The Exposure to Polypropylene Micro- and Nanoplastics Impairs Wound Healing and Tissue Regeneration in the Leech Hirudo verbana
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of PP-MPs and NPs by Means of Transmission Electron Microscopy (TEM)
2.2. Animals and Treatments
2.3. Embedding Tissue in Epoxy Resin for Morphological Analysis at Light and Transmission Electron Microscopy
2.4. Embedding Tissue in Paraffin and Masson’s Trichrome Staining
2.5. Embedding Tissue in Oct for Cryosections
2.6. Acid Phosphatase (ACP) Assay
2.7. Immunolocalizations on Cryosections
2.8. RNA Extraction and Quantitative PCR (qPCR)
2.9. Statistical Analysis
3. Results
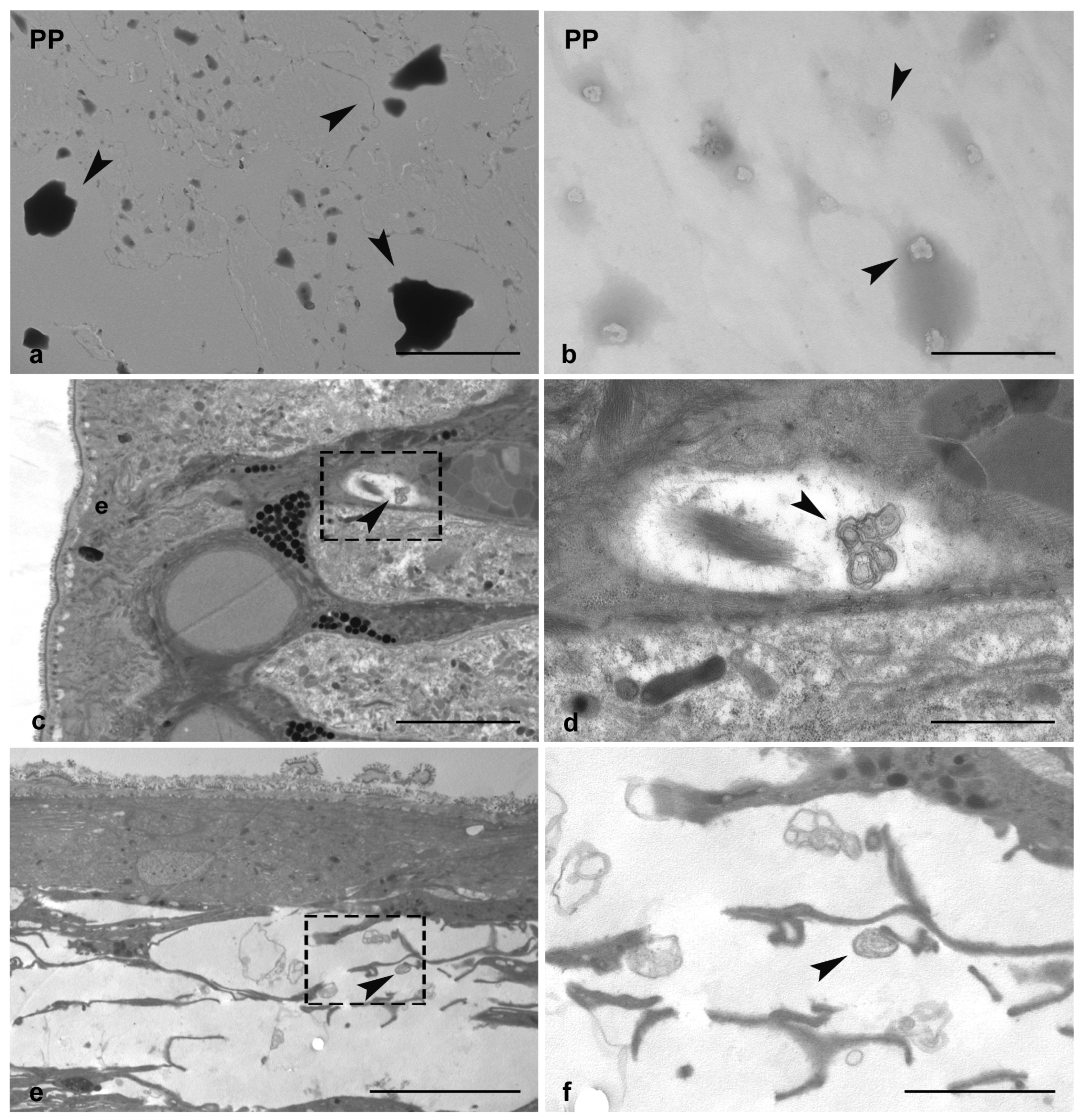
3.1. Determination of PP-MPs and NPs Presence in Leech Tissues
3.2. Morphological Analyses
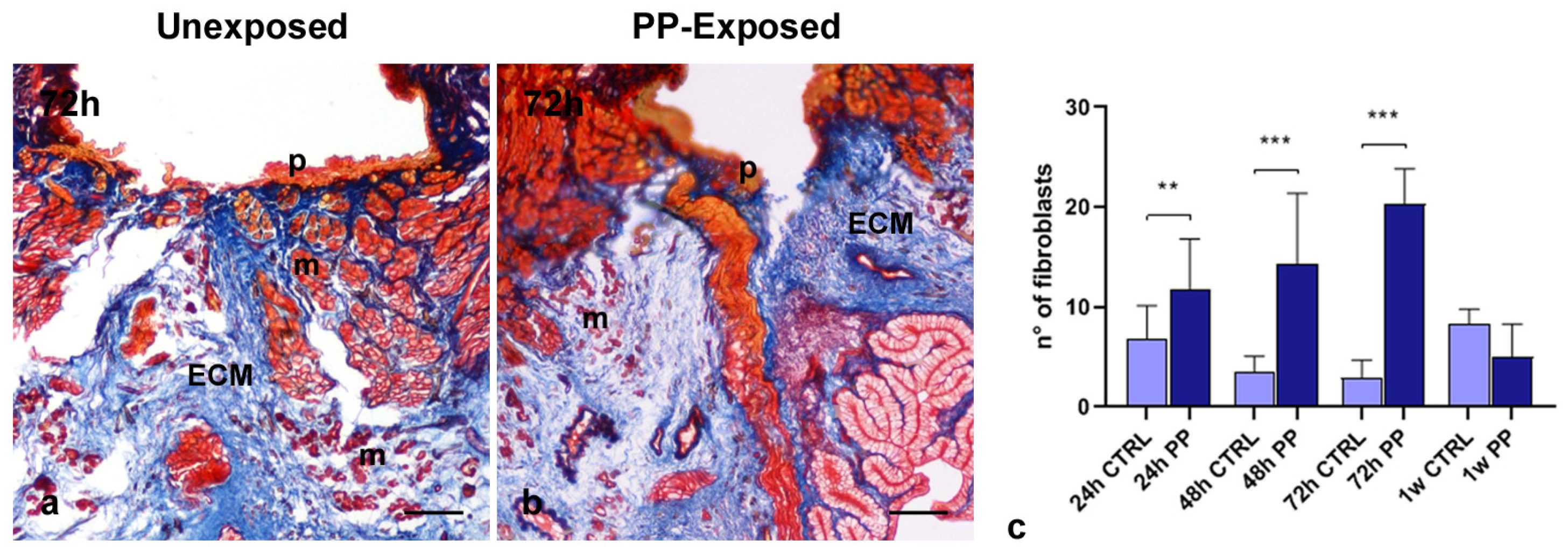
3.3. Colorimetric Staining of PP-Induced ECM
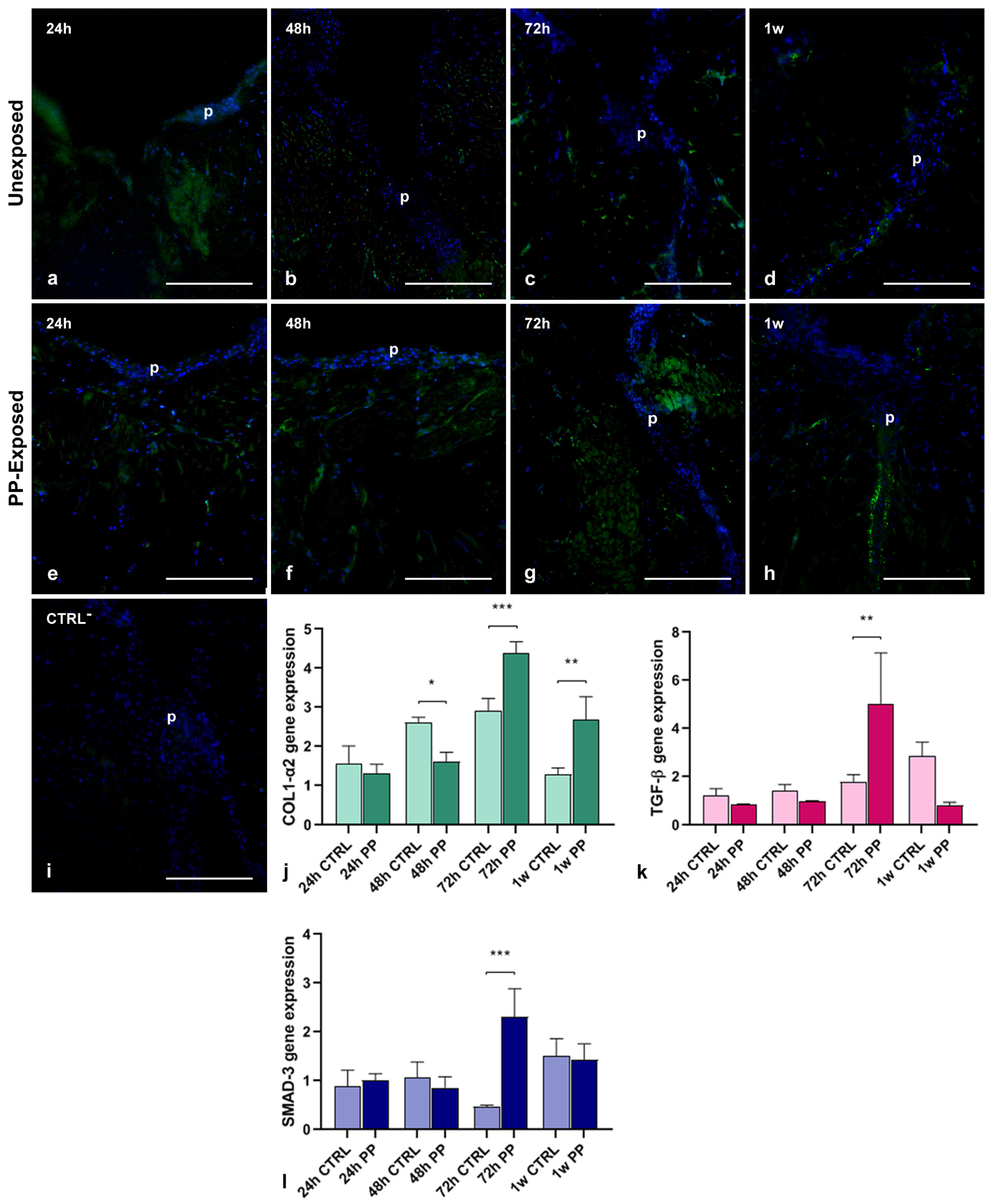
3.4. Effect of PP-MPs and NPs on Collagen I, TGF-β and SMAD-3 Expression
3.5. PP-MPs and NPs Effects on Angiogenesis and Muscle Regeneration
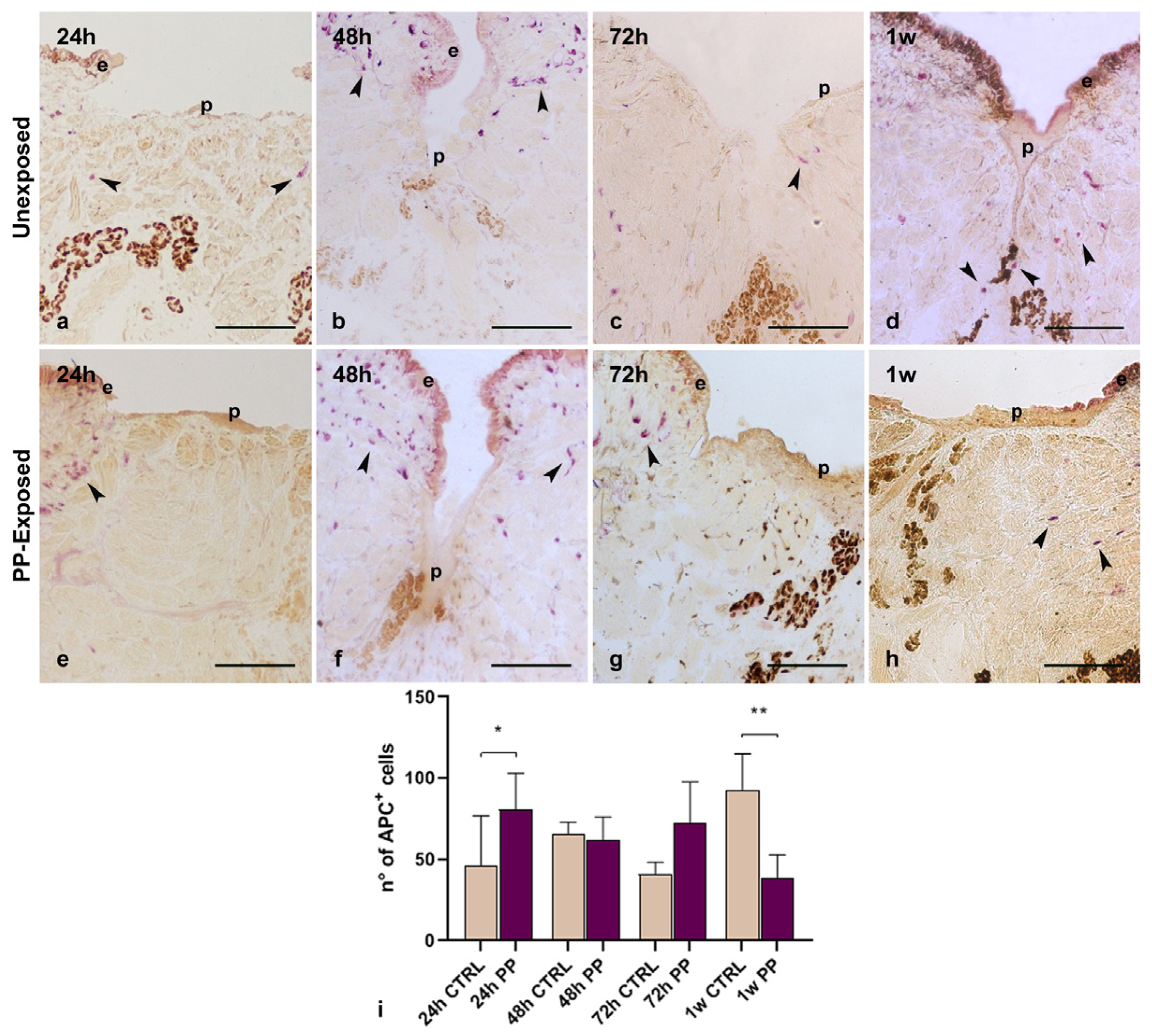
3.6. Effects of PP-MPs and NPs on Leech Macrophages Recruitment and Tissue Regeneration
3.7. HvRNASET2 and HmAIF-1 Expression Induced by PP-MPs and NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Acid Phosphatase |
| ANOVA | Analysis of Variance |
| BSA | Bovine Serum Albumin |
| COL1-α2 | Collagen Type I Alpha 2 Chain |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| ECM | Extracellular Matrix |
| FITC | Fluorescein Isothiocyanate |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| HmAIF-1 | Hirudo medicinalis Allograft Inflammatory Factor-1 |
| HSPCs | Hematopoietic Stem and Progenitor Cells |
| HvNASET2 | Hirudo verbana Ribonuclease T2 |
| MPs | Microplastics |
| NMR | Nuclear Magnetic Resonance |
| NPs | Nanoplastics |
| OsO4 | Osmium Tetroxide |
| PBS | Phosphate Buffered Saline |
| PP | Polypropylene |
| qPCR | Quantitative PCR (Polymerase Chain Reaction) |
| SEC | Size Exclusion Chromatography |
| SD | Standard Deviation |
| TEM | Transmission Electron Microscopy |
| TGF-β | Transforming Growth Factor Beta |
References
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Chandran, M.; Tamilkolundu, S.; Murugesan, C. Characterization studies: Waste plastic oil and its blends Characterization studies: Waste plastic oil and its blends. Energy Sources Part A 2019, 42, 281–291. [Google Scholar] [CrossRef]
- Sigler, M. The Effects of Plastic Pollution on Aquatic Wildlife: Current Situations and Future Solutions. Water Air Soil Pollut. 2014, 225, 2184. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.; Looi, C.K.; Lim, W.; Wong, S.; Kok, Y.; Tan, B.; Wong, C.; Leong, C. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Science of the Total Environment Microplastics in freshwater sediment: A review on methods, occurrence, and sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Barrick, A.; Boardwine, A.J.; Nguyen, N.H.A.; Sevcu, A.; Novotna, J.; Hoang, T.C. Acute toxicity of natural and synthetic clothing fibers towards Daphnia magna: Influence of fiber type and morphology. Sci. Total Environ. 2025, 967, 178751. [Google Scholar] [CrossRef]
- Engler, R.E. Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 302–315. [Google Scholar] [CrossRef]
- Gu, L.; Tian, L.; Gao, G.; Peng, S.; Zhang, J.; Wu, D.; Huang, J.; Hua, Q.; Lu, T.; Zhong, L.; et al. Inhibitory effects of polystyrene microplastics on caudal fin regeneration in zebrafish larvae. Environ. Pollut. 2020, 266, 114664. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Kang, S.; Yang, L.; Shi, H.; Tripathee, L.; Gao, T. Research progresses of microplastic pollution in freshwater systems. Sci. Total Environ. 2021, 795, 148888. [Google Scholar] [CrossRef]
- Raddadi, N.; Fava, F. Biodegradation of oil-based plastics in the environment: Existing knowledge and needs of research and innovation. Sci. Total Environ. 2019, 679, 148–158. [Google Scholar] [CrossRef]
- Mohan, P.; Shahul, F. Charting the microplastic menace: A bibliometric analysis of pollution in Malaysian mangroves and polypropylene bioaccumulation assessment in Anadara granosa. Mar. Pollut. Bull. 2024, 205, 116654. [Google Scholar] [CrossRef]
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef]
- Jahan, I.; Chowdhury, G.; Rafi, S.; Ashab, A.; Sarker, M.; Chakraborty, A.; Couetard, N.; Anamul, M.; Hossain, A.; Mahbub, M. Assessment of dietary polyvinylchloride, polypropylene and polyethylene terephthalate exposure in Nile tilapia, Oreochromis niloticus: Bioaccumulation, and effects on behaviour, growth, hematology. Environ. Pollut. 2024, 345, 123548. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Perono, G.; Tommasi, F.; Pagano, G.; Oral, R.; Buri, P.; Kova, I.; Toscanesi, M.; Trifuoggi, M.; Lyons, D.M. Resolving the effects of environmental micro- and nanoplastics exposure in biota: A knowledge gap analysis. Sci. Total Environ. 2021, 780, 146534. [Google Scholar] [CrossRef]
- Montero, D.; Rimoldi, S.; Torrecillas, S.; Rapp, J.; Moroni, F.; Herrera, A.; Gómez, M.; Fernández-montero, Á.; Terova, G. Impact of polypropylene microplastics and chemical pollutants on European sea bass (Dicentrarchus labrax) gut microbiota and health. Sci. Total Environ. 2022, 805, 150402. [Google Scholar] [CrossRef] [PubMed]
- Baranzini, N.; Pulze, L.; Bon, C.; Izzo, L.; Pragliola, S.; Venditto, V.; Grimaldi, A. Hirudo verbana as a freshwater invertebrate model to assess the effects of polypropylene micro and nanoplastics dispersion in freshwater. Fish Shellfish Immunol. 2022, 127, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Song, W.; Tian, H.; Zhang, K.; Li, B.; Du, Z.; Zhang, W.; Wang, J.; Wang, J.; Zhu, L. Chemosphere The effects of high-density polyethylene and polypropylene microplastics on the soil and earthworm Metaphire guillelmi gut microbiota. Chemosphere 2021, 267, 129219. [Google Scholar] [CrossRef]
- Gambino, G.; Falleni, A.; Nigro, M.; Salvetti, A.; Cecchettini, A.; Ippolito, C.; Guidi, P.; Rossi, L. Dynamics of interaction and effects of microplastics on planarian tissue regeneration and cellular homeostasis. Aquat. Toxicol. 2020, 218, 105354. [Google Scholar] [CrossRef]
- Gao, T.; Sun, B.; Xu, Z.; Chen, Q.; Yang, M.; Wan, Q.; Song, L.; Chen, G.; Jing, C.; Zeng, E.Y.; et al. Exposure to polystyrene microplastics reduces regeneration and growth in planarians. J. Hazard. Mater. 2022, 432, 128673. [Google Scholar] [CrossRef]
- Leung, J.; Chan, K.Y.K. Microplastics reduced posterior segment regeneration rate of the polychaete Perinereis aibuhitensis. Mar. Pollut. Bull. 2018, 129, 782–786. [Google Scholar] [CrossRef]
- Pragliola, S.; Grisi, F.; Vitale, V.; Sacco, O.; Venditto, V.; Izzo, L.; Grimaldi, A.; Baranzini, N. New fluorescence labeling isotactic polypropylenes as a tracer: A proof of concept. Polym. Chem. 2022, 13, 2685–2693. [Google Scholar] [CrossRef]
- Pulze, L.; Baranzini, N.; Congiu, T.; Acquati, F.; Grimaldi, A. Spatio-Temporal Changes of Extracellular Matrix (ECM) Stiffness in the Development of the Leech Hirudo verbana. Int. J. Mol. Sci. 2022, 23, 5953. [Google Scholar] [CrossRef]
- Baranzini, N.; Pulze, L.; Tettamanti, G.; Acquati, F.; Grimaldi, A. HvRNASET2 Regulate Connective Tissue and Collagen I Remodeling During Wound Healing Process. Front. Physiol. 2021, 12, 632506. [Google Scholar] [CrossRef]
- Grimaldi, A.; Banfi, S.; Gerosa, L.; Tettamanti, G.; Noonan, D.M.; Valvassori, R.; de Eguileor, M. Identification, isolation and expansion of myoendothelial cells involved in leech muscle regeneration. PLoS ONE 2009, 4, e7652. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Transportation fate and removal of microplastic pollution—A perspective on environmental pollution. Case Stud. Chem. Environ. Eng. 2020, 2, 100015. [Google Scholar] [CrossRef]
- Morrison, M.; Trevisan, R.; Ranasinghe, P.; Merrill, G.B.; Santos, J.; Hong, A.; Edward, W.C.; Jayasundara, N.; Somarelli, J.A. A growing crisis for One Health: Impacts of plastic pollution across layers of biological function. Front. Mar. Sci. 2022, 9, 980705. [Google Scholar] [CrossRef]
- Kukkola, A.; Krause, S.; Lynch, I.; Sambrook Smith, G.H.; Nel, H. Nano and microplastic interactions with freshwater biota—Current knowledge, challenges and future solutions. Environ. Int. 2021, 152, 106504. [Google Scholar] [CrossRef]
- Baranzini, N.; Pulze, L.; Acquati, F.; Grimaldi, A. Hirudo verbana as an alternative model to dissect the relationship between innate immunity and regeneration. Invertebr. Surviv. J. 2020, 17, 90–98. [Google Scholar]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Girardello, R.; Tasselli, S.; Baranzini, N.; Valvassori, R.; De Eguileor, M.; Grimaldi, A. Effects of carbon nanotube environmental dispersion on an aquatic invertebrate, Hirudo medicinalis. PLoS ONE 2015, 10, e0144361. [Google Scholar] [CrossRef] [PubMed]
- Bodó, K.; Baranzini, N.; Girardello, R.; Kokhanyuk, B.; Németh, P.; Hayashi, Y.; Grimaldi, A.; Engelmann, P. Nanomaterials and annelid immunity: A comparative survey to reveal the common stress and defense responses of two sentinel species to nanomaterials in the environment. Biology 2020, 9, 307. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Kong, L.; Xing, H.; Yang, Q.; Wu, J. A Review of Eco-Corona Formation on Micro/Nanoplastics and Its Effects on Stability, Bioavailability, and Toxicity. Water 2025, 17, 1124. [Google Scholar] [CrossRef]
- Bohaud, C.; Johansen, M.D.; Jorgensen, C.; Ipseiz, N.; Kremer, L.; Djouad, F. The Role of Macrophages During Zebrafish Injury and Tissue Regeneration Under Infectious and Non-Infectious Conditions. Front. Immunol. 2021, 12, 707824. [Google Scholar] [CrossRef]
- Mescher, A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 2017, 4, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Baranzini, N.; De Vito, A.; Orlandi, V.T.; Reguzzoni, M.; Monti, L.; de Eguileor, M.; Rosini, E.; Pollegioni, L.; Tettamanti, G.; Acquati, F.; et al. Antimicrobial Role of RNASET2 Protein During Innate Immune Response in the Medicinal Leech Hirudo verbana. Front. Immunol. 2020, 11, 370. [Google Scholar] [CrossRef]
- Seo, D.; Hare, J.M. The transforming growth factor-β/Smad3 pathway: Coming of age as a key participant in cardiac remodeling. Circulation 2007, 116, 2096–2098. [Google Scholar] [CrossRef]
- Branton, M.H.; Kopp, J.B. TGF-β and fibrosis. Microbes Infect. 1999, 1, 1349–1365. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF—β family in wound healing, burns and scarring: A review. IJBT 2012, 2, 18–28. [Google Scholar]
- Nacu, N.; Luzina, I.G.; Highsmith, K.; Pochetuhen, K.; Cooper, Z.A.; Michael, P.; Todd, N.W.; Atamas, S.P.; Lockatell, V.; Gillmeister, M.P.; et al. Macrophages Produce TGF-β-Induced (β-ig-h3) following Ingestion of Apoptotic Cells and Regulate MMP14 Levels and Collagen Turnover in Fibroblasts. J. Immunol. 2008, 180, 5036–5044. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages∗. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bon, C.; Maretti, A.; Pulze, L.; Paris, N.; Santoro, O.; Pragliola, S.; Izzo, L.; Baranzini, N.; Grimaldi, A. The Exposure to Polypropylene Micro- and Nanoplastics Impairs Wound Healing and Tissue Regeneration in the Leech Hirudo verbana. Microplastics 2025, 4, 56. https://doi.org/10.3390/microplastics4030056
Bon C, Maretti A, Pulze L, Paris N, Santoro O, Pragliola S, Izzo L, Baranzini N, Grimaldi A. The Exposure to Polypropylene Micro- and Nanoplastics Impairs Wound Healing and Tissue Regeneration in the Leech Hirudo verbana. Microplastics. 2025; 4(3):56. https://doi.org/10.3390/microplastics4030056
Chicago/Turabian StyleBon, Camilla, Alice Maretti, Laura Pulze, Nicolò Paris, Orlando Santoro, Stefania Pragliola, Lorella Izzo, Nicolò Baranzini, and Annalisa Grimaldi. 2025. "The Exposure to Polypropylene Micro- and Nanoplastics Impairs Wound Healing and Tissue Regeneration in the Leech Hirudo verbana" Microplastics 4, no. 3: 56. https://doi.org/10.3390/microplastics4030056
APA StyleBon, C., Maretti, A., Pulze, L., Paris, N., Santoro, O., Pragliola, S., Izzo, L., Baranzini, N., & Grimaldi, A. (2025). The Exposure to Polypropylene Micro- and Nanoplastics Impairs Wound Healing and Tissue Regeneration in the Leech Hirudo verbana. Microplastics, 4(3), 56. https://doi.org/10.3390/microplastics4030056







