Metal–Organic Frameworks (MOFs) for Adsorption and Degradation of Microplastics
Abstract
1. Introduction
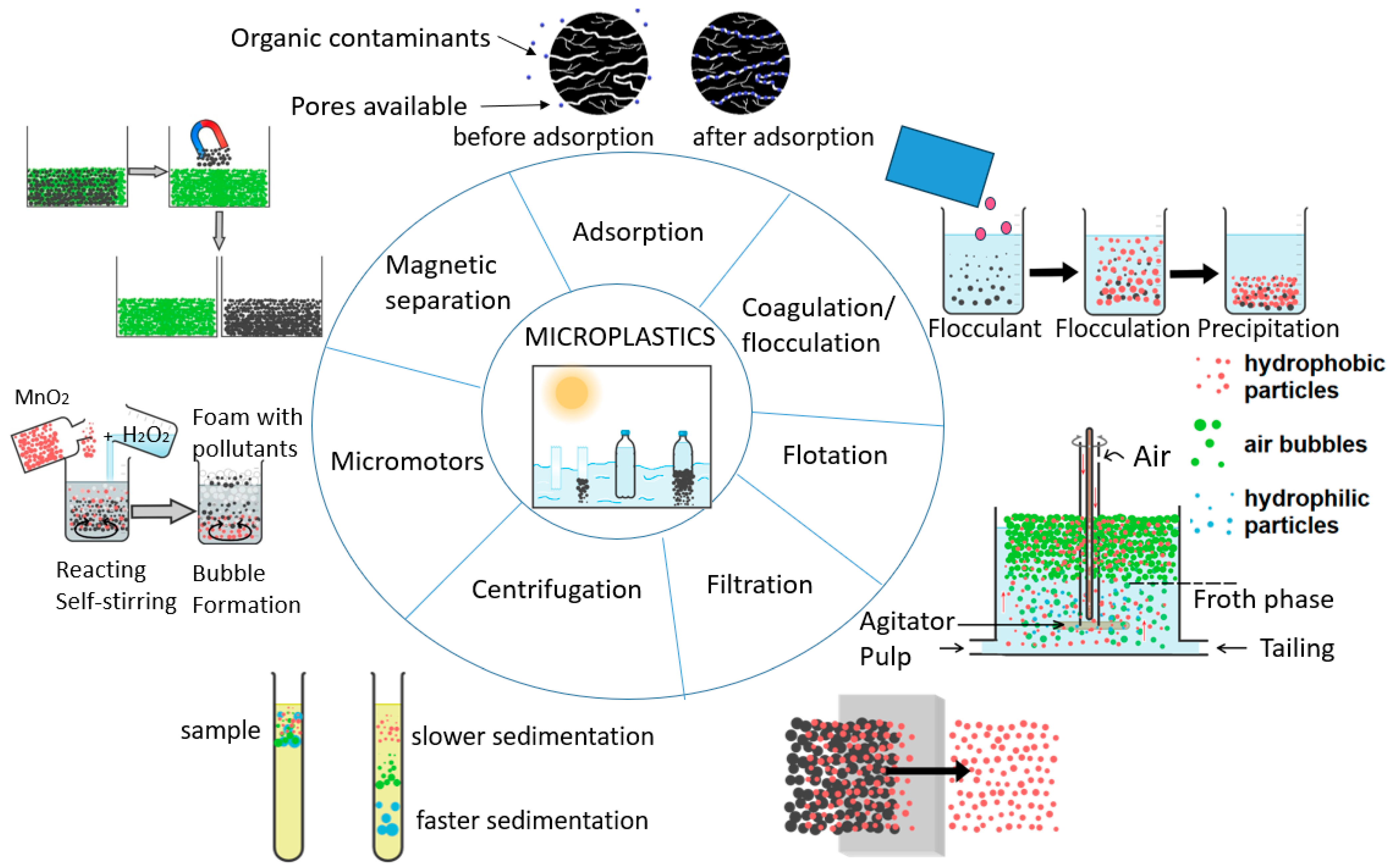
2. Separation Methods
2.1. Chemical Methods
2.1.1. Adsorption with Adsorbent Materials
2.1.2. Coagulation/Flocculation or Electrocoagulation
2.2. Physical Methods
2.2.1. Flotation by Density
2.2.2. Filtration
2.2.3. Centrifugation
2.2.4. Magnetic Separation
2.3. Biological Methods
2.3.1. Bioremediation
2.3.2. Biological Sponge
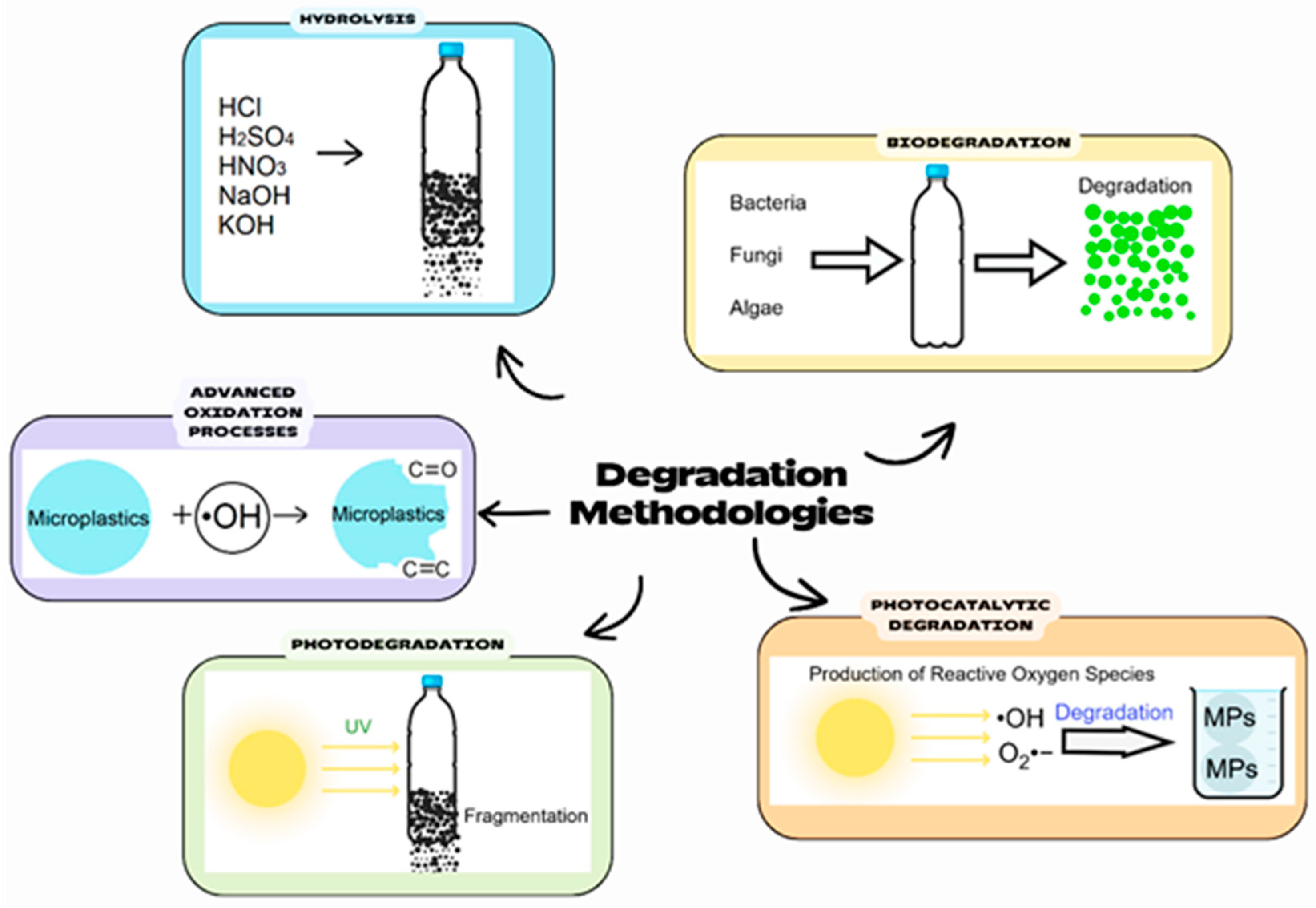
3. Microplastic Degradation
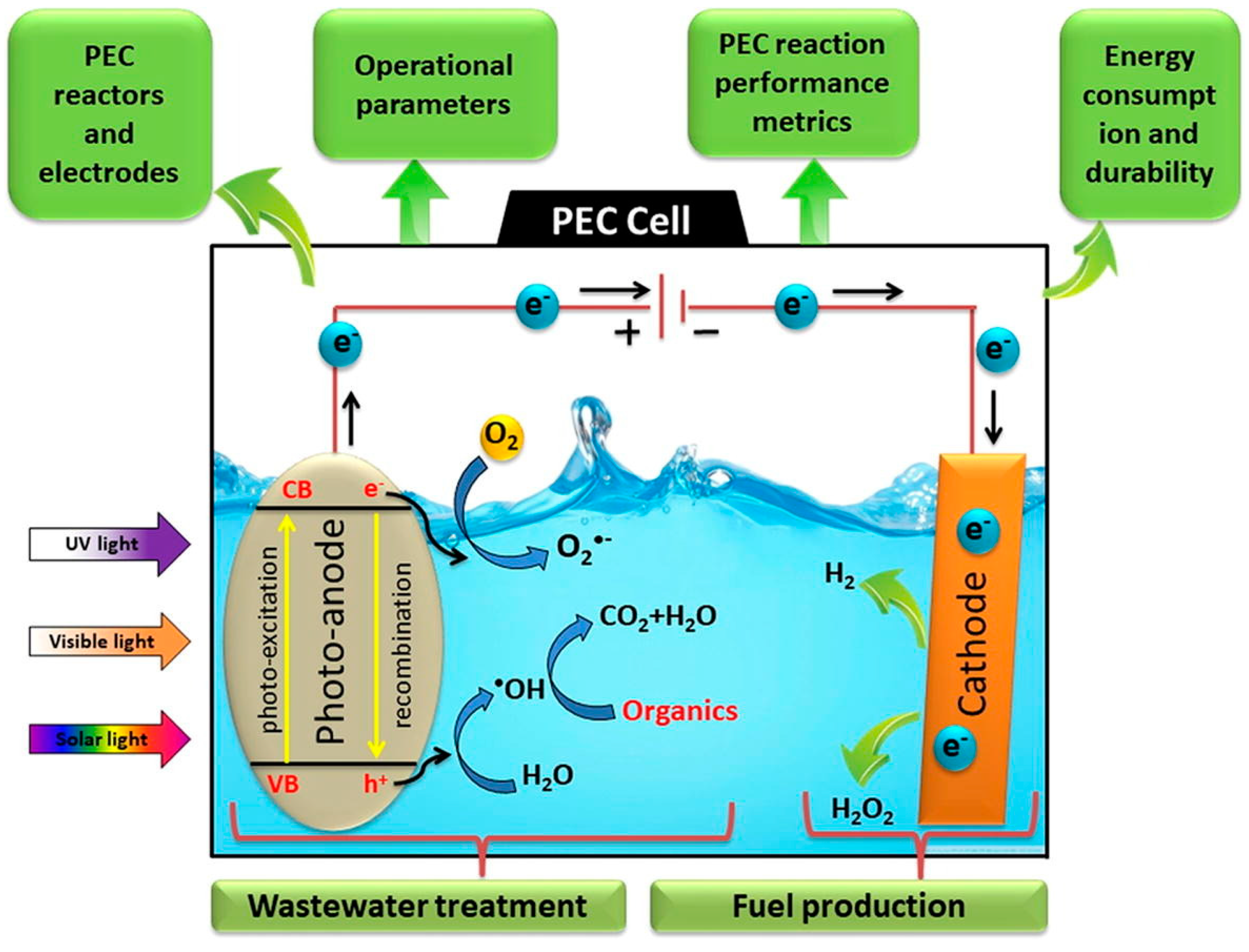
3.1. Photodegradation
3.2. Advanced Oxidation Processes (AOPs)
3.3. Biodegradation
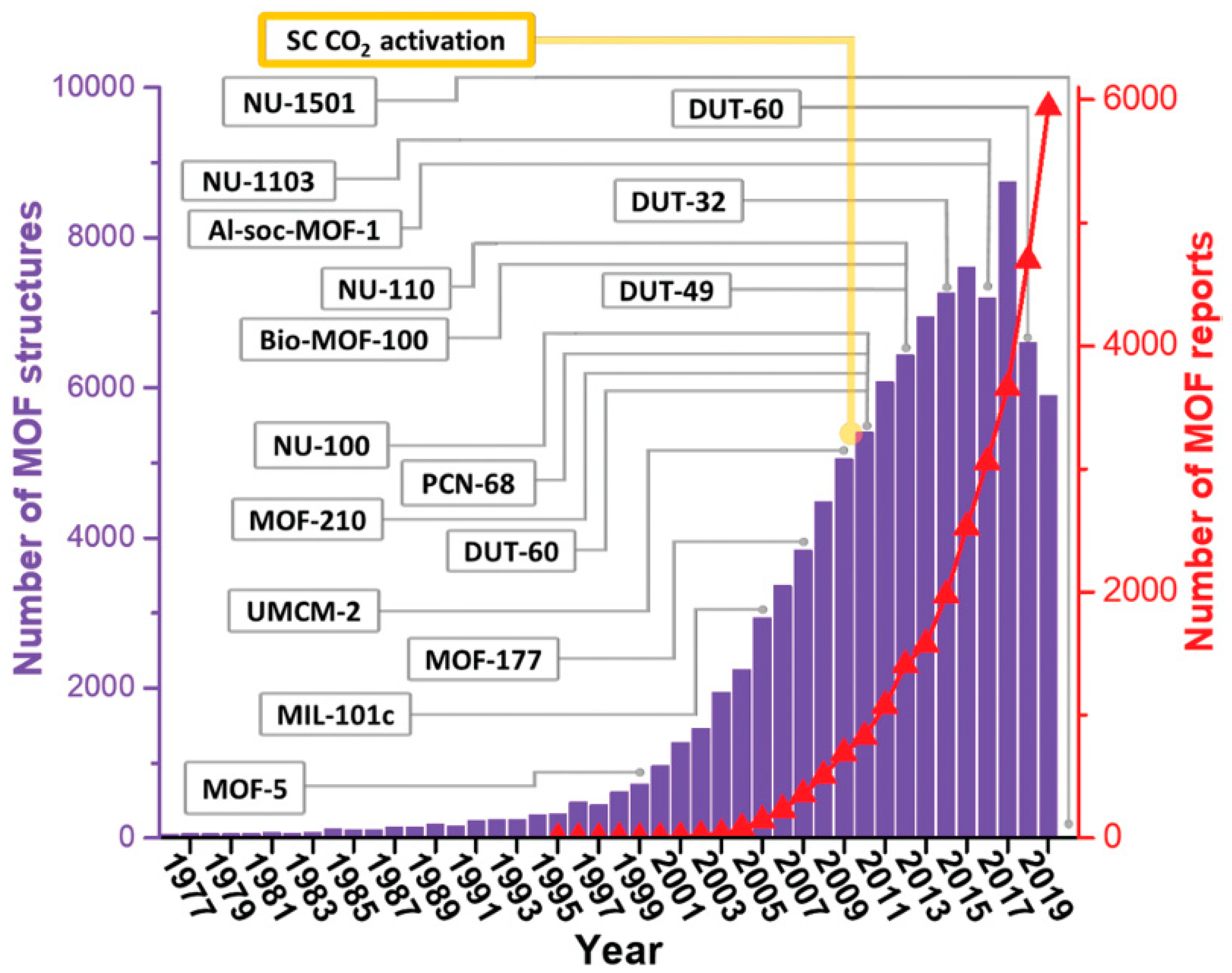
4. Metal–Organic Frameworks (MOFs)
- (a)
- Zeolitic Imidazolate Frameworks (ZIFs): This is an important subclass of MOFs, employing tetrahedrally coordinated divalent cations and imidazolate-based organic ligands [95]. They are characterized by their zeolite-like structure and thermal and chemical stability. ZIFs inherit the structural diversity of MOFs and the thermal and chemical robustness typical of zeolites, making them highly versatile materials that can be applied to energy storage, gas separation, and catalysis [96,97,98]. Their characteristics include acid sensibility, large surface area, and low toxicity [99,100].
- (b)
- Material Institute Lavoisier (MIL) MOFs: These materials, as the name indicates, were synthesized at the Lavoisier Institute [101,102]. They are constituted by trivalent metal cations and carboxylic acid ligands and contain large pores and permanent porosity [103,104]. They are widely used in biomedical applications [105,106,107], hydrogen storage, and remediation processes [108,109,110,111,112].
- (c)
- Porous Coordination Polymers (PCPs): These materials are MOFs with high specific surface areas and modulable pore sizes. Typically synthesized through carboxylic acids, pyridine, and its derivatives as primary building units (PBUs) and transition metal ions as secondary building units (SBUs) [113,114], these materials are employed in gas storage [115,116] and photocatalysis [117,118].
- (d)
- University of Oslo (UiO) MOFs: In this case, carboxylic acids are used as PBUs and Zr6(μ3-O)4(μ3-OH)4 as SBUs; these materials were synthesized for the first time by Cavka et al. [119]. UiO-MOFs can be found as UiO-66, UiO-67, UiO-68, and UiO-69. They have a large and relatively uniform pore size and specific surface area, with high strength and active ZrO groups, which have been widely studied and employed in post-modification and other applications. The post-modification of UiO-MOFs with functionalized specific groups in their linker is carried out to improve the active site for the adsorption of heavy metal ions [120]. UiO-MOFs and UiO-based materials are being studied as photocatalysts in the degradation of emergent contaminants or for CO2 reduction [121,122,123,124].
4.1. Metal–Organic Framework Synthesis
4.1.1. Solvothermal Method
4.1.2. Electrochemical Method
4.1.3. Other Methodologies
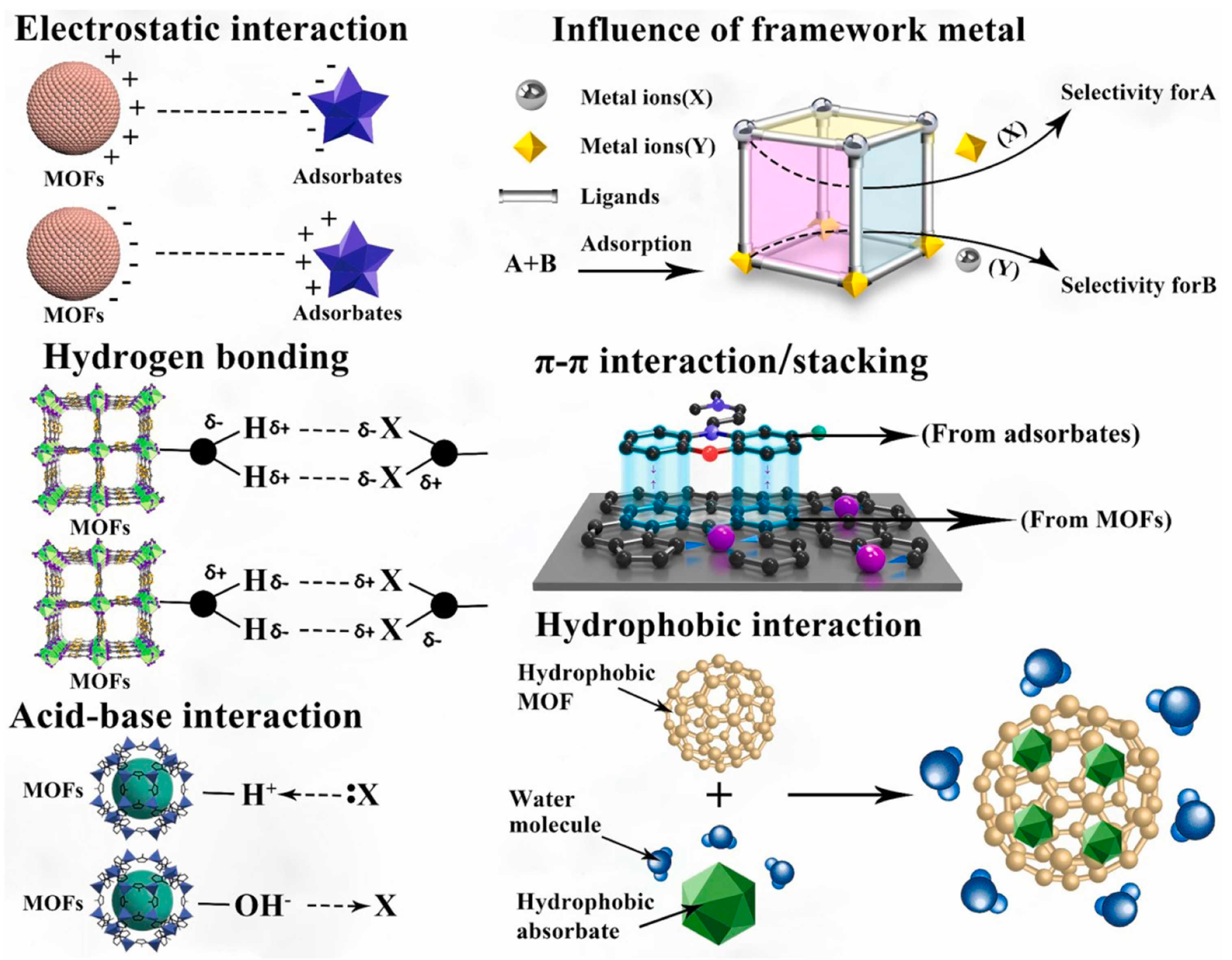
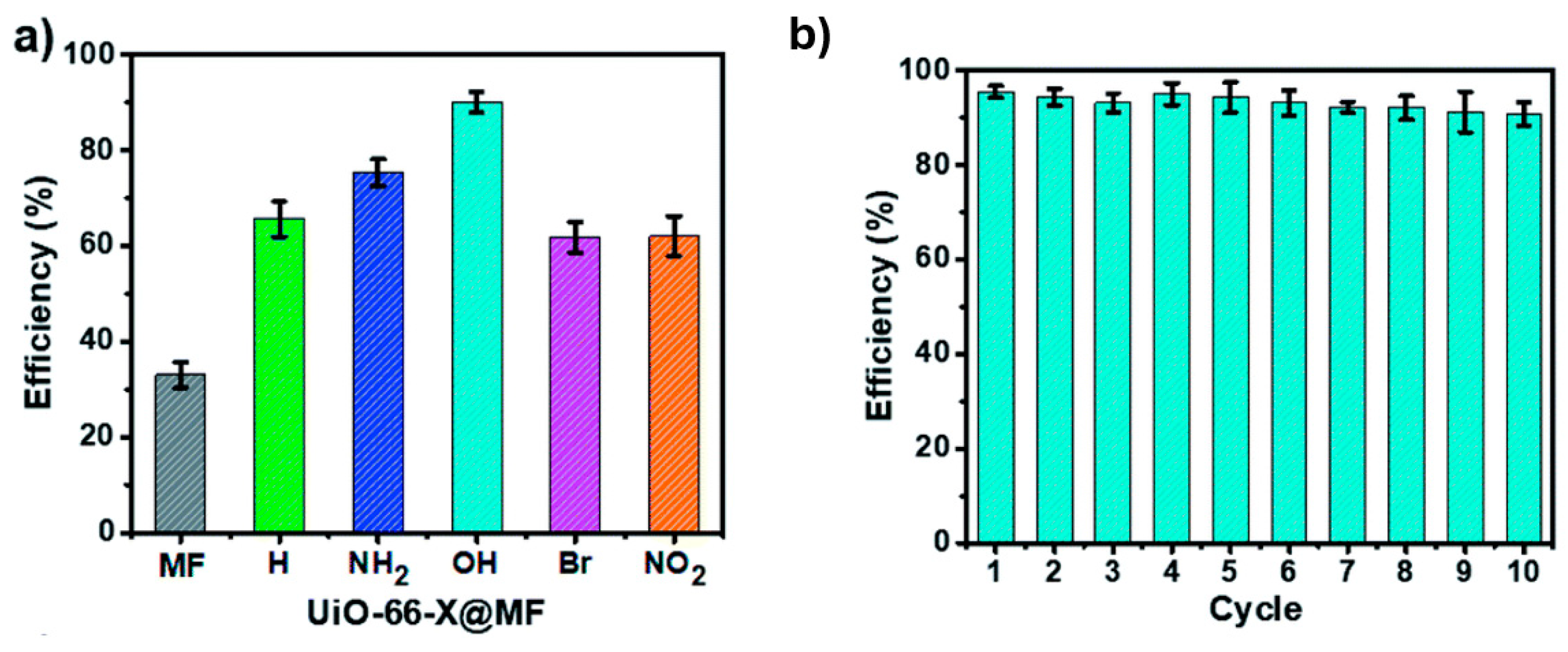
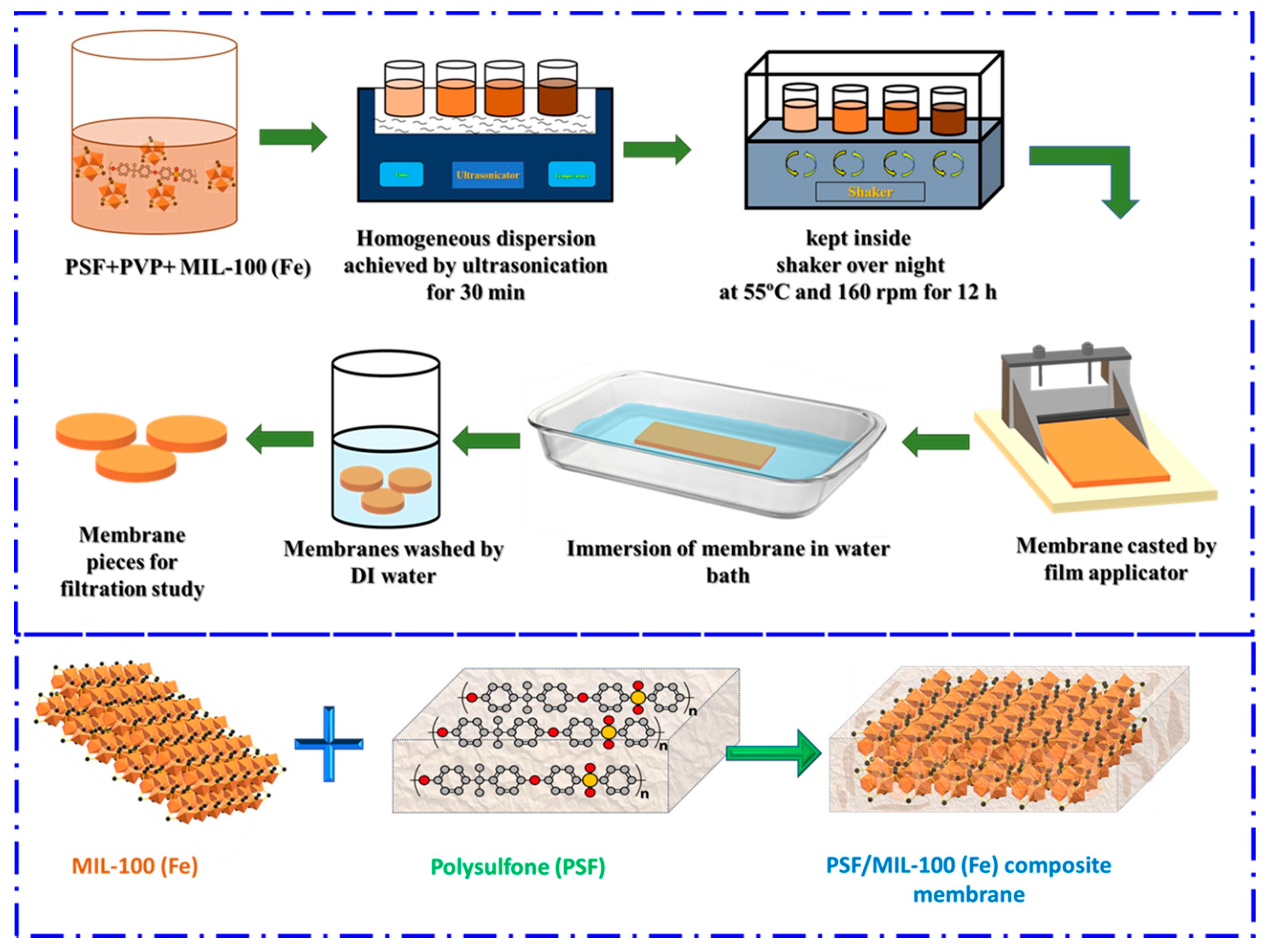
5. Use the MOFs to Adsorb Microplastics
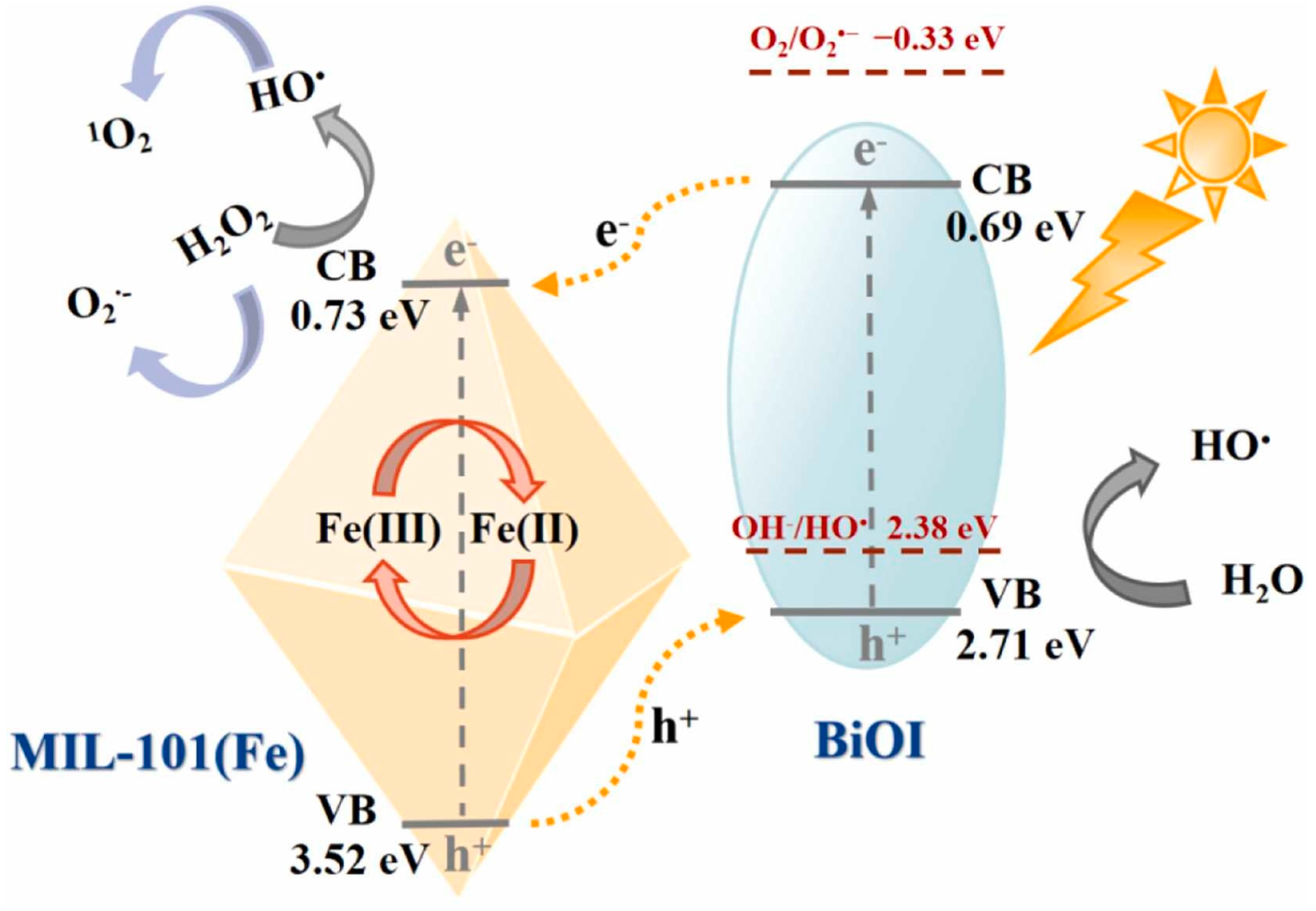
6. Degradation of Microplastics Employing MOFs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taha, Z.D.; Md Amin, R.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. Microplastics in Seawater and Zooplankton: A Case Study from Terengganu Estuary and Offshore Waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe AISBL Plastics—The Fast Facts 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 8 February 2025).
- Franzellitti, S. Editorial Overview: Plastic Pollution and Human Health: What We Know and What We Should Focus On. Curr. Opin. Toxicol. 2021, 28, 84–86. [Google Scholar] [CrossRef]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics Releasing from Personal Care and Cosmetic Products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Jemec Kokalj, A.; Horvat, P.; Skalar, T.; Kržan, A. Plastic Bag and Facial Cleanser Derived Microplastic Do Not Affect Feeding Behaviour and Energy Reserves of Terrestrial Isopods. Sci. Total Environ. 2018, 615, 761–766. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A Critical Overview of the Analytical Approaches to the Occurrence, the Fate and the Behavior of Microplastics in the Environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Ahmed, R.; Hamid, A.K.; Krebsbach, S.A.; He, J.; Wang, D. Critical Review of Microplastics Removal from the Environment. Chemosphere 2022, 293, 133557. [Google Scholar] [CrossRef]
- Casillas, G.; Hubbard, B.C.; Telfer, J.; Zarate-Bermudez, M.; Muianga, C.; Zarus, G.M.; Carroll, Y.; Ellis, A.; Hunter, C.M. Microplastics Scoping Review of Environmental and Human Exposure Data. Microplastics 2023, 2, 78–92. [Google Scholar] [CrossRef]
- Ahmad, M.; Li, J.-L.; Wang, P.-D.; Hozzein, W.N.; Li, W.-J. Environmental Perspectives of Microplastic Pollution in the Aquatic Environment: A Review. Mar. Life Sci. Technol. 2020, 2, 414–430. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Barceló, D.; Picó, Y.; Alfarhan, A.H. Microplastics: Detection in Human Samples, Cell Line Studies, and Health Impacts. Environ. Toxicol. Pharmacol. 2023, 101, 104204. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2022, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kang, J.; Lee, B.; Hong, S.; Jeon, Y.; Bak, M.; Im, S. Nonviable Carbon Neutrality with Plastic Waste-to-Energy. Energy Environ. Sci. 2023, 16, 3074–3087. [Google Scholar] [CrossRef]
- Sajid, M.; Ihsanullah, I.; Tariq Khan, M.; Baig, N. Nanomaterials-Based Adsorbents for Remediation of Microplastics and Nanoplastics in Aqueous Media: A Review. Sep. Purif. Technol. 2023, 305, 122453. [Google Scholar] [CrossRef]
- Singh, N.; Khandelwal, N.; Ganie, Z.A.; Tiwari, E.; Darbha, G.K. Eco-Friendly Magnetic Biochar: An Effective Trap for Nanoplastics of Varying Surface Functionality and Size in the Aqueous Environment. Chem. Eng. J. 2021, 418, 129405. [Google Scholar] [CrossRef]
- Yen, P.-L.; Hsu, C.-H.; Huang, M.-L.; Liao, V.H.-C. Removal of Nano-Sized Polystyrene Plastic from Aqueous Solutions Using Untreated Coffee Grounds. Chemosphere 2022, 286, 131863. [Google Scholar] [CrossRef]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Application of Zn/Al Layered Double Hydroxides for the Removal of Nano-Scale Plastic Debris from Aqueous Systems. J. Hazard. Mater. 2020, 397, 122769. [Google Scholar] [CrossRef]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (MagPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Dantas Neto, A.A.; Moura, M.C.P.A.; Castro Dantas, T.N. Use of Surfactant-Modified Adsorbents in the Removal of Microplastics from Wastewater. J. Environ. Chem. Eng. 2023, 11, 110827. [Google Scholar] [CrossRef]
- Tang, W.; Li, H.; Fei, L.; Wei, B.; Zhou, T.; Zhang, H. The Removal of Microplastics from Water by Coagulation: A Comprehensive Review. Sci. Total Environ. 2022, 851, 158224. [Google Scholar] [CrossRef]
- Tang, S.; Gao, L.; Tian, A.; Zhao, T.; Zou, D. The Coagulation Behavior and Removal Efficiency of Microplastics in Drinking Water Treatment. J. Water Process Eng. 2023, 53, 103885. [Google Scholar] [CrossRef]
- Xue, J.; Peldszus, S.; Van Dyke, M.I.; Huck, P.M. Removal of Polystyrene Microplastic Spheres by Alum-Based Coagulation-Flocculation-Sedimentation (CFS) Treatment of Surface Waters. Chem. Eng. J. 2021, 422, 130023. [Google Scholar] [CrossRef]
- Shammas, N.K.; Bennett, G.F. Principles of air flotation technology. In Flotation Technology: Volume 12; Wang, L.K., Shammas, N.K., Selke, W.A., Aulenbach, D.B., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 1–47. ISBN 978-1-60327-133-2. [Google Scholar]
- Shang, J.; Chai, M.; Zhu, Y. Photocatalytic Degradation of Polystyrene Plastic under Fluorescent Light. Environ. Sci. Technol. 2003, 37, 4494–4499. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of Microplastics from Secondary Wastewater Treatment Plant Effluent by Coagulation/Flocculation with Iron, Aluminum and Polyamine-Based Chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Bian, K.; Wang, H.; Wang, C. Is Froth Flotation a Potential Scheme for Microplastics Removal? Analysis on Flotation Kinetics and Surface Characteristics. Sci. Total Environ. 2021, 792, 148345. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Wang, C.; Wang, H. A Clean and Efficient Flotation towards Recovery of Hazardous Polyvinyl Chloride and Polycarbonate Microplastics through Selective Aluminum Coating: Process, Mechanism, and Optimization. J. Environ. Manag. 2021, 299, 113626. [Google Scholar] [CrossRef]
- Bai, W.; Wang, F.; Yan, L.; Sun, H.; Zhu, Z.; Chen, L.; Li, J.; Liang, W.; Li, A. Porous Organic Polymers (POPs) Membrane via Thiol-Yne Click Chemistry for Efficient Particulate Matter Capture and Microplastics Separation. Microporous Mesoporous Mater. 2022, 329, 111509. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Chen, Z.; Ma, B.; Chen, J.P. Ultrafiltration Membrane Fouling by Microplastics with Raw Water: Behaviors and Alleviation Methods. Chem. Eng. J. 2021, 410, 128174. [Google Scholar] [CrossRef]
- Kamaraj, P.; Vardhan Sridhar, V.; Vijaykumar Tharumasivam, S.; Parthasarathy, S.; Bupesh, G.; Kumar Raju, N.; Kumar Sahoo, U.; Nanda, A.; Saravanan, K.M. Carbon Nanoparticles Fabricated Microfilm: A Potent Filter for Microplastics Debased Water. Environ. Pollut. 2023, 336, 122502. [Google Scholar] [CrossRef]
- Acarer, S. A Review of Microplastic Removal from Water and Wastewater by Membrane Technologies. Water Sci. Technol. 2023, 88, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Conesa, J.A.; Ortuño, N. Reuse of Water Contaminated by Microplastics, the Effectiveness of Filtration Processes: A Review. Energies 2022, 15, 2432. [Google Scholar] [CrossRef]
- Sami, M.; Hedström, A.; Kvarnström, E.; Österlund, H.; Nordqvist, K.; Herrmann, I. Treatment of Greywater and Presence of Microplastics in On-Site Systems. J. Environ. Manag. 2024, 366, 121859. [Google Scholar] [CrossRef] [PubMed]
- Amirah Mohd Napi, N.N.; Ibrahim, N.; Adli Hanif, M.; Hasan, M.; Dahalan, F.A.; Syafiuddin, A.; Boopathy, R. Column-Based Removal of High Concentration Microplastics in Synthetic Wastewater Using Granular Activated Carbon. Bioengineered 2023, 14, 2276391. [Google Scholar] [CrossRef]
- Jakobs, A.; Gürkal, E.; Möller, J.N.; Löder, M.G.J.; Laforsch, C.; Lueders, T. A Novel Approach to Extract, Purify, and Fractionate Microplastics from Environmental Matrices by Isopycnic Ultracentrifugation. Sci. Total Environ. 2023, 857, 159610. [Google Scholar] [CrossRef]
- Jing, S.; Huang, Y.; Chen, Y.; He, X.; Chen, Z.; Lu, X.; Wu, M.; Wanger, T.C. Non-Destructive Extraction and Separation of Nano- and Microplastics from Environmental Samples by Density Gradient Ultracentrifugation. Anal. Chem. 2022, 94, 15280–15287. [Google Scholar] [CrossRef]
- Majcen, A.; Gohla, J.; Steinhoff, A.S.; Meißner, L.; Tassoti, S.; Spitzer, P. Fractionating Microplastics by Density Gradient Centrifugation: A Novel Approach Using LuerLock Syringes in a Low-Cost Density Gradient Maker. Chem. Teach. Int. 2024, 6, 259–267. [Google Scholar] [CrossRef]
- Vohl, S.; Kristl, M.; Stergar, J. Harnessing Magnetic Nanoparticles for the Effective Removal of Micro- and Nanoplastics: A Critical Review. Nanomaterials 2024, 14, 1179. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Aragón, D.; García-Merino, B.; Barquín, C.; Bringas, E.; Rivero, M.J.; Ortiz, I. Advanced Green Capture of Microplastics from Different Water Matrices by Surface-Modified Magnetic Nanoparticles. Sep. Purif. Technol. 2025, 354, 128813. [Google Scholar] [CrossRef]
- Martin, L.M.A.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials 2022, 12, 2348. [Google Scholar] [CrossRef] [PubMed]
- Miloloža, M.; Cvetnić, M.; Kučić Grgić, D.; Ocelić Bulatović, V.; Ukić, Š.; Rogošić, M.; Dionysiou, D.D.; Kušić, H.; Bolanča, T. Biotreatment Strategies for the Removal of Microplastics from Freshwater Systems: A Review. Environ. Chem. Lett. 2022, 20, 1377–1402. [Google Scholar] [CrossRef]
- Skariyachan, S.; Manjunatha, V.; Sultana, S.; Jois, C.; Bai, V.; Vasist, K.S. Novel Bacterial Consortia Isolated from Plastic Garbage Processing Areas Demonstrated Enhanced Degradation for Low Density Polyethylene. Environ. Sci. Pollut. Res. 2016, 23, 18307–18319. [Google Scholar] [CrossRef] [PubMed]
- Mary, O.A.; Venkateswarulu, T.C.; David, S. Bioremediation of plastics by microorganisms. Suranaree J. Sci. Technol. 2024, 31, 030204. [Google Scholar] [CrossRef]
- Swaminaathan, P.; Thamarai, P.; Yaashikaa, P.R.; Saravanan, A.; Vickram, A.S. Microbial Bioremediation of Dyes, Metals, and Microplastics for Ecological Sustainability. Energy Ecol. Environ. 2024. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, Q.; Xiao, Y.; Li, Z.; Xiong, S.; Ding, F.; He, J. Chitosan Based Aerogels with Low Shrinkage by Chemical Cross-Linking and Supramolecular Interaction. Gels 2022, 8, 131. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Wang, X.; Liu, Y.; Wu, W.; Xiao, H.; Huang, L.; Dai, H.; Zhou, X.; Bian, H. Aerogels Fabricated from Wood-Derived Functional Cellulose Nanofibrils for Highly Efficient Separation of Microplastics. ACS Sustain. Chem. Eng. 2023, 11, 13928–13938. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, S.; Wu, J.; Liu, F.; Chen, C.; Ding, B.; Zhou, X.; Deng, H. Revivable Self-Assembled Supramolecular Biomass Fibrous Framework for Efficient Microplastic Removal. Sci. Adv. 2024, 10, eadn8662. [Google Scholar] [CrossRef]
- Lv, L.; Zhou, F.; Wang, Z.; Wu, K.; Li, X.; Liao, W. The Current State and Future Opportunities of Micro- and Nano-Plastics Removal in Wastewater Treatment Plants. J. Water Process Eng. 2024, 63, 105462. [Google Scholar] [CrossRef]
- Wang, Z.; He, H.; Zhai, Y.; Chen, Y.; Xu, Z.; Wang, W. Microplastic Photoaging: A Critical Review on Occurrence, Influence Factors, Mechanism and Potential Effect. J. Clean. Prod. 2024, 464, 142783. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Sacco, N.A.; Zoppas, F.M.; Devard, A.; González Muñoz, M.d.P.; García, G.; Marchesini, F.A. Recent Advances in Microplastics Removal from Water with Special Attention Given to Photocatalytic Degradation: Review of Scientific Research. Microplastics 2023, 2, 278–303. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurišić, A.B. Photocatalytic Degradation of Different Types of Microplastics by TiOx/ZnO Tetrapod Photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef] [PubMed]
- Miranda Zoppas, F.; Sacco, N.; Soffietti, J.; Devard, A.; Akhter, F.; Marchesini, F.A. Catalytic Approaches for the Removal of Microplastics from Water: Recent Advances and Future Opportunities. Chem. Eng. J. Adv. 2023, 16, 100529. [Google Scholar] [CrossRef]
- Dos Santos, N.d.O.; Busquets, R.; Campos, L.C. Insights into the Removal of Microplastics and Microfibres by Advanced Oxidation Processes. Sci. Total Environ. 2023, 861, 160665. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Zhao, Y.; Xiang, Y.; Li, Y.; Pan, X. Effects of Advanced Oxidation Processes on Leachates and Properties of Microplastics. J. Hazard. Mater. 2021, 413, 125342. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Wang, Y.; Lv, Y.; Yang, L.; Chen, Z.; Ni, B.-J.; Chen, X. Efficient Degradation and Mineralization of Polyethylene Terephthalate Microplastics by the Synergy of Sulfate and Hydroxyl Radicals in a Heterogeneous Electro-Fenton-Activated Persulfate Oxidation System. J. Hazard. Mater. 2024, 478, 135635. [Google Scholar] [CrossRef]
- Ning, Z.; Duan, X.; Li, Y.; Zhao, X.; Chang, L. Degradation of Polyvinyl Chloride Microplastics via Electrochemical Oxidation with a CeO2–PbO2 Anode. J. Clean. Prod. 2023, 432, 139668. [Google Scholar] [CrossRef]
- Mais, L.; Melis, N.; Vacca, A.; Mascia, M. Electrochemical Removal of PET and PE Microplastics for Wastewater Treatment. Environ. Sci. 2024, 10, 399–407. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Liu, C.; Liu, X. Visible-Light-Driven Removal of Tetracycline Hydrochloride and Microplastics (HDPE) by Nano Flower Hybrid Heterojunction NH2-MIL-88B(Fe)/MoS2 via Enhanced Electron-Transfer. Sep. Purif. Technol. 2022, 302, 122138. [Google Scholar] [CrossRef]
- Piazza, V.; Uheida, A.; Gambardella, C.; Garaventa, F.; Faimali, M.; Dutta, J. Ecosafety Screening of Photo-Fenton Process for the Degradation of Microplastics in Water. Front. Mar. Sci. 2022, 8, 791431. [Google Scholar] [CrossRef]
- Torena, P.; Alvarez-Cuenca, M.; Reza, M. Biodegradation of Polyethylene Terephthalate Microplastics by Bacterial Communities from Activated Sludge. Can. J. Chem. Eng. 2021, 99, S69–S82. [Google Scholar] [CrossRef]
- Cunha, C.; Faria, M.; Nogueira, N.; Ferreira, A.; Cordeiro, N. Marine vs. Freshwater Microalgae Exopolymers as Biosolutions to Microplastics Pollution. Environ. Pollut. 2019, 249, 372–380. [Google Scholar] [CrossRef]
- Yolanda, D.M.; Anggiani, M.; Agung, M.U.K.; Anggraeni, S.R.; Afianti, N.F. Polystyrene Microplastics Degradation by Microbial Consortium from Jakarta Bay. Environ. Qual. Manag. 2024, 34, e22291. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- James, S.L. Metal-Organic Frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science (1979) 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the Structure and Function of Metal–Organic Frameworks via Linker Design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective Binding and Removal of Guests in a Microporous Metal–Organic Framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A Historical Overview of the Activation and Porosity of Metal–Organic Frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Feng, Y.; Ma, B.; Wang, Y.; Zhang, W. Mn-MOFs Derived Manganese-Based Oxide by Regulating Atmosphere as Zinc-Ion Battery Cathode. J. Mater. Sci. 2024, 59, 22166–22180. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.H.; Assadi, M.H.N. MOF-Derived Nanocrystalline ZnO with Controlled Orientation and Photocatalytic Activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef]
- Hong, Z.; Li, P.; Zou, Q.; Gu, L.; Wang, J.; Deng, L.; Wang, C.; Zhang, Y.; Li, M.; Chen, J.; et al. Metal Organic Framework (MOF-808) Incorporated Composite Polymer Electrolyte for Stable All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2024, 7, 11967–11976. [Google Scholar] [CrossRef]
- Pakizeh, M.; Bashghare, A.; Behroozi, M.; Kooshki, S. Synthesis and Characterization of MIL-101 Metal-Organic Framework and Its Application as Filler for Pebax2533 Membrane. J. Coord. Chem. 2024, 77, 2414–2439. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Lu, Y.; Azeez, N.A.; Zhang, L.; Krishnakumar, G.S.; Wang, M.-H. Exploring the Synthesis, Properties, and Potential of Chitosan-Functionalized Metal-Organic Frameworks in Emerging Applications. Prog. Mater. Sci. 2025, 148, 101387. [Google Scholar] [CrossRef]
- Borah, P.; McLeod, N.; Gupta, N.K.; Yeo, R.J.; Ghosh, T.; Aabdin, Z.; Li, L.; Bhatt, P.; Liu, Y.; Palgrave, R.; et al. Incarcerating Bismuth Nanoparticles into a Thiol-Laced Metal–Organic Framework for Electro and Photocatalysis. Mater. Horiz. 2025, 12, 1290–1302. [Google Scholar] [CrossRef]
- Sikma, R.E.; Reyes, R.A.; Richards, D.; Kotula, P.G.; Meyerson, M.L.; Schafer, D.P.; Romàn-Kustas, J.K.; Percival, S.J.; Sava Gallis, D.F. Monodisperse Cu Nanoparticles Supported on a Versatile Metal–Organic Framework for Electrocatalytic Reduction of CO2. ACS Appl. Nano Mater. 2024, 7, 26629–26635. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Guo, X.; Han, W.; Wang, S.; Zha, F.; Tian, H.; Chang, Y. CeO2/NH2-MIL-88B(Fe) Composites with Peroxidase-like Activity for Colorimetric Detection and Photo-Enzymatic Synergetic Degradation of Ciprofloxacin Hydrochloride. J. Environ. Chem. Eng. 2024, 12, 114734. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, B.; Wang, C.; Wang, Z.; Zhang, C. Green Synthesis of MIL-101/Au Composite Particles and Their Sensitivity to Raman Detection of Thiram. Chin. J. Inorg. Chem. 2024, 40, 2021–2030. [Google Scholar]
- Ghasemi, Z.; Beitollahi, H.; Garkani Nejad, F.; Dourandish, Z. MIL-101 (Fe)-NH2/Multi-Walled Carbon Nanotubes Nanocomposite Modified Glassy Carbon Electrode for Simultaneous Voltammetric Determination of Methotrexate and Calcium Folinate. Microchem. J. 2024, 207, 112252. [Google Scholar] [CrossRef]
- Loera-Serna, S.; Cortés-Suárez, J.; Sanchez-Salas, R.; Ramírez-Rosales, D.; Oliver-Tolentino, M.; Ramos-Fernández, E.V. CO2 Adsorption on a Water-Resist HKUST-1 by Incorporation of Graphene Oxide. Adsorption 2025, 31, 5. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Zikalala, S.A.; Mashiloane, K.C.; Nure, J.F.; Kebede, M.A.; Mokrani, T.; Nxumalo, E.N. Development of Engineered Zn-MOF/g-C3N4 Based Photoelectrochemical System for Real-Time Sensors and Removal of Naproxen in Wastewater. Talanta Open 2024, 10, 100371. [Google Scholar] [CrossRef]
- Sacourbaravi, R.; Ansari-Asl, Z.; Hoveizi, E.; Darabpour, E. Poly(Vinyl Alcohol)/Chitosan Hydrogel Containing Gallic Acid-Modified Fe, Cu, and Zn Metal–Organic Frameworks (MOFs): Preparation, Characterization, and Biological Applications. ACS Appl. Mater. Interfaces 2024, 16, 61609–61620. [Google Scholar] [CrossRef]
- Ali Tajwar, M.; Liu, Y.; Qi, L. Fabrication of Thermo-Responsive Polymer-MOF@cellulase Composites with Improved Catalytic Performance for Hydrolysis of Cellulose. Chem. Asian J. 2025, 20, e202400990. [Google Scholar] [CrossRef]
- Suhag, S.; Jain, U.; Chauhan, N.; Hooda, V. Cellulase Immobilization on Nano-Chitosan/Chromium Metal-Organic Framework Hybrid Matrix for Efficient Conversion of Lignocellulosic Biomass to Glucose. Prep. Biochem. Biotechnol. 2024, 1–21. [Google Scholar] [CrossRef]
- Cheng, C.; Guo, X.; Feng, Y.; Yu, J.; Huang, S.; Zhang, L.; Wu, Y.; Shao, L.; Xu, X.; Feng, L. Enhanced Activity of Enzymes Encapsulated in Spheres Metal Azolate Framework-7 with Defects. Int. J. Biol. Macromol. 2024, 283, 137689. [Google Scholar] [CrossRef]
- Cao, T.; Li, S.; Wang, X.; Sun, Y.; Luo, C. A Novel Target-Triggered Signal Chemiluminescence Kit for Thrombin Detection Based on Fusiform Au/MIL-53(Fe). Talanta 2024, 267, 125144. [Google Scholar] [CrossRef]
- Sheik, A.; Rethinasabapathy, M.; Kodiveri Muthukaliannan, G.; Safarkhani, M.; Kang, H.; Kim, D.; Alhammadi, M.; Jung, E.; Huh, Y.S. ZIF-8 Nanocarriers Synthesized by Co-Encapsulating Resveratrol and Cellulase for Biomedical Applications. Int. J. Biol. Macromol. 2024, 283, 137756. [Google Scholar] [CrossRef]
- Xing, P.; Li, C.; Chen, Y.; Zhang, R.-L. Preparation of 30%PMoV2@MOF@mSiO2 (MOF = MIL-101, HKUST-1, UiO-67, ZIF-8) Catalysts and Their Oxidative Desulfurization Performance. Mol. Catal. 2024, 569, 114613. [Google Scholar] [CrossRef]
- Chen, Z.; Shao, H.; Tang, R.; Gong, B.; Xu, B.; Ying, H.; Yang, W. Sulfide-Ion-Responsive Mesoporous Silica/ZIF-8 Nanocapsules to Inhibit Microbiologically Influenced Corrosion. ACS Appl. Nano Mater. 2023, 6, 20329–20337. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, J.; Gao, R.; Lin, D.-Y.; Wang, F.; Zhang, J. Design and Synthesis of Zeolitic Tetrazolate-Imidazolate Frameworks. Mater. Today Adv. 2021, 10, 100145. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Yue, Y.; Xiao, J.; Huang, X.; Ishag, A. Recent Advances in the Application of Zeolitic Imidazolate Frameworks (ZIFs) in Environmental Remediation: A Review. Environ. Sci. Nano 2022, 9, 4069–4092. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic Imidazolate Frameworks: Synthesis, Functionalization, and Catalytic/Adsorption Applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Tong, P.; Liang, J.; Jiang, X.; Li, J. Research Progress on Metal-Organic Framework Composites in Chemical Sensors. Crit. Rev. Anal. Chem. 2020, 50, 376–392. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Livage, C.; Egger, C.; Nogues, M.; Férey, G. Hybrid Open Frameworks (MIL-n). Part 5 Synthesis and Crystal Structure of MIL-9: A New Three-Dimensional Ferrimagnetic Cobalt(II) Carboxylate with a Two-Dimensional Array of Edge-Sharing Co Octahedra with 12-Membered Rings. J. Mater. Chem. 1998, 8, 2743–2747. [Google Scholar] [CrossRef]
- Riou-Cavellec, M.; Grenèche, J.-M.; Riou, D.; Férey, G. Hydrothermal Synthesis, Structure Determination, and Magnetic Properties of a New Layered Oxyfluorinated Iron Phosphate Templated by 1,4-Diaminobutane. Chem. Mater. 1998, 10, 2434–2439. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zeng, G.; Cheng, M.; Huang, D.; Liu, Y.; Zhou, C.; Xiong, W.; Yang, Y.; Wang, W.; et al. Materials Institute Lavoisier (MIL) Based Materials for Photocatalytic Applications. Coord. Chem. Rev. 2021, 438, 213874. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and More: Concepts, Properties and Applications for Porous Coordination Networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Larasati, L.; Dendy, D.; Lestari, W.W.; Suharbiansah, R.S.R.; Firdaus, M.; Masykur, A.; Wibowo, F.R. Rapid and Facile Electrochemical Synthesis of MIL-101(Fe)-NH2 and Its Curcumin Loading and Release Studies. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4039–4049. [Google Scholar] [CrossRef]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical Development of Simulated Body Fluids Used in Biomedical Applications: A Review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Deng, K.; Hou, Z.; Li, X.; Li, C.; Zhang, Y.; Deng, X.; Cheng, Z.; Lin, J. Aptamer-Mediated Up-Conversion Core/MOF Shell Nanocomposites for Targeted Drug Delivery and Cell Imaging. Sci. Rep. 2015, 5, 7851. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. Applications of Porous Metal–Organic Framework MIL-100(M) (M = Cr, Fe, Sc, Al, V). Cryst. Growth Des. 2018, 18, 7730–7744. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, F.; Wang, S. Adsorption and Catalytic Degradation by CoFe2O4 Coated on Metal–Organic Framework for the Removal of 4-Nitrophenol and 2,4-Dichlorophenol. Sep. Purif. Technol. 2025, 354, 129458. [Google Scholar] [CrossRef]
- Özcan, E.; Zorlu, Y. Iron-Based Metal–Organic Frameworks for Highly Efficient Photocatalytic Degradation of Reactive Orange 16 Textile Dye. ChemistrySelect 2024, 9, e202400471. [Google Scholar] [CrossRef]
- Dang Thi, M.H.; Hoang Thi, L.G.; Huynh, C.D.; Nguyen Thi, H.P.; La, D.D. La-Doped MIL-88B(Fe)–NH2: A Mixed-Metal–Organic Framework Photocatalyst for Highly Efficient Reduction of Cr(vi) in an Aqueous Solution. RSC Adv. 2024, 14, 20543–20552. [Google Scholar] [CrossRef]
- Solís, R.R.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Microwave-Assisted Synthesis of NH2-MIL-125(Ti) for the Solar Photocatalytic Degradation of Aqueous Emerging Pollutants in Batch and Continuous Tests. J. Environ. Chem. Eng. 2021, 9, 106230. [Google Scholar] [CrossRef]
- Behera, N.; Duan, J.; Jin, W.; Kitagawa, S. The Chemistry and Applications of Flexible Porous Coordination Polymers. EnergyChem 2021, 3, 100067. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Metal Cluster-Based Functional Porous Coordination Polymers. Coord. Chem. Rev. 2015, 293–294, 263–278. [Google Scholar] [CrossRef]
- Sánchez-Serratos, M.; Raziel Álvarez, J.; González-Zamora, E.; Ibarra, I.A. Porous Coordination Polymers (PCPs): New Platforms for Gas Storage. J. Mex. Chem. Soc. 2017, 60, 43–57. [Google Scholar] [CrossRef]
- Song, K.S.; Kim, D.; Polychronopoulou, K.; Coskun, A. Synthesis of Highly Porous Coordination Polymers with Open Metal Sites for Enhanced Gas Uptake and Separation. ACS Appl. Mater. Interfaces 2016, 8, 26860–26867. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Liu, H.; Wang, T.; Ye, J. Engineering Coordination Polymers for Photocatalysis. Nano Energy 2016, 22, 149–168. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Porphyrin-Based Nanomaterials for the Photocatalytic Remediation of Wastewater: Recent Advances and Perspectives. Molecules 2024, 29, 611. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO Series of Metal-Organic Frameworks Composites as Advanced Sorbents for the Removal of Heavy Metal Ions: Synthesis, Applications and Adsorption Mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Dionysiou, D.D.; Rodríguez, J.J.; Belver, C. Mixed Ti-Zr Metal-Organic-Frameworks for the Photodegradation of Acetaminophen under Solar Irradiation. Appl. Catal. B 2019, 253, 253–262. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Gómez-Avilés, A.; Zhang, S.; Rodriguez, J.J.; Bedia, J.; Belver, C. Metronidazole Photodegradation under Solar Light with UiO-66-NH2 Photocatalyst: Mechanisms, Pathway, and Toxicity Assessment. J. Environ. Chem. Eng. 2023, 11, 109744. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Guo, C.; Zhou, Y. Fabrication of a Direct Z-Scheme Heterojunction of UiO-66-NH2 and Tubular g-C3N4 for the Stable Photocatalytic Reduction of CO2 to CO and CH4. Catal. Sci. Technol. 2024, 14, 5938–5948. [Google Scholar] [CrossRef]
- Meng, Y.; Yue, F.; Zhang, S.; Zhang, L.; Li, C.; Shi, M.; Ma, Y.; Berrettoni, M.; Zhang, X.; Zhang, H. Zr-MOF/MXene Composite for Enhanced Photothermal Catalytic CO2 Reduction in Atmospheric and Industrial Flue Gas Streams. Carbon. Capture Sci. Technol. 2024, 13, 100274. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science (1979) 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Ahnfeldt, T.; Guillou, N.; Gunzelmann, D.; Margiolaki, I.; Loiseau, T.; Férey, G.; Senker, J.; Stock, N. [Al4(OH)2(OCH3)4(H2N-Bdc)3] x XH(2)O: A 12-Connected Porous Metal-Organic Framework with an Unprecedented Aluminum-Containing Brick. Angew. Chem. Int. Ed. 2009, 48, 5163–5166. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal–Organic Frameworks Meet Scalable and Sustainable Synthesis. Green. Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of Metal-Organic Frameworks: A Mini Review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.-C. Recent Progress in the Synthesis of Metal–Organic Frameworks. Sci. Technol. Adv. Mater. 2015, 16, 054202. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Han, Z.; Yang, Y.; Rushlow, J.; Huo, J.; Liu, Z.; Hsu, Y.-C.; Yin, R.; Wang, M.; Liang, R.; Wang, K.-Y.; et al. Development of the Design and Synthesis of Metal–Organic Frameworks (MOFs)—From Large Scale Attempts, Functional Oriented Modifications, to Artificial Intelligence (AI) Predictions. Chem. Soc. Rev. 2025, 54, 367–395. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional Metal–Organic Frameworks: From Academia to Industrial Applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef]
- Letwaba, J.; Uyor, U.O.; Mavhungu, M.L.; Achuka, N.O.; Popoola, P.A. A Review on MOFs Synthesis and Effect of Their Structural Characteristics for Hydrogen Adsorption. RSC Adv. 2024, 14, 14233–14253. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, K.; Li, Y. Greening the Processes of Metal–Organic Framework Synthesis and Their Use in Sustainable Catalysis. ChemSusChem 2017, 10, 3165–3187. [Google Scholar] [CrossRef] [PubMed]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline Metal-Organic Frameworks (MOFs): Synthesis, Structure and Function. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 3–10. [Google Scholar] [CrossRef]
- Martinez Joaristi, A.; Juan-Alcañiz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Electrochemical Synthesis of Some Archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–Organic Frameworks—Prospective Industrial Applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Li, W.-J.; Tu, M.; Cao, R.; Fischer, R.A. Metal–Organic Framework Thin Films: Electrochemical Fabrication Techniques and Corresponding Applications & Perspectives. J. Mater. Chem. A Mater. 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Al-Kutubi, H.; Gascon, J.; Sudhölter, E.J.R.; Rassaei, L. Electrosynthesis of Metal–Organic Frameworks: Challenges and Opportunities. ChemElectroChem 2015, 2, 462–474. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. (Regul. Ed.) 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Le, V.N.; Kwon, H.T.; Vo, T.K.; Kim, J.-H.; Kim, W.-S.; Kim, J. Microwave-Assisted Continuous Flow Synthesis of Mesoporous Metal-Organic Framework MIL-100 (Fe) and Its Application to Cu(I)-Loaded Adsorbent for CO/CO2 Separation. Mater. Chem. Phys. 2020, 253, 123278. [Google Scholar] [CrossRef]
- Quang, H.T.; Le Pham, H.A.; Van Cuong, N.; Dang, H.P.; Anh, N.T.H. Rapid Synthesis of Bismuth MOF@Carbon Nanotube Composite by Microwave-Assisted Solvothermal for Photodegrading RhB. Top. Catal. 2024, 67, 1155–1168. [Google Scholar] [CrossRef]
- Moradi, E.; Rahimi, R.; Safarifard, V. Sonochemically Synthesized Microporous Metal–Organic Framework Representing Unique Selectivity for Detection of Fe3+ Ions. Polyhedron 2019, 159, 251–258. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile Synthesis of Nanocrystals of a Microporous Metal–Organic Framework by an Ultrasonic Method and Selective Sensing of Organoamines. Chem. Commun. 2008, 31, 3642–3644. [Google Scholar] [CrossRef] [PubMed]
- Nurbayti, S.; Adawiah, A.; Bale, U.F.; Fadhilla, R.; Ramadhan, F.N.; Zulys, A.; Sukandar, D.; Saridewi, N.; Tulhusna, L. Sonochemical Assisted Synthesis of Cr-PTC Metal Organic Framework, ZnO, and Fe3O4 Composite and Their Photocatalytic Activity in Methylene Blue Degradation. Bull. Chem. React. Eng. Catal. 2024, 19, 318–326. [Google Scholar] [CrossRef]
- Abedi, S.; Tehrani, A.A.; Morsali, A. Mechanochemical Synthesis of Isoreticular Metal–Organic Frameworks and Comparative Study of Their Potential for Nitrobenzene Sensing. New J. Chem. 2015, 39, 5108–5111. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, J.; Hou, S.; Chen, R.; Wang, Z.; Kabtamu, D.M.; Zelekew, O.A.; Li, F. Room-Temperature Synthesis of a Zr–UiO-66 Metal–Organic Framework via Mechanochemical Pretreatment for the Rapid Removal of EDTA-Chelated Copper from Water. Dalton Trans. 2024, 53, 14098–14107. [Google Scholar] [CrossRef]
- Duan, J.-J.; Xin, X.; Guo, S.-J.; Wang, S.-L.; Chen, H.; Li, J.-L.; Qin, S.; Tao, G.-H. Regulating Defected Zirconium Metal–Organic Frameworks in Ionic Liquid for Sewage Treatment. J. Mol. Struct. 2023, 1286, 135607. [Google Scholar] [CrossRef]
- Yu, X.; Dutta, S.; Andreo, J.; Wuttke, S. Identifying Synthetic Variables Influencing the Reproducible Microfluidic Synthesis of ZIF Nano- and Micro-Particles. Commun. Mater. 2024, 5, 210. [Google Scholar] [CrossRef]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of Organic Pollutants from Aqueous Solution Using Metal Organic Frameworks (MOFs)-Based Adsorbents: A Review. Chemosphere 2021, 284, 131393. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Metawea, A.; Hammoud, M.; Ahmad, M.N.; Walker, G. Study of Process Parameters That Enable Direct Spray Drying Synthesis of UiO-66-NH2. Microporous Mesoporous Mater. 2024, 372, 113114. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Frameworks as Efficient Adsorbents for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, Y.; Miao, C.; Wang, Y.-R.; Gao, G.-K.; Yang, R.-X.; Zhu, H.-J.; Wang, J.-H.; Li, S.-L.; Lan, Y.-Q. Metal–Organic Framework-Based Foams for Efficient Microplastics Removal. J. Mater. Chem. A 2020, 8, 14644–14652. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Zeng, Y.; Li, H.; Hu, C.; Wang, C. Synthesis of a Novel Microplastic Trap with Abundant Oxime Groups Based on MOF-545 Post-Engineering for the Environmental Pollution Control and Water Remediation. J. Clean. Prod. 2023, 430, 139678. [Google Scholar] [CrossRef]
- Wan, H.; Wang, J.; Sheng, X.; Yan, J.; Zhang, W.; Xu, Y. Removal of Polystyrene Microplastics from Aqueous Solution Using the Metal–Organic Framework Material of ZIF-67. Toxics 2022, 10, 70. [Google Scholar] [CrossRef]
- Modak, S.; Kasula, M.; Esfahani, M.R. Nanoplastics Removal from Water Using Metal–Organic Framework: Investigation of Adsorption Mechanisms, Kinetics, and Effective Environmental Parameters. ACS Appl. Eng. Mater. 2023, 1, 744–755. [Google Scholar] [CrossRef]
- Pasanen, F.; Fuller, R.O.; Maya, F. Fast and Simultaneous Removal of Microplastics and Plastic-Derived Endocrine Disruptors Using a Magnetic ZIF-8 Nanocomposite. Chem. Eng. J. 2023, 455, 140405. [Google Scholar] [CrossRef]
- Haris, M.; Khan, M.W.; Zavabeti, A.; Mahmood, N.; Eshtiaghi, N. Self-Assembly of C@FeO Nanopillars on 2D-MOF for Simultaneous Removal of Microplastic and Dissolved Contaminants from Water. Chem. Eng. J. 2023, 455, 140390. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Chen, Z.; Xie, Y.; Yu, H.; Yuan, S.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Rapid Magnetization and Removal of Microplastics from Environment and Food Based on Magnetic Metal-Organic Framework Fe3O4@SiO2@MIL-53(Al). Environ. Sci. Pollut. Res. 2023, 30, 117373–117389. [Google Scholar] [CrossRef]
- You, D.; Zhao, Y.; Yang, W.; Pan, Q.; Li, J. Metal-Organic Framework-Based Wood Aerogel for Effective Removal of Micro/Nano Plastics. Chem. Res. Chin. Univ. 2022, 38, 186–191. [Google Scholar] [CrossRef]
- Peng, S.; Ji, X.; Dong, H.; Guo, Z.; Ma, H.; Han, R.; Pang, K.; Chen, X.; Wang, X. Designing MOFs/CuCo-LDHs Hybrid-Based Superhydrophobic Sponge for Speedy Eliminating Microplastics, Dyes, and Oils from High-Salinity Water. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133996. [Google Scholar] [CrossRef]
- Shahabi, M.; Ahmadpour, A.; Raissi, H. Assessment of Water Purification by IRMOF-1 Based on RGO as a New Nanoengineered Adsorbent: Insights from Adsorption Mechanism. J. Mol. Liq. 2024, 400, 124485. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-Organic Framework Membranes for Wastewater Treatment and Water Regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of Metal Organic Framework in Wastewater Treatment. Green. Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Mohana, A.A.; Rahman, M.; Sarker, S.K.; Haque, N.; Gao, L.; Pramanik, B.K. Nano/Microplastics: Fragmentation, Interaction with Co-Existing Pollutants and Their Removal from Wastewater Using Membrane Processes. Chemosphere 2022, 309, 136682. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Ji, X.; Han, R.; Pang, K.; Yang, Z.; Liu, Z.; Peng, S. Engineering Green MOF-Based Superhydrophobic Sponge for Efficiently Synchronous Removal of Microplastics and Pesticides from High-Salinity Water. Water Res. 2023, 243, 120314. [Google Scholar] [CrossRef]
- Gnanasekaran, G.; Arthanareeswaran, G.; Mok, Y.S. A High-Flux Metal-Organic Framework Membrane (PSF/MIL-100 (Fe)) for the Removal of Microplastics Adsorbing Dye Contaminants from Textile Wastewater. Sep. Purif. Technol. 2021, 277, 119655. [Google Scholar] [CrossRef]
- Peng, S.; Ma, H.; Hao, X.; Han, R.; Ji, X.; Wang, L.; Fang, Y.; Pang, K.; Il-Ho, K.; Chen, X. Constructing Green Superhydrophilic and Superoleophobic COFs-MOFs Hybrid-Based Membrane for Efficiently Emulsion Separation and Synchronous Removal of Microplastics, Dyes, and Pesticides. Environ. Res. 2024, 243, 117777. [Google Scholar] [CrossRef]
- Han, L.; Ma, J.; Lin, H.; Chen, C.; Teng, J.; Li, B.; Zhao, D.; Xu, Y.; Yu, W.; Shen, L. A Novel Flower-like Nickel-Metal-Organic Framework (Ni-MOF) Membrane for Efficient Multi-Component Pollutants Removal by Gravity. Chem. Eng. J. 2023, 470, 144311. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, P.; Yang, Y.; Hall, T.; Nie, G.; Yao, Y.; Duan, X.; Wang, S. Degradation of Microplastics by a Thermal Fenton Reaction. ACS EST Eng. 2022, 2, 110–120. [Google Scholar] [CrossRef]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s Reagent for the Rapid and Efficient Isolation of Microplastics from Wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, T.; Zhang, Q.; Wang, T.; Hou, X. Rapid Microwave Synthesis of Fe3O4-PVP@ZIF-67 as Highly Effective Peroxymonosulfate Catalyst for Degradation of Bisphenol F and Its Mechanism Analysis. Chem. Eng. J. 2021, 404, 126453. [Google Scholar] [CrossRef]
- Gu, X.; Li, L.; Wu, Y.; Dong, W. Enhancement of Microplastics Degradation with MIL-101 Modified BiOI Photocatalyst under Light and Dark Alternated System. J. Environ. Chem. Eng. 2024, 12, 112958. [Google Scholar] [CrossRef]
- Rincon, I.; Hidalgo, T.; Armani, G.; Rojas, S.; Horcajada, P. Enzyme_Metal-Organic Framework Composites as Novel Approach for Microplastic Degradation. ChemSusChem 2024, 17, e202301350. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Chen, Q.; Li, X.; Li, Y.; Shao, W.; Xu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Photocatalytic Conversion of Waste Plastics into C2 Fuels under Simulated Natural Environment Conditions. Angew. Chem. Int. Ed. 2020, 59, 15497–15501. [Google Scholar] [CrossRef]
- Jiang, Y.; Heinke, L. Photoswitchable Metal–Organic Framework Thin Films: From Spectroscopy to Remote-Controllable Membrane Separation and Switchable Conduction. Langmuir 2021, 37, 2–15. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Zanaty, M.; Zaki, A.H.; El-Dek, S.I.; Abdelhamid, H.N. Zeolitic Imidazolate Framework@hydrogen Titanate Nanotubes for Efficient Adsorption and Catalytic Oxidation of Organic Dyes and Microplastics. J. Environ. Chem. Eng. 2024, 12, 112547. [Google Scholar] [CrossRef]
- Martic, S.; Tabobondung, M.; Gao, S.; Lewis, T. Emerging Electrochemical Tools for Microplastics Remediation and Sensing. Front. Sens. 2022, 3, 958633. [Google Scholar] [CrossRef]
- Du, H.; Wang, Q.; Chen, G.; Wang, J. Photo/Electro-Catalytic Degradation of Micro- and Nano-Plastics by Nanomaterials and Corresponding Degradation Mechanism. TrAC Trends Anal. Chem. 2022, 157, 116815. [Google Scholar] [CrossRef]
- Rajput, H.; Kwon, E.E.; Younis, S.A.; Weon, S.; Jeon, T.H.; Choi, W.; Kim, K.-H. Photoelectrocatalysis as a High-Efficiency Platform for Pulping Wastewater Treatment and Energy Production. Chem. Eng. J. 2021, 412, 128612. [Google Scholar] [CrossRef]
- Kiendrebeogo, M.; Karimi Estahbanati, M.R.; Khosravanipour Mostafazadeh, A.; Drogui, P.; Tyagi, R.D. Treatment of Microplastics in Water by Anodic Oxidation: A Case Study for Polystyrene. Environ. Pollut. 2021, 269, 116168. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xia, Y. Excavating the Potential of Photo- and Electroupcycling Platforms Toward a Sustainable Future for Waste Plastics. Small Sci. 2024, 4, 2300096. [Google Scholar] [CrossRef]
- Qin, J.; Dou, Y.; Wu, F.; Yao, Y.; Andersen, H.R.; Hélix-Nielsen, C.; Lim, S.Y.; Zhang, W. In-Situ Formation of Ag2O in Metal-Organic Framework for Light-Driven Upcycling of Microplastics Coupled with Hydrogen Production. Appl. Catal. B 2022, 319, 121940. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinho, T.C.; Gomez-Aviles, A.; Herrasti, P. Metal–Organic Frameworks (MOFs) for Adsorption and Degradation of Microplastics. Microplastics 2025, 4, 11. https://doi.org/10.3390/microplastics4010011
Marinho TC, Gomez-Aviles A, Herrasti P. Metal–Organic Frameworks (MOFs) for Adsorption and Degradation of Microplastics. Microplastics. 2025; 4(1):11. https://doi.org/10.3390/microplastics4010011
Chicago/Turabian StyleMarinho, Thayna Campeol, Almudena Gomez-Aviles, and Pilar Herrasti. 2025. "Metal–Organic Frameworks (MOFs) for Adsorption and Degradation of Microplastics" Microplastics 4, no. 1: 11. https://doi.org/10.3390/microplastics4010011
APA StyleMarinho, T. C., Gomez-Aviles, A., & Herrasti, P. (2025). Metal–Organic Frameworks (MOFs) for Adsorption and Degradation of Microplastics. Microplastics, 4(1), 11. https://doi.org/10.3390/microplastics4010011





