A Novel Approach for Characterization of Microplastic Pollution in the Chesapeake Bay
Abstract
1. Introduction
2. Materials and Methods
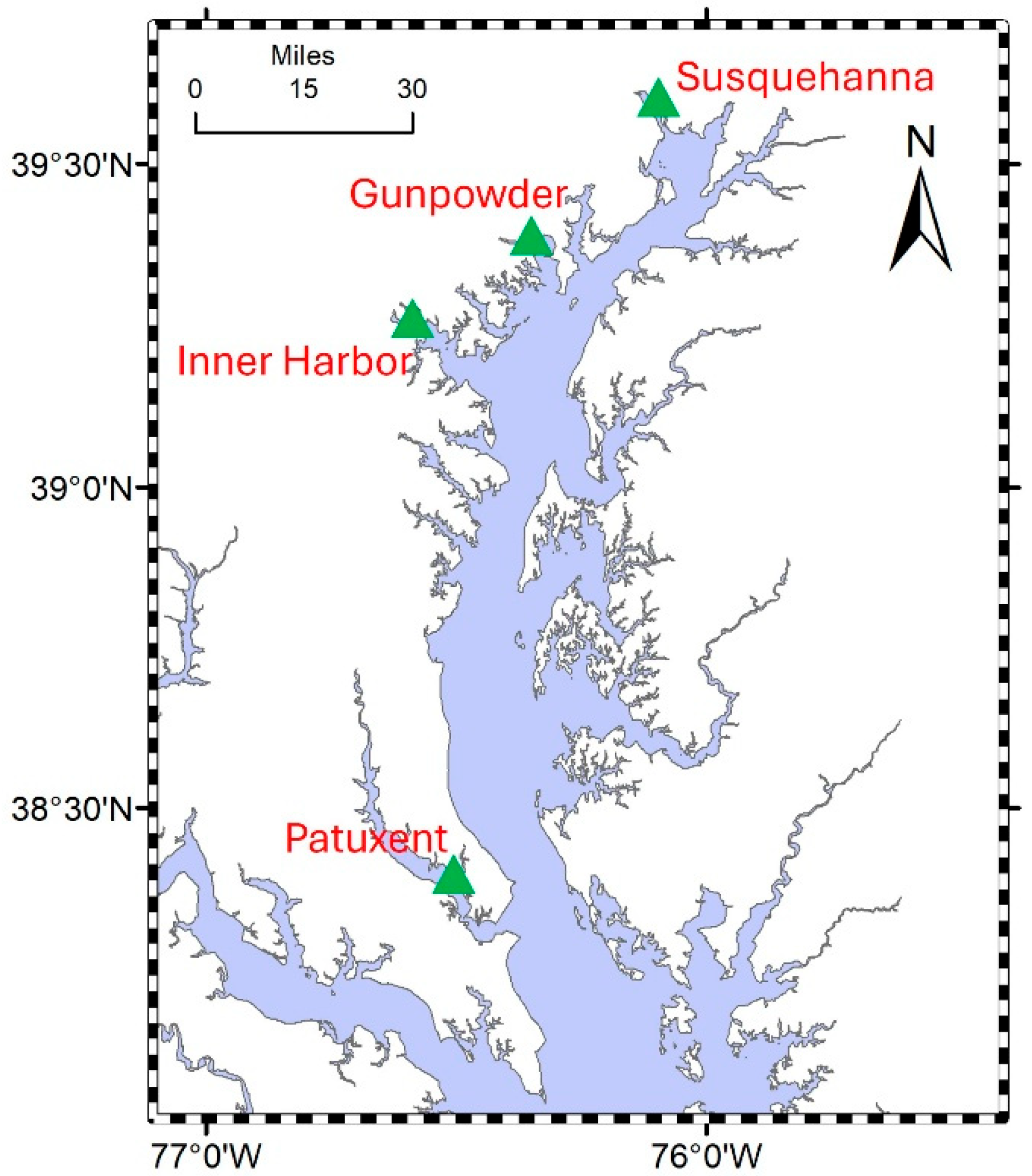
2.1. Study Site
2.2. Sample Collection and Processing
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results
3.1. Temporal and Spatial Distribution of Microplastics Abundance in the Chesapeake Bay
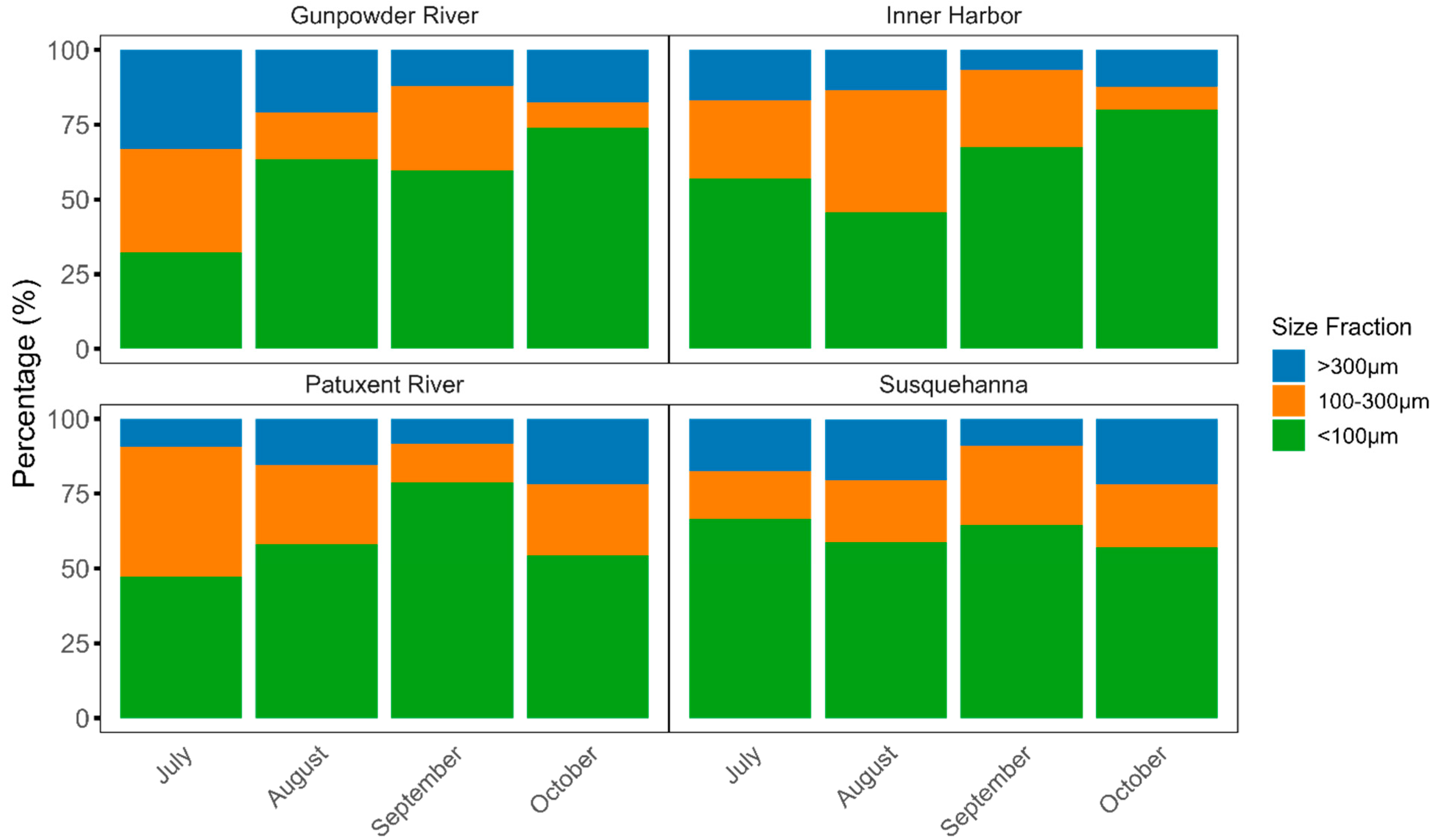
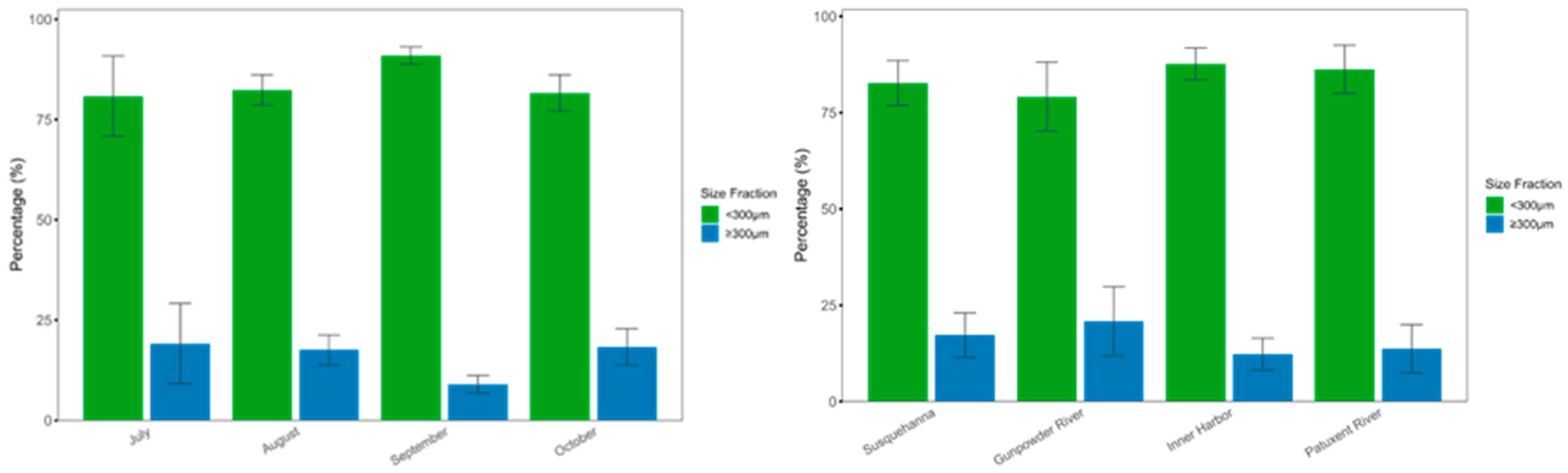
3.2. Spatiotemporal Distribution of the Microplastics Sizes in Chesapeake Bay Surface Water
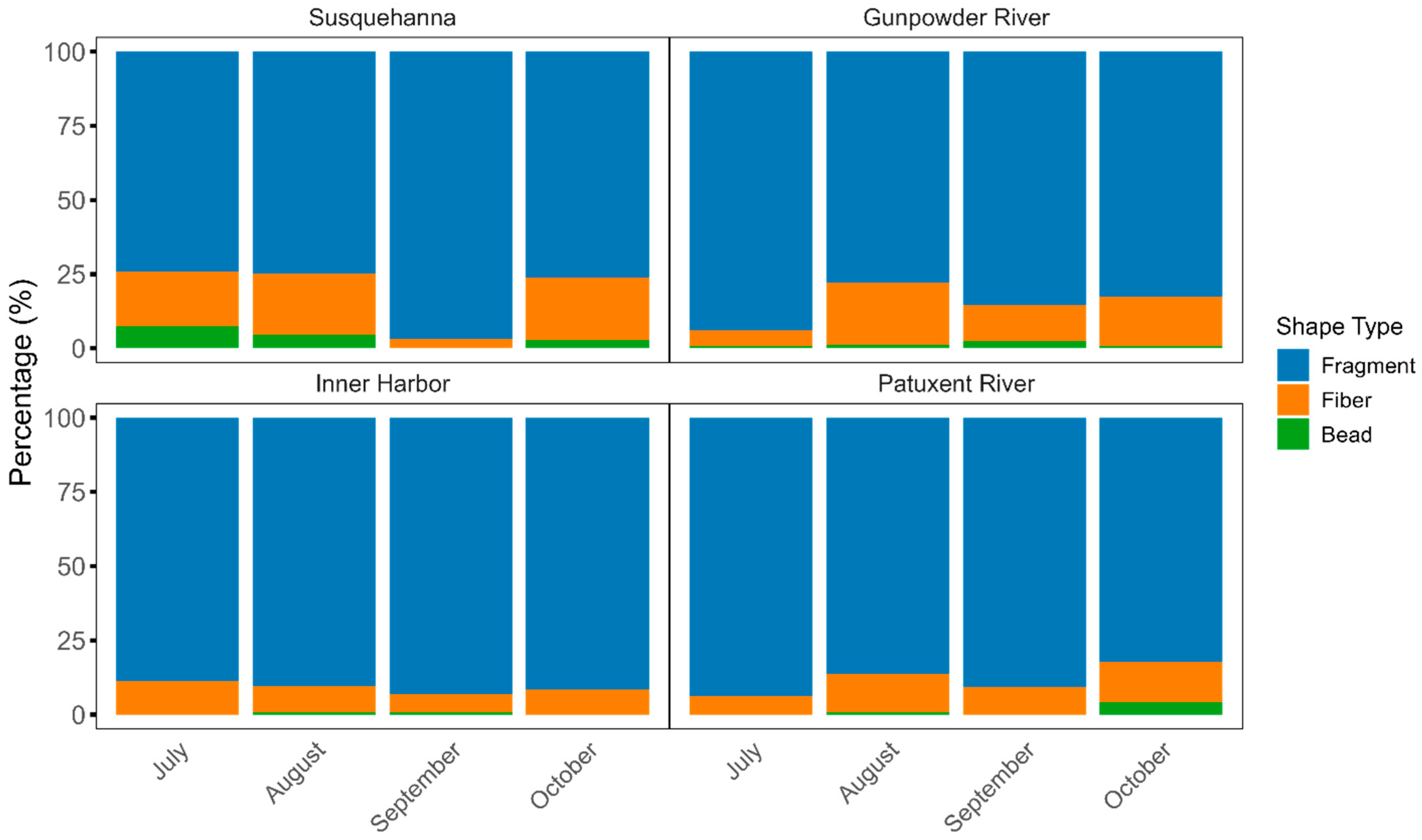
3.3. Spatiotemporal Distribution of Microplastics Morphology in Chesapeake Bay Surface Water
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iroegbu, A.O.C.; Sadiku, R.E.; Ray, S.S.; Hamam, Y. Plastics in municipal drinking water and wastewater treatment plant effluents: Challenges and opportunities for South Africa—A review. Environ. Sci. Pollut. Res. 2020, 27, 12953–12966. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Sonke, J.E.; Koenig, A.; Segur, T.; Yakovenko, N. Global environmental plastic dispersal under OECD policy scenarios toward 2060. Sci. Adv. 2025, 11, eadu2396. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Bejarano, S.; Diemel, V.; Feuring, A.; Ghilardi, M.; Harder, T. No short–term effect of sinking microplastics on heterotrophy or sediment clearing in the tropical coral Stylophora pistillata. Sci. Rep. 2022, 12, 1468. [Google Scholar] [CrossRef]
- Bikker, J.; Lawson, J.; Wilson, S.; Rochman, C.M. Microplastics and other anthropogenic particles in the surface waters of the Chesapeake Bay. Mar. Pollut. Bull. 2020, 156, 111257. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Liu, H.; Xu, X.; Xia, J. A review on the occurrence, distribution, characteristics, and analysis methods of microplastic pollution in ecosystems. Environ. Pollut. Bioavailab. 2021, 33, 227–246. [Google Scholar] [CrossRef]
- Rostampour, S.; Cook, R.; Jhang, S.-S.; Li, Y.; Fan, C.; Sung, L.-P. Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions. Polymers 2024, 16, 2249. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- López, A.G.; Najjar, R.G.; Friedrichs, M.A.M.; Hickner, M.A.; Wardrop, D.H. Estuaries as Filters for Riverine Microplastics: Simulations in a Large, Coastal–Plain Estuary. Front. Mar. Sci. 2021, 8, 715924. [Google Scholar] [CrossRef]
- Hood, R.R.; Shenk, G.W.; Dixon, R.L.; Smith, S.M.C.; Ball, W.P.; Bash, J.O.; Batiuk, R.; Boomer, K.; Brady, D.C.; Cerco, C.; et al. The Chesapeake Bay Program Modeling System: Overview and Recommendations for Future Development. Ecol. Model. 2021, 465, 109635. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Blomquist, J.D.; Bennett, M.; Berlin, A.; Blazer, V.; Claggett, P.R.; Faulkner, S.; Hyer, K.; Ladino, C.; Moyer, D.; et al. U.S. Geological Survey Chesapeake Science Strategy, 2015–2025—Informing Ecosystem Management of America’s Largest Estuary; Open–File Report 2015–1162; U.S. Geological Survey: Reston, VA, USA, 2015.
- Vasconcelos, R.P.; Reis–Santos, P.; Costa, M.J.; Cabral, H.N. Connectivity between estuaries and marine environment: Integrating metrics to assess estuarine nursery function. Ecol. Indic. 2011, 11, 1123–1133. [Google Scholar] [CrossRef]
- Brophy, L.S.; Greene, C.M.; Hare, V.C.; Holycross, B.; Lanier, A.; Heady, W.N.; O’Connor, K.; Imaki, H.; Haddad, T.; Dana, R. Insights into estuary habitat loss in the western United States using a new method for mapping maximum extent of tidal wetlands. PLoS ONE 2019, 14, e0218558. [Google Scholar] [CrossRef] [PubMed]
- Clayer, F.; Jartun, M.; Buenaventura, N.T.; Guerrero, J.-L.; Lusher, A. Bypass of Booming Inputs of Urban and Sludge–Derived Microplastics in a Large Nordic Lake. Env. Sci. Technol. 2021, 55, 7949–7958. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.-H.; Ho, Y.-S. A historical review and bibliometric analysis of research on estuary pollution. Mar. Pollut. Bull. 2012, 64, 13–21. [Google Scholar] [CrossRef]
- Curren, E.; Yew Leong, S.C. Spatiotemporal characterisation of microplastics in the coastal regions of Singapore. Heliyon 2023, 9, e12961. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Uy, C.A.; Johnson, D.W. Effects of microplastics on the feeding rates of larvae of a coastal fish: Direct consumption, trophic transfer, and effects on growth and survival. Mar. Biol. 2022, 169, 27. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Esiukova, E.E. Microplastics in a Marine Environment: Review of Methods for Sampling, Processing, and Analyzing Microplastics in Water, Bottom Sediments, and Coastal Deposits. Oceanology 2018, 58, 137–143. [Google Scholar] [CrossRef]
- Firdaus, M.; Trihadiningrum, Y.; Lestari, P. Microplastic pollution in the sediment of Jagir Estuary, Surabaya City, Indonesia. Mar. Pollut. Bull. 2020, 150, 110790. [Google Scholar] [CrossRef] [PubMed]
- Schall, M.K.; Smith, G.D.; Blazer, V.S.; Walsh, H.L.; Wagner, T. Factors Influencing the Prevalence of Hyperpigmented Melanistic Lesions in Smallmouth Bass Micropterus dolomieu in the Susquehanna River Basin, Pennsylvania. J. Fish Dis. 2025, 48, e14033. [Google Scholar] [CrossRef] [PubMed]
- Kiser, A.H. Patuxent River Basin Environmental Variable Rasters: Climate, Topography, Land Use and Land Cover; U.S. Geological Survey: Reston, VA, USA, 2024. [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015.
- Gabriel, A.D.; Amparado, R.F.; Lubguban, A.A.; Bacosa, H.P. Riverine Microplastic Pollution: Insights from Cagayan de Oro River, Philippines. Int. J. Environ. Res. Public Health 2023, 20, 6132. [Google Scholar] [CrossRef] [PubMed]
- Venrick, E.L.; McGowan, J.A.; Cayan, D.R.; Hayward, T.L. Climate and Chlorophyll a: Long–Term Trends in the Central North Pacific Ocean. Science 1987, 238, 70–72. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.-T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Chen, G.; Xu, K.; Gong, H.; Huang, K.; Yan, M.; Wang, J. Microplastics in Surface Waters and Sediments from Guangdong Coastal Areas, South China. Sustainability 2021, 13, 2691. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Neumann, C.A.; Berger, M.L.; Shaw, S.D. Grab vs. neuston tow net: A microplastic sampling performance comparison and possible advances in the field. Anal. Methods 2017, 9, 1446–1453. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Kang, J.-H.; Kwon, O.Y.; Han, G.M.; Shim, W.J. Large Accumulation of Micro–sized Synthetic Polymer Particles in the Sea Surface Microlayer. Environ. Sci. Technol. 2014, 48, 9014–9021. [Google Scholar] [CrossRef]
- Zaki, M.R.M.; Ying, P.X.; Zainuddin, A.H.; Razak, M.R.; Aris, A.Z. Occurrence, abundance, and distribution of microplastics pollution: An evidence in surface tropical water of Klang River estuary, Malaysia. Env. Geochem Health 2021, 43, 3733–3748. [Google Scholar] [CrossRef]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Microplastic sampling techniques in freshwaters and sediments: A review. Environ. Chem. Lett. 2021, 19, 4225–4252. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez–Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in Four Estuarine Rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Kooi, M.; Primpke, S.; Mintenig, S.M.; Lorenz, C.; Gerdts, G.; Koelmans, A.A. Characterizing the multidimensionality of microplastics across environmental compartments. Water Res. 2021, 202, 117429. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Deakin, K.; Savage, G.; Jones, J.S.; Porter, A.; Muñoz–Pérez, J.P.; Santillo, D.; Lewis, C. Sea surface microplastics in the Galapagos: Grab samples reveal high concentrations of particles <200 μm in size. Sci. Total Environ. 2024, 923, 171428. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Porter, A.; Santillo, D.; Simpson, H.; Lloyd–Williams, S.; Lewis, C. Particle characteristics of microplastics contaminating the mussel Mytilus edulis and their surrounding environments. Mar. Pollut. Bull. 2019, 146, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Brehm, J.; Vollmer, M.; Jasinski, J.; Xu, C.; Zainuddin, S.; Fröhlich, T.; Schott, M.; Greiner, A.; Scheibel, T.; et al. Shape, size, and polymer dependent effects of microplastics on Daphnia magna. J. Hazard. Mater. 2022, 426, 128136. [Google Scholar] [CrossRef]
- Rochman, C.M.; Grbic, J.; Earn, A.; Helm, P.A.; Hasenmueller, E.A.; Trice, M.; Munno, K.; De Frond, H.; Djuric, N.; Santoro, S.; et al. Local Monitoring Should Inform Local Solutions: Morphological Assemblages of Microplastics Are Similar within a Pathway, But Relative Total Concentrations Vary Regionally. Environ. Sci. Technol. 2022, 56, 9367–9378. [Google Scholar] [CrossRef]
- Liu, F.; Rasmussen, L.A.; Klemmensen, N.D.R.; Zhao, G.; Nielsen, R.; Vianello, A.; Rist, S.; Vollertsen, J. Shapes of Hyperspectral Imaged Microplastics. Environ. Sci. Technol. 2023, 57, 12431–12441. [Google Scholar] [CrossRef]
- Kooi, M.; Koelmans, A.A. Simplifying Microplastic via Continuous Probability Distributions for Size, Shape, and Density. Environ. Sci. Technol. Lett. 2019, 6, 551–557. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström–Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs. 2018, 17, e00097. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
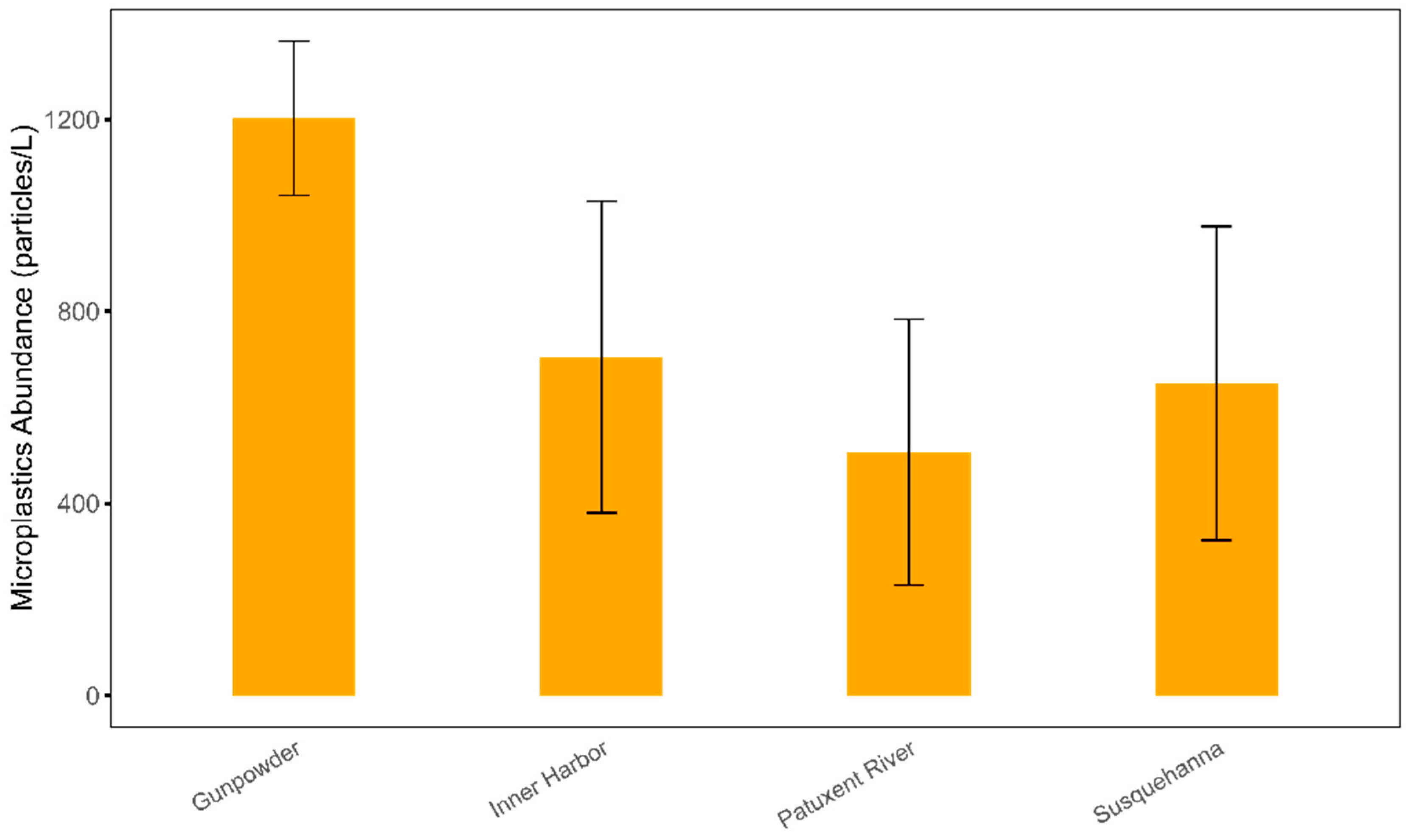
| Location | July (MP/L) | August (MP/L) | September (MP/L) | October (MP/L) |
|---|---|---|---|---|
| Susquehanna River | 603 | 351.6 | 1114.4 | 532.9 |
| Gunpowder River | 1349 | 1282 | 980.3 | 1198.2 |
| Inner Harbor | 484.3 | 703.9 | 1164.2 | 467.6 |
| Patuxent River | 507.8 | 152.8 | 537.1 | 829.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Bhatt, S.; Goswami, D.; Taylor, T. A Novel Approach for Characterization of Microplastic Pollution in the Chesapeake Bay. Microplastics 2025, 4, 53. https://doi.org/10.3390/microplastics4030053
Fan C, Bhatt S, Goswami D, Taylor T. A Novel Approach for Characterization of Microplastic Pollution in the Chesapeake Bay. Microplastics. 2025; 4(3):53. https://doi.org/10.3390/microplastics4030053
Chicago/Turabian StyleFan, Chunlei, Sulakshana Bhatt, Disha Goswami, and Tameka Taylor. 2025. "A Novel Approach for Characterization of Microplastic Pollution in the Chesapeake Bay" Microplastics 4, no. 3: 53. https://doi.org/10.3390/microplastics4030053
APA StyleFan, C., Bhatt, S., Goswami, D., & Taylor, T. (2025). A Novel Approach for Characterization of Microplastic Pollution in the Chesapeake Bay. Microplastics, 4(3), 53. https://doi.org/10.3390/microplastics4030053






