Detection and Quantification Limits for Polyethylene Particles Combining the Thermal Rock-Eval® Method with a Mathematical Extrapolation Procedure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Polyethylene (PE) Samples of Different Grain Sizes and Shapes
2.2. Methods: The Thermal Rock-Eval® Device
2.3. Sample Preparation for Rock-Eval® Analysis
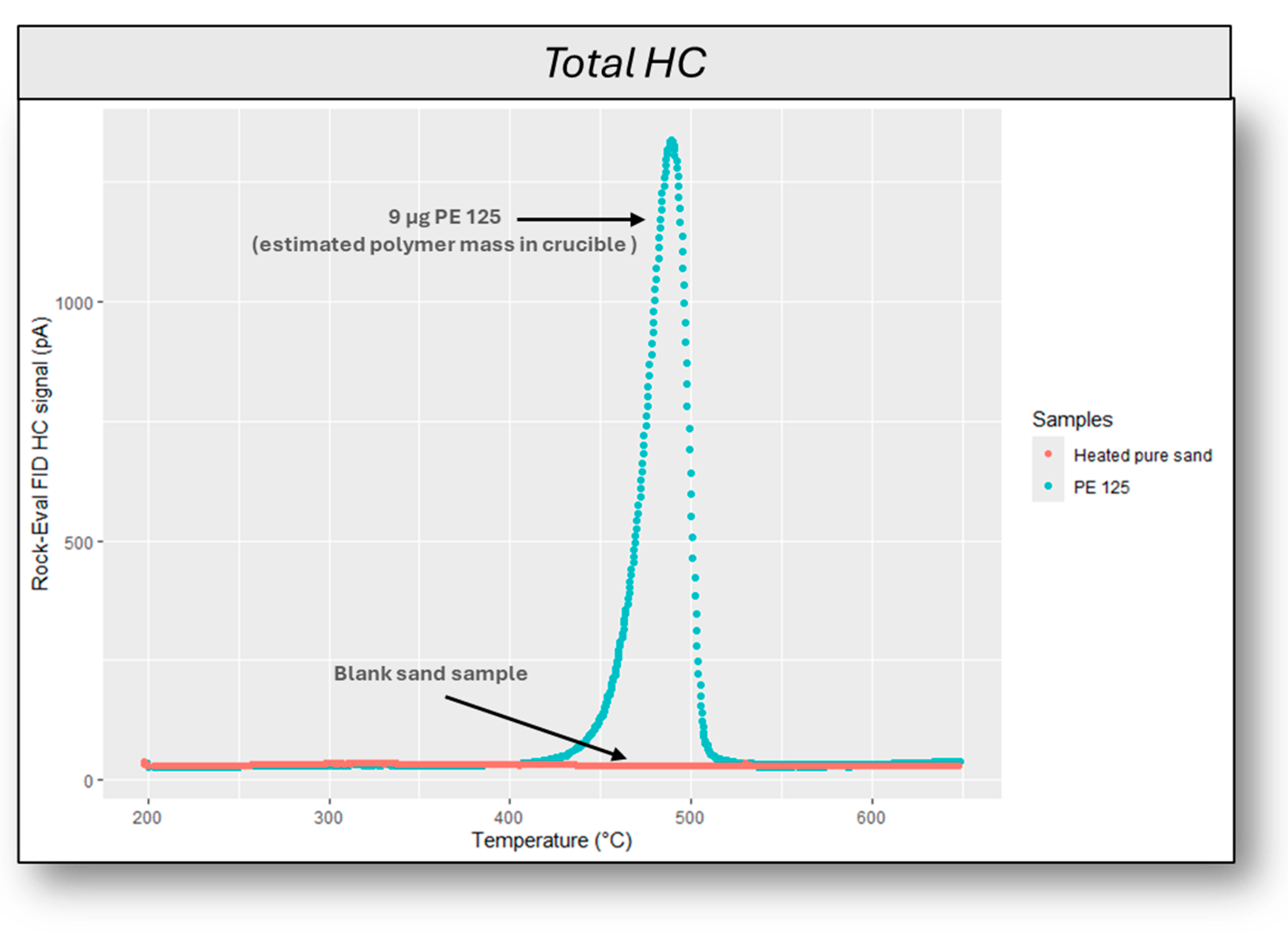
2.4. Limits of Blank (LOB), Detection (LOD), and Quantification (LOQ) Procedures
2.5. Modified Rock-Eval® Equations and Mathematical Extrapolation Procedures
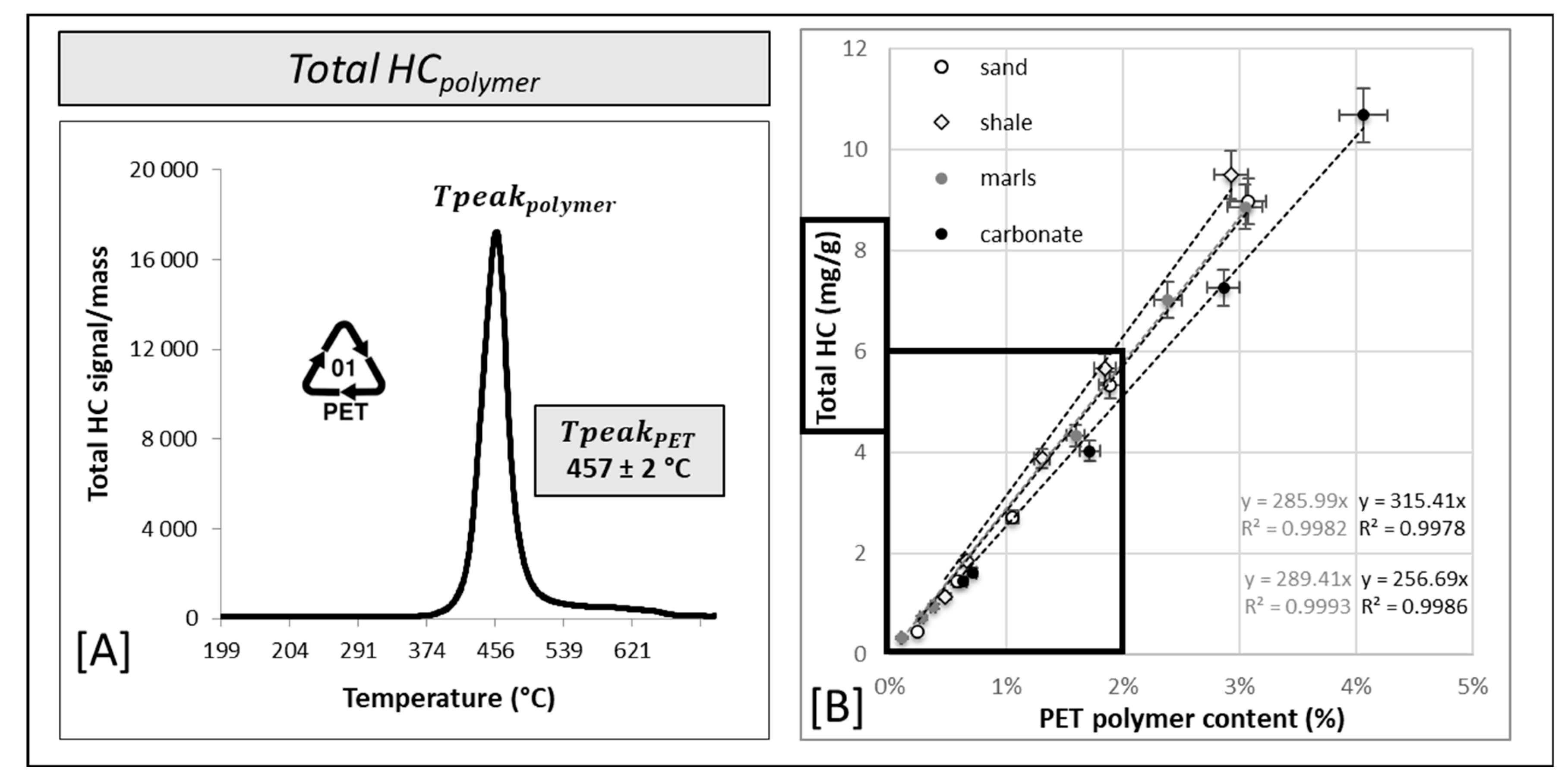
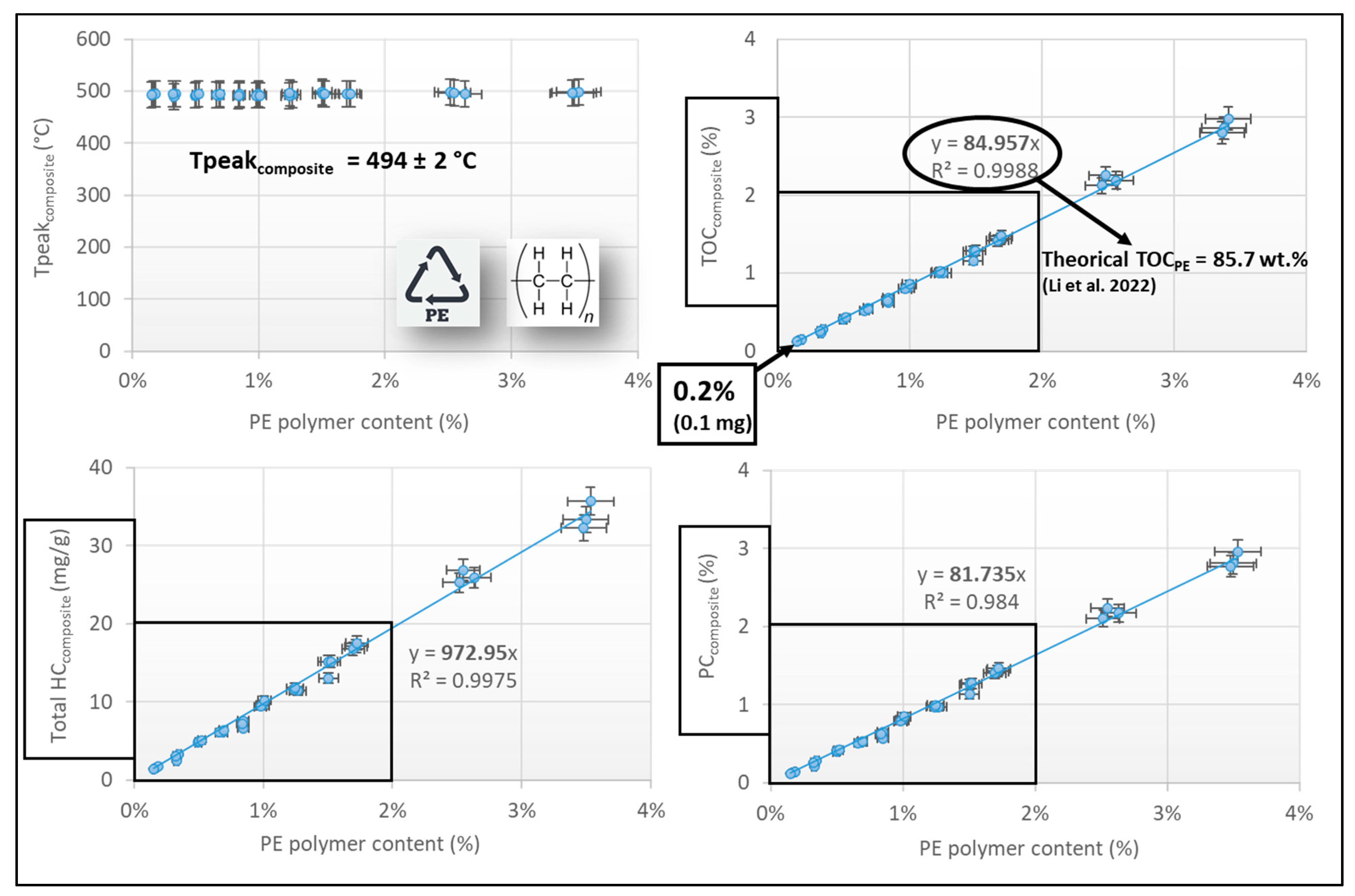
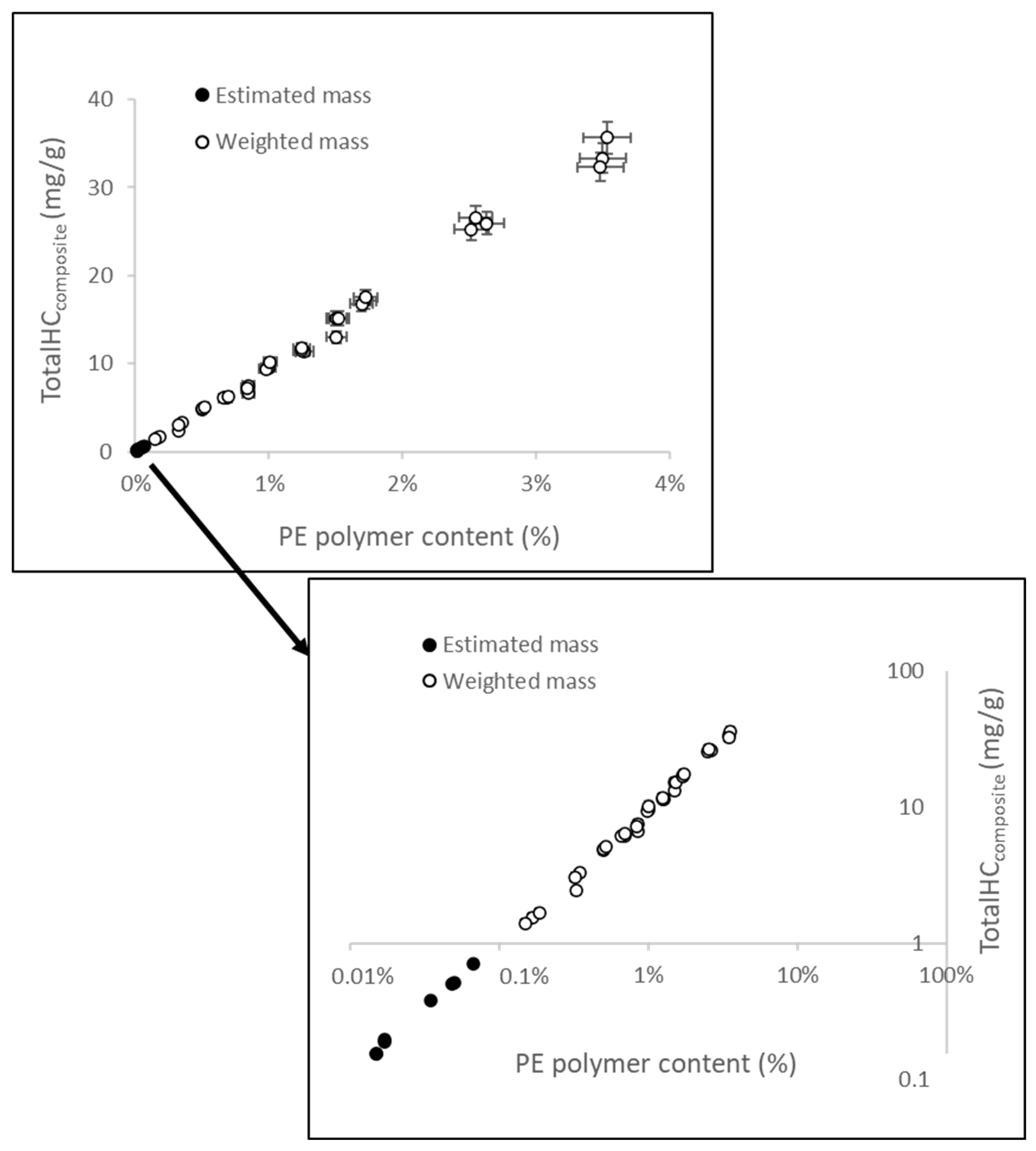
3. Results and Discussion
3.1. Limits of Detection (LOD) and Quantification (LOQ) of Polyethylene (PE) Combining the Thermal Rock-Eval® Method with a Mathematical Extrapolation Procedure
3.2. Influences of Particle Polymer Size and Shape on the Thermal Rock-Eval® Responses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| FTIR | Fourier transform infrared spectroscopy |
| TED-GC/MS | Thermal extraction desorption-gas chromatography/mass spectrometry |
| Py-GC-MS | Pyrolysis–gas chromatography–mass spectrometry |
| UHPLC | Ultrahigh-performance liquid chromatography |
| NMR | Nuclear magnetic resonance |
| FID | Flame ionization detector |
| IR | Infrared detector |
| BPC | Bulk polymer content |
| HC | Hydrocarbons |
References
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle polution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Baladima, F.; Phoenix, V.R.; Thomas, J.L.; Le Roux, G.; Sonke, J.E. Evidence of free tropospheric and long-range transport of microplastic at Pic du Midi Observatory. Nat. Commun. 2021, 12, 7242. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Hu, C.; Ge, T.; Wang, L.; Xing, J.; He, X.; Zhao, Y. Comparative evaluation of analytical techniques for quantifying and characterizing polyethylene microplastics in farmland soil samples. Agriculture 2024, 14, 554. [Google Scholar] [CrossRef]
- Goedecke, C.; Eisentraut, P.; Altmann, K.; Elert, A.M.; Bannick, C.G.; Ricking, M.; Obermaier, N.; Barthel, A.-K.; Schmitt, T.; Jekel, M.; et al. Development of a routine screening method for the microplastic mass content in a wastewater treatment plant effluent. Front. Environ. Chem. 2022, 3, 844633. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Insa, S.; Arxé, M.; Buttiglieri, G.; Rodríguez-Mozaz, S.; Barceló, D. Analysis of microplastics in the environment: Identification and quantification of trace levels of common types of plastic polymers using pyrolysis-GC/MS. MethodsX 2023, 10, 102143. [Google Scholar] [CrossRef]
- Ling, Y.; Bi, J.; Yong, W.; Yao, M.; Zhang, Y.; Zhang, F. Simultaneous determination of eight additives in polyethylene food contact materials by ultrahigh-performance liquid chromatography. Se Pu= Chin. J. Chromatogr. 2021, 39, 488–493. [Google Scholar] [CrossRef]
- Peez, N.; Janiska, M.C.; Imhof, W. The first application of quantitative 1H NMR spectroscopy as a simple and fast method of identification and quantification of microplastic particles (PE, PET, and PS). Anal. Bioanal. Chem. 2019, 411, 823–833. [Google Scholar] [CrossRef]
- Kaile, N.; Lindivat, M.; Elio, J.; Thuestad, G.; Crowley, Q.G.; Hoell, I.A. Preliminary results from detection of microplastics in liquid samples using flow cytometry. Front. Mar. Sci. 2020, 7, 552688. [Google Scholar] [CrossRef]
- Romero-Sarmiento, M.-F.; Ravelojaona, H.; Pillot, D.; Rohais, S. Polymer quantification using the Rock-Eval® device for identification of plastics in sediments. Sci. Total Environ. 2022, 807, 151068. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sarmiento, M.-F.; Rohais, S.; Dreillard, M. Quantification of textile microfibers from laundry wastewater using the Rock-Eval® device: Difference between natural and synthetic microfiber origin. Sci. Total Environ. 2024, 956, 177335. [Google Scholar] [CrossRef] [PubMed]
- Ricard, C.; Baudin, F.; Romero-Sarmiento, M.-F.; Bouton, N.; Copard, Y.; Friceau, L.; Lieunard, V.; Ludwig, W.; Rohais, S. The effect of the mineral matrix during thermal analysis of polymers: Implications for microplastics characterization. J. Anal. Appl. Pyrolysis 2025, 191, 107219. [Google Scholar] [CrossRef]
- Harris, P.T. The fate of microplastic in marine sedimentary environments: A review and synthesis. Mar. Pollut. Bull. 2020, 158, 111398. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; Ziajahromi, S. Converting mg/L to particles/L: Reconciling the occurrence and toxicity literature on microplastics. Environ. Sci. Technol. 2021, 55, 11470–11472. [Google Scholar] [CrossRef]
- Li, P.; Lai, Y.; Li, Q.; Dong, L.; Tan, Z.; Yu, S.; Chen, Y.; Sharma, V.K.; Liu, J.; Jiang, G. Total organic carbon as a quantitative index of micro- and nano-plastic pollution. Anal. Chem. 2022, 94, 740–747. [Google Scholar] [CrossRef]
- Espitalié, J.; Deroo, G.; Marquis, F. La pyrolyse Rock-Eval et ses applications. Rev. De L’institut Français Du Pétrole 1986, 41, 73–89. [Google Scholar] [CrossRef]
- Lafargue, E.; Marquis, F.; Pillot, D. Rock-Eval 6 applications in hydrocarbon exploration, production and soils contamination studies. Oil Gas Sci. Technol. 1998, 53, 421–437. [Google Scholar] [CrossRef]
- Behar, F.; Beaumont, V.I.; Penteado, H.L.; De, B. Rock-Eval 6 technology: Performances and developments. Oil Gas Sci. Technol. 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Disnar, J.R.; Guillet, B.; Keravis, D.; Di-Giovanni, C.; Sebag, D. Soil organic matter (SOM) characterization by Rock-Eval pyrolysis: Scope and limitations. Org. Geochem. 2003, 34, 327–343. [Google Scholar] [CrossRef]
- NCCLS. NCCLS Protocols for Determination of Limits of Detection and Limits of Quantitation: Approved Guideline; NCCLS Document EP17-A; NCCLS: Wayne, PA, USA, 2004; ISBN 1-56238-551-8. [Google Scholar]
- Currie, L.A. Detection and quantification limits: Basic concepts, international harmonization, and outstanding (“low-level”) issues. Appl. Radiat. Isot. 2004, 61, 145–149. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Try, P. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers: Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier Book: Amsterdam, The Netherlands, 2011; ISBN 9780444538932. [Google Scholar]
- Costa, J.P.; Avellan, A.; Mouneyrac, C.; Duerte, A.; Rocha-Santos, T. Plastic additives and microplastics as emerging contaminants: Mechanisms and analytical assessment. TrAC Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives presents in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]



| Analytical Technique | LOD | LOQ | Reference |
|---|---|---|---|
| TED-GC/MS | 1.6 µg (absolute polymer mass in crucible) | 3.2 µg | [6] |
| Py-GC/MS | 9.1 µg | 27.8 µg | [7] |
| UHPLC | 0.005% | 0.02% for additives in PE | [8] |
| NMR Spectroscopy | 19–21 µg/mL | 74–85 µg/mL | [9] |
| Flow Cytometry | The lowest spatial detectable limit for plastic particles was 200 nm | [10] | |
| PE Shapes | Grain Size (µm) | Molecular Weight (g/mol) | Density (g/mL) | Additives | Commercial Reference |
|---|---|---|---|---|---|
| Fine-grained powdered PE (PE 34-50) | 34–50 | 467.6 | 0.94 at 25 °C | No data available | 434272 |
| Fine-grained powdered PE (PE 110) | 110 | No data available | 0.94 at 25 °C | No data available | 429015 |
| Fine-grained powdered PE (PE 125) | 125 | Ultra-high molecular weight | 0.94 at 25 °C | No data available | 434264 |
| PE pellet (PE SPHERE) | >3000 | 872.0 | 0.918 at 25 °C | No data available | 428078 |
| PE fiber (PE FIBER) | Ø 5–20 | Ultra-high molecular weight | 0.94–0.965 | No data available | GF40985017 |
| High-density PE (HDPE) film (PE-HD FILM) | 500–2000 | No data available | 0.94–0.965 | No data available | 102.813.54 |
| PE foam, camping mattress (PE FOAM) | 500–2000 | No data available | Relative 0.03 | No data available | 1766-195 |
| HDPE fragment (PE-HD FRAGMENT) | 500–2000 | No data available | 0.94–0.965 | No data available | Montagne, Repère |
| Nano PE colloidal suspension (NANOPE) | 0.079 | No data available | No data available | No data available | Provided by CP2M, Lyon–France |
| Masspolymer (mg) | Masssediment (mg) | BPC (%) | Total HC (mg/g) | Total CO (mg/g) | Total CO2 (mg/g) | Tpeak (°C) | PC (%) | RC (%) | TOC (%) |
|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 60.30 | 0.2 | 1.54 | 0.13 | 0.81 | 495 | 0.13 | 0.01 | 0.14 |
| 0.11 | 59.49 | 0.2 | 1.68 | 0.10 | 0.85 | 495 | 0.14 | 0.01 | 0.15 |
| 0.09 | 60.06 | 0.2 | 1.40 | 0.10 | 0.64 | 493 | 0.12 | 0.00 | 0.12 |
| 0.21 | 60.67 | 0.3 | 3.27 | 0.13 | 1.05 | 494 | 0.28 | 0.01 | 0.29 |
| 0.20 | 60.97 | 0.3 | 2.42 | 0.36 | 2.30 | 489 | 0.21 | 0.02 | 0.23 |
| 0.19 | 59.27 | 0.3 | 3.04 | 0.17 | 0.45 | 494 | 0.26 | 0.01 | 0.27 |
| 0.30 | 60.51 | 0.5 | 4.78 | 0.10 | 1.10 | 492 | 0.40 | 0.01 | 0.41 |
| 0.30 | 60.03 | 0.5 | 4.88 | 0.08 | 0.77 | 493 | 0.41 | 0.01 | 0.42 |
| 0.31 | 59.48 | 0.5 | 5.08 | 0.09 | 0.82 | 495 | 0.42 | 0.01 | 0.43 |
| 0.41 | 59.78 | 0.7 | 6.13 | 0.64 | 2.11 | 492 | 0.52 | 0.03 | 0.55 |
| 0.40 | 60.76 | 0.7 | 6.09 | 0.30 | 1.34 | 493 | 0.51 | 0.01 | 0.52 |
| 0.41 | 59.57 | 0.7 | 6.34 | 0.35 | 1.40 | 494 | 0.53 | 0.01 | 0.54 |
| 0.50 | 59.41 | 0.8 | 7.56 | 0.62 | 4.26 | 492 | 0.65 | 0.03 | 0.68 |
| 0.50 | 59.43 | 0.8 | 6.64 | 0.71 | 3.92 | 494 | 0.57 | 0.05 | 0.62 |
| 0.50 | 60.18 | 0.8 | 7.22 | 0.56 | 2.06 | 491 | 0.62 | 0.03 | 0.65 |
| 0.59 | 59.71 | 1.0 | 9.56 | 0.10 | 0.95 | 495 | 0.80 | 0.01 | 0.81 |
| 0.58 | 59.83 | 1.0 | 9.40 | 0.23 | 1.89 | 492 | 0.79 | 0.01 | 0.80 |
| 0.60 | 60.08 | 1.0 | 10.20 | 0.16 | 0.58 | 492 | 0.85 | 0.01 | 0.86 |
| 0.75 | 59.92 | 1.2 | 11.40 | 1.26 | 1.72 | 493 | 0.97 | 0.03 | 1.00 |
| 0.75 | 61.13 | 1.2 | 11.51 | 0.57 | 2.33 | 491 | 0.97 | 0.03 | 1.00 |
| 0.74 | 60.15 | 1.2 | 11.75 | 0.73 | 2.05 | 496 | 0.99 | 0.03 | 1.02 |
| 0.89 | 60.09 | 1.5 | 15.16 | 0.28 | 0.74 | 498 | 1.26 | 0.01 | 1.27 |
| 0.89 | 60.05 | 1.5 | 13.04 | 1.39 | 5.47 | 494 | 1.13 | 0.03 | 1.16 |
| 0.90 | 60.00 | 1.5 | 15.14 | 0.40 | 1.83 | 494 | 1.27 | 0.03 | 1.30 |
| 1.53 | 59.68 | 2.5 | 25.93 | 0.69 | 2.50 | 494 | 2.17 | 0.02 | 2.19 |
| 1.49 | 60.72 | 2.5 | 25.27 | 0.28 | 1.37 | 498 | 2.10 | 0.02 | 2.12 |
| 1.49 | 59.98 | 2.5 | 26.89 | 0.17 | 1.22 | 497 | 2.24 | 0.01 | 2.25 |
| 1.01 | 59.82 | 1.7 | 17.10 | 0.33 | 1.58 | 495 | 1.43 | 0.01 | 1.44 |
| 0.99 | 59.41 | 1.7 | 16.79 | 0.38 | 1.67 | 495 | 1.40 | 0.01 | 1.42 |
| 1.01 | 59.55 | 1.7 | 17.51 | 0.26 | 1.52 | 494 | 1.46 | 0.01 | 1.47 |
| 2.02 | 59.77 | 3.4 | 33.35 | 1.89 | 5.02 | 497 | 2.81 | 0.05 | 2.86 |
| 2.04 | 59.78 | 3.4 | 35.67 | 0.18 | 0.79 | 499 | 2.96 | 0.01 | 2.97 |
| 2.01 | 59.77 | 3.4 | 32.30 | 2.91 | 5.74 | 496 | 2.77 | 0.03 | 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Sarmiento, M.-F.; Bauer, D.; Rohais, S. Detection and Quantification Limits for Polyethylene Particles Combining the Thermal Rock-Eval® Method with a Mathematical Extrapolation Procedure. Microplastics 2025, 4, 71. https://doi.org/10.3390/microplastics4040071
Romero-Sarmiento M-F, Bauer D, Rohais S. Detection and Quantification Limits for Polyethylene Particles Combining the Thermal Rock-Eval® Method with a Mathematical Extrapolation Procedure. Microplastics. 2025; 4(4):71. https://doi.org/10.3390/microplastics4040071
Chicago/Turabian StyleRomero-Sarmiento, Maria-Fernanda, Daniela Bauer, and Sébastien Rohais. 2025. "Detection and Quantification Limits for Polyethylene Particles Combining the Thermal Rock-Eval® Method with a Mathematical Extrapolation Procedure" Microplastics 4, no. 4: 71. https://doi.org/10.3390/microplastics4040071
APA StyleRomero-Sarmiento, M.-F., Bauer, D., & Rohais, S. (2025). Detection and Quantification Limits for Polyethylene Particles Combining the Thermal Rock-Eval® Method with a Mathematical Extrapolation Procedure. Microplastics, 4(4), 71. https://doi.org/10.3390/microplastics4040071






