Microplastic Filtration by a Coastal Mangrove Wetland as a Novel Ecosystem Service
Abstract
1. Introduction
2. Study Area
3. Methods
3.1. Field Sampling
3.2. Bulk Density and Organic Matter Content
3.3. Method Validation Tests
3.4. R.O.S.E.S. Microplastic Isolation and Identification
3.5. Contamination Prevention and Correction
3.6. Statistical Tests of MP Concentrations and Size
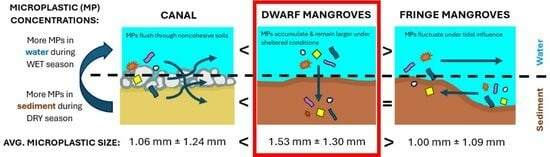
4. Results
4.1. Method Validation and Spiked Test Samples
4.2. Contamination
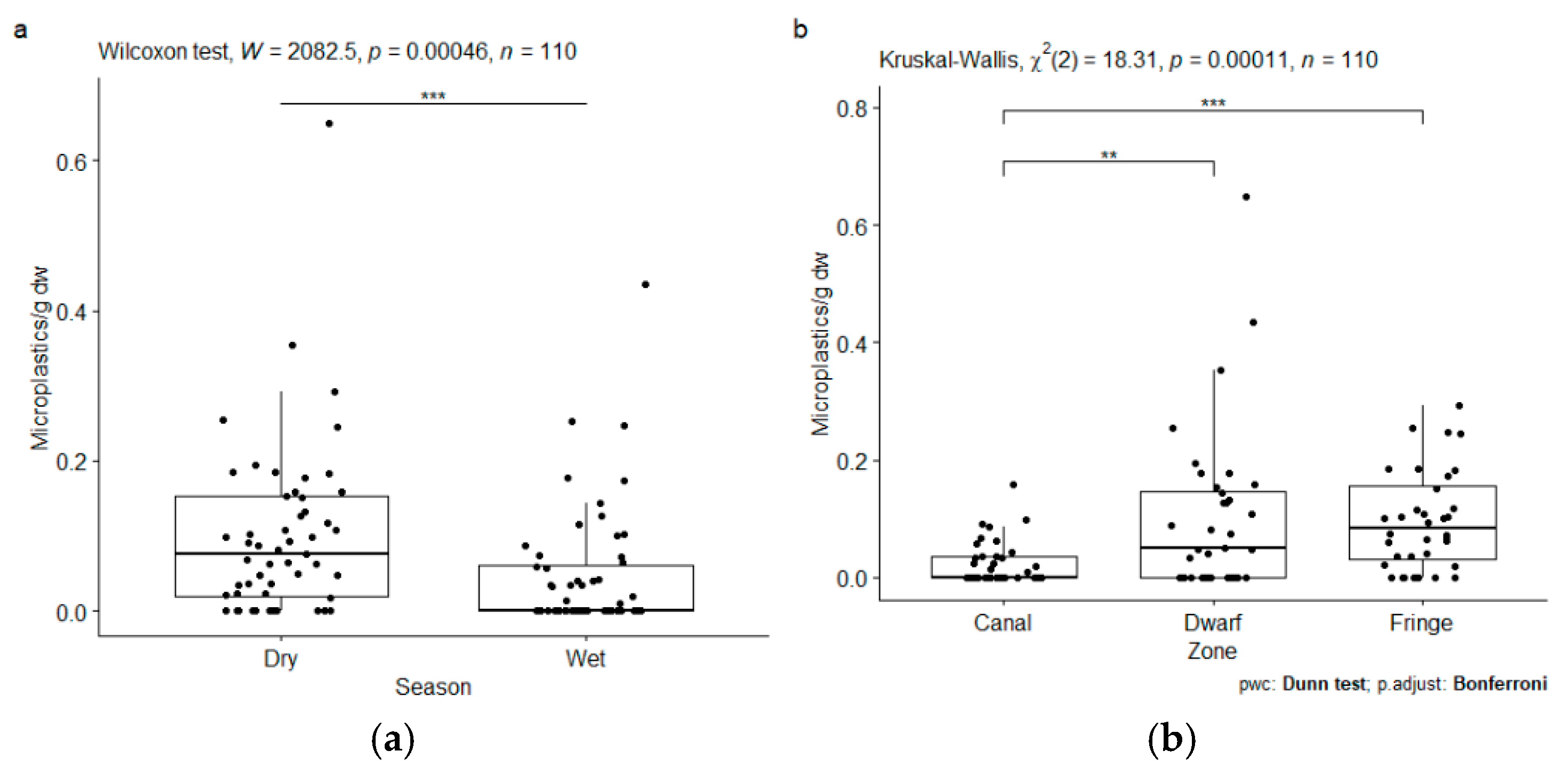
4.3. Microplastic Concentrations in Water
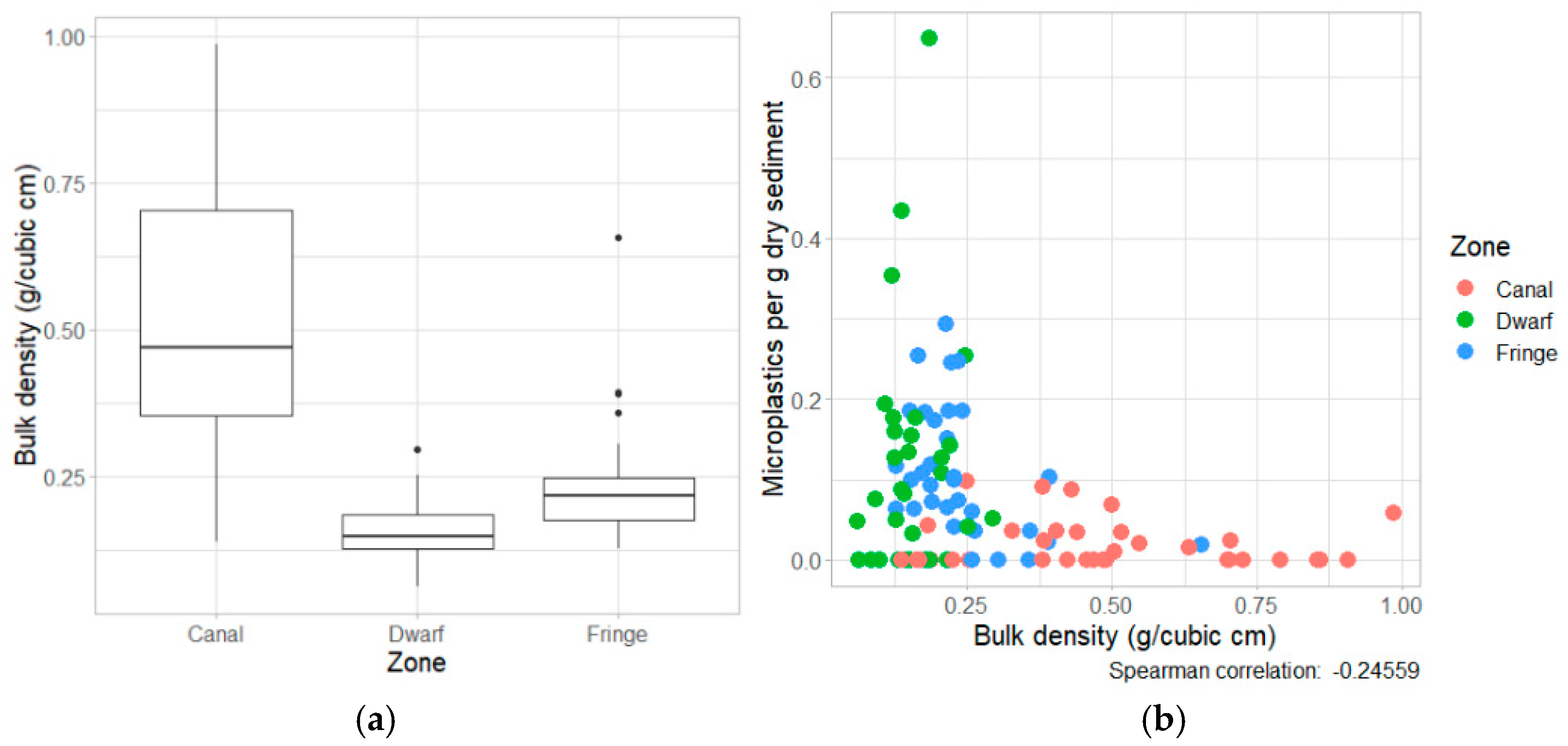
4.4. Microplastic Concentrations in Sediment
4.5. Microplastic Size
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Habitat; WHO. Progress on Wastewater Treatment—Global Status and Acceleration Needs for SDG Indicator 6.3.1.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kumar, R.; Sharma, P.; Bandyopadhyay, S. Evidence of microplastics in wetlands: Extraction and quantification in Freshwater and coastal ecosystems. J. Water Process. Eng. 2021, 40, 101966. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Abalansa, S.; El Mahrad, B.; Vondolia, G.K.; Icely, J.; Newton, A. The Marine Plastic Litter Issue: A Social-Economic Analysis. Sustainability 2020, 12, 8677. [Google Scholar] [CrossRef]
- Khan, A.; Jie, Z.; Wang, J.; Nepal, J.; Ullah, N.; Zhao, Z.-Y.; Wang, P.-Y.; Ahmad, W.; Khan, A.; Wang, W.; et al. Ecological risks of microplastics contamination with green solutions and future perspectives. Sci. Total Environ. 2023, 899, 165688. [Google Scholar] [CrossRef]
- Ismanto, A.; Hadibarata, T.; Kristanti, R.A.; Zainuri, M.; Sugianto, D.N.; Kusumastuti, W.; Anindita, M.A. Microplastics in ecological system: Their prevalence, health effects, and remediation. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101007. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Viehman, S.; Pluym, J.L.V.; Schellinger, J. Characterization of marine debris in North Carolina salt marshes. Mar. Pollut. Bull. 2011, 62, 2771–2779. [Google Scholar] [CrossRef]
- Brennan, E.; Wilcox, C.; Hardesty, B.D. Connecting flux, deposition and resuspension in coastal debris surveys. Sci. Total Environ. 2018, 644, 1019–1026. [Google Scholar] [CrossRef]
- Cordeiro, C.; Costa, T. Evaluation of solid residues removed from a mangrove swamp in the São Vicente Estuary, SP, Brazil. Mar. Pollut. Bull. 2010, 60, 1762–1767. [Google Scholar] [CrossRef]
- Paduani, M.; Ross, M.; Odom, G. Mangrove Forests of Biscayne Bay, FL, USA may Act as Sinks for Plastic Debris. Wetlands 2024, 44, 32. [Google Scholar] [CrossRef]
- Martin, C.; Almahasheer, H.; Duarte, C.M. Mangrove forests as traps for marine litter. Environ. Pollut. 2019, 247, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, G.; Scalici, M. Riparian vegetation as a trap for plastic litter. Environ. Pollut. 2022, 292, 118410. [Google Scholar] [CrossRef]
- Olivelli, A.; Hardesty, B.D.; Wilcox, C. Coastal margins and backshores represent a major sink for marine debris: Insights from a continental-scale analysis. Environ. Res. Lett. 2020, 15, 074037. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; González-Fernández, D.; Defeo, O.; Neal, W.; Galgani, F. Rethinking plastic entrapment: Misconceptions and implications for ecosystem services in coastal habitats. Mar. Pollut. Bull. 2024, 205, 116665. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yuan, J.; Lu, Z.; Xu, J.; He, Y. An enlarging ecological risk: Review on co-occurrence and migration of microplastics and microplastic-carrying organic pollutants in natural and constructed wetlands. Sci. Total Environ. 2022, 837, 155772. [Google Scholar] [CrossRef]
- Noman, A.; Adyel, T.M.; Trevathan-Tackett, S.; Macreadie, P.I. Plastic Paradox in Blue Carbon Ecosystems. Environ. Sci. Technol. 2024, 58, 4469–4475. [Google Scholar] [CrossRef]
- Watson, S.C.; Paterson, D.M.; Queirós, A.M.; Rees, A.P.; Stephens, N.; Widdicombe, S.; Beaumont, N.J. A conceptual framework for assessing the ecosystem service of waste remediation: In the marine environment. Ecosyst. Serv. 2016, 20, 69–81. [Google Scholar] [CrossRef]
- Deng, J.; Guo, P.; Zhang, X.; Su, H.; Zhang, Y.; Wu, Y.; Li, Y. Microplastics and accumulated heavy metals in restored mangrove wetland surface sediments at Jinjiang Estuary (Fujian, China). Mar. Pollut. Bull. 2020, 159, 111482. [Google Scholar] [CrossRef]
- Vogelsang, C.; Lusher, A.; Dadkhah, M.E.; Sundvor, I.; Umar, M.; Ranneklev, S.B.; Eidsvoll, D.; Meland, S. Microplastics in Road Dust—Characteristics, Pathways and Measures. 7361. 2019. Available online: https://toi.brage.unit.no/toi-xmlui/handle/11250/2670146 (accessed on 28 January 2024).
- Harris, P.T. The fate of microplastic in marine sedimentary environments: A review and synthesis. Mar. Pollut. Bull. 2020, 158, 111398. [Google Scholar] [CrossRef]
- Paduani, M. Microplastics as novel sedimentary particles in coastal wetlands: A review. Mar. Pollut. Bull. 2020, 161, 111739. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic Disguising As Soil Carbon Storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef] [PubMed]
- Hall, E. Blue Carbon and Microplastic Sequestration in Coastal Habitats of the Philippines; University of Rhode Island: Kingston, RI, USA, 2023. [Google Scholar]
- Martin, C.; Baalkhuyur, F.; Valluzzi, L.; Saderne, V.; Cusack, M.; Almahasheer, H.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.; Arias-Ortiz, A.; et al. Exponential increase of plastic burial in mangrove sediments as a major plastic sink. Sci. Adv. 2020, 6, eaaz5593. [Google Scholar] [CrossRef] [PubMed]
- Adaro, M.E.; Ronda, A.C. Natural filters of marine microplastic pollution: Implications for plants and submerged environments. Environ. Adv. 2024, 16, 100535. [Google Scholar] [CrossRef]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.; Jin, W.; He, X. From source to sink: Review and prospects of microplastics in wetland ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.R.; Lu, H.-C.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Canals, M.; de Haan, W.P.; Romero, J.; Veny, M. Seagrasses provide a novel ecosystem service by trapping marine plastics. Sci. Rep. 2021, 11, 254. [Google Scholar] [CrossRef]
- Ogbuagu, C.C.; Kassem, H.; Udiba, U.U.; Stead, J.L.; Cundy, A.B. Role of saltmarsh systems in estuarine trapping of microplastics. Sci. Rep. 2022, 12, 15546. [Google Scholar] [CrossRef]
- Nguyễn, T.N.; Trương, V.V.; Phạm, T.T. Methodology for Assessing the Role of Mangroves in Trace Metal(loid) Filtration to Develop a Mechanism of Payments for Environmental Services for Mangroves in Vietnam (268); Center for International Forestry Research (CIFOR) and World Agroforestry Centre (ICRAF): Bogor, Indonesia, 2020. [Google Scholar]
- Chen, H.; Ivanoff, D.; Pietro, K. Long-term phosphorus removal in the Everglades stormwater treatment areas of South Florida in the United States. Ecol. Eng. 2015, 79, 158–168. [Google Scholar] [CrossRef]
- Armstrong, C.; Piccone, T.; Dombrowski, J. The largest constructed treatment wetland project in the world: The story of the Everglades stormwater treatment areas. Ecol. Eng. 2023, 193, 107005. [Google Scholar] [CrossRef]
- Vymazal, J. The Historical Development of Constructed Wetlands for Wastewater Treatment. Land 2022, 11, 174. [Google Scholar] [CrossRef]
- Harris, P.; Westerveld, L.; Nyberg, B.; Maes, T.; Macmillan-Lawler, M.; Appelquist, L. Exposure of coastal environments to river-sourced plastic pollution. Sci. Total Environ. 2021, 769, 145222. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, X.; Xu, C.; Perianen, Y.D.; Hu, J.; Holmer, M. Seagrass beds acting as a trap of microplastics—Emerging hotspot in the coastal region? Environ. Pollut. 2020, 257, 113450. [Google Scholar] [CrossRef] [PubMed]
- López, A.G.; Najjar, R.G.; Friedrichs, M.A.M.; Hickner, M.A.; Wardrop, D.H. Estuaries as Filters for Riverine Microplastics: Simulations in a Large, Coastal-Plain Estuary. Front. Mar. Sci. 2021, 8, 1200. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Lindeque, P.K.; Miliou, A.; Tolhurst, T.J.; Cole, M. Microplastic shape influences fate in vegetated wetlands. Environ. Pollut. 2024, 345, 123492. [Google Scholar] [CrossRef]
- Xia, F.; Liu, H.; Zhang, J.; Wang, D. Migration characteristics of microplastics based on source-sink investigation in a typical urban wetland. Water Res. 2022, 213, 118154. [Google Scholar] [CrossRef]
- Drummond, J.D.; Nel, H.A.; Packman, A.I.; Krause, S. Significance of Hyporheic Exchange for Predicting Microplastic Fate in Rivers. Environ. Sci. Technol. Lett. 2020, 7, 727–732. [Google Scholar] [CrossRef]
- Iribarne, O.; Botto, F.; Martinetto, P.; Gutierrez, J.L. The Role of Burrows of the SW Atlantic Intertidal Crab Chasmagnathus granulata in Trapping Debris. Mar. Pollut. Bull. 2000, 40, 1057–1062. [Google Scholar] [CrossRef]
- Accinelli, C.; Saccà, M.L.; Mencarelli, M.; Vicari, A. Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere 2012, 89, 136–143. [Google Scholar] [CrossRef]
- Kathiresan, K. Polythene and Plastics-Degrading Microbes from the Mangrove Soil. Revista de Biología Tropical, 51. 2003. Available online: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0034-77442003000300003 (accessed on 28 January 2024).
- Sridewi, N.; Bhubalan, K.; Sudesh, K. Degradation of commercially important polyhydroxyalkanoates in tropical mangrove ecosystem. Polym. Degrad. Stab. 2006, 91, 2931–2940. [Google Scholar] [CrossRef]
- Kumar, S.; Hatha, A.; Christi, K. Diversity and effectiveness of tropical mangrove soil microflora on the degradation of polythene carry bags. Rev. De Biol. Trop. 2007, 55, 777–786. [Google Scholar] [CrossRef][Green Version]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef] [PubMed]
- Stang, C.; Mohamed, B.A.; Li, L.Y. Microplastic removal from urban stormwater: Current treatments and research gaps. J. Environ. Manag. 2022, 317, 115510. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Ziajahromi, S.; Locke, A.; Neale, P.A.; Leusch, F.D. Microplastics profile in constructed wetlands: Distribution, retention and implications. Environ. Pollut. 2022, 313, 120079. [Google Scholar] [CrossRef] [PubMed]
- García-Haba, E.; Hernández-Crespo, C.; Martín, M.; Andrés-Doménech, I. The role of different sustainable urban drainage systems in removing microplastics from urban runoff: A review. J. Clean. Prod. 2023, 411, 137197. [Google Scholar] [CrossRef]
- Österlund, H.; Blecken, G.; Lange, K.; Marsalek, J.; Gopinath, K.; Viklander, M. Microplastics in urban catchments: Review of sources, pathways, and entry into stormwater. Sci. Total Environ. 2023, 858, 159781. [Google Scholar] [CrossRef]
- Koutnik, V.S.; Leonard, J.; Glasman, J.B.; Brar, J.; Koydemir, H.C.; Novoselov, A.; Bertel, R.; Tseng, D.; Ozcan, A.; Ravi, S.; et al. Microplastics retained in stormwater control measures: Where do they come from and where do they go? Water Res. 2022, 210, 118008. [Google Scholar] [CrossRef]
- Gilbreath, A.; McKee, L.; Shimabuku, I.; Lin, D.; Werbowski, L.M.; Zhu, X.; Grbic, J.; Rochman, C. Multiyear Water Quality Performance and Mass Accumulation of PCBs, Mercury, Methylmercury, Copper, and Microplastics in a Bioretention Rain Garden. J. Sustain. Water Built Environ. 2019, 5, 04019004. [Google Scholar] [CrossRef]
- Ocean Conservancy; International Coastal Cleanup. We Clean On 2021 Report. 2021. Available online: https://oceanconservancy.org/wp-content/uploads/2021/09/2020-ICC-Report_Web_FINAL-0909.pdf (accessed on 12 February 2024).
- Millette, N.C.; Kelble, C.; Linhoss, A.; Ashby, S.; Visser, L. Using Spatial Variability in the Rate of Change of Chlorophyll a to Improve Water Quality Management in a Subtropical Oligotrophic Estuary. Estuaries Coasts 2019, 42, 1792–1803. [Google Scholar] [CrossRef]
- Caccia, V.G.; Boyer, J.N. A nutrient loading budget for Biscayne Bay, Florida. Mar. Pollut. Bull. 2007, 54, 994–1008. [Google Scholar] [CrossRef]
- Troxell, K.; Ceccopieri, M.; Gardinali, P. Unraveling the chemical fingerprint of the Miami River sources: Insights from high-resolution mass spectrometry and nontarget analysis. Chemosphere 2024, 349, 140863. [Google Scholar] [CrossRef]
- Kleinschmidt, J.M.; Janosik, A.M. Microplastics in Florida, United States: A Case Study of Quantification and Characterization with Intertidal Snails. Front. Ecol. Evol. 2021, 9, 638. [Google Scholar] [CrossRef]
- Walters, L.J.; Craig, C.A.; Dark, E.; Wayles, J.; Encomio, V.; Coldren, G.; Sailor-Tynes, T.; Fox, D.W.; Zhai, L. Quantifying Spatial and Temporal Trends of Microplastic Pollution in Surface Water and in the Eastern Oyster Crassostrea virginica for a Dynamic Florida Estuary. Environments 2022, 9, 131. [Google Scholar] [CrossRef]
- McEachern, K.; Alegria, H.; Kalagher, A.L.; Hansen, C.; Morrison, S.; Hastings, D. Microplastics in Tampa Bay, Florida: Abundance and variability in estuarine waters and sediments. Mar. Pollut. Bull. 2019, 148, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Durako, M.J.; Hall, M.O. Multivariate Analysis of Water Quality and Benthic Macrophyte Communities in Florida Bay, USA Reveals Hurricane Effects and Susceptibility to Seagrass Die-Off. Front. Plant Sci. 2018, 9, 630. [Google Scholar] [CrossRef]
- Yan, Z.; Kamanmalek, S.; Alamdari, N. Predicting coastal harmful algal blooms using integrated data-driven analysis of environmental factors. Sci. Total Environ. 2024, 912, 169253. [Google Scholar] [CrossRef]
- Wester, J. Public perception of an important urban estuary: Values, attitudes, and policy support in the Biscayne Bay-Miami Social Ecological System. PLoS ONE 2023, 18, e0287930. [Google Scholar] [CrossRef]
- Biscayne Bay Task Force. A Unified Approach to Recovery for a Healthy & Resilient Biscayne Bay. 2020. Available online: https://www.scribd.com/document/474305052/Recommendations-of-the-Biscayne-Bay-Task-Force#download&from_embed (accessed on 3 May 2023).
- U.S. Army Corps of Engineers; South Florida Water Management District. Central and Southern Florida Project Comprehensive Everglades Restoration Plan Project Management Plan. 2020. Available online: https://www.saj.usace.army.mil/BBSEER/ (accessed on 12 February 2024).
- Kibria, G. Impacts of microplastic on fisheries and seafood security—Global analysis and synthesis. Sci. Total Environ. 2023, 904, 166652. [Google Scholar] [CrossRef]
- McAfee, D.; Leung, J.Y.; Connell, S.D. Improving ecological function of polluted coasts under a tide of plastic waste. Front. Ecol. Environ. 2023, 21, 435–442. [Google Scholar] [CrossRef]
- Chiappone, M.; White, A.; Swanson, D.; Miller, S. Occurrence and biological impacts of fishing gear and other marine debris in the Florida Keys. Mar. Pollut. Bull. 2002, 44, 597–604. [Google Scholar] [CrossRef]
- Dario, C.; Molina, R.; Kelly, D.L. Public preferences for coastal adaptation: Economic evidence from a discrete choice experiment for hard structures and nature-based solutions in Miami, Florida. Mar. Policy 2024, 165, 106217. [Google Scholar] [CrossRef]
- Deng, H.; He, J.; Feng, D.; Zhao, Y.; Sun, W.; Yu, H.; Ge, C. Microplastics pollution in mangrove ecosystems: A critical review of current knowledge and future directions. Sci. Total Environ. 2021, 753, 142041. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.; Phlips, E.; Batich, C.; Jackson, M.; Wachnicka, A. Polystyrene microplastic contamination versus microplankton abundances in two lagoons of the Florida Keys. Sci. Rep. 2021, 11, 6029. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Sáez-Zamacona, I.; Silva, D.M.; Rodrigues, S.M.; Pereira, R.; Ramos, S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. [Google Scholar] [CrossRef]
- Davis, S.M.; Childers, D.L.; Lorenz, J.J.; Wanless, H.R.; Hopkins, T.E. A Conceptual Model of Ecological Interactions in the Mangrove Estuaries of the Florida Everglades. Wetlands 2005, 25, 832–842. [Google Scholar] [CrossRef]
- Caccia, V.G.; Boyer, J.N. Spatial patterning of water quality in Biscayne Bay, Florida as a function of land use and water management. Mar. Pollut. Bull. 2005, 50, 1416–1429. [Google Scholar] [CrossRef]
- Ng, B.; Quinete, N.; Maldonado, S.; Lugo, K.; Purrinos, J.; Briceño, H.; Gardinali, P. Understanding the occurrence and distribution of emerging pollutants and endocrine disruptors in sensitive coastal South Florida Ecosystems. Sci. Total Environ. 2021, 757, 143720. [Google Scholar] [CrossRef]
- Charkhian, B.; Baldwin, L.; Deal, C.; Hazelton, D.; Niemeyer, N.; Hill, S.; Possley, J. South Florida Environmental Report-Volume III Appendix 2–3: Annual Permit Report for the Biscayne Bay Coastal Wetlands Project; South Florida Water Management District: West Palm Beach, FL, USA, 2023. [Google Scholar]
- Ross, M.S. The L-31E Surface Water Rediversion Project Final Report: Implementation, Results, and Recommendations. 2003. Available online: http://serc.fiu.edu/ (accessed on 1 September 2020).
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H.; Zhang, K.; Davis, S.E.; Ross, M. Sediment and Nutrient Deposition Associated with Hurricane Wilma in Mangroves of the Florida Coastal Everglades. Estuaries Coasts 2010, 33, 45–58. [Google Scholar] [CrossRef]
- Charles, S.P.; Kominoski, J.S.; Armitage, A.R.; Guo, H.; Weaver, C.A.; Pennings, S.C. Quantifying how changing mangrove cover affects ecosystem carbon storage in coastal wetlands. Ecology 2020, 101, e02916. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Xue, B.; Wang, Y. Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: New implications for location, rhizosphere, and sediment compositions. Environ. Pollut. 2019, 244, 685–692. [Google Scholar] [CrossRef]
- Lo, H.-S.; Xu, X.; Wong, C.-Y.; Cheung, S.-G. Comparisons of microplastic pollution between mudflats and sandy beaches in Hong Kong. Environ. Pollut. 2018, 236, 208–217. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Almuhtaram, H.; McKie, M.J.; Andrews, R.C. Microplastics for Use in Environmental Research. J. Polym. Environ. 2022, 30, 4320–4332. [Google Scholar] [CrossRef]
- Li, R.; Zhang, S.; Zhang, L.; Yu, K.; Wang, S.; Wang, Y. Field Study of the Microplastic Pollution in Sea Snails (Ellobium Chinense) from Mangrove Forest and Their Relationships with Microplastics in Water/Sediment Located on the North of Beibu Gulf. Environ. Pollut. 2020, 263, 114368. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Waddell, E.N.; Lascelles, N.; Conkle, J.L. Microplastic contamination in Corpus Christi Bay blue crabs, Callinectes sapidus. Limnol. Oceanogr. Lett. 2020, 5, 92–102. [Google Scholar] [CrossRef]
- Johnson, A.C.; Ball, H.; Cross, R.; Horton, A.A.; Jürgens, M.D.; Read, D.S.; Vollertsen, J.; Svendsen, C. Identification and Quantification of Microplastics in Potable Water and Their Sources within Water Treatment Works in England and Wales. Environ. Sci. Technol. 2020, 54, 12326–12334. [Google Scholar] [CrossRef]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-automated analysis of microplastics in complex wastewater samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef]
- Tsering, T.; Viitala, M.; Hyvönen, M.; Reinikainen, S.-P.; Mänttäri, M. The assessment of particle selection and blank correction to enhance the analysis of microplastics with Raman microspectroscopy. Sci. Total Environ. 2022, 842, 156804. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 11 December 2023).
- Kassambara, A. _ggpubr: ‘ggplot2’ Based Publication Ready Plots_. R package version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 11 December 2023).
- Way, C.; Hudson, M.D.; Williams, I.D.; Langley, G.J. Evidence of underestimation in microplastic research: A meta-analysis of recovery rate studies. Sci. Total Environ. 2022, 805, 150227. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef]
- Lourenço, P.M.; Serra-Gonçalves, C.; Ferreira, J.L.; Catry, T.; Granadeiro, J.P. Plastic and other microfibers in sediments, macroinvertebrates and shorebirds from three intertidal wetlands of southern Europe and west Africa. Environ. Pollut. 2017, 231, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial Patterns of Plastic Debris along Estuarine Shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Lusher, A.L.; Munno, K.; Hermabessiere, L.; Carr, S. Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization. Appl. Spectrosc. 2020, 74, 1049–1065. [Google Scholar] [CrossRef]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Fraser, M.A.; Chen, L.; Ashar, M.; Huang, W.; Zeng, J.; Zhang, C.; Zhang, D. Occurrence and distribution of microplastics and polychlorinated biphenyls in sediments from the Qiantang River and Hangzhou Bay, China. Ecotoxicol. Environ. Saf. 2020, 196, 110536. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Esmaili, Z.; Mason, S.A.; Vethaak, A.D. The occurrence of microplastic contamination in littoral sediments of the Persian Gulf, Iran. Environ. Sci. Pollut. Res. 2017, 24, 20459–20468. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Drapper, D.; Hornbuckle, A.; Rintoul, L.; Leusch, F.D. Microplastic pollution in a stormwater floating treatment wetland: Detection of tyre particles in sediment. Sci. Total Environ. 2020, 713, 136356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, H.; Xiong, X.; Zhao, E.; Wang, K.; Wu, C. Urban natural wetland as a sink for microplastics: A case from Lalu Wetland in Tibet, China. Sci. Total Environ. 2022, 828, 154399. [Google Scholar] [CrossRef]
- Rasta, M.; Sattari, M.; Taleshi, M.S.; Namin, J.I. Identification and distribution of microplastics in the sediments and surface waters of Anzali Wetland in the Southwest Caspian Sea, Northern Iran. Mar. Pollut. Bull. 2020, 160, 111541. [Google Scholar] [CrossRef]
- Yu, X.; Ladewig, S.; Bao, S.; Toline, C.A.; Whitmire, S.; Chow, A.T. Occurrence and distribution of microplastics at selected coastal sites along the southeastern United States. Sci. Total Environ. 2018, 613–614, 298–305. [Google Scholar] [CrossRef]
- Tunnell, J.W.; Dunning, K.H.; Scheef, L.P.; Swanson, K.M. Measuring plastic pellet (nurdle) abundance on shorelines throughout the Gulf of Mexico using citizen scientists: Establishing a platform for policy-relevant research. Mar. Pollut. Bull. 2020, 151, 110794. [Google Scholar] [CrossRef]
- Grace, J.K.; Duran, E.; Ottinger, M.A.; Woodrey, M.S.; Maness, T.J. Microplastics in the Gulf of Mexico: A Bird’s Eye View. Sustainability 2022, 14, 7849. [Google Scholar] [CrossRef]
- Helcoski, R.; Yonkos, L.T.; Sanchez, A.; Baldwin, A.H. Wetland soil microplastics are negatively related to vegetation cover and stem density. Environ. Pollut. 2020, 256, 113391. [Google Scholar] [CrossRef]
- Yao, W.; Di, D.; Wang, Z.; Liao, Z.; Huang, H.; Mei, K.; Dahlgren, R.A.; Zhang, M.; Shang, X. Micro- and macroplastic accumulation in a newly formed Spartina alterniflora colonized estuarine saltmarsh in southeast China. Mar. Pollut. Bull. 2019, 149, 110636. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Sahoo, B.K.; Das, A.; Nag, S.K.; Manna, R.K.; Behera, B.K.; Samanta, S. Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res. 2021, 192, 116853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Y.; Pang, G.; Jia, X.; Song, Y.; Guo, A.; Wang, A.; Zhang, S.; Ji, M. Microplastic abundance, characteristics and removal in large-scale multi-stage constructed wetlands for effluent polishing in northern China. Chem. Eng. J. 2022, 430, 132752. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuarine Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Ochoa, L.; Chan, J.; Auguste, C.; Arbuckle-Keil, G.; Fahrenfeld, N. Stormwater runoff microplastics: Polymer types, particle size, and factors controlling loading rates. Sci. Total Environ. 2024, 929, 172485. [Google Scholar] [CrossRef]
- Nolte, S.; Koppenaal, E.C.; Esselink, P.; Dijkema, K.S.; Schuerch, M.; De Groot, A.V.; Bakker, J.P.; Temmerman, S. Measuring sedimentation in tidal marshes: A review on methods and their applicability in biogeomorphological studies. J. Coast. Conserv. 2013, 17, 301–325. [Google Scholar] [CrossRef]
- Dierberg, F.E.; DeBusk, T.A.; Kharbanda, M.D.; Potts, J.A.; Grace, K.A.; Jerauld, M.J.; Ivanoff, D.B. Long-term sustainable phosphorus (P) retention in a low-P stormwater wetland for Everglades restoration. Sci. Total Environ. 2021, 756, 143386. [Google Scholar] [CrossRef]
- Walker, W.W. Design basis for Everglades Stormwater Treatment Areas. JAWRA J. Am. Water Resour. Assoc. 1995, 31, 671–685. [Google Scholar] [CrossRef]
- California Water Boards. Policy Handbook Establishing a Standard Method of Testing and Reporting of Microplastics in Drinking Water. 2022. Available online: https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/docs/2022/mp-hndbk.pdf (accessed on 3 May 2023).
- Tetra Tech. Preliminary Conceptual Model for an Ecological Risk Assessment for Microplastics on Striped Bass in the Potomac River Estuary. 2021. Available online: https://d18lev1ok5leia.cloudfront.net/chesapeakebay/documents/FINAL_ERA_02102021.pdf (accessed on 3 May 2023).
- Bodus, B.; O’Malley, K.; Dieter, G.; Gunawardana, C.; McDonald, W. Review of emerging contaminants in green stormwater infrastructure: Antibiotic resistance genes, microplastics, tire wear particles, PFAS, and temperature. Sci. Total Environ. 2024, 906, 167195. [Google Scholar] [CrossRef]
- Alarcon, V.J.; Linhoss, A.C.; Kelble, C.R.; Mickle, P.F.; Fine, A.; Montes, E. Potential challenges for the restoration of Biscayne Bay (Florida, USA) in the face of climate change effects revealed with predictive models. Ocean Coast. Manag. 2023, 247, 106929. [Google Scholar] [CrossRef]
- 125. Public-Sector Green Infrastructure on the Rise, New Report Finds—Stormwater Report. (n.d.). Available online: https://stormwater.wef.org/2023/05/public-sector-green-infrastructure-on-the-rise-new-report-finds/ (accessed on 3 May 2023).
- Burgis, C.R.; Hayes, G.M.; Zhang, W.; Henderson, D.A.; Macko, S.A.; Smith, J.A. Tracking denitrification in green stormwater infrastructure with dual nitrate stable isotopes. Sci. Total Environ. 2020, 747, 141281. [Google Scholar] [CrossRef] [PubMed]
- 2022–2024 Biennial Assessment Draft Verified and Delist Lists|Florida Department of Environmental Protection. (n.d.). Available online: https://floridadep.gov/dear/watershed-assessment-section/content/2022-2024-biennial-assessment-draft-verified-and-delist (accessed on 28 January 2024).
- Peller, J.; Nevers, M.B.; Byappanahalli, M.; Nelson, C.; Babu, B.G.; Evans, M.A.; Kostelnik, E.; Keller, M.; Johnston, J.; Shidler, S. Sequestration of microfibers and other microplastics by green algae, Cladophora, in the US Great Lakes. Environ. Pollut. 2021, 276, 116695. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.G.; Wang, L.-F.; Kuppusamy, S.; Li, Y. Periphytic biofilm: An innovative approach for biodegradation of microplastics. Sci. Total Environ. 2020, 717, 137064. [Google Scholar] [CrossRef] [PubMed]
- Fla. Stat. § 376 et seq. Available online: http://www.leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0300-0399/0376/0376.html (accessed on 10 October 2024).







| Microplastic Identification Criteria |
|---|
| Distinct shape, clean edges, glows brightly, may look translucent |
| No scales, hairs, spots/stripes, bark, or cellular structures |
| Fibers equally thick and not twisted along their length; clean-cut ends |
| Non-natural color (optional) |
| Relatively even or consistent scratch marks/human-made indentations or markings (optional) |
| Zone | n | Min | Max | Mean | sd |
|---|---|---|---|---|---|
| Canal | 47 | 0.077 | 7.26 | 1.06 | 1.24 |
| Dwarf | 113 | 0.102 | 6.18 | 1.53 | 1.30 |
| Fringe | 91 | 0.062 | 6.31 | 1.00 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduani, M.; Ross, M.; Gardinali, P. Microplastic Filtration by a Coastal Mangrove Wetland as a Novel Ecosystem Service. Microplastics 2025, 4, 15. https://doi.org/10.3390/microplastics4020015
Paduani M, Ross M, Gardinali P. Microplastic Filtration by a Coastal Mangrove Wetland as a Novel Ecosystem Service. Microplastics. 2025; 4(2):15. https://doi.org/10.3390/microplastics4020015
Chicago/Turabian StylePaduani, Melinda, Michael Ross, and Piero Gardinali. 2025. "Microplastic Filtration by a Coastal Mangrove Wetland as a Novel Ecosystem Service" Microplastics 4, no. 2: 15. https://doi.org/10.3390/microplastics4020015
APA StylePaduani, M., Ross, M., & Gardinali, P. (2025). Microplastic Filtration by a Coastal Mangrove Wetland as a Novel Ecosystem Service. Microplastics, 4(2), 15. https://doi.org/10.3390/microplastics4020015