Occurrence and Control of Microplastics and Emerging Technological Solutions for Their Removal in Freshwaters: A Comprehensive Review
Abstract
1. Introduction
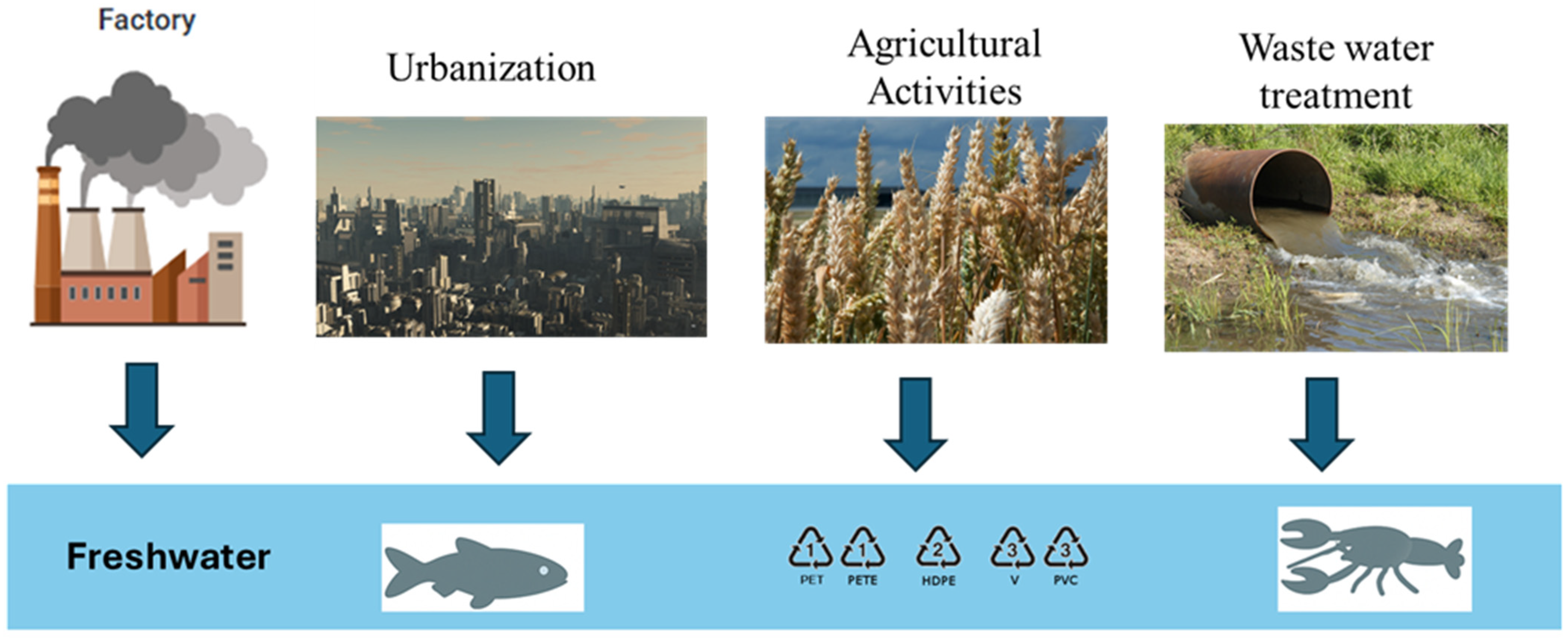
2. Distribution of Microplastics in Freshwater
3. Techniques Used for Analysing Microplastics in Environmental Samples
3.1. Instruments Used for Microplastic Analysis and Polymer Identification
3.2. Machine Learning (ML) and Internet of Things (IoT)
3.3. Geographic Information Systems and Remote Sensing
4. Toxicity Mechanisms and Effects
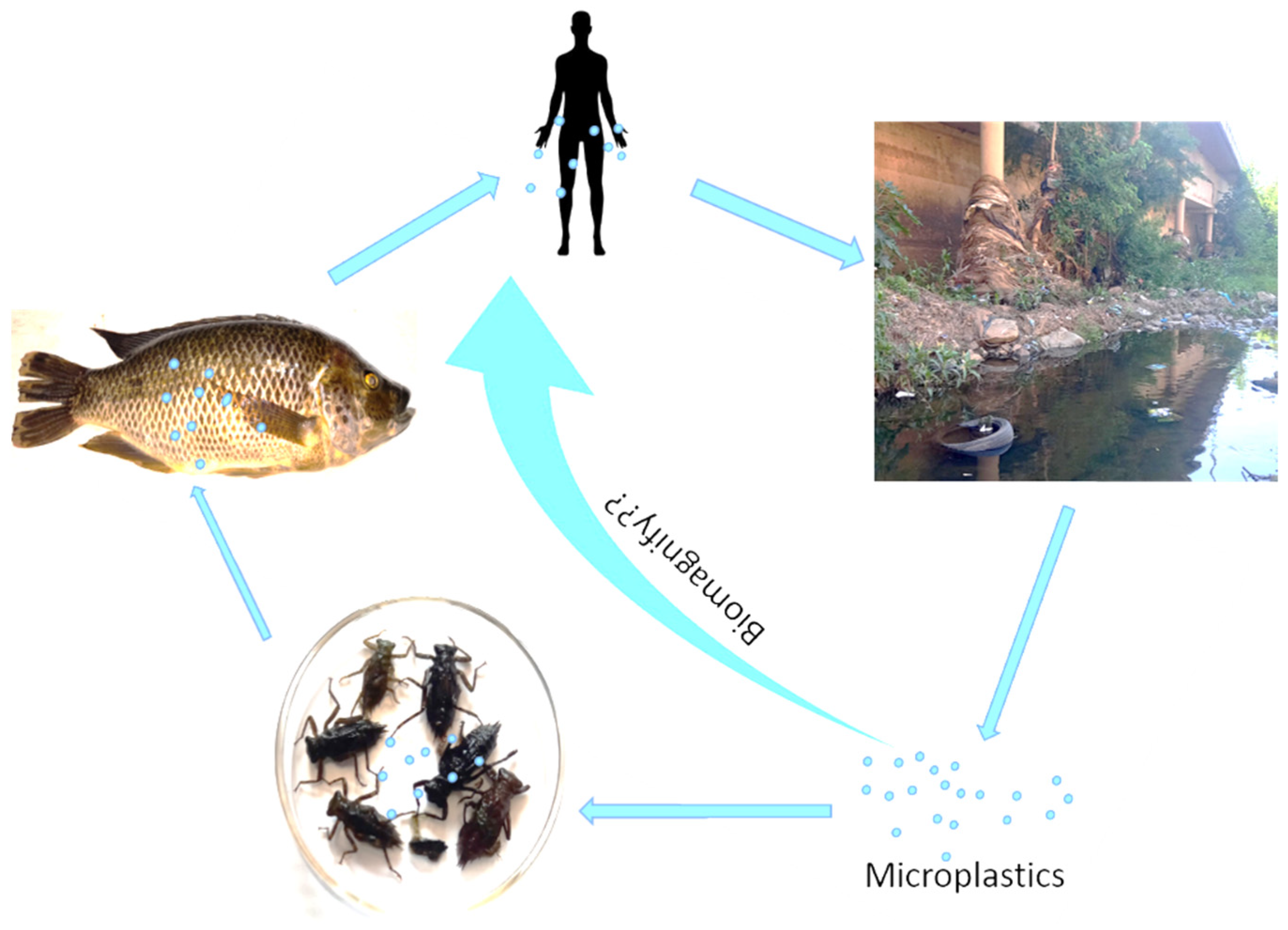
5. Microplastic Interaction with Microbial Communities
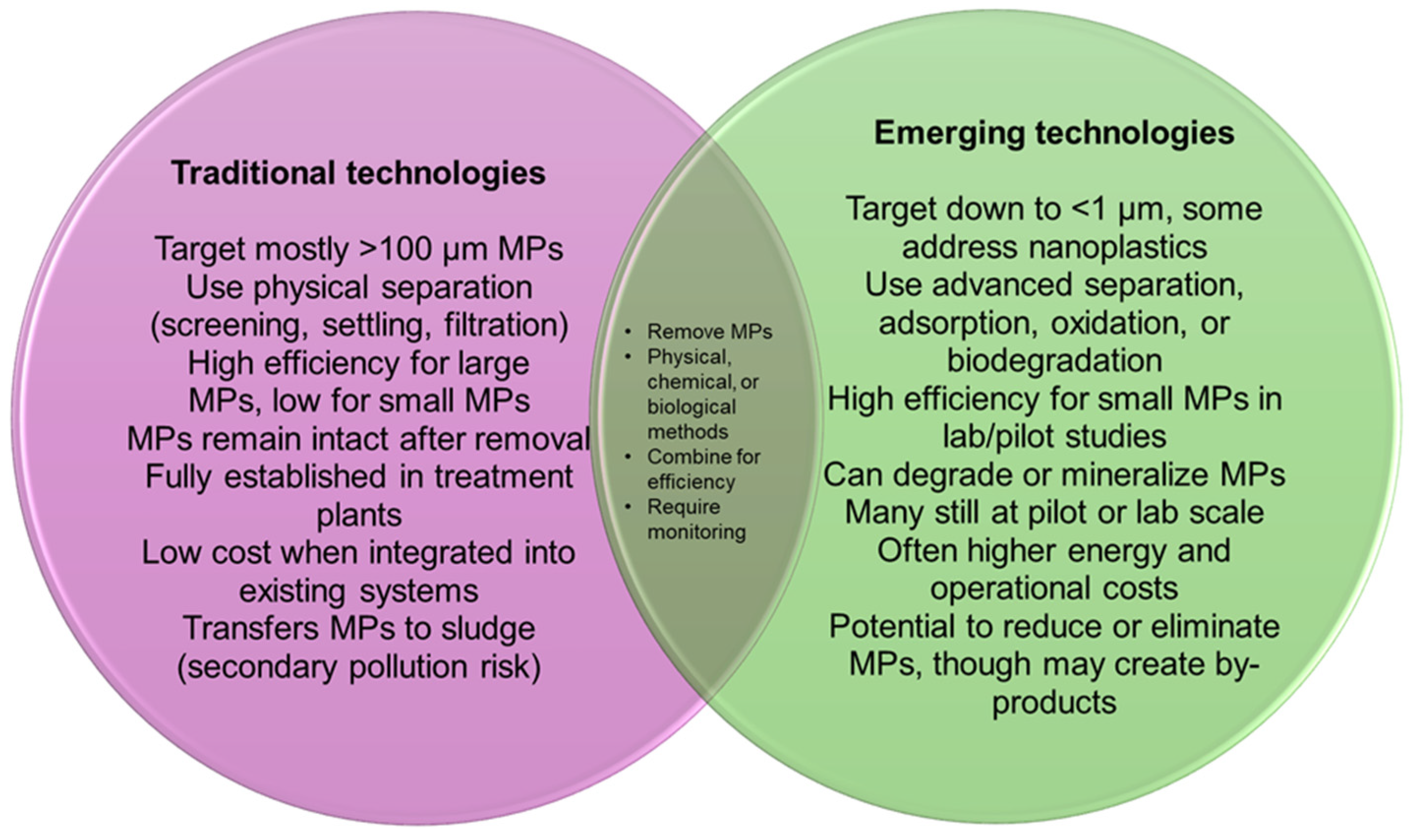
6. Control and Management of Microplastics in Freshwaters
6.1. Bioremediation of Microplastic Pollution
6.2. Nanotechnology-Based Solutions for Microplastic Removal
6.2.1. MXene
6.2.2. Zeolites
6.2.3. Carbon Nanomaterials
6.2.4. Metals and Metal Oxides
7. Innovative Technologies to Reduce Microplastics’ Release into the Environment
7.1. Plastic-to-Fuel Technologies
7.2. Use of Biodegradable Plastics
Biodegradation of Microplastics in Freshwater Environments
7.3. Use of Recyclable Products
8. Conclusions and Way Forward
- ➢
- The lack of globally standardised techniques, which can enhance the comparability of results between different regions;
- ➢
- The lack of guidelines for microplastic load in different environmental compartments;
- ➢
- Poor enforcement of legislation or policies regarding microplastic pollution, especially in low- and middle-income countries.
- ➢
- The dynamics governing the leaching of plasticisers and their effect on freshwater ecosystems;
- ➢
- The main and combined effect of plasticiser and metals, as well as physical variables influencing toxicity;
- ➢
- The integration of AI into field-based microplastic assessments;
- ➢
- The potential for microplastics to biomagnify along the food web;
- ➢
- Spatio-temporal dynamics in rivers, wetlands, and pans to allow for modelling and prediction through different exposure routes;
- ➢
- The use of mesocosm experimental systems for cause-and-effect studies to enhance the comparability of findings to those of a natural/field environment;
- ➢
- Microplastic partitioning between different matrices in aquatic environments;
- ➢
- Technological advancements to enhance the resilience of filtration systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, X.; Zhang, X.-H.; Yu, M. Microbial colonization and degradation of marine microplastics in the plastisphere: A review. Front. Microbiol. 2023, 14, 1127308. [Google Scholar] [CrossRef]
- Burgess, R.M.; Ho, K.T.; Mallos, N.J.; Leonard, G.H.; Hidalgo-Ruz, V.; Cook, A.M.; Christman, K. Microplastics in the aquatic environment—Perspectives on the scope of the problem. Environ. Toxicol. Chem. 2017, 36, 2259–2265. [Google Scholar] [CrossRef]
- Sipe, J.M.; Bossa, N.; Berger, W.; von Windheim, N.; Gall, K.; Wiesner, M.R. From bottle to microplastics: Can we estimate how our plastic products are breaking down? Sci. Total Environ. 2022, 814, 152460. [Google Scholar] [CrossRef] [PubMed]
- Castro-Castellon, A.T.; Horton, A.A.; Hughes, J.M.; Rampley, C.; Jeffers, E.S.; Bussi, G.; Whitehead, P. Ecotoxicity of microplastics to freshwater biota: Considering exposure and hazard across trophic levels. Sci. Total Environ. 2022, 816, 151638. [Google Scholar] [CrossRef]
- Khaki, Q.Z.; Kumar, P. Ecological Impacts of Microplastics and Their Additives: Exposure Risk/Toxicity Assessment and Fate/Transport of Persistent, Bio-Accumulative and Toxic Substances. In Microplastics in the Environment: Fate, Impacts, Removal, and Management; Wiley Online Library: Hoboken, NJ, USA, 2025; pp. 259–282. [Google Scholar]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef]
- Topcu, A.; Turgut, İ.M. Microplastics: Large-scale problems from small particles. Agric. For. Aquac. Sci. 2024, 18, 263. [Google Scholar]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms—An overview of their environmental implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079. [Google Scholar] [CrossRef]
- Araújo, A.P.d.C.; Malafaia, G. Can short exposure to polyethylene microplastics change tadpoles’ behavior? A study conducted with neotropical tadpole species belonging to the order anura (Physalaemus cuvieri). J. Hazard. Mater. 2020, 391, 122214. [Google Scholar] [CrossRef] [PubMed]
- Preda, O.-T.; Vlasceanu, A.-M.; Andreescu, C.V.; Tsatsakis, A.; Mezhuev, Y.; Negrei, C.; Baconi, D.L. Health Implications of Widespread Micro-and Nanoplastic Exposure: Environmental Prevalence, Mechanisms, and Biological Impact on Humans. Toxics 2024, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Rossi, M.; Vergara, A.; Troisi, R.; Alberico, M.; Carraturo, F.; Salamone, M.; Giordano, S.; Capozzi, F.; Spagnuolo, V.; de Magistris, F.A. Microplastics, microfibers and associated microbiota biofilm analysis in seawater, a case study from the Vesuvian Coast, southern Italy. J. Hazard. Mater. 2025, 488, 137468. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Wang, T.; Chen, Q.; Ji, R. Microplastics as vectors of chemicals and microorganisms in the environment. In Particulate Plastics in Terrestrial and Aquatic Environments; CRC Press: Boca Raton, FL, USA, 2020; pp. 209–230. [Google Scholar]
- Di Pippo, F.; Crognale, S.; Levantesi, C.; Vitanza, L.; Sighicelli, M.; Pietrelli, L.; Di Vito, S.; Amalfitano, S.; Rossetti, S. Plastisphere in lake waters: Microbial diversity, biofilm structure, and potential implications for freshwater ecosystems. Environ. Pollut. 2022, 310, 119876. [Google Scholar] [CrossRef]
- Lai, K.P.; Tsang, C.F.; Li, L.; Yu, R.M.K.; Kong, R.Y.C. Microplastics act as a carrier for wastewater-borne pathogenic bacteria in sewage. Chemosphere 2022, 301, 134692. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, Q.; Zhao, S.; Zhao, P.; Yang, X.; Huang, Q.; Su, J. Watershed urbanization enhances the enrichment of pathogenic bacteria and antibiotic resistance genes on microplastics in the water environment. Environ. Pollut. 2022, 313, 120185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, X.; Guo, J.; Jia, W.; Wang, Q.; Zhang, M.; Huang, Y. Evidence of selective enrichment of bacterial assemblages and antibiotic resistant genes by microplastics in urban rivers. Water Res. 2020, 183, 116113. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Sabatino, R.; Sathicq, M.B.; Eckert, E.M.; Fontaneto, D.; Dalla Fontana, G.; Mossotti, R.; Corno, G.; Volta, P.; Di Cesare, A. Contribution of microplastic particles to the spread of resistances and pathogenic bacteria in treated wastewaters. Water Res. 2021, 201, 117368. [Google Scholar] [CrossRef]
- Chen, X.; Tao, G.; Wang, Y.; Wei, W.; Lian, X.; Shi, Y.; Chen, S.; Sun, Y. Interactive impacts of microplastics and chlorine on biological stability and microbial community formation in stagnant water. Water Res. 2022, 221, 118734. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Jeyasanta, K.I.; Sathish, N.; Patterson, J.; Edward, J.K.P. Macro-, meso- and microplastic debris in the beaches of Tuticorin district, Southeast coast of India. Mar. Pollut. Bull. 2020, 154, 111055. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Samandra, S.; Singh, J.; Plaisted, K.; Mescall, O.J.; Symons, B.; Xie, S.; Ellis, A.V.; Clarke, B.O. Quantifying environmental emissions of microplastics from urban rivers in Melbourne, Australia. Mar. Pollut. Bull. 2023, 189, 114709. [Google Scholar] [CrossRef]
- Blankson, E.R.; Tetteh, P.N.; Oppong, P.; Gbogbo, F. Microplastics prevalence in water, sediment and two economically important species of fish in an urban riverine system in Ghana. PLoS ONE 2022, 17, e0263196. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, H.; Wang, J.; Su, L.; Wang, X.; Zhu, J.; Lan, W. The distribution of microplastics in water, sediment, and fish of the Dafeng River, a remote river in China. Ecotoxicol. Environ. Saf. 2021, 228, 113009. [Google Scholar] [CrossRef] [PubMed]
- Mutshekwa, T.; Motitsoe, S.N.; Naidoo, T.; Majingo, Z.; Mlambo, M.C. Plastics underground: Microplastic pollution in South African freshwater caves and associated biota. Hydrobiologia 2025, 1–19. [Google Scholar] [CrossRef]
- Balestra, V.; Vigna, B.; De Costanzo, S.; Bellopede, R. Preliminary investigations of microplastic pollution in karst systems, from surface watercourses to cave waters. J. Contam. Hydrol. 2023, 252, 104117. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Kutlu, B.; Özcan, T.; Büyükdeveci, F.; Blettler, M.C. Microplastic pollution in two remote rivers of Türkiye. Environ. Monit. Assess. 2023, 195, 791. [Google Scholar] [CrossRef]
- Townsend, K.R.; Lu, H.C.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Ramaremisa, G.; Ndlovu, M.; Saad, D. Comparative Assessment of Microplastics in Surface Waters and Sediments of the Vaal River, South Africa: Abundance, Composition, and Sources. Environ. Toxicol. Chem. 2022, 41, 3029–3040. [Google Scholar] [CrossRef]
- Graham, P.; Pattinson, N.; Bakir, A.; McGoran, A.; Nel, H. Determination of microplastics in sediment, water, and fish across the Orange-Senqu River basin. Water Res. 2024, 266, 122394. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, W.; Hu, Y.; Zhang, J.; Shen, W. Horizontal and vertical distribution of microplastics in dam reservoir after impoundment. Sci. Total Environ. 2022, 832, 154962. [Google Scholar] [CrossRef]
- Shen, J.; Gu, X.; Liu, R.; Feng, H.; Li, D.; Liu, Y.; Jiang, X.; Qin, G.; An, S.; Li, N.; et al. Damming has changed the migration process of microplastics and increased the pollution risk in the reservoirs in the Shaying River Basin. J. Hazard. Mater. 2023, 443, 130067. [Google Scholar] [CrossRef]
- Pojar, I.; Dobre, O.; Lazăr, C.; Baboș, T.; Ristea, O.; Constantin, A.; Cristoiu, N. Microplastic Evaluation in Water and Sediments of a Dam Reservoir–Riverine System in the Eastern Carpathians, Romania. Sustainability 2024, 16, 4541. [Google Scholar] [CrossRef]
- Varg, J.E.; Outomuro, D.; Kunce, W.; Kuehrer, L.; Svanbäck, R.; Johansson, F. Microplastic exposure across trophic levels: Effects on the host–microbiota of freshwater organisms. Environ. Microbiome 2022, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, Y.; Song, K.; Du, W.; Huang, W.; Gu, Z.; Feng, Z. Microplastics in different tissues of wild crabs at three important fishing grounds in China. Chemosphere 2021, 271, 129479. [Google Scholar] [CrossRef]
- Shetu, M.H.; Parvin, F.; Tareq, S.M. Identifying the presence of microplastics in frogs from the largest delta of the world. Environ. Adv. 2023, 11, 100355. [Google Scholar] [CrossRef]
- Dahms, H.T.; Tweddle, G.P.; Greenfield, R. Gastric microplastics in Clarias gariepinus of the upper Vaal River, South Africa. Front. Environ. Sci. 2022, 10, 931073. [Google Scholar] [CrossRef]
- Saad, D.; Chauke, P.; Cukrowska, E.; Richards, H.; Nikiema, J.; Chimuka, L.; Tutu, H. First biomonitoring of microplastic pollution in the Vaal river using Carp fish (Cyprinus carpio) “as a bio-indicator”. Sci. Total Environ. 2022, 836, 155623. [Google Scholar] [CrossRef]
- Queiroz, L.G.; de Oliveira Hallai, L.; Rocha de Moraes, B.; Ando, R.A.; Pompêo, M.; Rani-Borges, B. A straightforward protocol for extracting microplastics from freshwater sediment with high organic content. Knowl. Manag. Aquat. Ecosyst. 2025, 426, 6. [Google Scholar] [CrossRef]
- Zhao, P.; ShafieiDarabi, M.; Wang, X.; Slowinski, S.; Li, S.; Abbasi, Z.; Rezanezhad, F.; Van Cappellen, P.; Ren, C.L. Detection of microplastics by microfluidic microwave sensing: An exploratory study. Sens. Actuators A Phys. 2025, 383, 116154. [Google Scholar] [CrossRef]
- Wen, H.; Zhao, Y.; Shi, T.; Li, M.; Li, T.; Xu, Y.; Jia, H.; Zhu, W.; Han, L.; Yan, S.; et al. Microfluidic Microwave Sensor for Rapid Detection of Microplastics in Water: Optimization, Modeling, and Performance Evaluation. IEEE Sens. J. 2024, 24, 35599–35609. [Google Scholar] [CrossRef]
- Barrancos, A.; Macedo, V.; Ramos, P.M.; Rosado, L.S. Microplastics detection with microfluidics integrated with a microwave sensor. Meas. Sens. 2025, 38, 101435. [Google Scholar] [CrossRef]
- Alvarado-Zambrano, D.; Rivera-Hernández, J.R.; Green-Ruiz, C. First insight into microplastic groundwater pollution in Latin America: The case of a coastal aquifer in Northwest Mexico. Environ. Sci. Pollut. Res. 2023, 30, 73600–73611. [Google Scholar] [CrossRef]
- Saad, D.; Ndlovu, M.; Ramaremisa, G.; Tutu, H. Microplastics in freshwater environment: The first evaluation in sediment of the Vaal River, South Africa. Heliyon 2022, 8, e11118. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chèvre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 2021, 403, 124055. [Google Scholar] [CrossRef]
- Azari, A.; Ronsmans, S.; Vanoirbeek, J.A.J.; Hoet, P.H.M.; Ghosh, M. Challenges in Raman spectroscopy of (micro)Plastics: The interfering role of colourants. Environ. Pollut. 2024, 363, 125250. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sui, M.; Wang, T.; Teng, X.; Sun, J.; Chen, M. Detection and quantification of various microplastics in human endometrium based on laser direct infrared spectroscopy. Sci. Total Environ. 2024, 906, 167760. [Google Scholar] [CrossRef]
- Nizamali, J.; Mintenig, S.M.; Koelmans, A.A. Assessing microplastic characteristics in bottled drinking water and air deposition samples using laser direct infrared imaging. J. Hazard. Mater. 2023, 441, 129942. [Google Scholar] [CrossRef]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Guidelines or the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; Kershaw, P.J., Turra, A., Galgani, F., Eds.; (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Rep. Stud. GESAMP No. 99; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2019; 130p, Available online: http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean (accessed on 12 July 2025).
- Cowger, W.; Booth, A.M.; Hamilton, B.M.; Thaysen, C.; Primpke, S.; Munno, K.; Lusher, A.L.; Dehaut, A.; Vaz, V.P.; Liboiron, M.; et al. Reporting Guidelines to Increase the Reproducibility and Comparability of Research on Microplastics. Appl. Spectrosc. 2020, 74, 1066–1077. [Google Scholar] [CrossRef]
- Tran, H.-T.; Hadi, M.; Nguyen, T.T.H.; Hoang, H.G.; Nguyen, M.-K.; Nguyen, K.N.; Vo, D.-V.N. Machine learning approaches for predicting microplastic pollution in peatland areas. Mar. Pollut. Bull. 2023, 194, 115417. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Balasubadra, K.; Vadivel, G.; Arunfred, N.; Ishwarya, M.; Murugan, S. IoT-Driven Image Recognition for Microplastic Analysis in Water Systems using Convolutional Neural Networks. In Proceedings of the 2024 2nd International Conference on Computer, Communication and Control (IC4), Indore, India, 8–10 February 2024; pp. 1–6. [Google Scholar]
- Guo, P.; Wang, Y.; Moghaddamfard, P.; Meng, W.; Wu, S.; Bao, Y. Artificial intelligence-empowered collection and characterization of microplastics: A review. J. Hazard. Mater. 2024, 8, 134405. [Google Scholar] [CrossRef]
- Khanam, M.M.; Uddin, M.K.; Kazi, J.U. Advances in machine learning for the detection and characterization of microplastics in the environment. Front. Environ. Sci. 2025, 13, 1573579. [Google Scholar] [CrossRef]
- Fazil, A.Z.; Gomes, P.I.A.; Sandamal, R.M.K. Applicability of machine learning techniques to analyze Microplastic transportation in open channels with different hydro-environmental factors. Environ. Pollut. 2024, 357, 124389. [Google Scholar] [CrossRef]
- Sajan, R.I.; Manchu, M.; Felsy, C.; Joselin Kavitha, M. Microplastic predictive modelling with the integration of Artificial Neural Networks and Hidden Markov Models (ANN-HMM). J. Environ. Health Sci. Eng. 2024, 22, 579–592. [Google Scholar] [CrossRef]
- Sharma, E.; Surendra, K.C.; Thanh, D.T.; Koottatep, T. A geospatial investigation of microplastics leaching in Ubon Ratchathani province, Thailand: Fuzzy logic-based analysis. Environ. Monit. Assess. 2025, 197, 821. [Google Scholar] [CrossRef]
- Tajwar, M.; Yousuf Gazi, M.; Saha, S.K. Characterization and spatial abundance of microplastics in the coastal regions of Cox’s Bazar, Bangladesh: An integration of field, laboratory, and GIS techniques. Soil Sediment Contam. Int. J. 2022, 31, 57–80. [Google Scholar] [CrossRef]
- El-Alfy, M.A.; El-Hamid, H.T.A.; Keshta, A.E.; Elnaggar, A.A.; Darwish, D.H.; Basiony, A.I.; Alzeny, A.M.; Abou-Hadied, M.M.; Toubar, M.M.; Shalby, A.; et al. Assessing microplastic pollution vulnerability in a protected coastal lagoon in the Mediterranean Coast of Egypt using GIS modeling. Sci. Rep. 2025, 15, 11557. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.U.; Sofiany, I.R.; Fashani, Y.; Al Naday, Q.; Sulistiowati. Application of Geographic Information System (GIS) and Remote Sensing (RS) in Microplastic Studies Around Asia. In Microplastics in African and Asian Environments: The Influencers, Challenges, and Solutions; Springer: Berlin/Heidelberg, Germany, 2024; pp. 581–599. [Google Scholar]
- Gulizia, A.M.; Patel, K.; Philippa, B.; Motti, C.A.; van Herwerden, L.; Vamvounis, G. Understanding plasticiser leaching from polystyrene microplastics. Sci. Total Environ. 2023, 857, 159099. [Google Scholar] [CrossRef]
- Sendra, M.; Pereiro, P.; Figueras, A.; Novoa, B. An integrative toxicogenomic analysis of plastic additives. J. Hazard. Mater. 2021, 409, 124975. [Google Scholar] [CrossRef]
- Keshu; Rani, M.; Shanker, U. Recent Advances in Photocatalytic Degradation of Plastics and Plastic-Based Chemicals. In Plastic Degradation and Conversion by Photocatalysis (Volume 1): A Sustainable Approach; ACS Publications: Washington, DC, USA, 2024; pp. 163–183. [Google Scholar]
- Andjelković, T.; Bogdanović, D.; Kostić, I.; Kocić, G.; Nikolić, G.; Pavlović, R. Phthalates leaching from plastic food and pharmaceutical contact materials by FTIR and GC-MS. Environ. Sci. Pollut. Res. 2021, 28, 31380–31390. [Google Scholar] [CrossRef]
- Do, A.T.N.; Ha, Y.; Kwon, J.-H. Leaching of microplastic-associated additives in aquatic environments: A critical review. Environ. Pollut. 2022, 305, 119258. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Wuethrich, T.; Mayer, P.; Escher, B.I. Development of a dynamic delivery method for in vitro bioassays. Chemosphere 2009, 76, 83–90. [Google Scholar] [CrossRef]
- Gwenzi, W. Emerging Contaminants in the Terrestrial-Aquatic-Atmosphere Continuum: Occurrence, Health Risks and Mitigation; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Zink, L.; Wood, C.M. The effects of microplastics on ionoregulatory processes in the gills of freshwater fish and invertebrates: A prospective review. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 295, 111669. [Google Scholar] [CrossRef] [PubMed]
- Mbugani, J.J.; Machiwa, J.F.; Shilla, D.A.; Kimaro, W.; Joseph, D.; Khan, F.R. Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration. Microplastics 2022, 1, 240–253. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Schwarz, A.; Gomez-Mestre, I.; Meyer, R.; Domscheit, M.; Bartels, F.; Schaeffer, S.-M.; Engelkes, K. Life in plastic, it’s not fantastic: Sublethal effects of polyethylene microplastics ingestion throughout amphibian metamorphosis. Sci. Total Environ. 2023, 885, 163779. [Google Scholar] [CrossRef]
- Gupta, P.; Mahapatra, A.; Suman, A.; Ray, S.S.; Malafaia, G.; Singh, R.K. Polystyrene microplastics disrupt female reproductive health and fertility via sirt1 modulation in zebrafish (Danio rerio). J. Hazard. Mater. 2023, 460, 132359. [Google Scholar] [CrossRef]
- Fu, L.; Xi, M.; Nicholaus, R.; Wang, Z.; Wang, X.; Kong, F.; Yu, Z. Behaviors and biochemical responses of macroinvertebrate Corbicula fluminea to polystyrene microplastics. Sci. Total Environ. 2022, 813, 152617. [Google Scholar] [CrossRef]
- Zang, H.; Zhao, C.; Cai, R.; Wu, H.; Wei, L.; Zhou, C.; Chai, J.; Teng, X.; Liu, T. Vital role of oxidative stress in tadpole liver damage caused by polystyrene nanoparticles. Ecotoxicol. Environ. Saf. 2024, 277, 116331. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Zhang, C.; Wang, Y.; Yang, F.; Zhao, Y.; Jiang, Y. Microplastics shift macrobenthic community structures near a coastal nuclear power plant under construction in North East China. J. Hazard. Mater. 2022, 437, 129335. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.M.; Machado, A.L.; Campos, D.; Rodrigues, A.C.M.; Patrício Silva, A.L.; Soares, A.M.V.M.; Pestana, J.L.T. Microplastics in freshwater sediments: Effects on benthic invertebrate communities and ecosystem functioning assessed in artificial streams. Sci. Total Environ. 2022, 804, 150118. [Google Scholar] [CrossRef]
- Ceschin, S.; Mariani, F.; Di Lernia, D.; Venditti, I.; Pelella, E.; Iannelli, M.A. Effects of Microplastic Contamination on the Aquatic Plant Lemna minuta (Least Duckweed). Plants 2023, 12, 207. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Cao, X.; Wang, Y.; Zhang, Z.; Xu, Y.; Qi, W. Effects of microplastics and glyphosate on growth rate, morphological plasticity, photosynthesis, and oxidative stress in the aquatic species Salvinia cucullata. Environ. Pollut. 2021, 279, 116900. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, P.; Zhang, X.; Zhang, Y.; Xie, S.; Deng, J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Pourmoshtagh, H.; Sabour, S.; Hadi, N.; Azimi, T.; Soleiman-Meigooni, S. Biofilm formation in mycobacterial genus; mechanism of biofilm formation and anti-mycobacterial biofilm agents. Curr. Pharm. Biotechnol. 2025, 26, 982–991. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Onda, D.F.L.; Sharief, K.M. Identification of microorganisms related to microplastics. In Handbook of Microplastics in the Environment; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–34. [Google Scholar]
- Deo, R.; Lakra, U.; Ojha, M.; Nigam, V.K.; Sharma, S.R. Exopolysaccharides in microbial interactions: Signalling, quorum sensing, and community dynamics. Nat. Prod. Res. 2024, 39, 3224–3239. [Google Scholar] [CrossRef]
- Singh, A.; Lalbiaktluangi, C.; Zomuansangi, R.; Srivastava, S.; Yadav, M.K.; Gupta, A.K. Cell-to-cell interaction and cell signaling in biofilm formation. In Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2024; pp. 177–214. [Google Scholar]
- Hashem, Z.S. Bacterial Metabolites in Defense: A Crucial Aspect of Microbial Interaction and Host Protection. In Metabolic Dynamics in Host-Microbe Interaction; Springer: Berlin/Heidelberg, Germany, 2025; pp. 101–120. [Google Scholar]
- Duraisamy, S.; Balakrishnan, S.; Ranjith, S.; Husain, F.; Sathyan, A.; Peter, A.S.; Prahalathan, C.; Kumarasamy, A. Bacteriocin—A potential antimicrobial peptide towards disrupting and preventing biofilm formation in the clinical and environmental locales. Environ. Sci. Pollut. Res. 2020, 27, 44922–44936. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Gonçalves, A.L.; Canhoto, C. Salinization effects on stream biofilm functioning. Hydrobiologia 2020, 847, 1453–1459. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Khan, M.T.; Hossain, M.F.; Bilal, M.; Ali Shah, I. Eco-Friendly Solutions to Emerging Contaminants: Unveiling the Potential of Bioremediation in Tackling Microplastic Pollution in Water. Adv. Sustain. Syst. 2024, 8, 2400172. [Google Scholar] [CrossRef]
- Rani, R.; Malik, S.; Kumar, D.; Kumar, R.; Mukherjee, S.; Saharan, B.S.; Duhan, J.S. Advances in the role of microorganisms, waste management strategies and policies on microplastic abatement in the era of bio-circular economy. Sustain. Chem. Pharm. 2024, 39, 101595. [Google Scholar] [CrossRef]
- Ferreira, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Nanoplastics and marine organisms: What has been studied? Environ. Toxicol. Pharmacol. 2019, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khoiriyah, S.; Syaputra, M.D. Bioremediation to Overcome Microplastic Contamination in The Water Environment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; p. 012027. [Google Scholar]
- Taipale, S.; Rigaud, C.; Calderini, M.; Kainz, M.; Pilecky, M.; Uusi-Heikkilä, S.; Vesamäki, J.; Vuorio, K.; Tiirola, M. The second life of terrestrial and plastic carbon as nutritionally valuable food for aquatic consumers. Ecol. Lett. 2023, 26, 1336–1347. [Google Scholar] [CrossRef]
- Payanthoth, N.S.; Mut, N.N.N.; Samanta, P.; Li, G.; Jung, J. A review of biodegradation and formation of biodegradable microplastics in soil and freshwater environments. Appl. Biol. Chem. 2024, 67, 110. [Google Scholar] [CrossRef]
- El-Kurdi, N.; El-Shatoury, S.; ElBaghdady, K.; Hammad, S.; Ghazy, M. Biodegradation of polystyrene nanoplastics by Achromobacter xylosoxidans M9 offers a mealworm gut-derived solution for plastic pollution. Arch. Microbiol. 2024, 206, 238. [Google Scholar] [CrossRef]
- Pratap, J.K.; Krishnan, K. Microbial Nanobioremediation of Micro-Nanoplastics: Current Strategies, Challenges, and Future Prospects. In Management of Micro and Nano-plastics in Soil and Biosolids: Fate, Occurrence, Monitoring, and Remedies; Bhat, S.A., Kumar, V., Li, F., Kumar, S., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 419–445. [Google Scholar]
- Miao, L.; Guo, S.; Liu, Z.; Liu, S.; You, G.; Qu, H.; Hou, J. Effects of Nanoplastics on Freshwater Biofilm Microbial Metabolic Functions as Determined by BIOLOG ECO Microplates. Int. J. Environ. Res. Public Health 2019, 16, 4639. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Taniguchi, I.; Oda, K. Ideonella sakaiensis, PETase, and MHETase: From identification of microbial PET degradation to enzyme characterization. In Methods Enzymol.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 648, pp. 187–205. [Google Scholar]
- Roberts, C.; Edwards, S.; Vague, M.; León-Zayas, R.; Scheffer, H.; Chan, G.; Swartz, N.A.; Mellies, J.L. Environmental consortium containing Pseudomonas and Bacillus species synergistically degrades polyethylene terephthalate plastic. Msphere 2020, 5, e01151-20. [Google Scholar] [CrossRef]
- Liu, X.; Dong, X.; Wang, D.; Xie, Z. Biodeterioration of polyethylene by Bacillus cereus and Rhodococcus equi isolated from soil. Int. Microbiol. 2024, 27, 1795–1806. [Google Scholar] [CrossRef]
- Caruso, G. Plastic degrading microorganisms as a tool for bioremediation of plastic contamination in aquatic environments. J. Pollut. Eff. Cont. 2015, 3, 3. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Bharti, D.; Singh, S.; Banoo, R.; Bundela, V.; Nain, P.; Sahgal, M. Genetically engineered plastic munching microbes: Recent advancements and perspectives. In Advanced Strategies for Biodegradation of Plastic Polymers; Springer: Berlin/Heidelberg, Germany, 2024; pp. 193–224. [Google Scholar]
- Brott, S.; Pfaff, L.; Schuricht, J.; Schwarz, J.N.; Böttcher, D.; Badenhorst, C.P.; Wei, R.; Bornscheuer, U.T. Engineering and evaluation of thermostable IsPETase variants for PET degradation. Eng. Life Sci. 2022, 22, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Goswami, L.; Singhvi, M.; Kim, B.S. Biodegradation of poly (Ethylene terephthalate): Mechanistic insights, advances, and future innovative strategies. Chem. Eng. J. 2023, 457, 141230. [Google Scholar] [CrossRef]
- Vojnovic, S.; Aleksic, I.; Ilic-Tomic, T.; Stevanovic, M.; Nikodinovic-Runic, J. Bacillus and Streptomyces spp. as hosts for production of industrially relevant enzymes. Appl. Microbiol. Biotechnol. 2024, 108, 185. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2022, 297, 118773. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.-L.; Guan, Y.; Zhu, C.; Gong, G.; Hu, Y. Novel RO membranes fabricated by grafting sulfonamide group: Improving water permeability, fouling resistance and chlorine resistant performance. J. Membr. Sci. 2022, 641, 119919. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecinska-Mydlak, A. Application of nanotechnology and nanomaterials in water and wastewater treatment: Membranes, photocatalysis and disinfection. Desalination Water Treat. 2020, 186, 88–106. [Google Scholar] [CrossRef]
- Li, Y.; Lan, S.; Zhu, T. Recent advances of graphene-based sorptive materials in extraction: A review. TrAC Trends Anal. Chem. 2021, 142, 116319. [Google Scholar] [CrossRef]
- Khalil, H.A.; Dungani, R.; Hossain, M.; Suraya, N.; Aprilia, S.; Astimar, A.; Hayawin, Z.N.; Davoudpour, Y. Mechanical properties of oil palm biocomposites enhanced with micro to nanobiofillers. In Biocomposites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 401–435. [Google Scholar]
- Vijayshanthy, S.; Priyanka, E.B.; Thangavel, S.; Anand, R.; Bhavana, G.B.; Khan, B.; Jeyanthi, K. Performance of polyvinyl alcohol graphene oxide membrane for microplastic removal in wastewater with an IoT based monitoring approach. Sci. Rep. 2025, 15, 20774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y. Integrated forward osmosis-adsorption process for strontium-containing water treatment: Pre-concentration and solidification. J. Hazard. Mater. 2021, 414, 125518. [Google Scholar] [CrossRef]
- Tchalala, M.; Bhatt, P.; Chappanda, K.; Tavares, S.; Adil, K.; Belmabkhout, Y.; Shkurenko, A.; Cadiau, A.; Heymans, N.; De Weireld, G. Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat. Commun. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Shahid, K.; Srivastava, V.; Sillanpää, M. Protein recovery as a resource from waste specifically via membrane technology—From waste to wonder. Environ. Sci. Pollut. Res. 2021, 28, 10262–10282. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. In MXenes; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2023; pp. 677–722. [Google Scholar]
- Yang, K.; White, C.E. Multiscale pore structure determination of cement paste via simulation and experiment: The case of alkali-activated metakaolin. Cem. Concr. Res. 2020, 137, 106212. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Sagadevan, S.; Johan, R.B.; Shah, S.T.; Adebesi, A.; Md, S.I.; Rafique, R.F. A review on electrochemically modified carbon nanotubes (CNTs) membrane for desalination and purification of water. Mater. Res. Express 2018, 5, 102001. [Google Scholar] [CrossRef]
- Adewuyi, A.; Campbell, A.J.; Adeyemi, O.G. The potential role of membrane technology in the removal of microplastics from wastewater. J. Appl. Membr. Sci. Technol. 2021, 25, 31–53. [Google Scholar] [CrossRef]
- Xuen, L.R.; Isa, N.; Razak, K.A.; Jaafar, M.; Lockman, Z. Silver Nanoparticles/Titanium Dioxide Nanowires Photocatalyst Formation for Microplastic Removal Using Ultraviolet Radiation. Solid State Phenom. 2023, 352, 67–74. [Google Scholar] [CrossRef]
- Bodzek, M.; Bodzek, P. Remediation of Micro- and Nanoplastics by Membrane Technologies. Membranes 2025, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Evaluation of membranes performance for microplastic removal in a simple and low-cost filtration system. Case Stud. Chem. Environ. Eng. 2021, 3, 100075. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Kudlek, E.; Juszczyk, J.; Moraczewska-Majkut, K.; Pieczykolan, B.; Nocoń, W.K. Analysis of membrane surface after the filtration of surface water containing microplastic. Desalination Water Treat. 2025, 322, 101139. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Golgoli, M.; Khiadani, M.; Razmjou, A.; Zargar, M. Microplastics fouling mitigation in forward osmosis membranes by the molecular assembly of sulfobetaine zwitterion. Desalination 2024, 575, 117300. [Google Scholar] [CrossRef]
- Colakoglu, E.B.; Uyanik, I.; Elbir, H.; Sahinkaya, E.; Yurtsever, A. A novel gravity-driven dynamic membrane filtration reactor for microplastic removal from plastic recycling facility wastewater. J. Environ. Chem. Eng. 2025, 13, 115793. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Yang, L.; Cao, X.; Cui, J.; Wang, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, J.; Li, A. Holey Ti3C2 nanosheets based membranes for efficient separation and removal of microplastics from water. J. Colloid Interface Sci. 2022, 617, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Yu, S.; Wang, J. Degradation of pyridine and quinoline in aqueous solution by gamma radiation. Radiat. Phys. Chem. 2018, 144, 322–328. [Google Scholar] [CrossRef]
- Babalar, M.; Siddiqua, S.; Sakr, M.A. A novel polymer coated magnetic activated biochar-zeolite composite for adsorption of polystyrene microplastics: Synthesis, characterization, adsorption and regeneration performance. Sep. Purif. Technol. 2024, 331, 125582. [Google Scholar] [CrossRef]
- SPONZA, D.; Öztekin, R. Zeolitic Imidazolate/Fe3O4 Nanocomposite for Removal of Polystyrene and 4-tert-butylphenol via Adsorption. WSEAS Trans. Environ. Dev. 2023, 19, 1071–1082. [Google Scholar] [CrossRef]
- Indrawirawan, S.; Sun, H.; Duan, X.; Wang, S. Nanocarbons in different structural dimensions (0–3D) for phenol adsorption and metal-free catalytic oxidation. Appl. Catal. B Environ. 2015, 179, 352–362. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Dey, T.K.; Jamal, M.; Uddin, M.E. Fabrication and performance analysis of graphene oxide-based composite membrane to separate microplastics from synthetic wastewater. J. Water Process Eng. 2023, 52, 103554. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska-Mydlak, A. Nanotechnology in water and wastewater treatment. Graphene–the nanomaterial for next generation of semipermeable membranes. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1515–1579. [Google Scholar] [CrossRef]
- Kamble, S.B.; Bhore, R.K. Versatile nanomaterials for remediation of microplastics from the environment. In Advances in Nano and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 107–126. [Google Scholar]
- Deline, A.R.; Frank, B.P.; Smith, C.L.; Sigmon, L.R.; Wallace, A.N.; Gallagher, M.J.; Goodwin Jr, D.G.; Durkin, D.P.; Fairbrother, D.H. Influence of oxygen-containing functional groups on the environmental properties, transformations, and toxicity of carbon nanotubes. Chem. Rev. 2020, 120, 11651–11697. [Google Scholar] [CrossRef]
- Sajid, M.; Asif, M.; Baig, N.; Kabeer, M.; Ihsanullah, I.; Mohammad, A.W. Carbon nanotubes-based adsorbents: Properties, functionalization, interaction mechanisms, and applications in water purification. J. Water Process Eng. 2022, 47, 102815. [Google Scholar] [CrossRef]
- Rozman, U.; Klun, B.; Marolt, G.; Imperl, J.; Kalčíková, G. A study of the adsorption of titanium dioxide and zinc oxide nanoparticles on polyethylene microplastics and their desorption in aquatic media. Sci. Total Environ. 2023, 888, 164163. [Google Scholar] [CrossRef]
- Tosco, T.; Papini, M.P.; Viggi, C.C.; Sethi, R. Nanoscale zerovalent iron particles for groundwater remediation: A review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Shamsuddin, A.S.; Othman, N.M.I.; Zakaria, N.H.; Mutalib, M.A.; Ismail, S.N.S.; Muhamad, N. Plastic waste-to-fuel and sustainable development goals. In Advances in Energy from Waste; Elsevier: Amsterdam, The Netherlands, 2024; pp. 525–550. [Google Scholar]
- Alam, S.S.; Husain Khan, A.; Khan, N.A. Plastic waste management via thermochemical conversion of plastics into fuel: A review. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 1–20. [Google Scholar] [CrossRef]
- Olatunji, O. Re-Envisioning Plastics Role in the Global Society; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Nanda, S.; Sarker, T.R.; Kang, K.; Li, D.; Dalai, A.K. Perspectives on thermochemical recycling of end-of-life plastic wastes to alternative fuels. Materials 2023, 16, 4563. [Google Scholar] [CrossRef] [PubMed]
- Acaroğlu, H.; Güllü, M.; Sivri, N.; Marquez, F.P.G. How can there be an economic transition to a green ecosystem by adapting plastic-to-fuel technologies through renewable energy? Sustain. Energy Technol. Assess. 2024, 64, 103691. [Google Scholar] [CrossRef]
- Tang, L.; Su, C.; Chen, Y.; Xian, Y.; Hui, X.; Ye, Z.; Chen, M.; Zhu, F.; Zhong, H. Influence of biodegradable polybutylene succinate and non-biodegradable polyvinyl chloride microplastics on anammox sludge: Performance evaluation, suppression effect and metagenomic analysis. J. Hazard. Mater. 2021, 401, 123337. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.-W.; Huber, G.W. A review of biodegradable plastics: Chemistry, applications, properties, and future research needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef]
- Roohi; Srivastava, P.; Bano, K.; Zaheer, M.R.; Kuddus, M. Biodegradable smart biopolymers for food packaging: Sustainable approach toward green environment. In Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Springer: Singapore, 2018; pp. 197–216. [Google Scholar]
- Zhu, J.; Wang, C. Biodegradable plastics: Green hope or greenwashing? Mar. Pollut. Bull. 2020, 161, 111774. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar] [CrossRef]
- Guicherd, M.; Ben Khaled, M.; Guéroult, M.; Nomme, J.; Dalibey, M.; Grimaud, F.; Alvarez, P.; Kamionka, E.; Gavalda, S.; Noël, M.; et al. An engineered enzyme embedded into PLA to make self-biodegradable plastic. Nature 2024, 631, 884–890. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Hagelskjær, O.; Crézé, A.; Le Roux, G.; Sonke, J.E. Investigating the correlation between morphological features of microplastics (5–500 µm) and their analytical recovery. Microplast. Nanoplast. 2023, 3, 22. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Cucina, M.; de Nisi, P.; Tambone, F.; Adani, F. The role of waste management in reducing bioplastics’ leakage into the environment: A review. Bioresour. Technol. 2021, 337, 125459. [Google Scholar] [CrossRef] [PubMed]
- Eronen-Rasimus, E.L.; Nakki, P.P.; Kaartokallio, H.P. Degradation rates and bacterial community compositions vary among commonly used bioplastic materials in a brackish marine environment. Environ. Sci. Technol. 2022, 56, 15760–15769. [Google Scholar] [CrossRef]
- Nabeoka, R.; Suzuki, H.; Akasaka, Y.; Ando, N.; Yoshida, T. Evaluating the ready biodegradability of biodegradable plastics. Environ. Toxicol. Chem. 2021, 40, 2443–2449. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of so-called biodegradable polymers in seawater and freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Ribba, L.; Lopretti, M.; de Oca-Vasquez, G.M.; Batista, D.; Goyanes, S.; Vega-Baudrit, J.R. Biodegradable plastics in aquatic ecosystems: Latest findings, research gaps, and recommendations. Environ. Res. Lett. 2022, 17, 033003. [Google Scholar] [CrossRef]
- Volova, T.; Gladyshev, M.; Trusova, M.Y.; Zhila, N. Degradation of polyhydroxyalkanoates in eutrophic reservoir. Polym. Degrad. Stab. 2007, 92, 580–586. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Sridewi, N.; Bhubalan, K.; Sudesh, K. Degradation of commercially important polyhydroxyalkanoates in tropical mangrove ecosystem. Polym. Degrad. Stab. 2006, 91, 2931–2940. [Google Scholar] [CrossRef]
- Syahirah, W.N.; Azami, N.A.; Huong, K.-H.; Amirul, A. Preparation, characterization and biodegradation of blend films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with natural biopolymers. Polym. Bull. 2021, 78, 3973–3993. [Google Scholar] [CrossRef]
- Dey, S.; Veerendra, G.T.N.; Babu, P.S.S.A.; Manoj, A.V.P.; Nagarjuna, K. Degradation of Plastics Waste and Its Effects on Biological Ecosystems: A Scientific Analysis and Comprehensive Review. Biomed. Mater. Devices 2024, 2, 70–112. [Google Scholar] [CrossRef]
- Cicconi, P. Eco-design and Eco-materials: An interactive and collaborative approach. Sustain. Mater. Technol. 2020, 23, e00135. [Google Scholar] [CrossRef]
- Tang, X.; Hong, M.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E.Y.-X. The quest for converting biorenewable bifunctional α-methylene-γ-butyrolactone into degradable and recyclable polyester: Controlling vinyl-addition/ring-opening/cross-linking pathways. J. Am. Chem. Soc. 2016, 138, 14326–14337. [Google Scholar] [CrossRef] [PubMed]
- Westlie, A.H.; Quinn, E.C.; Parker, C.R.; Chen, E.Y.-X. Synthetic biodegradable polyhydroxyalkanoates (PHAs): Recent advances and future challenges. Prog. Polym. Sci. 2022, 134, 101608. [Google Scholar] [CrossRef]
- Tiwari, R. Effect of Polyglycolic Acid and Liquid Crystal Polymer on the Performance of Polyethylene Terephthalate Films; Michigan State University: East Lansing, MI, USA, 2024. [Google Scholar]
- Gonçalves, A.; Henriques, E.; Ribeiro, I. Towards plastics circular economy: Sustainability assessment of mono-material design for recycling. Procedia CIRP 2024, 122, 401–406. [Google Scholar] [CrossRef]
- Guerritore, M.; Olivieri, F.; Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M.E.; Galdi, M.R.; Carfagna, C.; Gentile, G. Recyclable-by-design mono-material flexible packaging with high barrier properties realized through graphene hybrid coatings. Resour. Conserv. Recycl. 2022, 179, 106126. [Google Scholar] [CrossRef]
- Ajmal, M.; Asad, M.; Huo, W.; Shao, Y.; Lu, W. Enhancing degradation of PLA-made rigid biodegradable plastics with non-thermal plasma treatment. J. Clean. Prod. 2024, 479, 143985. [Google Scholar] [CrossRef]
- Li, Q.; Cao, J.; Li, J.; Li, D.; Jing, B.; Zhou, J.; Ao, Z. Novel insights into photoaging mechanisms and environmental persistence risks of polylactic acid (PLA) microplastics: Direct and indirect photolysis. Sci. Total Environ. 2024, 954, 176350. [Google Scholar] [CrossRef]
- Chen, Q.; Fei, X. Effective reduction of land-to-ocean plastic leakage in Thailand from 2000 to 2019 and implications for low- and middle-income countries. Resour. Conserv. Recycl. 2023, 198, 107204. [Google Scholar] [CrossRef]
| Environmental Parameter | Impact on Microbial Communities | Implications for Plastisphere Ecology |
|---|---|---|
| Temperature | Modifies microbial metabolism, enzyme activity, and community succession. | Influences biofilm growth rate and biodiversity; may drive ecosystem-specific profiles. |
| Salinity | Selects for halotolerant and halophilic species; inhibits sensitive organisms. | Shapes community composition based on habitat (marine vs. freshwater). |
| pH | Affects microbial viability, gene expression, and EPS production; extreme pH limits diversity. | Controls biofilm stability and species compatibility in various environments. |
| UV Exposure | Causes oxidative stress, DNA damage, and microbial mortality; favours UV-resistant species. | Alters surface colonization and may reduce primary producers in the plastisphere. |
| Nutrient Availability | Stimulates colonization, metabolic activity, and biofilm thickness; scarcity triggers stress response. | Dictates biofilm development, succession, and potential for pollutant degradation. |
| Filtration | Pros | Cons |
|---|---|---|
| Microfiltration | Pore sizes require low pressure (0.1–10 µm) Good pre-treatment | Efficiency may be low compared to UF, NF, and RO Abrasion can easily occur |
| Ultrafiltration | Removes smaller particles (1–100 nm) | Allows for membrane scaling Repulsion between ultrafiltration manufacturing materials and microplastics affects the removal efficiency |
| Nanofiltration | High efficiency, particles smaller than 10 nm (1–10 nm) | Allows for membrane scaling High pressure requirements |
| Reverse osmosis | High efficiency, smaller pore sizes (<1 nm) Low water flux | High energy requirements Fibres may pass through |
| Forward osmosis | Fouling resistance Water flux enhanced | Expensive |
| Dynamic membrane filtration | Effective for fibre removal | Low efficiency for fragments |
| Membrane bioreactor | May function efficiently in the absence of a secondary settling tank | High operational cost Membrane fouling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebepe, J.; Buthelezi, N.M.D.; Manganyi, M.C. Occurrence and Control of Microplastics and Emerging Technological Solutions for Their Removal in Freshwaters: A Comprehensive Review. Microplastics 2025, 4, 70. https://doi.org/10.3390/microplastics4040070
Lebepe J, Buthelezi NMD, Manganyi MC. Occurrence and Control of Microplastics and Emerging Technological Solutions for Their Removal in Freshwaters: A Comprehensive Review. Microplastics. 2025; 4(4):70. https://doi.org/10.3390/microplastics4040070
Chicago/Turabian StyleLebepe, Jeffrey, Nana M. D. Buthelezi, and Madira C. Manganyi. 2025. "Occurrence and Control of Microplastics and Emerging Technological Solutions for Their Removal in Freshwaters: A Comprehensive Review" Microplastics 4, no. 4: 70. https://doi.org/10.3390/microplastics4040070
APA StyleLebepe, J., Buthelezi, N. M. D., & Manganyi, M. C. (2025). Occurrence and Control of Microplastics and Emerging Technological Solutions for Their Removal in Freshwaters: A Comprehensive Review. Microplastics, 4(4), 70. https://doi.org/10.3390/microplastics4040070






