Comparative Analysis of Airborne Particle Concentrations in Textile Industry Environments Throughout the Workday
Abstract
1. Introduction
- Routes of Entry into the Body
- 2.
- Potential Health Impacts
- 3.
- How Nanoparticles Affect the Body
- 4.
- Studies and Evidence
- 5.
- Figures on Nanoparticles and Health Risks
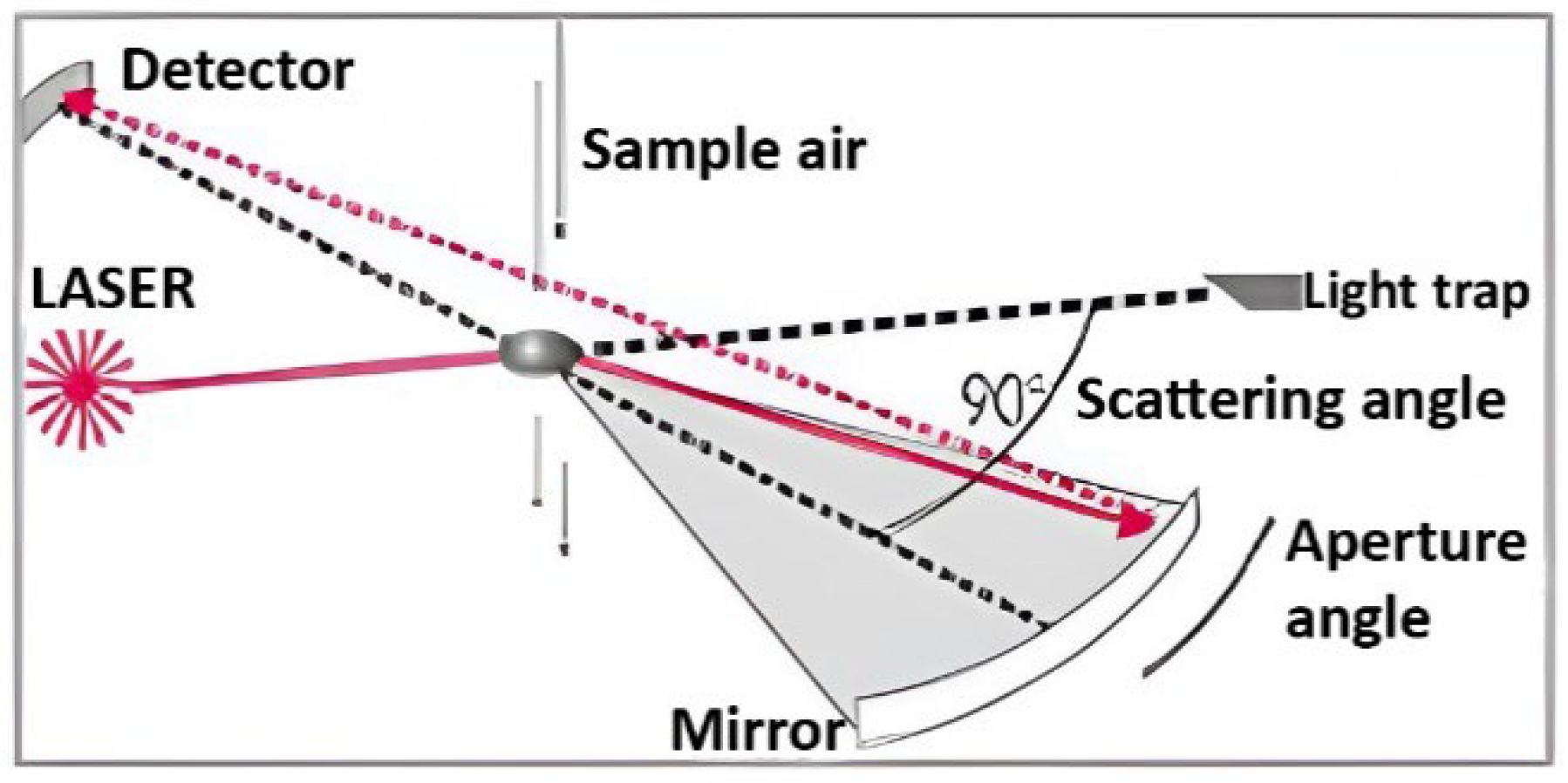
2. Materials and Methods
System Configuration and Components Are the Following
- (a)
- Thermal extraction/furnace: a device capable of heating samples to high temperatures (typically up to 600 °C) to facilitate the release of VOCs;
- (b)
- Solid-phase adsorber: a material that traps the volatile compounds released during thermal extraction;
- (c)
- Thermal desorption unit (TDU): a component that heats the adsorber to release the trapped compounds into a carrier gas;
- (d)
- Gas chromatograph (GC): an instrument that separates compounds based on their interaction with a stationary phase within a column;
- (e)
- Mass spectrometer (MS): an analytical device that ionizes and detects compounds based on their mass-to-charge ratio.
3. Results and Discussion
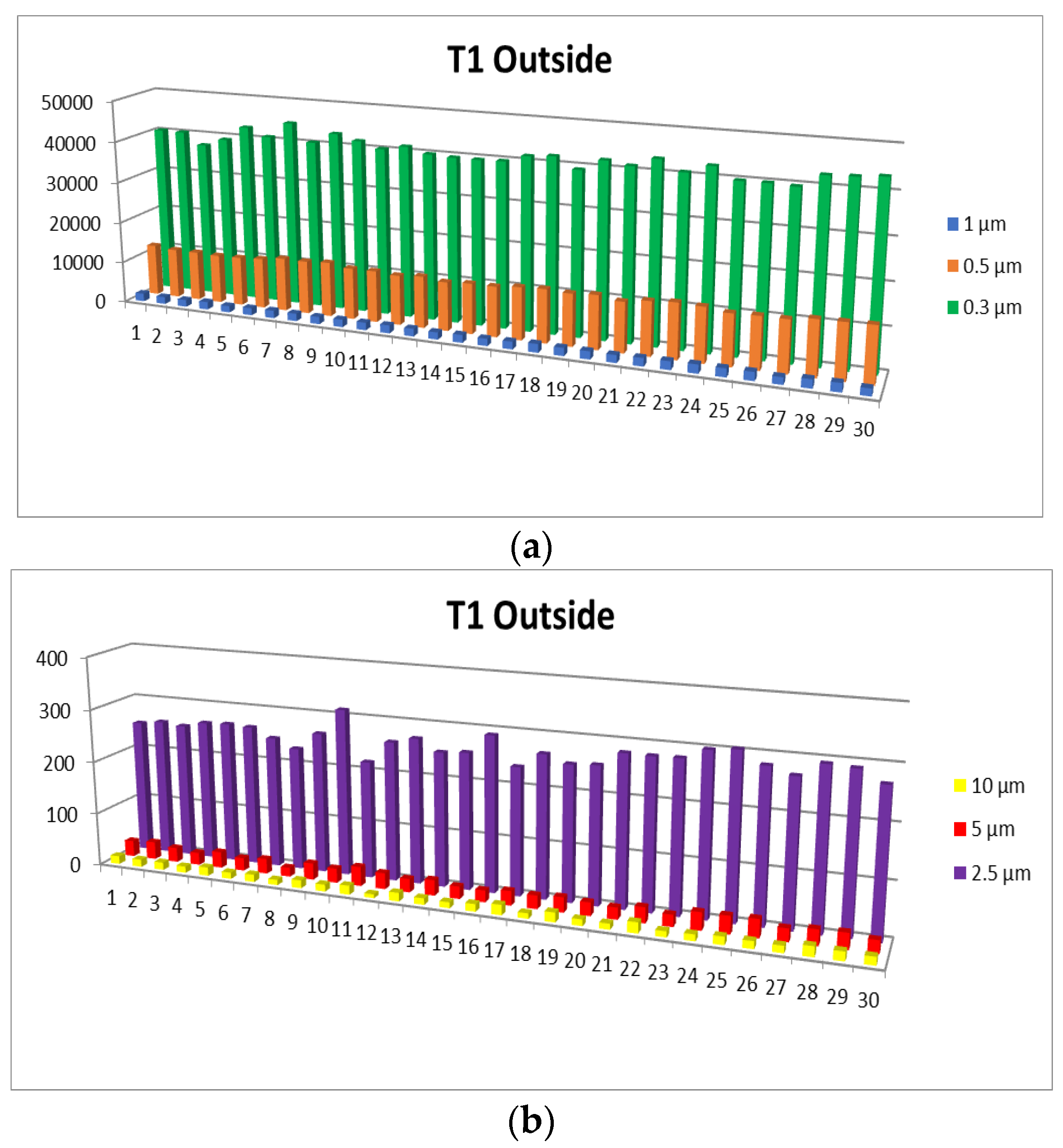
3.1. Airborne Particle Concentration
- Particle concentration: 0.3 µm particles have the highest concentration range, followed by 0.5 µm, 2.5 µm, 1.0 µm, and finally 5.0 µm and 10 µm, which have the lowest concentrations.
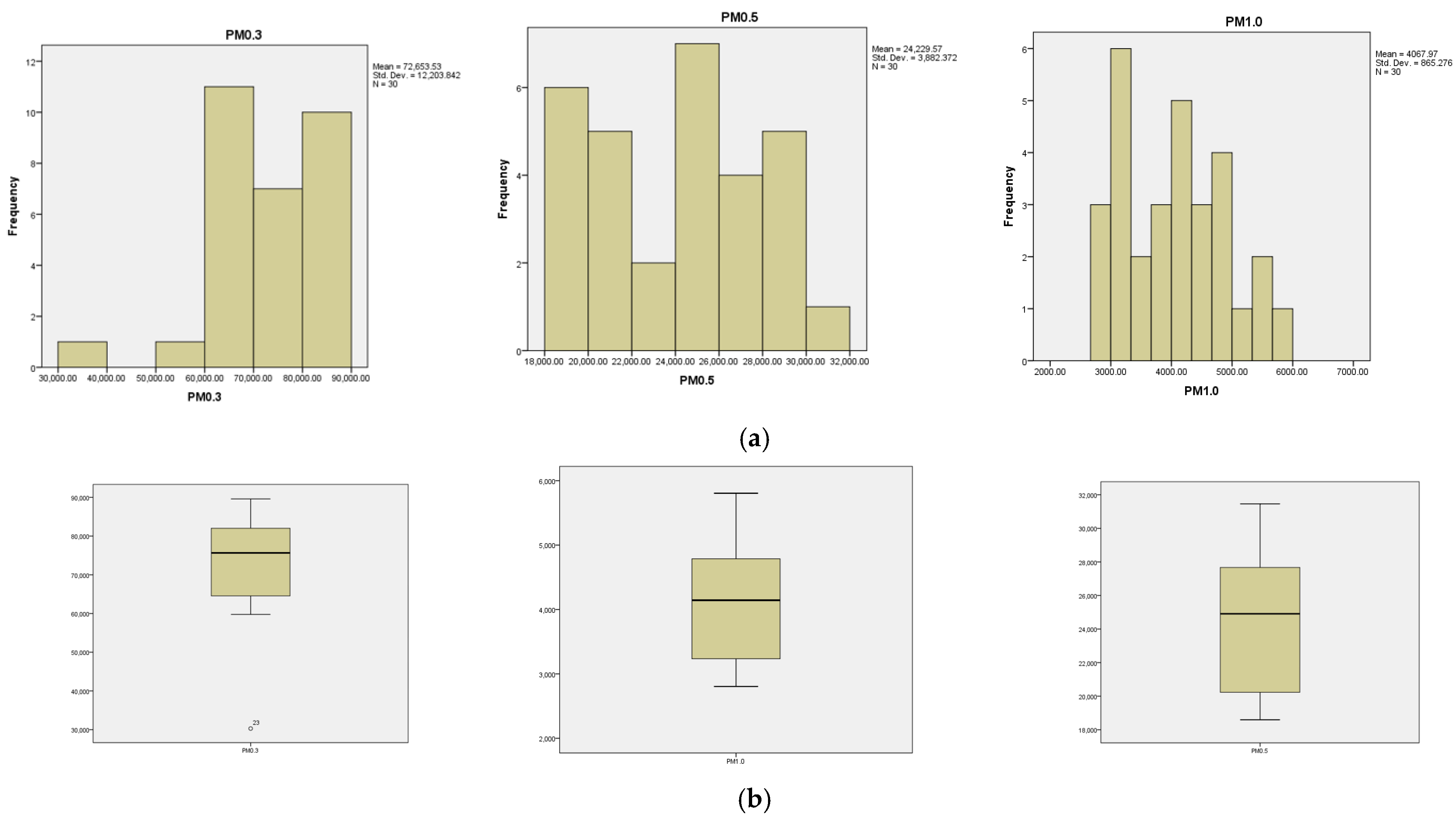
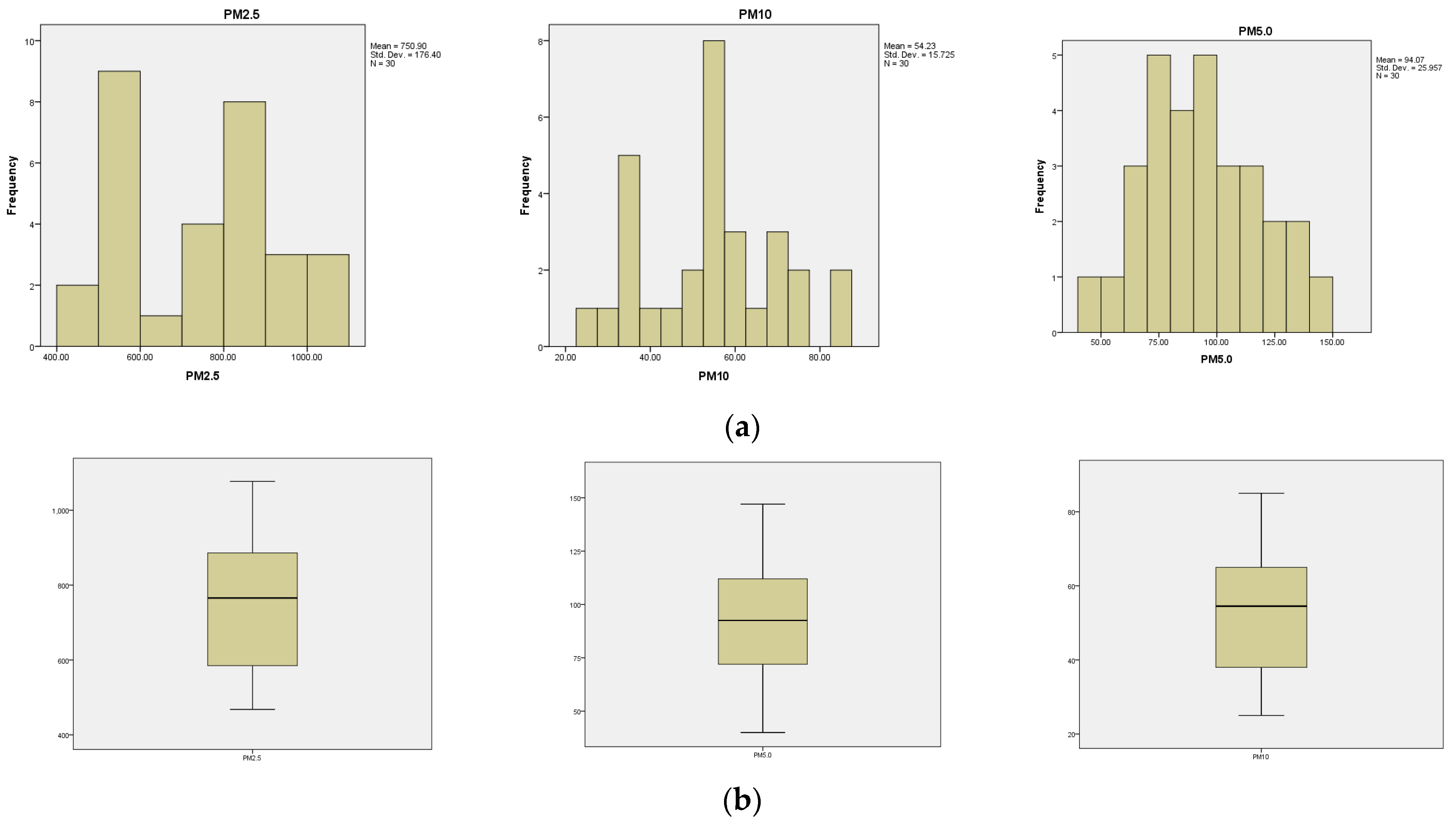
- Distribution patterns: In total, 0.3 µm, 0.5 µm, and 2.5 µm particles exhibit skewed distributions to the left, indicating a predominance of smaller values. Additionally, 1.0 µm particles show a balanced distribution with the median in the center, meaning there is a more equal proportion of small and large values. Further, 5.0 µm and 10 µm particles show right-skewed distributions, suggesting that larger values are more common.
- Size vs. distribution: as particle size increases, the concentration generally decreases, and the distribution shifts from skewed left (small particles) to more balanced (1.0 µm) and then skewed right (larger particles).
- The kurtosis indicators have negative values for the variable’s particle numbers 0.3, 0.5, 2.5, 5, and 10 µm: −0.544, −0.048, −0.0430, −0.990, and −0.230, with the curves being platykurtic in shape. On the other hand, they have a positive value for the 1.0 µm particle variable, 0.254, with the curve being leptokurtic.
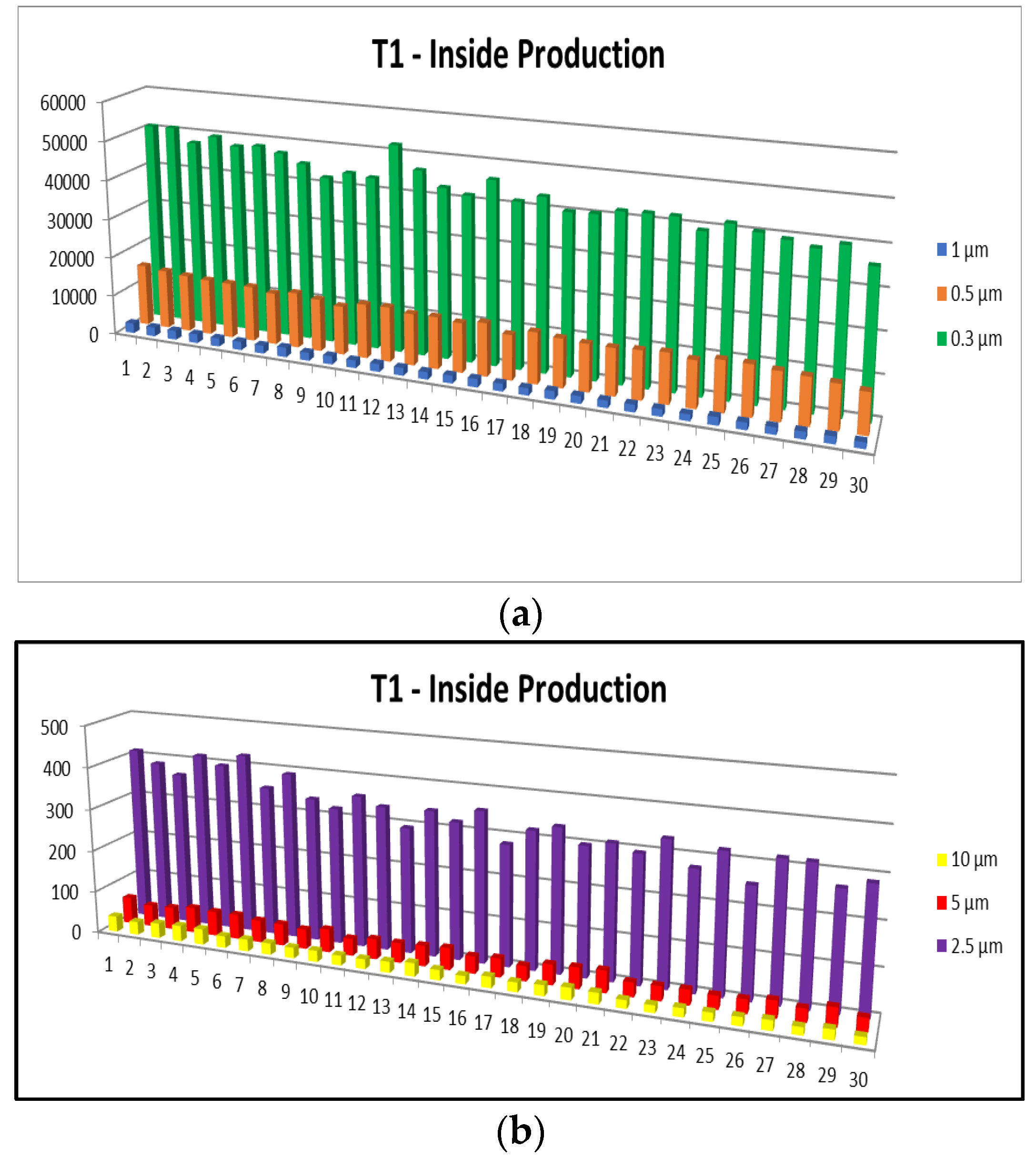
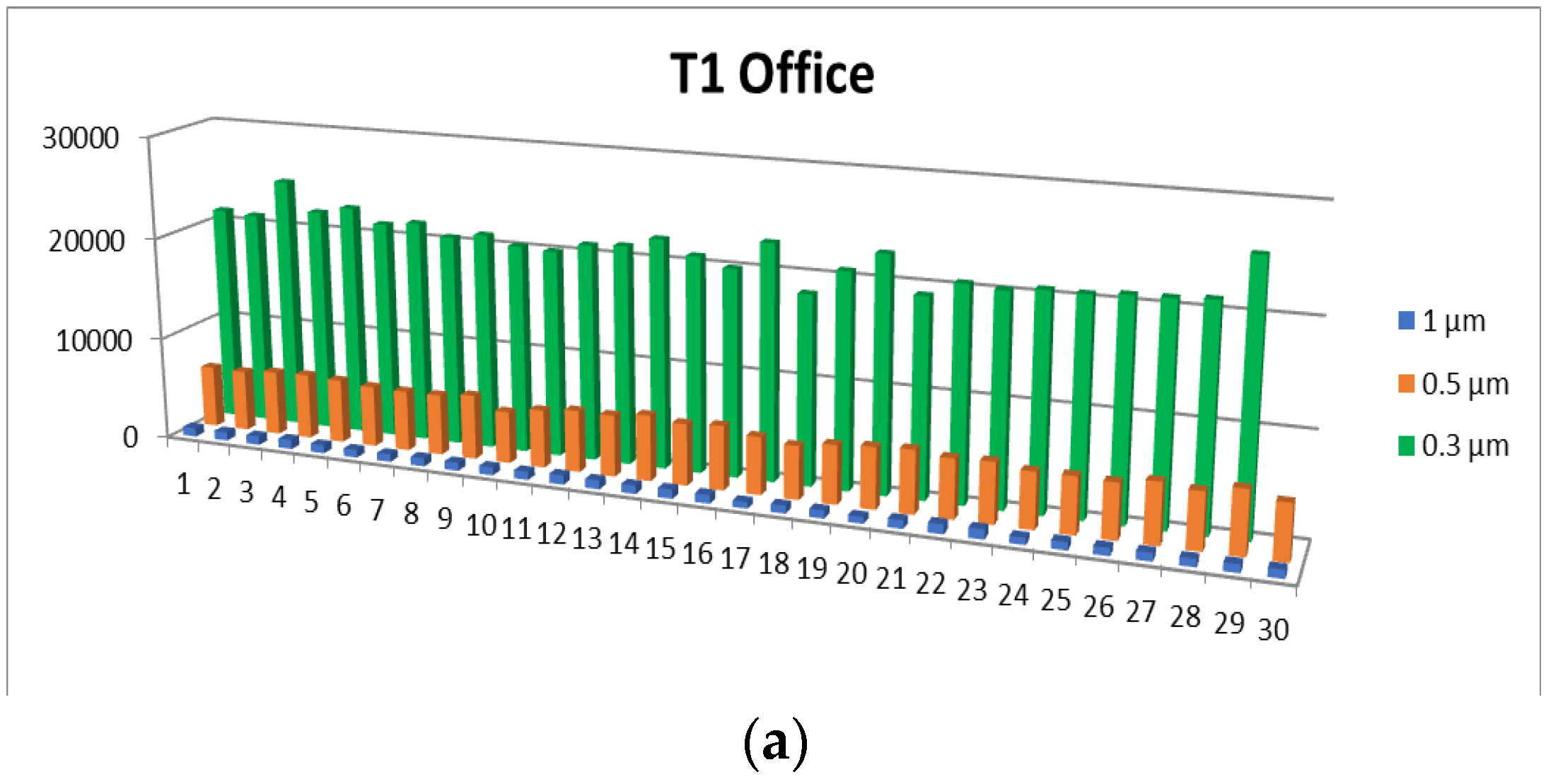
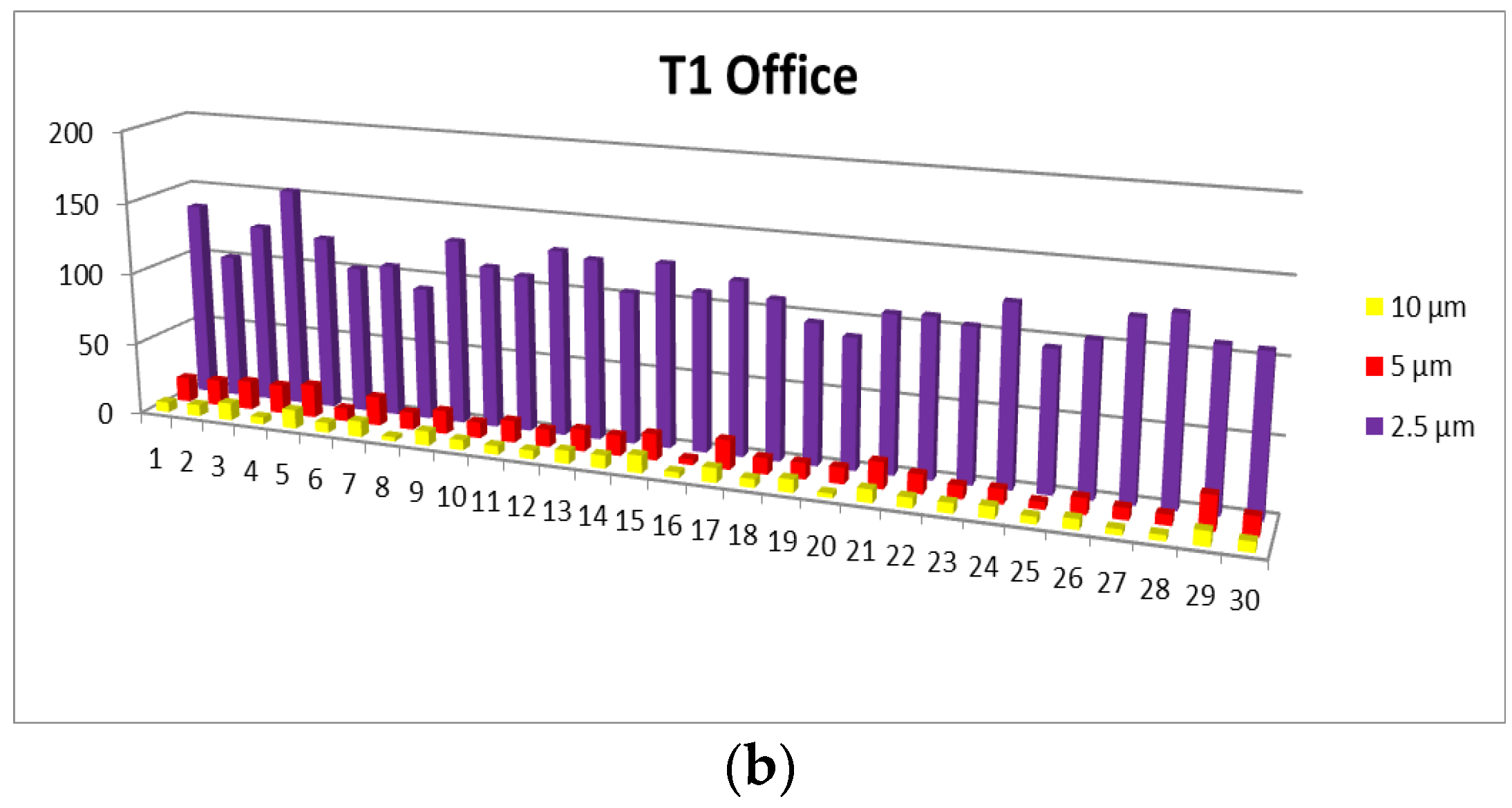
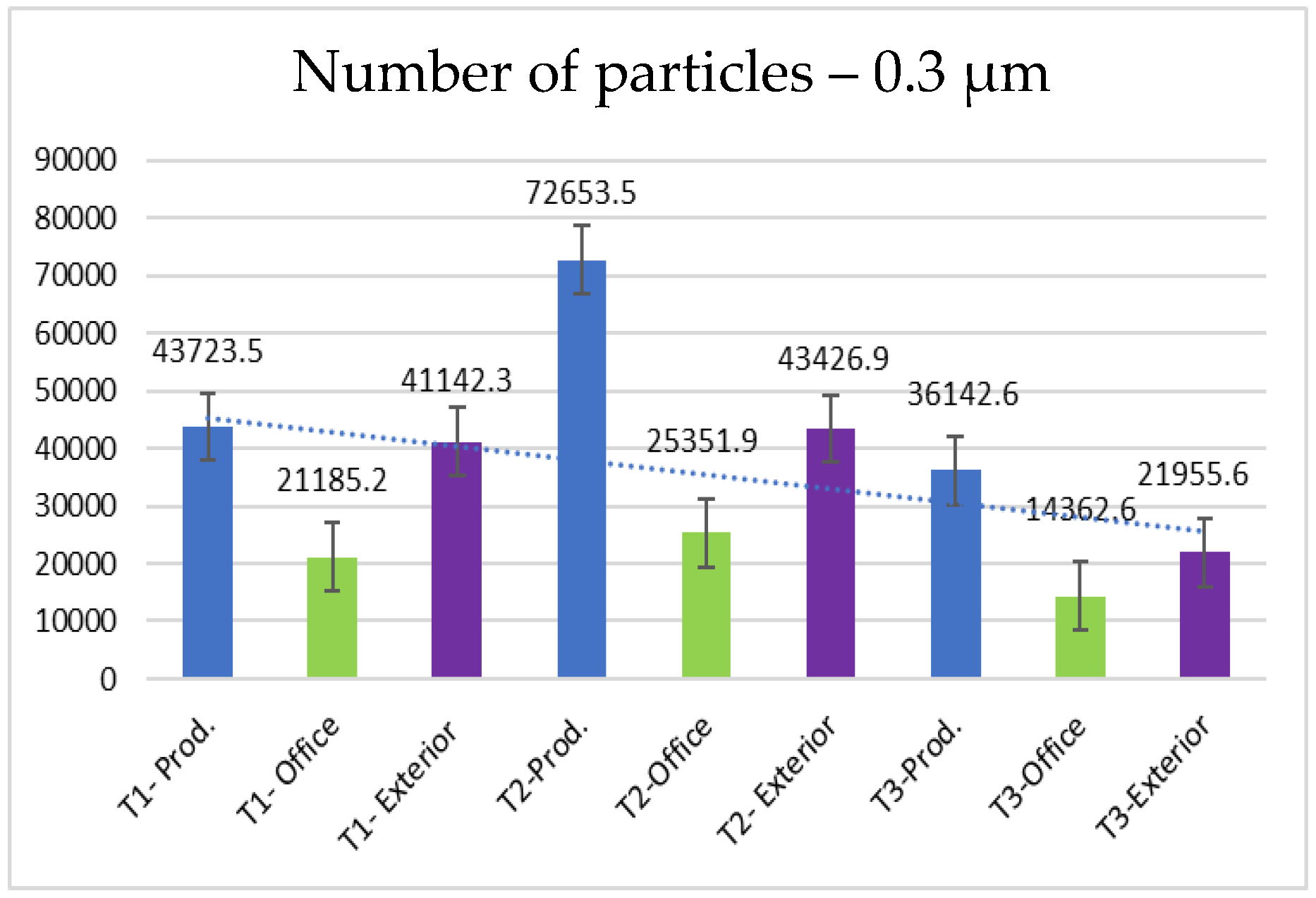
- 0.3 µm particles (Figure 7): These particles are the most numerous in the production area compared to other sizes, with a maximum of 72,653.5 at T2. They are much more frequent than the larger particles. These particles show significantly higher concentrations compared to the company’s exterior.
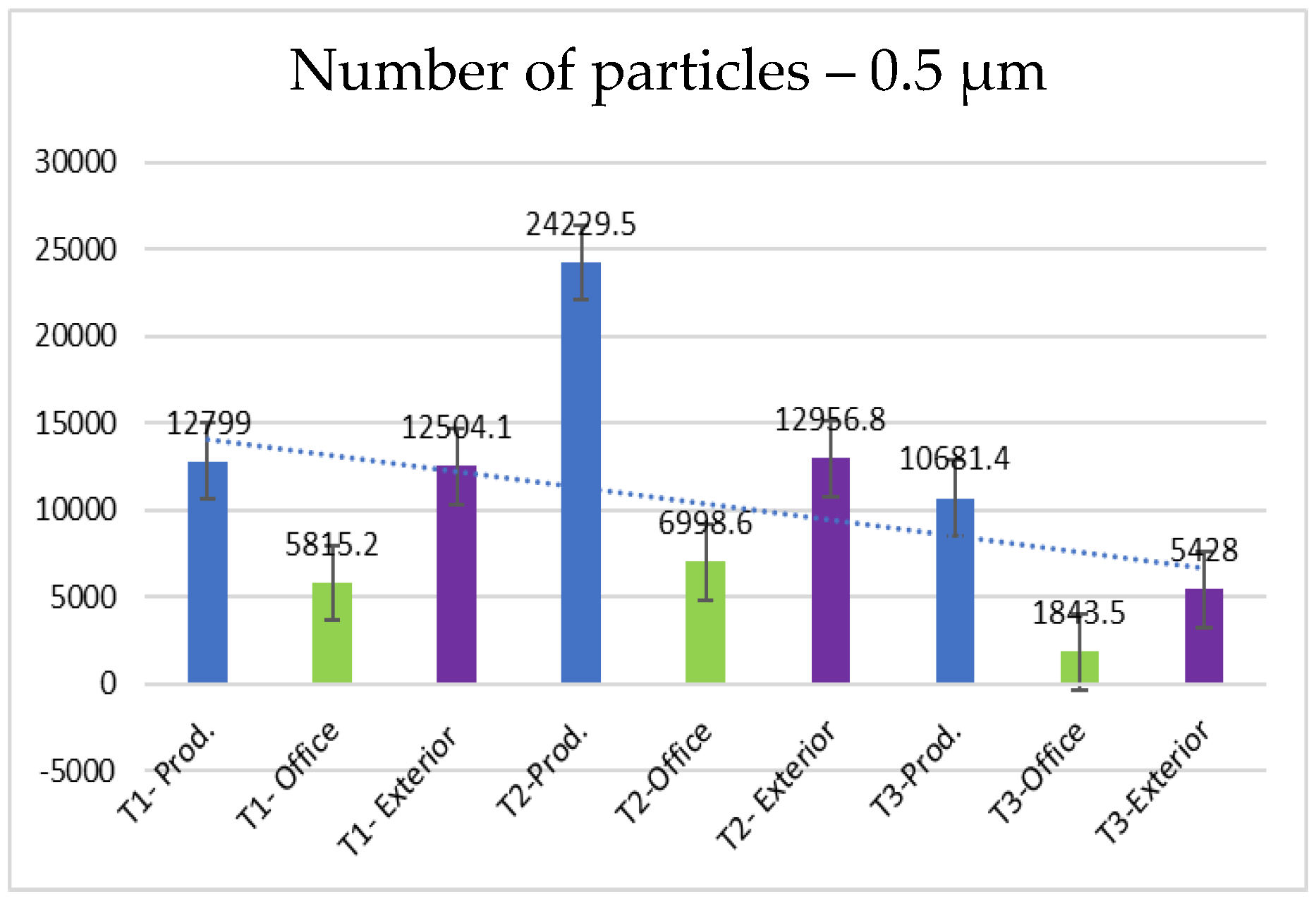
- 0.5 µm particles (Figure 8): These particles also have a large number in the production area (24,229.5 particles at T2), but their concentration is lower than that of the 0.3 µm particles. Compared to the 0.3 µm particles, their number decreases significantly.
- 1.0 µm particles (Figure 9): The concentrations of 1.0 µm particles are lower than those of the 0.3 µm and 0.5 µm particles, with a maximum of 4067.8 particles at T2 in the production area. These are significantly less frequent than the smaller particles, but still more numerous than in the exterior.
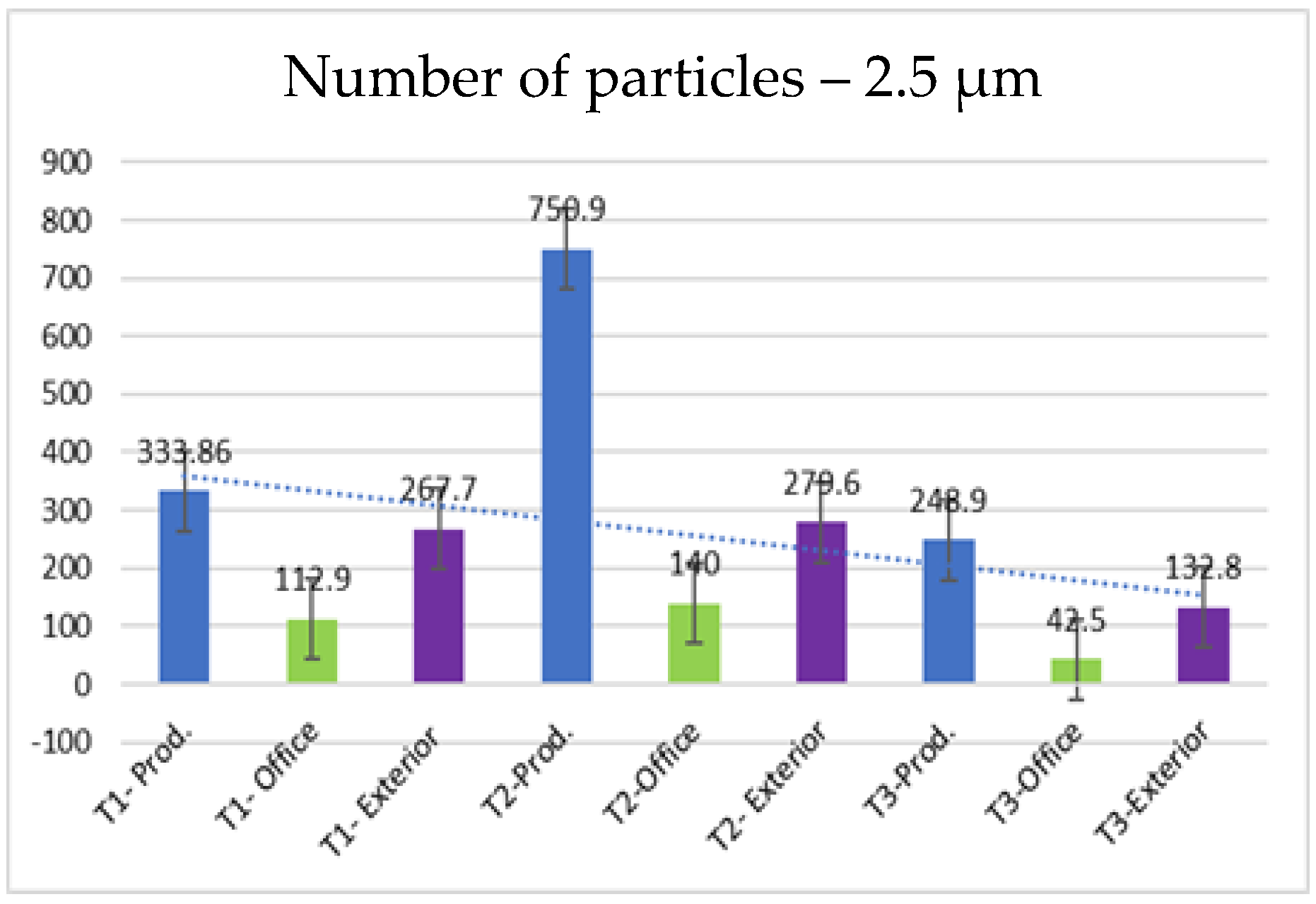
- 2.5 µm particles (Figure 10): The number of 2.5 µm particles is significantly lower than that of 0.3 µm and 0.5 µm particles, but higher than that of 5.0 µm and 10.0 µm particles. At T2, 750.9 particles were recorded in the production area, and the differences with the exterior are not as significant as with smaller particles
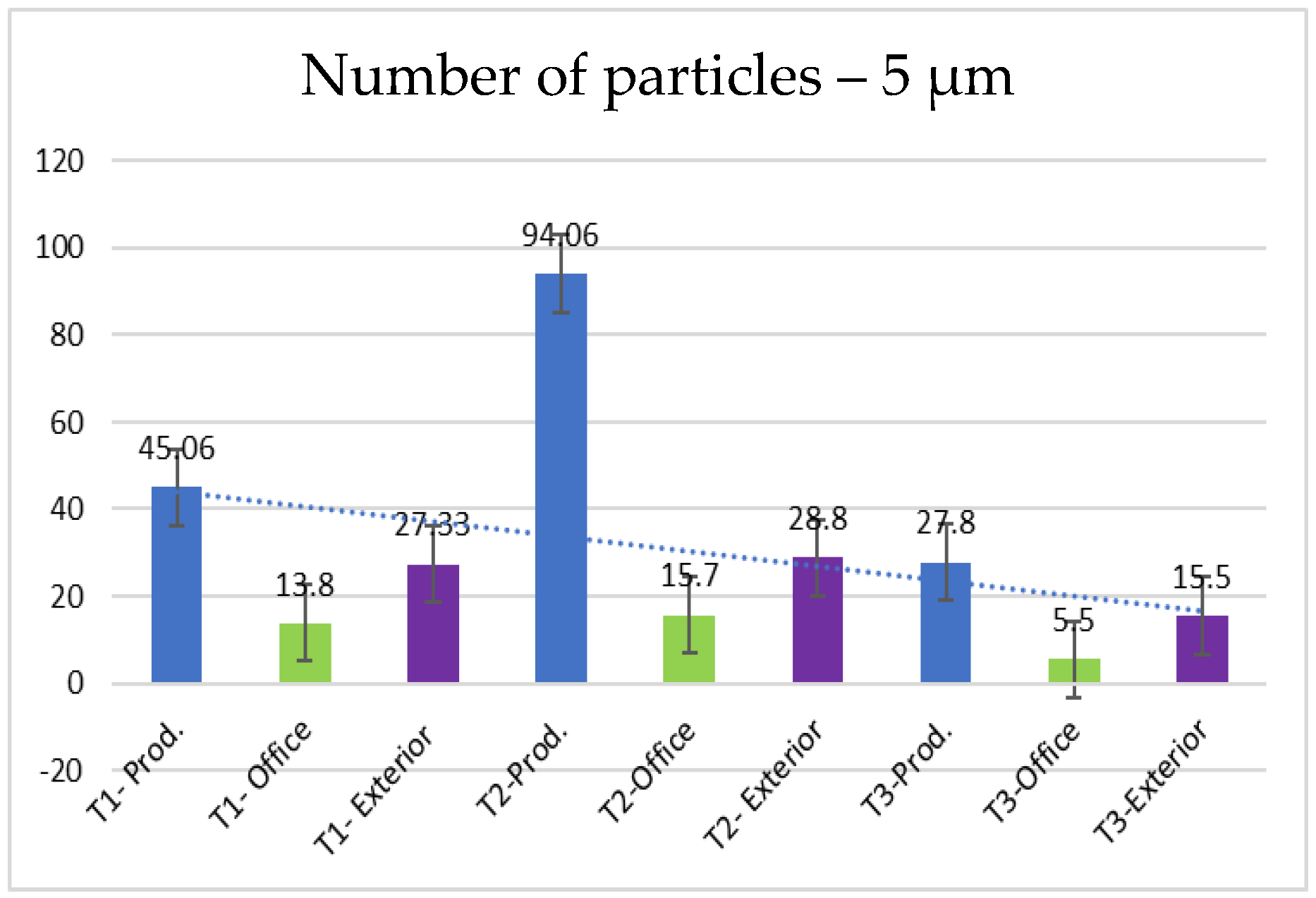
- 5.0 µm particles (Figure 11): The concentrations of 5.0 µm particles are lower than those of 2.5 µm particles, with a maximum of 94.06 particles at T2 in the production area. Compared to smaller particles, these are much less frequent but still more frequent than the 10.0 µm particles.
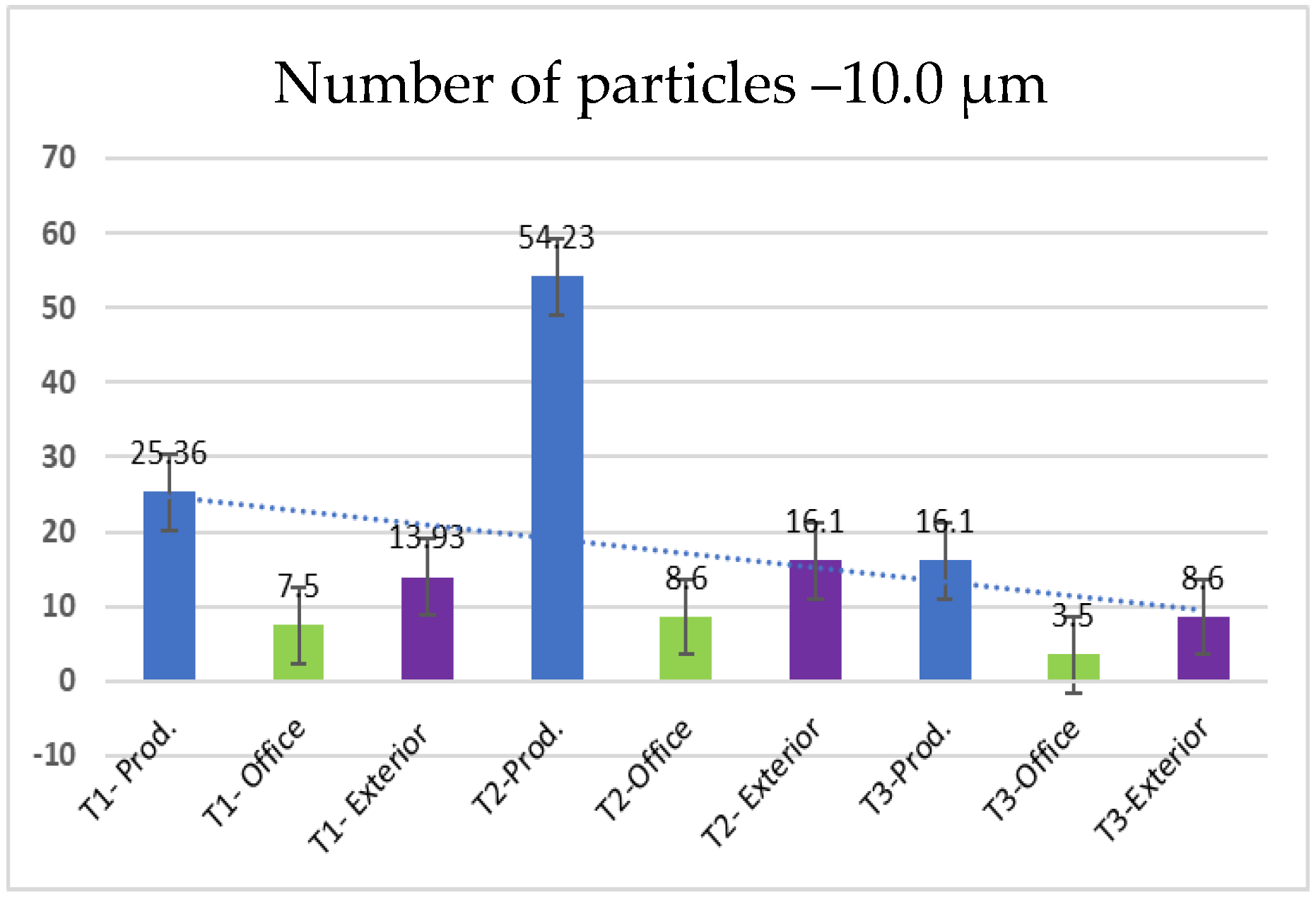
- 10.0 µm particles (Figure 12): The least number of particles is those of 10.0 µm, with a maximum of 56.23 particles at T2 in the production area. Compared to other sizes, their number is much lower, indicating an unequal distribution of particles as their size increases.
3.2. Particles Collection
3.3. TED GC-MS Analysis
4. Conclusions
- ⮚
- The concentration of particles in the production area is significantly higher than in both the office and the exterior for all time intervals, especially for T2 (for all sizes). The smallest particles (0.3 µm) dominate the production environment, with the highest concentration observed during the mid-day shift (T2). This suggests that the textile manufacturing processes, such as cutting and sewing, contribute to a substantial amount of particulate pollution, primarily consisting of fine particles and microfibers, that accumulate as the working day evolves due to poor ventilation.
- ⮚
- Smaller particles (0.3 µm and 0.5 µm) are more abundant in the production environment compared to larger particles (5.0 µm and 10.0 µm). As the particle size increases, the concentration tends to decrease, indicating that smaller particles are more prevalent during the textile manufacturing process. The distribution of particle sizes also exhibits distinct skewness: Smaller particles are more frequent with a left-skewed distribution, while larger particles show a right-skewed distribution. It is hard to tell if this is caused by the work processes, because smaller particles tend to stay in the air longer, or both.
- ⮚
- The concentration of airborne particles varies throughout the day (T1, T2, and T3) and between locations (production, office, and exterior). For example, particle levels in the production area peak around and after midday (T2) and show a significant drop in the morning of Day II (T3). This variation emphasizes the dynamic nature of particle concentration, being influenced by production activities and the time of day.
- ⮚
- The study showed that the filtration method for particle collection was efficient, with collection efficiencies ranging between 71.5% and 96.9%. However, smaller particles (sub-micron-sized) tend to pass through the filters more easily than larger ones, and fluctuations in the airflow rate of the pumps might contribute to discrepancies in the sampled air volume.
- ⮚
- Analysis using TED-GS-MS confirmed the presence of PET and polystyrene (PS) markers in airborne particles, with PET being the most predominant polymer detected. This finding correlates with the use of polyester materials in the manufacturing of sports t-shirts. The detection of these substances suggests that textile processing contributes significantly to the release of polymer-based pollutants into the air: 2.5–16.1% of the sampled particles.
- ⮚
- Although the concentration of collected particles is lower than the legal exposure limit for the respirable/inhalable fraction, the high share of very small particles indicates considerable risks to workers’ health. Such very small particles can penetrate deep into the respiratory system and cause health problems. Some research is currently in progress, and the results and additional information will be available to provide more details regarding particle concentration in various textile workplaces.
- ⮚
- The findings highlight the need for air quality control measures, particularly in the production areas of textile factories. The high concentration of fine particles, especially in the 0.3 µm and 0.5 µm range, calls for effective filtration systems and ventilation strategies to reduce airborne particle exposure, protecting workers’ health and improving air quality in industrial environments. The study suggests that the manufacturing processes in textile factories, especially those dealing with polyester materials, are significant sources of airborne particulate pollution, with implications for both environmental and workplace health. Further studies and better air quality management strategies are necessary to mitigate these risks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, X.; Xue, Y.; Li, J.; Zou, L.; Tang, M. Potential health impact of environmental micro-and nanoplastics pollution. J. Appl. Toxicol. 2020, 40, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, H.R.; Jeon, E.; Yu, H.C.; Lee, S.; Li, J.; Kim, D.H. Evaluation of the biodegradation efficiency of four various types of plastics by Pseudomonas aeruginosa isolated from the gut extract of superworms. Microorganisms 2020, 8, 1341. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.; Narayan Vaidya, A.; Kumar, A.R. Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environ. Sci. Pollut. Res. 2022, 29, 49490–49512. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Microplastics from Textiles—Towards a Circular Economy. EEA Report No. 12/2022. 2022. Available online: https://www.eea.europa.eu/publications/microplastics-from-textiles-towards-a (accessed on 30 March 2025).
- Yang, T.; Gao, M.; Nowack, B. Formation of microplastic fibers and fibrils during abrasion of a representative set of 12 polyester textiles. Sci. Total Environ. 2023, 862, 160758. [Google Scholar] [CrossRef] [PubMed]
- NILU. Micro and Nano Plastics in the Marine-Atmospheric Environment. Available online: https://nilu.com/2022/05/micro-and-nano-plastics-in-the-marine-atmospheric-environment/ (accessed on 30 March 2025).
- Sheraz, M.; Kim, J.; Kim, J. Nano/microplastics in indoor air: A critical review of synthesis routes for toxicity testing and preventative measure strategies. Process Saf. Environ. Prot. 2023, 180, 274–304. [Google Scholar] [CrossRef]
- Shojaee Barjoee, S.; Azimzadeh, H.; Hosseini Sangchi, S. Study of Suspended Air Particles and Health Risk Assessment of Exposure to PM10 and PM2.5 in One of the Textile Industries. J. Health 2020, 11, 144–155. Available online: http://healthjournal.arums.ac.ir/article-1-2159-en.html (accessed on 30 March 2025). [CrossRef]
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef] [PubMed]
- Visileanu, E.; Ene, A.G.; Grosu, M.C.; Miclea, P.T.; Scarlat, R.V. The Structural Influence of Airborne Particles on their Toxicity. Adv. Mater. Lett. 2023, 14, 2302-1718. [Google Scholar] [CrossRef]
- Adegeest, D.-A. European Commission Urged to Deal with Microplastics from Synthetic Textiles, FashionUnited. 2023. Available online: https://fashionunited.com/news/fashion/european-commission-urged-to-deal-with-microplastics-from-synthetic-textiles/2023041853372 (accessed on 30 March 2025).
- Feron, V.J.; de Jong, W.H. Health risks of nanoparticles in the workplace. Lancet 2015, 385, 2299–2300. [Google Scholar]
- Fitzgerald, D.C.; Russo, D. Inhalation and systemic distribution of inhaled nanoparticles: Implications for human health. Nanomedicine 2019, 14, 2409–2422. [Google Scholar]
- Schwabe, S.; Pohl, C. Microplastics in food and the human body: A review. Environ. Int. 2020, 141, 105755. [Google Scholar]
- Prado, J.M.; Vieira, E.A. Nanoparticles in the environment: Distribution, exposure, and biological impact on human health. J. Nanosci. Nanotechnol. 2019, 19, 3914–3926. [Google Scholar]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Song, Z.; Xu, Z.; Zhang, Q. Nanoplastics in the environment: Sources, fate, and impacts. Environ. Sci. Nano 2018, 5, 3–28. [Google Scholar]
- Abdolahpur Monikh, F.; Baun, A.; Hartmann, N.B.; Kortet, R.; Akkanen, J.; Lee, J.S.; Shi, H.; Lahive, E.; Uurasjärvi, E.; Tufenkji, N.; et al. Exposure protocol for ecotoxicity testing of microplastics and nanoplastics. Nat. Protoc. 2023, 18, 3534–3564. [Google Scholar] [CrossRef] [PubMed]


| Time | Temperature, T °C | Moisture, RH% | ||||
|---|---|---|---|---|---|---|
| Office | Production | Exterior | Office | Production | Exterior | |
| T1—7:00 AM—Day 1 | 21.4 | 22.2 | 9.5 | 49.2 | 47.6 | 50.3 |
| T2—14:00 PM—Day 1 | 22.1 | 22.2 | 8.8 | 47.3 | 47.9 | 50.8 |
| T3—7:00 AM—Day 2 | 21.0 | 21.7 | 4.8 | 52.3 | 47.4 | 55.6 |
| Size of the Particle (µm) | T1 | T2 | T3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Production | Office | Exterior | Production | Office | Exterior | Production | Office | Exterior | |
| 0.3 | 43,723.5 | 21,185.2 | 41,142.3 | 72,653.5 | 25,351.6 | 43,426.9 | 36,142.36 | 14,362.6 | 21,955.6 |
| 0.5 | 12,799.0 | 5815.2 | 12,504.1 | 24,229.5 | 6998.6 | 12,956.8 | 10,681.4 | 1843.5 | 5428 |
| 1.0 | 1975.8 | 802.0 | 1866.7 | 4067.9 | 958.3 | 1867.2 | 1536.4 | 263.6 | 857.7 |
| 2.5 | 333.86 | 112.9 | 267.5 | 750.9 | 140.0 | 279.6 | 248.9 | 42.5 | 132.8 |
| 5.0 | 45.066 | 13.8 | 27.33 | 94.06 | 15.7 | 28.8 | 27.8 | 5.5 | 15.5 |
| 10.0 | 25.366 | 7.5 | 13.93 | 54.23 | 8.6 | 16.1 | 16.1 | 3.5 | 8.6 |
| Place and Time of the Particle Collection | Filter Mass (g) | Air Volume, (L) | Airflow, (L/min) | Airborne Particle Concentration (µg/m3) | Collection Efficiency, % | |||
|---|---|---|---|---|---|---|---|---|
| G Initial | G Final | ΔG | ||||||
| Office | T1 | 0.043978 | 0.044026 | 0.000048 | 303.0 | 3.0 | 165.97 | 96.9 |
| T2 | 0.041988 | 0.042055 | 0.000067 | 570.0 | 3.0 | 146.53 | 80.0 | |
| T3 | 0.041005 | 0.041061 | 0.000056 | 480.0 | 3.0 | 137.19 | 86.0 | |
| Production | T1 | 0.041539 | 0.041585 | 0.000046 | 418.2 | 2.5 | 153.84 | 71.2 |
| T2 | 0.041579 | 0.041630 | 0.000051 | 309.1 | 2.5 | 219.13 | 76.0 | |
| T3 | 0.043159 | 0.043170 | 0.000011 | 442.5 | 2.5 | 34.86 | 71.4 | |
| Polymer Mass in the Production Area (µg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | ||||||
| PET | PS | Total | PET | PS | Total | PET | PS | Total |
| 19.9 | 2.4 | 22.3 | 5.4 | 2.5 | 7.9 | 5.6 | 2.5 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visileanu, E.; Altmann, K.; Stepa, R.; Haiducu, M.; Miclea, P.T.; Vladu, A.; Dondea, F.; Grosu, M.C.; Scarlat, R. Comparative Analysis of Airborne Particle Concentrations in Textile Industry Environments Throughout the Workday. Microplastics 2025, 4, 34. https://doi.org/10.3390/microplastics4020034
Visileanu E, Altmann K, Stepa R, Haiducu M, Miclea PT, Vladu A, Dondea F, Grosu MC, Scarlat R. Comparative Analysis of Airborne Particle Concentrations in Textile Industry Environments Throughout the Workday. Microplastics. 2025; 4(2):34. https://doi.org/10.3390/microplastics4020034
Chicago/Turabian StyleVisileanu, Emilia, Korinna Altmann, Raluca Stepa, Maria Haiducu, Paul Tiberiu Miclea, Alina Vladu, Felicia Dondea, Marian Catalin Grosu, and Razvan Scarlat. 2025. "Comparative Analysis of Airborne Particle Concentrations in Textile Industry Environments Throughout the Workday" Microplastics 4, no. 2: 34. https://doi.org/10.3390/microplastics4020034
APA StyleVisileanu, E., Altmann, K., Stepa, R., Haiducu, M., Miclea, P. T., Vladu, A., Dondea, F., Grosu, M. C., & Scarlat, R. (2025). Comparative Analysis of Airborne Particle Concentrations in Textile Industry Environments Throughout the Workday. Microplastics, 4(2), 34. https://doi.org/10.3390/microplastics4020034






