N. Y. Acad. Sci. 2005, 1051, 340–350. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs). 2017. Available online: http://www.who.int/cardiovascular_diseases/en/ (accessed on 25 July 2022).
- Shamsuzzaman, A.S.; Gersh, B.J.; Somers, V.K. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA 2003, 290, 1906–1914. [Google Scholar] [CrossRef]
- Gupta, R.M.; Libby, P.; Barton, M. Linking regulation of nitric oxide to endothelin-1: The Yin and Yang of vascular tone in the atherosclerotic plaque. Atherosclerosis 2020, 292, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Maines, M.D. Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38. Cell. Mol. Biol. (Noisy-le-Grand) 2000, 46, 609–617. [Google Scholar] [PubMed]
- Bain, A.R.; Weil, B.R.; Diehl, K.J.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Atherosclerosis 2017, 265, 41–46. [Google Scholar] [CrossRef]
- Huang, P.L. Endothelial nitric oxide synthase and endothelial dysfunction. Curr. Hypertens. Rep. 2003, 5, 473–480. [Google Scholar] [CrossRef]
- Durante, W. Targeting heme oxygenase-1 in vascular disease. Curr. Drug Targets 2010, 11, 1504–1516. [Google Scholar] [CrossRef]
- Ishikawa, K.; Sugawara, D.; Wang, X.; Suzuki, K.; Itabe, H.; Maruyama, Y.; Lusis, A.J. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ. Res. 2001, 88, 506–512. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.Y.; Bach, F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef]
- Ahmad, M.; Turkseven, S.; Mingone, C.J.; Gupte, S.A.; Wolin, M.S.; Abraham, N.G. Heme oxygenase-1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell. Mol. Biol. (Noisy-le-Grand) 2005, 51, 371–376. [Google Scholar]
- Peng, Y.J.; Zhang, X.; Gridina, A.; Chupikova, I.; McCormick, D.L.; Thomas, R.J.; Scammell, T.E.; Kim, G.; Vasavda, C.; Nanduri, J.; et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc. Natl. Acad. Sci. USA 2017, 114, 1413–1418. [Google Scholar] [CrossRef]
- Fortuna, A.M.; Miralda, R.; Calaf, N.; Gonzalez, M.; Casan, P.; Mayos, M. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir. Med. 2011, 105, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nagai, Y.; Umemura, K.; Kimura, Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005, 7, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.; Webb, G.D.; Bian, J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Li, L.; Kostetski, I.; Chu, S.H.; Siau, J.L.; Bhatia, M.; Moore, P.K. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006, 343, 303–310. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.X.; et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef]
- Nagpure, B.V.; Bian, J.S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell. Longev. 2016, 2016, 6904327. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.J.; Quan, S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2007. [Google Scholar]
- Sparatore, A.; Perrino, E.; Tazzari, V.; Giustarini, D.; Rossi, R.; Rossoni, G.; Erdmann, K.; Schroder, H.; Del Soldato, P. Pharmacological profile of a novel H2S-releasing aspirin. Free. Radic. Biol. Med. 2009, 46, 586–592. [Google Scholar] [CrossRef]
- Kartha, R.V.; Zhou, J.; Basso, L.; Schroder, H.; Orchard, P.J.; Cloyd, J. Mechanisms of Antioxidant Induction with High-Dose N-Acetylcysteine in Childhood Cerebral Adrenoleukodystrophy. CNS Drugs 2015, 29, 1041–1047. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Durante, W.; Kroll, M.H.; Christodoulides, N.; Peyton, K.J.; Schafer, A.I. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ. Res. 1997, 80, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Kato, M.; Roberts-Thomson, P.; Phillips, B.G.; Haynes, W.G.; Winnicki, M.; Accurso, V.; Somers, V.K. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000, 102, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkoski, M.; Soriano, R.N.; Francescato, H.D.; Batalhao, M.E.; Coimbra, T.M.; Carnio, E.C.; Branco, L.G. Hydrogen sulfide as a cryogenic mediator of hypoxia-induced anapyrexia. Neuroscience 2012, 201, 146–156. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid. Redox Signal. 2010, 12, 1219–1234. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; St Leger, J.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R51–R60. [Google Scholar] [CrossRef]
- Ali, M.Y.; Ping, C.Y.; Mok, Y.Y.; Ling, L.; Whiteman, M.; Bhatia, M.; Moore, P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006, 149, 625–634. [Google Scholar] [CrossRef]
- Peter, E.A.; Shen, X.; Shah, S.H.; Pardue, S.; Glawe, J.D.; Zhang, W.W.; Reddy, P.; Akkus, N.I.; Varma, J.; Kevil, C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013, 2, e000387. [Google Scholar] [CrossRef]
- Dang-Thi-Mai, K.; Le-Dong, N.N.; Le-Thuong, V.; Tran-Van, N.; Duong-Quy, S. Exhaled Nitric Oxide as a Surrogate Marker for Obstructive Sleep Apnea Severity Grading: An In-Hospital Population Study. Nat. Sci. Sleep 2021, 13, 763–773. [Google Scholar] [CrossRef]
- Al-Alawi, A.; Mulgrew, A.; Tench, E.; Ryan, C.F. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2006, 2, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).